Abstract
Temperature-sensitive transient receptor potential ion channels (thermoTRPs) expressed in epidermal keratinocytes and sensory afferents play an important role as peripheral pain detectors for our body. Many natural and synthetic compounds have been found to act on the thermoTRPs leading to altered nociception, but little is known about endogenous painful molecules activating TRPV3. Here, we show that farnesyl pyrophosphate (FPP), an intermediate metabolite in the mevalonate pathway, specifically activates TRPV3 among six thermoTRPs using Ca2+ imaging and electrophysiology with cultured keratinocytes and TRPV3-overexpressing cells. Agonistic potencies of related compounds in the FPP metabolism were ignorable. Voltage-dependence of TRPV3 was shifted by FPP, which appears to be the activation mechanism. An intraplantar injection of FPP acutely elicits nociceptive behaviors in inflamed animals, indicating that FPP is a novel endogenous pain-producing substance via TRPV3 activation. Co-culture experiments demonstrated that this FPP-evoked signal in the keratinocytes is transmitted to sensory neurons. In addition, FPP reduced TRPV3 heat threshold resulting in heightened behavioral sensitivity to noxious heat. Taken together, our data suggest that FPP is the firstly identified endogenous TRPV3 activator that causes nociception. Our results may provide useful chemical information to elucidate TRPV3 physiology and novel pain-related metabolisms.
Keywords: Drug Action, Neuron, Neurotransmitters, Skin, TRP Channels, TRPV3, Endogenous Activator, Farnesyl Pyrophosphate, Pain
Introduction
Skin keratinocytes and sensory neurons express sensor ion channels that detect changes in the external or internal environments. TRPV3 channel has been found to be expressed in the skin keratinocytes of mice and humans and in the sensory neurons of humans and is activated by temperatures exceeding 33 °C (1–3). Studies using TRPV3-knock-out mice or TRPV3-overexpressing transgenic mice have demonstrated that TRPV3 is involved in behavioral responses to innocuous and noxious heat (4–5).
Searching for pharmacological tools to modulate TRPV3 activity seems active (see review, Ref. 6). Several phytosubstances and synthetic compounds, such as camphor, menthol, carvacrol, citral, incensole acetate, and 2-aminoethoxydiphenyl borate (2-APB)2 are shown to activate TRPV3 (4, 7–13). It has been reported that some of endogenous molecules are able to modulate TRPV3 activation. For example, unsaturated fatty acids potentiated TRPV3 activation by 2-APB (14). S-Nitrosylation of the channel protein activates TRPV3, as well as other TRPVs (15). Ca2+ and calmodulin suppressed the sensitization of TRPV3 to recurrent stimuli (16). However, little is known about endogenous compounds that are able to directly and specifically activate TRPV3.
A variety of metabolites are formed in the human body as a result of the diverse biochemical processes and some of the metabolites are potentially involved in pain development (17). Farnesyl pyrophosphate (FPP) is an intermediate molecule in the cholesterol synthesis pathway. In the present study, we examined whether FPP is an activator for TRPV3. We performed Ca2+ imaging and patch clamp experiments with TRPV3 and other related thermoTRPs for the pharmacological characterization of the FPP action. Animal behavioral studies were also carried out to test whether FPP elicits pain via a TRPV3-mediated mechanism.
EXPERIMENTAL PROCEDURES
Cell Cultures
HEK293T cells were cultured as previously (18–19). Briefly, the cells were maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin. The HEK293T cells were transiently transfected with 3 μg of individual TRP channel plasmid DNA (hTRPA1, rTRPV1, rTRPV2, or mTRPV4 in pcDNA3.1; hTRPV3 or mTRPM8 in PCDNA5/FRT) per 35-mm dish using Fugene HD (Roche Diagnostics Corp., Indianapolis, IN). The human keratinocyte cell line HaCaT was maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin. In vitro transfection of HaCaT keratinocytes with 8 μg of small hairpin RNA (shRNA) was performed according to the above protocols for the HEK293T cells. Normal human epidermal keratinocytes in the first passage were purchased from Cambrex Bioscience Walkersville, Inc. (Walkersville, MD). The keratinocytes were maintained in serum-free keratinocyte growth medium (KGM, Cambrex Bioscience Walkersville, Inc.). All the experiments were conducted using the 3–5 times passed keratinocytes. Mouse dorsal root ganglion (DRG) neurons were cultured. Briefly, DRG was dissected out of adult ICR mice in cold phosphate-buffered saline and treated with 1.5 mg/ml collagenase/dispase (Roche Diagnostics Corp.) at 37 °C for 45 min and were then treated with 0.25% trypsin (Invitrogen, Carlsbad, CA) for 15 min. Neurons were then plated onto poly-l-lysine-coated glass cover slips in DMEM/F12 containing 10% FBS, 1% penicillin/streptomycin, and 5–50 ng/ml 2.5S NGF (Invitrogen). Experiments with DRG neurons were performed 48–72 h after plating. HaCaT cells and mouse DRG neurons were co-cultured in serum-free keratinocyte growth medium (KGM, Cambrex Bioscience, Walkersville Inc.) with 2.5 S NGF for some experiments. All cells were grown at 37 °C and 5% CO2. DRG neuron and keratinocyte co-cultures were stained with rabbit polyclonal anti-TRPV3 (Alomon Labs, Jerusalem, Israel) for TRPV3-expressing cells, and Hoechst 33258 (Molecular Probes, Eugene, OR) for nuclei. The secondary antibody for the anti-TRPV3 was Alexa Fluor 488 anti-rabbit IgG (Molecular Probes). All cultures were performed at least in triplicate for Ca2+ imaging and electrophysiology experiments.
Ca2+ Imaging Experiments
Ca2+ imaging experiments were carried out as described previously (20). Briefly, HEK293T cells were plated onto poly-l-lysine-coated 35-mm glass coverslips and used for Fluo-3 Ca2+ imaging 16–48 h later. The bath solution was composed of (in mm) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, titrated to pH 7.4 with NaOH. Cells were loaded with Fluo-3AM (5 μm, at 37 °C for 1 h) in bath solution containing 0.02% pluronic acid (Invitrogen). The imaging experiments were performed with a confocal microscope (LSM5 Pascal, Carl Zeiss, Germany) and images at 488 nm excitation/514 nm emission were collected every 3 s using Carl Zeiss ratio tool software. Keratinocytes were loaded with 5 μm Fura-2AM for 30 min, and the cells were resuspended in (in mm) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, titrated to pH 7.4 with NaOH. Images of Fura-2 loaded cells with the excitation wavelength alternating between 340 nm and 380 nm were captured with a cooled CCD camera (Retiga-SRV, Q-imaging Corp., Burnaby, BC, Canada). The ratio of fluorescence intensity of the two wavelengths in each experiment was analyzed using MetaFluor (Molecular Devices, Sunnyvale, CA). Values from different experiments were normalized to baseline of the ratio 340/380 nm.
