Abstract
Hypoxic pulmonary vasoconstriction (HPV) is a physiological response to a decrease in airway O2 tension, but the underlying mechanism is incompletely understood. We studied the contribution of glucose-6-phosphate dehydrogenase (Glc-6-PD), an important regulator of NADPH redox and production of reactive oxygen species, to the development of HPV. We found that hypoxia (95% N2, 5% CO2) increased contraction of bovine pulmonary artery (PA) precontracted with KCl or serotonin. Depletion of extracellular glucose reduced NADPH, NADH, and HPV, substantiating the idea that glucose metabolism and Glc-6-PD play roles in the response of PA to hypoxia. Our data also show that inhibition of glycolysis and mitochondrial respiration (indicated by an increase in NAD+ and decrease in the ATP-to-ADP ratio) by hypoxia, or by inhibitors of pyruvate dehydrogenase or electron transport chain complexes I or III, increased generation of reactive oxygen species, which in turn activated Glc-6-PD. Inhibition of Glc-6-PD decreased Ca2+ sensitivity to the myofilaments and diminished Ca2+-independent and -dependent myosin light chain phosphorylation otherwise increased by hypoxia. Silencing Glc-6-PD expression in PA using a targeted small interfering RNA abolished HPV and decreased extracellular Ca2+-dependent PA contraction increased by hypoxia. Similarly, Glc-6-PD expression and activity were significantly reduced in lungs from Glc-6-PDmut(−/−) mice, and there was a corresponding reduction in HPV. Finally, regression analysis relating Glc-6-PD activity and the NADPH-to-NADP+ ratio to the HPV response clearly indicated a positive linear relationship between Glc-6-PD activity and HPV. Based on these findings, we propose that Glc-6-PD and NADPH redox are crucially involved in the mechanism of HPV and, in turn, may play a key role in increasing pulmonary arterial pressure, which is involved in the development of pulmonary hypertension.
Keywords: Calcium, Glucose, Hypoxia, Pentose Pathway, Smooth Muscle, Calcium, Glucose Metabolism, Hypoxic Pulmonary Vasoconstriction, NADPH, Pulmonary Artery
Introduction
Pulmonary hypertension (PH)2 is a major cause of morbidity and mortality, the incidence of which is increasing around the world. The mechanisms underlying the development of PH remain unclear, however, and current medical treatment is inadequate. That said, it has been noted that in people living at high altitude and in patients suffering from chronic obstructive pulmonary disease, the chronic reduction in inhaled O2 induces sustained hypoxic pulmonary vasoconstriction (HPV), which eventually leads to development of PH (1, 2). HPV is a phenomenon that balances the ventilation-to-respiration quotient by constricting the pulmonary artery (PA) and redirecting blood flow in the lungs in response to local reductions in inspired pO2 (3). Since its discovery nearly a century ago, numerous studies have been conducted to identify the mechanisms responsible for HPV. To date, however, no unifying mechanism has been identified.
In 1982, McMurtry et al. (4) proposed that inhibition of glycolysis and oxidative phosphorylation mimics HPV and suggested that inhibition of the mitochondrial electron transport chain (ETC) caused by a reduction in pO2 is involved in initiating HPV (5). Since then, several theories on how a reduction in pO2 could lead to HPV have been developed. The redox hypothesis presumes that a decrease in mitochondrial reactive oxygen species (ROS) levels triggers acute HPV (6), whereas the ROS hypothesis argues that an increase in free radical generation via the mitochondrial ETC is a major cause of HPV (7). Consistent with the latter, inhibition of mitochondrial ETC complex III using antimycin and myxothiazol induces ROS production and PA contraction (8, 9). Although the Qo site of complex III appears to be potentially important for O2 sensing (10), how a decrease in pO2 at this site enhances ROS production and activates ion channels and arterial smooth muscle contraction remains unclear. Considering the importance of HPV in lung physiology and in the development of PH, it is important to identify the O2 sensor in pulmonary artery smooth muscle cells (PASMCs) and the mechanisms responsible for the HPV response.
Hypoxic stimuli rapidly and significantly enhance glucose uptake in the PA but inhibit glycolysis (11, 12). We recently reported that glucose-6-phosphate dehydrogenase (Glc-6-PD), the rate-limiting enzyme in the pentose phosphate pathway (PPP), catalyzes the production of NADPH, thereby increasing the NADPH-to-NADP+ ratio in the PA and lungs exposed to hypoxia (13, 14). These studies also showed that inhibition of Glc-6-PD reduces NADPH oxidase-derived production of superoxide anion ( ) in PA, relaxes precontracted PA, and significantly reduces HPV in rat lungs. All of these observations suggest that Glc-6-PD could be a link between O2 sensing, altered glucose metabolism, and initiation of HPV. Moreover, because glucose metabolism through glycolysis is reduced and mitochondrial ETC is shut down by hypoxia in the PA (11), we postulated that under those conditions glucose accumulating in PASMCs is likely shunted through the PPP. Therefore, our first objective was to determine whether Glc-6-PD is activated by hypoxia and whether it plays a role in contracting isolated PA in response to a decrease in pO2.
) in PA, relaxes precontracted PA, and significantly reduces HPV in rat lungs. All of these observations suggest that Glc-6-PD could be a link between O2 sensing, altered glucose metabolism, and initiation of HPV. Moreover, because glucose metabolism through glycolysis is reduced and mitochondrial ETC is shut down by hypoxia in the PA (11), we postulated that under those conditions glucose accumulating in PASMCs is likely shunted through the PPP. Therefore, our first objective was to determine whether Glc-6-PD is activated by hypoxia and whether it plays a role in contracting isolated PA in response to a decrease in pO2.
Glc-6-PD-derived NADPH is a co-factor in many enzymatic reactions in vascular tissue. In addition, cellular NADPH redox has been implicated in regulating ion channel activity. For instance, NAD(P)H inhibits and NAD(P)+ activates BKca and Kv channels in rabbit PASMCs (15, 16), and the binding of NADPH or NADP+ to the β-subunit of Kv1.4 or Kv1.5 channels modulates their currents (17, 18). Moreover, the closing of Kv1.5 in response to a reduction in H2O2 levels caused by an acute drop in pO2 induces contraction of dog and rat PASMC, whereas down-regulation of Kv1.5 by chronic hypoxia in rat PASMCs has been proposed to induce HPV and PH (6, 19). We have shown that inhibition of Glc-6-PD, which reduces the NADPH-to-NADP+ ratio in the PA and lungs, modestly activates Kv channels in PASMCs and contributes somewhat to the relaxation of precontracted PA (13). But these effects cannot fully explain why inhibition of Glc-6-PD almost completely abolishes HPV and PH (13, 20–22). Therefore, we speculate that inhibition of Ca2+ influx or reduction of Ca2+ sensitivity may be the link between inhibition of Glc-6-PD and attenuation of HPV. Thus, our second objective was to determine the mechanism by which activation of Glc-6-PD leads to PA contraction under hypoxic conditions.
EXPERIMENTAL PROCEDURES
Bovine lungs were purchased from a local slaughterhouse (Stuckey's Meat Packer, Semmes, AL). After quickly removing the left and right lungs from the animal, they were placed in normal Tyrode solution (in mmol/liter: 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1.0 MgCl2, 5 HEPES and 11 glucose; pH was adjusted to 7.40 with NaOH) and transported to the laboratory on ice. All chemicals were purchased from Sigma. Glc-6-PD and nontargeting siRNA were from Dharmacon (Lafayette, CO). All transfection reagents were purchased from Invitrogen.
Western Blot Analysis
Proteins were extracted from frozen tissue in lysis buffer containing 50 mmol/liter Tris-HCl (pH 7.4), 150 mmol/liter NaCl, 0.5% Nonidet P-40, 100 mmol/liter phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 200 mmol/liter pepstatin. Western blot analysis was performed as described previously (23).