Patch-clamp Electrophysiology
Whole-cell voltage clamp recordings were performed as described previously (18). Briefly, the bath solution contained (in mm) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, titrated to pH 7.4 with NaOH. The pipette solution contained (in mm) 140 CsCl, 5 EGTA, 10 HEPES, 2 MgATP, 0.2 NaGTP titrated to pH 7.2 with CsOH. The holding potential was −60 mV and for the current-voltage analysis, 800-ms voltage-ramp pulses from −80 to +80 mV were used. For whole cell voltage-dependence analysis, a step protocol composed of 32 episodes of 800-ms step pulses with a 10-mV increment starting from −120 mV was used. Each step pulse was followed by a 400-ms invariant step to −120 mV. The peak inward tail currents at −120 mV were collected for each step, normalized to the maximum tail current, and fitted using the Boltzmann equation. For single channel recordings with cell-attached and inside-out configurations, the whole cell bath solution was used as the pipette solution and the holding potential was −60 mV. For the experiments for the in vitro channel activation by heat, the solution temperature of the bath chamber was controlled and monitored using a digital temperature controller (CL-100, Warner Instruments, Hamden, CT). Heat activation thresholds were obtained from Q10 (increase in reaction rate per 10 °C increase in temperature) analysis in which the intersection temperature point of the nonspecific reaction phase and the steeply increasing phase is referred to as the temperature threshold (21).
Behavioral Studies
The study was performed in accordance with protocols approved by the University Committee on Laboratory Animals. Hind paw licking and lifting behavior was quantitated every minute for 20 min as previously described (4, 18). 6-week-old male ICR mice and TRPV1-knock-out mice (generously provided by Dr. Uhtaek Oh) were used. Animals were acclimated for 1 h to the test environment prior to performing the experiments. For inflammation, 2% carrageenan in 10 μl was injected into a hind paw 3 h prior to the FPP injection. Drugs in 10-μl vehicle (phosphate-buffered saline containing 0.5% Tween 80) were injected into mice hind paws intradermally at the doses detailed in the results. For shRNA administration, 10 μg of shRNA in 20 μl of saline with 20 μl EzWayTM transfection reagent (Komabiotech, Korea) were injected into a hind paw 48 h prior to the FPP injection. Hargreaves assay apparatus (Plantar Analgesia meter, for noxious heat sensitivity) were from UGO Basile (Italy) and the assay for changes in thermal behaviors was performed as described previously (4, 22). The tests were performed under unconstrained condition. Baseline responses were measured 5 min prior to FPP administration. Paw withdrawal latencies of the contralateral hind paws were also measured both before and after drug injection. Data were analyzed using the two-tailed Student's t test (***, p < 0.001; **, p < 0.01; *, p < 0.05) and shown as means ± S.E. For the comparison of the accumulating licking/lifting time or flinch numbers, one-way analysis of variance (ANOVA) with Bonferroni post hoc test was performed and for the comparison of the data at each time point, the two-tailed Student's t test was performed.
Analyses of TRPV3 mRNA and Protein Expression
To examine the epidermal expression of TRPV3, cultured human keratinocytes or epidermal keratinocytes that were collected from mice hind paws were used. For collecting the mice hind paw keratinocytes, ∼0.2 cm2 pieces of the plantar skin epidermal layers were isolated from sacrificed mice and the cells were lysed in radioimmune precipitation assay cell lysis buffer (Elpis Biotech, Korea) containing 1 mm phenylmethylsulfonyl fluoride on ice. The cell lysate was centrifuged at 15,000 × g for 15 min and was followed by SDS-PAGE for Western blotting (Bio-Rad). The transferred membranes were probed with rabbit polyclonal anti-TRPV3 antibodies (1:300, Alomon Labs, Israel) and with rabbit polyclonal anti-β-actin (1:1000, Abcam, Cambridge, UK), followed by horseradish peroxidase-conjugated goat anti-rabbit antibodies (1:2000, Abcam) before visualizing the proteins by ECL system (GE Healthcare, Buckinghamshire, UK). The extraction of total RNA from isolated keratinocytes was carried out using RNA preparation kit (Axygen, Union City, CA). Reverse transcriptase-PCR (RT-PCR) was performed using a PCR thermal cycler (Takara, Japan). Reverse transcription was performed using the amfiRivert single-step RT-PCR kit (GenDEPOT, Barker, TX) according to the manufacturer's protocol. PCR primers were as follows: mTRPV3, 5′-CCCCATCCTCTTTCTCTTCC-3′ and 5′-CGACGTTTCTGGGAATTCAT-3′; hTRPV3, 5′-CCTCGTGCTCAGTGCTCAGT-3′ and 5′-CTGGCAGGAGGAAAGACTCC-3′. The PCR products were electrophoresed on agarose gels and stained with ethidium bromide.
Compounds
All chemicals were purchased from Sigma-Aldrich unless otherwise described. FPP, farnesol, geranyl pyrophosphate (GPP), and geranylgeranyl pyrophosphate (GGPP) were purchased from Biomol (Plymouth Meeting). Isopentenyl pyrophosphate (IPP) was purchased from Echelon Research Laboratories (Salt Lake City, UT). mTRPV3-shRNA (5′-CCGGGCTGGAAATCATCGTCTACAACTCGAGTTGTAGACGATGATTTCCAGCTTTTTG-3′) and hTRPV3-shRNA (5′-CCGGGCTGCATATGAAGTGGAAGAACTCGAGTTCTTCCACTTCATATGCAGCTTTTTG-3′) were purchased from Open Biosystems (Huntsville, AL). A non-target scrambled shRNA (5′-CCTAAGGTTAAGTCGCCCTCGCTCTAGCGAGGGCGACTTAACCTTAGG-3′) was purchased from Sigma-Aldrich. Stock solutions were made using water or ethanol and were diluted with test solutions before use.
RESULTS
FPP Activates TRPV3 in HEK293T Cells
Fluo-3 intracellular Ca2+ imaging experiments were performed with TRPV3-expressing HEK293T cells to examine whether FPP activates TRPV3. As soon as 100 nm FPP was applied to the bath, increases in the intracellular Ca2+ levels were detected (Fig. 1A). To test if the same cells expressed TRPV3, camphor, a TRPV3 agonist was added subsequently and all of the FPP-sensitive cells showed Ca2+ influx response to camphor (Fig. 1A). FPP failed to evoke such a response from untransfected HEK293T cells (data not shown, n = 45). In whole-cell voltage-clamp experiments, 1 μm FPP application also elicited rapid current responses in the TRPV3-expressing HEK cells (Fig. 1B). The current in response to FPP was outwardly rectifying as that in response to camphor, and was obviously blunted by addition of 20 μm ruthenium red (RR), a TRP channel blocker (Fig. 1, B and C), which indicates that the FPP-evoked current was mediated by TRPV3 activation. Because phosphorylated intermediates in the mevalonate pathway (3-hydroxy-3-methyl-glutaryl-coenzyme reductase pathway) hardly cross the plasma membrane (23), the binding site of FPP would be located in the extracellular side of the channel according to the above results with extracellular drug application methods. To further confirm whether FPP acts on the extracellular side of the TRPV3 channel, we carried out single channel patch recordings with cell-attached and inside-out configurations. In the cell-attached patches, single channel currents relevant to the TRPV3 conductance were detected as soon as the seal formation when the recording pipette contained 300 nm FPP (Fig. 1, D, upper trace and E). On the other hand, with the recording pipette free from FPP, delayed onset of the single channel activation with low open probability was observed in the inside-out patch when FPP was applied to the bath (intracellular side of the channel) (Fig. 1, D, lower trace and E). These data indicate that FPP binds to the extracellular side of TRPV3 and activate this channel. The results from the whole cell and inside-out experiments also suggest that FPP unlikely acts as a donor for farnesylation of TRPV3 proteins for activating the channel because cytosolic enzymes are diluted or removed in the two experiments.
FIGURE 1.
TRPV3 is activated by FPP. A, 100 nm FPP elevated intracellular Ca2+ levels in hTRPV3-transfected HEK293T cells (n = 75) in the Fluo-3 Ca2+ imaging experiments. A camphor response was also shown by the same cells. Responses during all Ca2+-imaging experiments are displayed as means ± S.E. B, 1 μm FPP evoked an outwardly rectifying current increase in the whole cell voltage clamp study (n = 8). The current was inhibited by co-application of the thermoTRP blocker, RR (20 μm). C, current-voltage curves obtained from the trace in B (points labeled with letters) were displayed. D, 300 nm FPP in the recording pipette evoked the immediate response of single channel current in the cell-attached patch of the hTRPV3-transfected HEK293T cells (n = 24, upper trace). In the inside-out patch, intracellular 300 nm FPP (applied to the bath) evoked delayed and smaller single channel responses (n = 11, lower trace). It took 1 min 19 s ±11 s on average for the current responses to occur in the inside-out patches (n = 11). E, summary of the mean open probabilities (NPo) of single channel TRPV3 activation at cell-attached (C/A) and inside-out (I/O) configurations. The mean values of C/A (0–30 s) (for 30 s from the patch formation), I/O (0–30 s) (for 30 s from the start of FPP application), I/O (70–100 s) (for 30 s before the end of FPP application), and camphor (during camphor application at I/O) were demonstrated. The NPo value of C/A is significantly higher than those of I/O during FPP application (NPoC/A(0–30s) = 1.84 ± 0.48, NPoI/O(70–100s) = 0.48 ± 0.16, ***, p < 0.001). Student's t test was performed.
We next examined the thermoTRP channel specificity for FPP activation using HEK293T cells expressing individual TRPs (TRPV1, TRPV2, TRPV4, TRPA1, and TRPM8) and using the Ca2+ imaging. Of the six TRP channels, only TRPV3 clearly showed a significant sensitivity to FPP in terms of its activation (Fig. 2A). Other substances chemically related to FPP were also tested for the thermoTRP activation. IPP and GPP are the precursors of FPP and GGPP is the downstream product of FPP in the mevalonate pathway. Farnesol is the alcoholic form of FPP with the same carbon backbone (Fig. 2E) and is known as a ligand of nuclear Farnesoid X receptor (24). None of the substances elevated intracellular Ca2+ levels in the TRPV3-HEK cells at micromolar ranges (Fig. 2A). From the TRPV3-mediated Ca2+ influx, the dose-response curve for FPP was obtained (Fig. 2B). The EC50 of FPP on TRPV3 was 131.1 nm, indicating that FPP exerts its action on the TRPV3 activity at the nanomolar and micromolar levels. To further investigate TRPV3 activation mechanisms by FPP, we performed voltage-dependence analysis as TRPV3 exhibits voltage-dependent activation (16, 25). The peak inward tail currents at −120 mV after a 800-ms voltage step protocol of test potentials ranging from −120 mV to +200 mV were measured with or without FPP application (Fig. 2C). As a result, the voltage dependence of TRPV3 activation is shifted toward more negative voltages upon FPP application (Fig. 2D). The V½ decreased from 143 mV at resting state to −66 mV during FPP application. The results suggest that TRPV3 activation by FPP is caused via a shift in its voltage dependence.
FIGURE 2.
FPP exerts its potency and specificity on TRPV3 activation. A, summary of the intracellular Ca2+ increases in the cells transfected with TRPV3 or other thermoTRP channels upon drug treatments in the Fluo-3 Ca2+ imaging. Untransfected cells did not show intracellular Ca2+ increases upon treatment with any test compound (data not shown). FPP elicited Ca2+ influx in the TRPV3-expressing HEK293T cells at 1 μm. IPP, GPP, GGPP, and farnesol were tested at 10 μm. IPP, GPP, GGPP, and farnesol failed to elevated intracellular Ca2+ levels in the TRPV3 cells (n = 28, n = 41 and n = 25 and n = 28, respectively). Greater than 25 cells was used for the tests of each thermoTRP activity with each of the four compounds (n = 25–98). B, dose-response curve for FPP on TRPV3 in the Fluo-3 Ca2+ imaging. The curve was fitted by Hill equation (EC50 = 131.1 nm and n = 2.4). Open circles represent mean values of responses of Ca2+ influx upon FPP application (n = 22–53 for each point). C, whole-cell currents in response to the indicated voltage step protocol applied with or without FPP application (1 μm). D, average peak inward tail currents at −120 mV from the step protocol were plotted by a function of test voltage steps (n = 7 with 1 μm FPP (diamonds), n = 8 without FPP (squares) and n = 6 with heat (36 °C; circles), respectively). Symbols represent the data collected from every 20 mV. The curves were fitted by the Boltzmann equation. E, structures of the FPP and related substances tested in the present study were displayed.