CPI-17 and Myosin Light Chain Phosphorylation
The phosphorylation status of CPI-17 and myosin light chain (MLC) in PA was determined as described previously (24). Tissue samples were collected into acetone + 10% trichloroacetic acid solution at the end of each treatment described under “Results.” Levels of phosphorylated and total MLC or CPI-17 were determined by Western blot analysis using antibodies against phospho-MLC, total MLC, phospho-CPI-17, and total CPI-17 purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Glc-6-PD Activity
Glc-6-PD activity was measured in PA homogenates by following the reduction of NADP+ to NADPH (23). NADPH fluorescence was detected using a Flx800 microplate and Synergy 2 fluorescence detectors (excitation, 340 nm; emission, 460 nm; BioTek Instruments, Winooski, VT). To determine the effect of hypoxia on Glc-6-PD activity, we homogenized PA rings exposed to hypoxia for 10 min in Tris-MgCl2 buffers bubbled with 95% N2, 5% CO2 for 20 min and performed the assay in hypoxic environment.
Glutathione Reductase Activity
GSH reductase activity was measured in PA homogenates using a kit purchased from Cayman Chemical Co. Reduction in NADPH fluorescence was detected using a Flx800 microplate and Synergy 2 fluorescence detectors (excitation, 340 nm; emission, 460 nm; BioTek Instruments).
Pyridine Nucleotide Levels
NADP(H) and NAD(H) was extracted from PA, and the levels were determined using HPLC as described previously (25). Briefly, PA rings were incubated in individually thermostated (37 °C) 10-ml baths (World Precision Instruments, FL) for 2 h in Krebs bicarbonate buffer (pH 7.4) containing the following (in mm): 118 NaCl, 4.7 KCl, 1.5 CaCl2, 25 NaHCO3, 1.1 MgSO4, 1.2 KH2PO4, and 5.6 glucose, gassed with 21% O2, 5% CO2, 5% N2. After a 2-h equilibration period, PA were treated with KCl (30 mmol/liter) in normoxia (21% O2, 5% CO2, 5% N2) or the rings were exposed hypoxia (95% N2, 5% CO2) for various time points and immediately frozen in liquid nitrogen. The frozen tissues were crushed and homogenized in an extraction medium consisting of 0.02 n NaOH, containing 0.5 mm cysteine at 0 °C. The extracts were then heated at 60 °C for 10 min and neutralized with 2 ml of 0.25 m glycylglycine buffer (pH 7.6). Acidic extracts were prepared by homogenizing the tissues in hot 0.1 n HCl followed by neutralization. The NAD(P)H was eluted on a reverse-phase HPLC column (4.6 × 250 mm; C18 column, Supelco) at room temperature using La Chrome Elite HPLC system (Hitachi Inc., Tokyo) and buffer system consisting of 100 mm potassium phosphate (pH 6.0) (buffer A), and 100 mm potassium phosphate (pH 6.0) containing 5% methanol (buffer B). The column was eluted with 100% buffer A from 0 to 8.5 min, 80% buffer A plus 20% buffer B from 8.5 to 14.5 min, and 100% buffer B from 14.5 to 40 min. The flow rate was 1.0 ml/min, and the ultraviolet absorbance was monitored at 260 nm.
In a separate set of experiments, NADPH was extracted from PA rings treated with or without mitochondrial ETC and pyruvate dehydrogenase inhibitors in a tissue bath for 30 min at 37 °C, and the NADPH levels were determined using a kit from Biovision (Mountain View, CA). Briefly, ∼20 mg of tissue was washed with cold phosphate-buffered saline, homogenized with 400 μl of NADP/NADPH extraction buffer in microcentrifuge tubes, and centrifuged at 14,000 rpm for 5 min. To detect NADPH, 200 μl of sample was aliquoted into Eppendorf tubes and heated at 60 °C for 30 min. Subsequently, samples were centrifuged at 14,000 rpm for 5 min, and NADPH levels were determined in 50 μl of supernatant using a colorimetry method. The absorbance of the developed color was measured at 450 nm.