FPP Activates TRPV3 in Human Keratinocytes
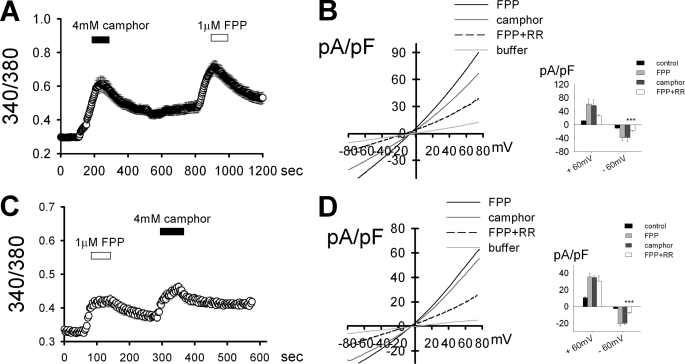
With the results of TRPV3-specific activation by FPP in the heterologous expression system, we hypothesized that the skin keratinocyte, which is a TRPV3-abundant cell type, is also able to show similar responses to FPP. In Ca2+-imaging experiments using HaCaT keratinocyte cell lines and normal human keratinocytes, rapid intracellular Ca2+ elevation were induced upon FPP application (Fig. 3, A and C). In all the same cells, camphor responses were also detected. In whole cell voltage clamp experiments evident current responses (in which the outwardly rectifying current-voltage relationships were similar to those upon camphor application) were detected upon FPP application and blocked by RR co-application, both with HaCaT keratinocytes and with normal human keratinocytes (Fig. 3, B and D). Earlier, FPP was reported to activate GPR92, leading to intracellular Ca2+ elevation (26). Another activator for GPR92, N-arachidonoyl glycine (NAG) failed to elicit such Ca2+ response (data not shown). Thus the Ca2+ influx of the keratinocytes are mediated via TRPV3 activation and not via GPR92 activation.
FIGURE 3.
FPP activates TRPV3 expressed in keratinocytes. A and C, 1 μm FPP elevated intracellular Ca2+ levels in HaCaT cell lines (n = 72; A), and in normal human epidermal keratinocytes (n = 114; C) in the Fura-2 Ca2+-imaging experiments. A camphor response was also shown by the same cells. B and D, 1 μm FPP evoked an outwardly rectifying current increase in the whole cell voltage clamp study using HaCaT cell lines (n = 5; B) and using normal human epidermal keratinocytes (n = 5; D). The current responses to FPP were blocked by 20 μm RR, and those with the similar current-voltage relationship were evoked by 4 mm camphor treatment in the HaCaT cell lines (B) and normal human epidermal keratinocytes (D). Insets B–D, average current densities through each TRPV3 activation at ±60 mV. As reported earlier (7), the FPP-evoked currents were significantly inhibited by RR at −60 mV. *, p < 0.05; ***, p < 0.001; Student's t test.
FPP Elicits Pain via Communication between Keratinocytes and Sensory Neurons
Thermal activation of TRPV3 results in pain behaviors (4–5). To check if FPP is also able to evoke pain behaviors, we performed acute pain behavior assays used in a previous study (18). When FPP was intradermally injected into a hind paw, no significant change in behavior was observed in normal mice (Fig. 4B). Animals locally inflamed by carrageenan for 3 h, however, exhibited significant increases in time consumed in acute licking and lifting behaviors in response to the FPP injections (Fig. 4, A and C) whereas injections with vehicle or GPP (the structurally closest precursor to FPP without activity on thermoTRPs) were without effect (Fig. 4A). Moreover, the effects of FPP were attenuated when intradermally pretreated with RR, which indicates that the acute pain induced by FPP was mediated by TRPV3 activation (Fig. 4, A and B). For further examination, mice were pretreated with mTRPV3-shRNA in a hind paw 48 h prior to the behavioral test. The mTRPV3-shRNA-treated mice showed suppressed epidermal TRPV3 expression in the pretreated hind paw (Fig. 4, D and E). Like the pharmacological blockade with RR, the shRNA treatment significantly attenuated FPP-induced licking/lifting responses (Fig. 4, B and C). The mTRPV3-shRNA did not affect capsaicin-evoked licking behaviors (n = 5, data not shown). No significant behavioral suppression was observed in mice treated with a scrambled shRNA (Fig. 4, B and C), which indicates that only the mTRPV3-shRNA specifically down-regulated TRPV3 expression and that it subsequently blocked FPP-action on TRPV3.
FIGURE 4.
FPP induces acute nociception under inflammation and lowered the thermal threshold of TRPV3. A, summary of the time course of licking/lifting behaviors in mice treated with FPP (1 mm in 10 μl) administered intradermally into hind paws for the 20-min period immediately following the injection (n = 5, filled circles). Carrageenan was injected to the hind paws 3 h prior to the experiments. Pretreatment with RR (1 mm in 10 μl) 5 min prior to the FPP administration prevented such behaviors (n = 5, open circles). Neither intradermal injection with vehicle without drugs (n = 5, triangles) nor the 1 mm GPP (in 10 μl) administration elicited licking/lifting behaviors (n = 6, diamonds). Mice with an FPP-injected hind paw showed no responses from their non-injected hind paw similar to the vehicle-injected controls (n = 5, data not shown). B, summary of the time course of licking/lifting behaviors in mice subplantarly pretreated with mTRPV3-shRNA (KD) for the 20-min period immediately following FPP injection (n = 6, open triangles). Carrageenan (CAR) was injected to the hind paws 3 h prior to the experiments. FPP-evoked behaviors occurred with scrambled shRNA pretreatment (n = 6, open circles). Without carrageenan pretreatment, no significant time consumption in licking/lifting behaviors evoked by FPP administration was detected (n = 6, open triangles). C, sums of the time spent in licking/lifting behaviors of carrageenan-inflamed mice for 20 min immediately after drug injection. Intradermal pretreatment with RR and mTRPV3 knockdown with shRNA significantly suppressed FPP-evoked behaviors. ANOVA followed by Bonferroni post hoc test was performed for the statistical comparison (*, p < 0.05; **, p < 0.01). D, reverse-transcriptase PCR results of TRPV3 mRNA in the mouse hind paw epidermis. Control and SC indicates no treatment and scrambled shRNA treatment, respectively. E, Western blot results from the mouse hind paw epidermis. For A–E, scrambled shRNA or TRPV3-shRNA was subplantarly injected into hind paws 48 h prior to the behavioral assays or mRNA or protein isolation.
We hypothesized that this FPP-induced nociception is relayed by the sensory neurons. Thus we examined the in vitro neuronal response upon FPP application using co-culture of HaCaT keratinocytes and the mouse DRG sensory neurons. When co-cultured, a close proximity of neuronal terminals and keratinocytes was observed (Fig. 5A). In the Ca2+ imaging experiments, the DRG sensory neurons showed significant Ca2+ influx responses following the keratinocyte responses upon FPP application in the same dish (Fig. 5B). Interestingly, 38% of the neurons responding to FPP was also capsaicin-sensitive (n = 16), which indicates that nociceptive populations relay the FPP signals. The onsets of the DRG responses were behind those of keratinocyte responses (Fig. 5, B and C), indicating that the sensory neurons do not contain functional TRPV3 or other excitatory components directly sensitive to FPP. RR application completely ablated the keratinocyte FPP responses and subsequent DRG responses (Fig. 5D). Moreover, no change in the intracellular Ca2+ levels of the mouse sensory neurons by FPP was detected in cultures without keratinocytes or cultures with keratinocytes treated with hTRPV3-shRNA, which further demonstrates that the mouse sensory neurons are lacking in functional TRPV3 or other direct FPP-sensing machinery on their own (Fig. 5, E and F) (1,4,27). We also confirmed the effect of the hTRPV3-shRNA on TRPV3 expression of the keratinocytes using RT-PCR and Western blot analyses (Fig. 5, G and H). These results suggest that FPP excites keratinocytes via TRPV3 activation and that signals instantly released from the keratinocytes activate the adjoined sensory neurons.
FIGURE 5.
FPP excites DRG neurons via keratinocyte activation. A, a representative image of the DRG neurons and keratinocyte co-cultures with staining: anti-TRPV3 antibody (green) for TRPV3 expressors and Hoechst dye for nuclei (blue). Arrows indicate DRG neurons, and arrowheads indicate keratinocytes. Scale bar, 100 μm. B, in the Fura-2 Ca2+-imaging experiments, 1 μm FPP elevated intracellular Ca2+ levels both in HaCaT keratinocytes and DRG neurons in the same dish (n = 118 for HaCaT cells and n = 48 for DRG neurons). Application of 60 mm KCl for depolarization-induced Ca2+ influx in the excitable cells functionally confirms identities of the HaCaT keratinocytes and the DRG neurons. C, summary of the onset times of FPP response of TRPV3 (***, p < 0.001). Student's t test was performed. D, 1 μm FPP failed to elevate intracellular Ca2+ levels in the keratinocytes or the DRG neurons during 20 μm RR application in the co-cultures (n = 231 for HaCaT cells and n = 7 for DRG neurons). E, 1 μm FPP failed to elevate intracellular Ca2+ levels in the keratinocytes or the DRG neurons in the co-cultures incubated with hTRPV3-shRNA (48 h) (n = 122 for HaCaT cells and n = 33 for DRG neurons). F, 1 μm FPP failed to elevate intracellular Ca2+ levels in the DRG neurons in cultures without keratinocytes (n = 37). G, reverse-transcriptase PCR of TRPV3 mRNA in HaCaT keratinocytes. H, Western blot results from HaCaT keratinocytes.
We also ascertained whether FPP acutely affects in vivo heat thresholds. After FPP was intradermally injected into a hind paw, paw withdrawal latencies upon radiant heat were significantly decreased in the Hargreaves test (Fig. 6A). We hypothesized that an increase in heat sensitivity of TRPV3 or TRPV1 may cause this phenomenon. TRPV1-knock-out mice exhibited the similar reduction in the Hargreaves threshold (Fig. 6A), indicating that FPP affects the heat sensitivity in a TRPV1-independent manner. By contrast, no significant reduction in the heat threshold of mice pretreated with mTRPV3-shRNA was observed, suggesting that TRPV3 mediates the in vivo FPP-induced thermal hypersensitivity. We further examined the in vitro heat thresholds of these two TRP channels with or without FPP incubation. TRPV3 but not TRPV1 (n = 4, data not shown), exhibited significantly lower temperature thresholds for its activation under FPP incubation even at a 10 nm, which is a marginal concentration to activate TRPV3 (n = 8, 3.5 °C ± 0.5 decrease at average, Fig. 6, B and C). FPP also failed to potentiate capsaicin response of TRPV1 (data not shown. n = 160 in Ca2+ imaging). We asked whether not only the threshold shift, but also a change in the temperature coefficient Q10 values contribute to this sensitization. The Q10 values of the specifically heat-sensitive phase of TRPV3 activation were increased by FPP but it was not statistically different (16.5 ± 5.4 without FPP versus 21.8 ± 9.7 with FPP), indicating that the thermal threshold shift is the major contributor to the sensitization of TRPV3 response. Besides the heat threshold shift, peak responses of heat-activated TRPV3 also seems to be potentiated by the 10 nm FPP incubation (Fig. 6B). To ascertain whether the effects on the TRPV3 responses of FPP and heat are additive or synergistic when applied together, we tested a series of FPP concentrations (10 nm, 100 nm, and 1 μm) with heat stimulation. Upon the application with 10 nm FPP plus heat, significantly larger current responses of TRPV3 were detected than the sum of those upon FPP alone and heat alone. On the other hand, additive responses upon higher concentrations (100 nm and 1 μm) of FPP plus heat were observed (Fig. 6, E and F). Thus, largely, the sensitizing effects of FPP on the heat responses are likely to depend on an increase in the heat potency (left shifts of the voltage dependence, Fig. 6F) rather than on a synergistic increase in the efficacy. Consequently, in addition to directly activating TRPV3, FPP is able to positively modify the TRPV3 heat threshold. Throughout the overall experiments, our results in the present study suggest that FPP is able to activate and sensitize TRPV3, resulting in pain sensation.
FIGURE 6.