Lactate and Pyruvate Levels
Endothelium-denuded PA segments were equilibrated in 21% O2, 5% CO2, and 74% N2-gassed Krebs bicarbonate buffer for 1 h at 37 °C. After fresh Krebs buffer was added, PAs were incubated in normoxic or hypoxic conditions for 10 min in the presence of 30 mm KCl. At the end of this incubation, PAs were then quickly frozen in liquid nitrogen. Powdered frozen tissue was deproteinized by 10% trichloroacetic acid and neutralized with a mixture containing 2 n KOH, 0.4 m imidazole base, and 0.4 m KCl. The supernatant was used for assay of the metabolites studied using a kit from Biovision, Inc.
Silencing of Glc-6-PD in Pulmonary Artery
PA rings (4th order branches) were incubated overnight in Dulbecco's modified Eagle medium in a 6-well plate and then transfected for 60 h with 100 nmol/liter SMARTpool siRNA targeting Glc-6-PD (Dharmacon; see Table 1) using 10 μg of Lipofectamine2000 reagent (Invitrogen). Control experiments were performed using a nontargeting/scrambled (NT) siRNA (negative control).
TABLE 1.
Sequence for four siRNA-targeting Glc-6-PD gene (NM_000402) used to down-regulate Glc-6-PD protein expression in pulmonary artery tissue
P identifies 5′-phosphate at the start of the antisense sequence.
| Sequence for siRNA 1 | Sense | GAGAGUGGGUUUCCAGUAUUU |
|---|---|---|
| Antisense | 5′-PAUACUGGAAACCCACUCUCUU | |
| Sequence for siRNA 2 | Sense | CAACAUCGCCUGCGUUAUCUU |
| Antisense | 5′-PGAUAACGCAGGCGAUGUUGUU | |
| Sequence for siRNA 3 | Sense | CGUGAGGCCUGGCGUAUUUUU |
| Antisense | 5′-PAAAUACGCCAGGCCUCACGUU | |
| Sequence for siRNA #4 | Sense | UGACCUACGGCAACAGAUAUU |
| Antisense | 5′-PUAUCUGUUGCCGUAGGUCAUU |
PA Contraction
Fourth and fifth order PAs were prepared from bovine lungs and studied for changes in isometric force as described previously (26). To study the effect of hypoxia on PA force generation, arteries were initially precontracted with KCl (30 mmol/liter) in the presence of 21% O2, 5% CO2. Once the isometric force reached a steady state, the muscle baths were aerated with 95% N2, 5% CO2. This typically reduced the medium PO2 from 140 to 10–20 torr. PAs were exposed to hypoxia for 20 min and then reoxygenated for 20 min.
α-Toxin Skinning Procedure
PAs were permeabilized as described previously with slight modification (27, 28). Briefly, PA rings (3 × 3 mm) were cut from isolated PA, and a control contraction was elicited with 120 mmol/liter KCl, after which the rings were relaxed by washing with normal Krebs solution. The rings were then washed three times at 5-min intervals with modified relaxing solution (in mmol/liter: 118 NaCl, 4.7 KCl, 25 NaHCO3, 1.1 MgSO4, 1.2 KH2PO4, 5.6 glucose, and 0.1 EGTA; pH was adjusted to 7.4 by gassing with 21% O2, 5% CO2, 74% N2), after which they were treated with α-toxin (25 μg/ml) for 30 min at 37 °C in relaxing solution. Contraction was induced using activating solution (in mmol/liter: 2 Na2ATP, 5 creatine phosphate, 0.001 leupeptin, 0.002 carbonyl cyanide p-trifluoromethoxyphenylhydrazone, and 10 units/ml creatine phosphokinase) containing various concentrations of free Ca2+ under normoxic and hypoxic conditions. In preliminary experiments, the stability of the preparations was tested by sequentially constructing two full pCa-force curves in normoxia. We found that the concentration-response curve and maximum response were reproducible and stable between first and second curves. Thereafter, we studied the pCa-force relationship first in normoxia and then hypoxia.