FPP reduces TRPV3 thermal threshold leading to heightened heat sensitivity. A, summary of the changes in the paw withdrawal latencies from Hargreaves tests with or without FPP treatment. Hargreaves latencies were decreased by 42.3 ± 9.5% for wild-type mice, by 36.2 ± 3.5% for TRPV1-null mice and by 43.2 ± 5.2% for mice treated with scrambled shRNA (SC). No significant decrease in the latencies was detected in mice pretreated with mTRPV3-shRNA. The Hargreaves latencies were examined 30 min after the hind paw intradermal administration of FPP (1 mm in 10 μl). n = 10 for each histogram (*, p < 0.05; **, p < 0.01, Student's t test). B, representative heat-induced current responses of TRPV3-transfected HEK293T cells. When temperature elevated, the TRPV3-mediated currents were elicited (top: 10 nm FPP was incubated; bottom: without FPP incubation). C, summary of the changes in the heat thresholds of TRPV3 in experiments of B. The mean heat threshold of TRPV3 from the group without FPP incubation was 31.8 ± 0.8 °C (n = 7), and the value from the group with FPP incubation was 28.8 ± 0.5 °C (n = 8). D, summary of the Q10 values of the heat-sensitive phases of TRPV3 activation in experiments of B. The mean Q10 with or without FPP incubation was 16.5 ± 5.4 and 21.9 ± 9.7, respectively. No statistical difference was found. E, summary of the peak TRPV3 current densities activated by heat, FPP, or heat plus FPP at ± 60 mV (n = 8 with heat alone, n = 6 with 10 nm FPP, n = 7 with heat plus 10 nm FPP, n = 12 with 100 nm FPP, n = 5 with heat plus 100 nm FPP, n = 7 with 1 μm FPP, and n = 17 with heat plus 1 μm FPP. Filled bars for current densities at −60 mV, and open bars at + 60mV). TRPV3-transfected HEK293T cells were used. Dotted lines indicate the average peak currents activated by heat alone. F, average peak inward tail currents at −120 mV from the step protocol as shown in Fig. 2C were plotted by a function of test voltage steps (n = 10 with 10 nm FPP (open circles), n = 8 with heat (36 °C) plus 10 nm FPP (closed circles), n = 8 with heat plus 100 nm FPP (gray triangles), n = 5 with heat plus 1 μm FPP (open triangles), and n = 9 without stimulation (gray circles)). Symbols represent the data collected from every 20 mV. The curves were fitted by Boltzmann equation. V½ was 143 mV at the resting state, 116 mV during 10 nm FPP, 30 mV during heat plus 10 nm FPP, −27 mV during heat plus 100 nm FPP, and −122 mV during heat plus 1 μm FPP applications. *, p < 0.05; **, p < 0.01, Student's t test.
DISCUSSION
The present study demonstrates that FPP activates TRPV3. None of other five sensory neuronal thermoTRPs is activated by FPP. Therefore, TRPV3 appears to be the sole detector of FPP among the thermoTRPs. Steric stringency appears to be required for the activation according to our results with FPP-related metabolites. The TRPV3 activation is repeatable in the experiments using the skin keratinocytes.
Surprisingly, FPP is shown to be a pain-inducing substance in the present study. We demonstrated that treatment with FPP acutely evoked nociceptive behaviors, which was prevented by RR pretreatment or by TRPV3 knockdown. Recent studies suggested that activated keratinocytes release signal messengers like ATP or prostaglandin E2 to the vicinal sensory neurons, leading to pain perception (5, 27–28). The same transduction mechanism may account for FPP-evoked pain. In our hands, no significant behavioral change was observed in normal mice, but the mice with inflamed hind paws exhibited FPP-evoked nociceptive behaviors. It is likely that primed condition is required for the in vivo release of putative messengers from the keratinocytes in response to FPP. In this respect, it is possible that our co-culture system using cultured DRG neurons that may not perfectly escape a pathological status caused by their acute dissociation mimics the in vivo inflamed conditions. Interestingly, FPP was able to enhance the in vitro heat sensitivity of TRPV3 and moreover, the behavioral hypersensitivity to noxious heat also occurred by the short-term intradermal incubation of FPP in normal mice. A lower concentration range appears to be sufficient for a shift of the in vitro heat sensitivity than for the excitation mediated by direct TRPV3 activation according to our result that FPP at a marginal concentration robustly sensitized and potentiated the TRPV3 responses to heat. It is possible to speculate that higher dose of FPP by itself might evoke acute nociceptive behavior in normal mice, but the limited solubility prevented the verification. Collectively, FPP seems to acquire direct in vivo nociceptive potential under inflammation while it affects the heat threshold even under quiescent condition.
The search for TRPV3 agonists becomes more active and many exogenous ligands for TRPV3 have been found (6). First, a synthetic chemical 2-APB was shown to activate TRPV3 (7–8). Among natural compounds, camphor is the firstly identified as a TRPV3 activator (4). Interestingly, Vogt-Eisele et al. (11) examined a library of monoterpenoids including many natural plant chemicals, showing that about 30 compounds have agonistic activities on TRPV3 activation. Although quantitative analyses for the potency and specificity of the chemicals were not fully performed in these earlier studies, those compounds largely have relatively high EC50 (hundreds of micromolar to millimolar concentrations) and low specificity (also activating other thermoTRPs). For example, 2-APB also activates TRPV1 and TRPV2 (8). The lowest EC50 in the monoterpenoid study was 370 μm (11). Camphor was shown to weakly activate TRPV1 with an immediate desensitization effect (9, 29). Thus to date, FPP is the most potent with a nanomolar EC50 and is specific only for TRPV3. The chemical structure of FPP is out of the prediction that Vogt-Eisele et al. (11) proposed, where a cyclic structure seems required for activation. Rather, according to our data, it is possible that the three isoprenyl repeats of FPP confer a flexibility to fit the TRPV3 binding pocket. Alternatively, FPP may only partly share the binding region with cyclic monoterpenes. Further studies on the TRPV3 structure combined with pharmacology are needed to answer this question. In addition, it is notable that the optimal size of the isoprenyl repeats and the pyrophosphate residue are also important to acquire the potency according to our results with IPP, GPP, GGPP, and farnesol.
FPP is an intermediate molecule in the mevalonate pathway. FPP is generated via one isomerization and subsequent condensation processes with IPP by IPP isomerase and FPP synthase. FPP is then used for cholesterol synthesis or protein prenylation. Though FPP is basically synthesized in the cytosol, it can be detected in human plasma. The steady-state plasma level was reported to be 6.6 ng/ml (17 nm) (30). Thus, it is possible that hyperemic or bleeding conditions may enable TRPV3 to expose to nanomolar FPP, leading to the channel sensitization according to our heat-threshold data. ∼17 nm appears lower than to directly activate TRPV3. Therefore, rather than at the quiescent state, pathological conditions in which internal contents such as FPP can be released via cellular damage are likely to be important to direct TRPV3 activation and subsequent nociception. Otherwise, FPP is possibly secreted extracellularly through a selective transporter system at a particular event, resulting in the elevation of the local FPP level outside the cells. Further studies will explore the presence of this releasing mechanism. Lessons from the studies on the secretion of other soluble cellular components (for example, via cell lysis or via opening of transporters like connexin, ATP is released and acts on adjacent purinergic receptors) may provide an insight (31).
FPP acts on other membrane receptors: it activates GPR92 and inhibits LPA2 and LPA3 receptors (26, 32). In the present study, another activating ligand for GPR92, NAG failed to elevate intracellular Ca2+ level in the keratinocyte experiments. On the other hand a TRP inhibitor RR and TRPV3-shRNA were able to block the FPP-induced Ca2+ influx in the keratinocytes, which suggests that GPR92 is not involved in the native responses from keratinocytes. RR and the shRNA also prevented FPP-evoked pain behavior, supporting this notion. As well, it is not possible that LPA2 or LPA3 receptor is involved because FPP leads to the opposite result through the receptor inhibition (FPP blocked the LPA-induced intracellular Ca2+ increase in the observations of Liliom et al. (32)) Differential roles of FPP may be expected in the different receptor expressers.
FPP synthase is inhibited by nitrogen-containing bisphosphonates, which is a mechanism that explains the therapy of osteoporosis with these drugs (33). It has been reported that the treatments with bisphosphonates were effective for some types of bone cancer pain and neuropathic pain (34–35). It would be interesting to investigate whether certain painful states involve abnormally balanced FPP metabolism and also whether the inhibition of FPP synthase contributes to the analgesic mechanisms, at least in part, via prevention from the FPP effect on TRPV3. As well as in pain, TRPV3 is proposed to be involved in other physiological events such as emotional regulation, hair growth, and dermatitis (13, 36–37). It remains to elucidate the possible roles of FPP in these phenomena and whether artificial manipulation of the body FPP level using drugs can lead to beneficial outcomes in this context.
In the present study, we successfully repeated voltage-dependent activation of TRPV3 (16, 25). As in the previous studies using 2-APB and camphor, the voltage-dependence curve is left-shifted upon FPP application, which indicates that the common mechanism can explain TRPV3 activation by FPP. Furthermore, like camphor (4), FPP was able to sensitize and potentiate heat-induced activation of TRPV3. As previously observed in the sensitization by repeated heat, Q10 values were consistent with or without FPP and only the threshold shift appears to consequently affect the outcome of TRPV3 potentiation (3). These FPP effects on the heat response seem to shift from a synergistic one to an additive one along increasing concentrations (Fig. 6E), probably because the response becomes saturated at higher concentrations. Though TRPV1 is referred to as a major contributor to the heat nociception, no threshold change in heat-induced TRPV1 activation by FPP was observed here. Capsaicin response of TRPV1 was also insensitive to FPP incubation. Moreover, TRPV1-null mice showed the similar in vivo heat threshold reduction upon the FPP treatment whereas TRPV3-kockdown mice showed marked insensitivity to FPP in the threshold change. Therefore, the crucial component that derives the change in the temperature sensitivity by FPP in this study is likely to be TRPV3.
One of the challenges in the TRPV3 research has been the absence of potent and specific agonists. The present study demonstrates that FPP specifically activates TRPV3 among six thermoTRPs. Of the TRPV3 ligands found to date, FPP is the most potent and selective one, and is a uniquely sole endogenous substance. Moreover, we show that FPP evokes pain behavior which is mediated by TRPV3. This information may offer useful tools to understanding TRPV3-related physiology and unknown pain-related signalings, and designing synthetic TRPV3-ligands.
This work was supported by Korea Research Foundation Grants (Codes KRF-2008-331-E00457 and 2009-0076543), the Republic of Korea.
- 2-APB
- 2-aminoethoxydiphenyl borate
- CAR
- carrageenan
- C/A
- cell-attached
- DRG
- dorsal root ganglion
- FPP
- farnesyl pyrophosphate
- GGPP
- geranylgeranyl pyrophosphate
- GPP
- geranyl pyrophosphate
- HEK
- human embryonic kidney
- IPP
- isopentenyl pyrophosphate
- I/O
- inside-out
- KD
- knockdown
- NAG
- N-arachidonoyl glycine
- ND
- no difference
- NPo
- open probability
- RR
- ruthenium red
- SC
- scrambled shRNA
- shRNA
- small hairpin RNA
- thermoTRP
- temperature-sensitive transient receptor potential ion channel
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum.