Detection of  Using Immunofluorescence Microscopy
Using Immunofluorescence Microscopy
Groups of PA rings prepared as described for the force generation studies were incubated for 30 min at 37 °C with either dihydroethidium (5 μmol/liter) or MitoSOX (5 μmol/liter) in relaxing solution and were then contracted with KCl (30 mmol/liter) under normoxic or hypoxic conditions. The PA rings were fixed in acetone, quickly removed from the wire hooks, and then embedded in OCT media and frozen in liquid nitrogen, after which cryosections (5-μm-thick cross-sections) were cut and mounted in Aquamount (Vector Laboratories, Burlingame, CA) at room temperature. Tissue samples were excited with 480 nm, and emission spectra was recorded at 567 nm. Image data were collected using a Leica Plan 63× APO 0.25 ϕ objective. All data were collected at identical imaging settings.
Statistical Analysis
Analysis of variance with post hoc Fisher's protected t test was used for analysis of all studies of vascular contractility. All enzyme activity and Western blot data were analyzed using Student's t tests. The number of experimental determinations (n) was always equal to the number of lungs from which the PAs were harvested. Values of p < 0.05 were considered significant.
RESULTS
HPV and NADPH Levels Are Reduced by Depletion of Extracellular Glucose
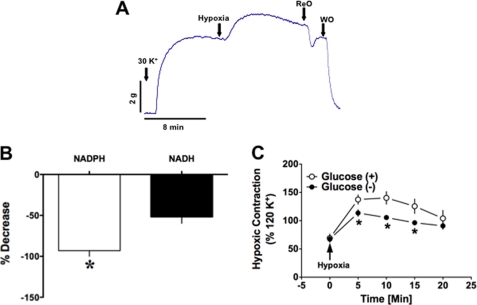
When PA rings precontracted with KCl (30 mmol/liter) under normoxia (21% O2, 5% CO2; 140 torr) were exposed to hypoxia (95% N2, 5% CO2; 10–20 torr), isometric force generation increased 30–50% (Fig. 1A). Incubating PA rings in Krebs-bicarbonate buffer in which the glucose was replaced with n-mannitol almost completely eliminated NADPH from the tissue, and NADH levels were also significantly reduced (Fig. 1B). These arteries also produced less force in response to hypoxia than PAs incubated in normal glucose-containing buffer (Fig. 1C).
FIGURE 1.
Bovine PA is contracted upon reduction of pO2. A, representative trace illustrating the typical response of adult bovine PA to hypoxia (pO2 = 10–20 torr). PA precontracted with KCl (30 mmol/liter; n = 20) was exposed to hypoxia (20 min) followed by reoxygenation (ReO) and then washout (WO) with normal buffer. *, p < 0.05. B and C, incubating PA in glucose-free Krebs buffer containing KCl (30 mmol/liter; n = 6) significantly reduced NAD(P)H levels (B) and suppressed HPV (C).
Glc-6-PD Is Activated, and NADP+-to-NADPH Ratios Are Decreased in PAs Exposed to Hypoxia
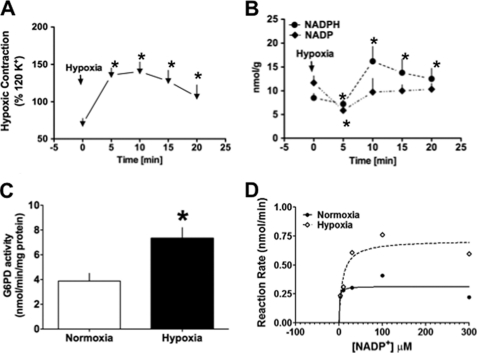
Glc-6-PD is a major source of NADPH, and the NADP+-to-NADPH ratio is an established indicator of Glc-6-PD activity. We therefore measured NADP+ and NADPH levels at several time points (5, 10, 15, and 20 min) during the development of HPV to establish the temporal relationship between changes in Glc-6-PD activity or pyridine nucleotide levels and hypoxic contraction of PA (Fig. 2, A and B). We found that NADP+ levels in PA declined substantially within the first 5 min of exposure to hypoxia and that the levels remained low for up to 20 min in hypoxia. Correspondingly, NADPH levels were increased at 5 min, and they remained elevated over the next 15 min of hypoxic contraction. Consistent with these findings, we found that hypoxia augmented Glc-6-PD activity (Fig. 2C) by increasing Vmax (Fig. 2D). Interestingly, we found that the apparent Km value (in μmol/liter: normoxia, 1.1; hypoxia, 7.9) was lower in normoxia as compared with hypoxia.