REFERENCES
- 1.Peier A. M., Reeve A. J., Andersson D. A., Moqrich A., Earley T. J., Hergarden A. C., Story G. M., Colley S., Hogenesch J. B., McIntyre P., Bevan S., Patapoutian A. (2002) Science 296, 2046–2049 [DOI] [PubMed] [Google Scholar]
- 2.Smith G. D., Gunthorpe M. J., Kelsell R. E., Hayes P. D., Reilly P., Facer P., Wright J. E., Jerman J. C., Walhin J. P., Ooi L., Egerton J., Charles K. J., Smart D., Randall A. D., Anand P., Davis J. B. (2002) Nature 418, 186–190 [DOI] [PubMed] [Google Scholar]
- 3.Xu H., Ramsey I. S., Kotecha S. A., Moran M. M., Chong J. A., Lawson D., Ge P., Lilly J., Silos-Santiago I., Xie Y., DiStefano P. S., Curtis R., Clapham D. E. (2002) Nature 418, 181–186 [DOI] [PubMed] [Google Scholar]
- 4.Moqrich A., Hwang S. W., Earley T. J., Petrus M. J., Murray A. N., Spencer K. S., Andahazy M., Story G. M., Patapoutian A. (2005) Science 307, 1468–1472 [DOI] [PubMed] [Google Scholar]
- 5.Huang S. M., Lee H., Chung M. K., Park U., Yu Y. Y., Bradshaw H. B., Coulombe P. A., Walker J. M., Caterina M. J. (2008) J. Neurosci. 28, 13727–13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vriens J., Appendino G., Nilius B. (2009) Mol. Pharmacol. 75, 1262–1279 [DOI] [PubMed] [Google Scholar]
- 7.Chung M. K., Lee H., Mizuno A., Suzuki M., Caterina M. J. (2004) J. Neurosci. 24, 5177–5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H. Z., Gu Q., Wang C., Colton C. K., Tang J., Kinoshita-Kawada M., Lee L. Y., Wood J. D., Zhu M. X. (2004) J. Biol. Chem. 279, 35741–35748 [DOI] [PubMed] [Google Scholar]
- 9.Macpherson L. J., Hwang S. W., Miyamoto T., Dubin A. E., Patapoutian A., Story G. M. (2006) Mol. Cell Neurosci. 32, 335–343 [DOI] [PubMed] [Google Scholar]
- 10.Xu H., Delling M., Jun J. C., Clapham D. E. (2006) Nat Neurosci 9, 628–635 [DOI] [PubMed] [Google Scholar]
- 11.Vogt-Eisele A. K., Weber K., Sherkheli M. A., Vielhaber G., Panten J., Gisselmann G., Hatt H. (2007) Br. J. Pharmacol. 151, 530–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stotz S. C., Vriens J., Martyn D., Clardy J., Clapham D. E. (2008) PLoS One 3, e2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moussaieff A., Rimmerman N., Bregman T., Straiker A., Felder C. C., Shoham S., Kashman Y., Huang S. M., Lee H., Shohami E., Mackie K., Caterina M. J., Walker J. M., Fride E., Mechoulam R. (2008) FASEB J. 22, 3024–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu H. Z., Xiao R., Wang C., Gao N., Colton C. K., Wood J. D., Zhu M. X. (2006) J. Cell. Physiol. 208, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida T., Inoue R., Morii T., Takahashi N., Yamamoto S., Hara Y., Tominaga M., Shimizu S., Sato Y., Mori Y. (2006) Nat. Chem. Biol. 2, 596–607 [DOI] [PubMed] [Google Scholar]
- 16.Xiao R., Tang J., Wang C., Colton C. K., Tian J., Zhu M. X. (2008) J. Biol. Chem. 283, 6162–6174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland S. P., Cook S. P., McCleskey E. W. (2000) Prog. Brain Res. 129, 21–38 [DOI] [PubMed] [Google Scholar]
- 18.Bang S., Kim K. Y., Yoo S., Kim Y. G., Hwang S. W. (2007) Eur. J. Neurosci. 26, 2516–2523 [DOI] [PubMed] [Google Scholar]
- 19.Bang S., Kim K. Y., Yoo S., Lee S. H., Hwang S. W. (2007) Neurosci. Lett. 425, 120–125 [DOI] [PubMed] [Google Scholar]
- 20.Kim K. Y., Bang S., Han S., Nguyen Y. H., Kang T. M., Kang K. W., Hwang S. W. (2008) Biochem. Biophys. Res. Commun. 370, 295–300 [DOI] [PubMed] [Google Scholar]
- 21.Vyklický L., Vlachová V., Vitásková Z., Dittert I., Kabát M., Orkand R. K. (1999) J. Physiol. 517, 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo S., Han S., Park Y. S., Lee J. H., Oh U., Hwang S. W. (2009) Mol. Cell 27, 417–422 [DOI] [PubMed] [Google Scholar]
- 23.Henneman L., van Cruchten A. G., Denis S. W., Amolins M. W., Placzek A. T., Gibbs R. A., Kulik W., Waterham H. R. (2008) Anal. Biochem. 383, 18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forman B. M., Goode E., Chen J., Oro A. E., Bradley D. J., Perlmann T., Noonan D. J., Burka L. T., McMorris T., Lamph W. W., Evans R. M., Weinberger C. (1995) Cell 81, 687–693 [DOI] [PubMed] [Google Scholar]
- 25.Hu H., Grandl J., Bandell M., Petrus M., Patapoutian A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh D. Y., Yoon J. M., Moon M. J., Hwang J. I., Choe H., Lee J. Y., Kim J. I., Kim S., Rhim H., O'Dell D. K., Walker J. M., Na H. S., Lee M. G., Kwon H. B., Kim K., Seong J. Y. (2008) J. Biol. Chem. 283, 21054–21064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandadi S., Sokabe T., Shibasaki K., Katanosaka K., Mizuno A., Moqrich A., Patapoutian A., Fukumi-Tominaga T., Mizumura K., Tominaga M. (2009) Pflugers Arch 458, 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koizumi S., Fujishita K., Inoue K., Shigemoto-Mogami Y., Tsuda M. (2004) Biochem. J. 380, 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H., Blair N. T., Clapham D. E. (2005) J. Neurosci. 25, 8924–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saisho Y., Morimoto A., Umeda T. (1997) Anal. Biochem. 252, 89–95 [DOI] [PubMed] [Google Scholar]
- 31.Lazarowski E. R., Boucher R. C., Harden T. K. (2003) Mol Pharmacol. 64, 785–795 [DOI] [PubMed] [Google Scholar]
- 32.Liliom K., Tsukahara T., Tsukahara R., Zelman-Femiak M., Swiezewska E., Tigyi G. (2006) Biochim. Biophys. Acta 1761, 1506–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell R. G., Xia Z., Dunford J. E., Oppermann U., Kwaasi A., Hulley P. A., Kavanagh K. L., Triffitt J. T., Lundy M. W., Phipps R. J., Barnett B. L., Coxon F. P., Rogers M. J., Watts N. B., Ebetino F. H. (2007) Ann. N.Y. Acad. Sci. 1117, 209–257 [DOI] [PubMed] [Google Scholar]
- 34.von Moos R., Strasser F., Gillessen S., Zaugg K. (2008) Support Care Cancer 16, 1105–1115 [DOI] [PubMed] [Google Scholar]
- 35.Sharma A., Williams K., Raja S. N. (2006) Curr. Opin. Anaesthesiol. 19, 566–572 [DOI] [PubMed] [Google Scholar]
- 36.Asakawa M., Yoshioka T., Matsutani T., Hikita I., Suzuki M., Oshima I., Tsukahara K., Arimura A., Horikawa T., Hirasawa T., Sakata T. (2006) J. Invest. Dermatol. 126, 2664–2672 [DOI] [PubMed] [Google Scholar]
- 37.Yoshioka T., Imura K., Asakawa M., Suzuki M., Oshima I., Hirasawa T., Sakata T., Horikawa T., Arimura A. (2009) J. Invest. Dermatol. 129, 714–722 [DOI] [PubMed] [Google Scholar]