FIGURE 2.
NADPH redox status is altered in PA by hypoxia. A, time course of hypoxia-induced PA contraction (n = 5). B, estimated NADP+ and NADPH levels from PA pre-contracted with KCl (30 mmol/liter; n = 5) at the indicated time points during the course of HPV. A reduction in NADP+ levels from base line coincided with initiation of the HPV response, and a corresponding increase in NADPH was sustained for up to 20 min. C and D, in PA exposed to hypoxia for 10-min Glc-6-PD (G6PD) activity assay (C, n = 5), Michaelis-Menten graph (D, average of three experiments) shows Glc-6-PD activity was increased in PA exposed to hypoxia for 10 min. *, p < 0.05.
Hypoxia Inhibits Both Glycolysis and Oxidative Phosphorylation, and Glc-6-PD Is Activated by Inhibition of Pyruvate Dehydrogenase and Mitochondrial Complex I or III
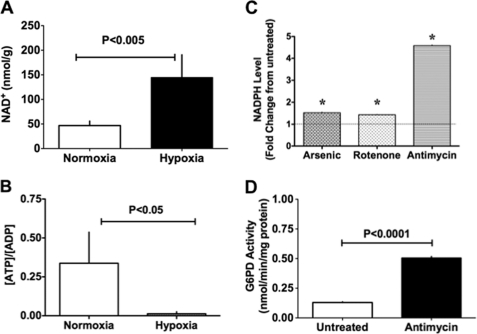
NADH and ATP are produced in the glycolytic pathway, Krebs cycle, and ETC. In PA, hypoxia increases glucose uptake by PASMCs but decreases aerobic glycolysis (12). Consistent with those findings, we observed that NAD+ levels (Fig. 3A), NAD+-to-NADH ratios (from 5.3 ± 0.1 to 23.7 ± 0.9), and lactate-to-pyruvate ratios (from 0.12 ± 0.04 to 0.51 ± 0.15) were increased in PA within 5 min after exposure to hypoxia. At the same time, the ATP-to-ADP ratio was significantly reduced (Fig. 3B). In addition, NAD(P)H levels were increased in PA homogenates pretreated with arsenic (10 μmol/liter), which inhibits pyruvate dehydrogenase, or with rotenone (10 μmol/liter) or antimycin (10 μmol/liter), which inhibit complexes I and III, respectively (Fig. 3C). In each case, a further increase in NAD(P)H was elicited by addition of 6-phosphogluconate (200 μmol/liter), a PPP metabolite produced by conversion of Glc-6-P. This suggests that Glc-6-P accumulating in cells due to inhibition of glycolysis and ETC was shunted into the PPP. To test that idea, we inhibited complex III using antimycin and then measured Glc-6-PD activity. As expected, Glc-6-PD activity in homogenates of PA pretreated with antimycin was also significantly increased (Fig. 3D).
FIGURE 3.
Effect of hypoxia on the PA metabolic status. A and B, NAD+, ADP, and ATP estimated in PA pre-contracted with KCl (30 mmol/liter; n = 5) and exposed to hypoxia for 5 min. Hypoxia increased NAD+ levels (A) and decreased ATP-to-ADP ratios (B), as compared with normoxia. C, NAD(P)H levels in homogenates of PA exposed to arsenic (10 μmol/liter; n = 5), rotenone (10 μmol/liter; n = 5), or antimycin (10 μmol/liter; n = 5). *, p < 0.05. D, effect of antimycin (10 μmol/liter; n = 5) on Glc-6-PD (G6PD) activity in PA.
Hypoxia Increases NADPH and ROS Generation in PA
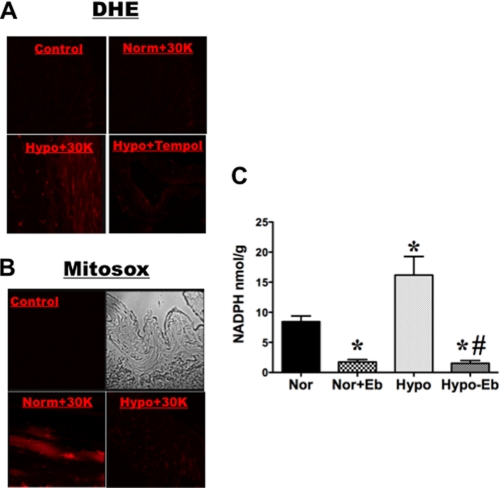
Because ROS are reported to stimulate Glc-6-PD activity (3), we tested whether elevation of ROS levels by mitochondrial or extramitochondrial oxidases was stimulating Glc-6-PD in PA exposed to hypoxia. Extramitochondrial and mitochondrial ROS generation was examined by loading PA with DHE or MitoSOX, respectively. DHE fluorescence, signaling extramitochondrial ROS, was higher in precontracted PAs exposed to hypoxia than in normoxic PAs, with or without KCl (30 mmol/liter) (Fig. 4A). By contrast, MitoSOX fluorescence, signaling mitochondrial ROS, was increased in KCl-contracted PAs under normoxia (Fig. 4B). Interestingly, the punctate MitoSOX signal disappeared, and nuclear fluorescence resembling DHE signals appeared when PAs were exposed to hypoxia. We also found that pretreating PAs with ebselen (100 μmol/liter), a glutathione peroxidase mimic that scavenges H2O2 (29), significantly reduced NADPH levels by about 69% in PAs exposed to normoxia (Fig. 4C). Ebselen also reduced NADPH levels under hypoxic conditions, decreasing them by about 70%, as compared with basal normoxic levels.
FIGURE 4.
ROS generation increases NADPH levels in PA. A and B, ROS production in PA under normoxic and hypoxic conditions was determined using DHE and MitoSOX probes. PA rings were loaded with either DHE (5 μmol/liter; n = 5) or MitoSOX (5 μmol/liter; n = 5) and contracted with KCl (30 mmol/liter) for 10 min in 21% O2 (Norm+30K), 0% O2 (Hypo+30K), or Tempol (10 μmol/liter) + 0% O2 (Hypo+30K), after which the rings were quickly embedded in OCT media. Untreated PA rings served as time-matched controls (Control). B immunofluorescence (left panels) and bright field (right panels) of control are shown. C, ebselen (Eb) (100 μmol/liter; n = 8) treatment reduced NADPH levels in normoxic (Nor) and hypoxic (Hypo) PA rings pre-treated with KCl (30 mmol/liter). *, p < 0.05 versus normoxia; #, p < 0.05 versus hypoxia.
HPV Is Inhibited by Silencing Glc-6-PD Expression
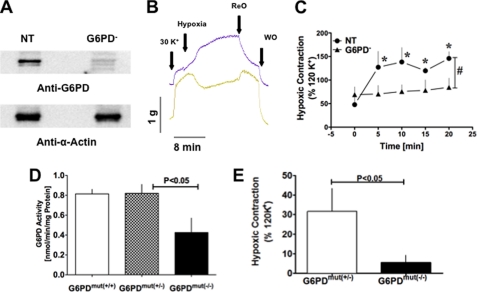
To assess the involvement of Glc-6-PD in the development of HPV, we knocked down Glc-6-PD expression in PA rings by transfecting them with a pool of siRNAs (see sequence in Table 1) targeting four exons in the Glc-6-PD gene. Transfection with Glc-6-PD siRNA for 60 h almost completely eliminated expression of the Glc-6-PD protein, whereas scrambled (nontargeting; NT) siRNA had no effect (Fig. 5A). Notably, silencing Glc-6-PD completely abolished HPV, and in some cases PAs actually dilated in response to hypoxia (Fig. 5, B and C).
FIGURE 5.
Effect of reduced PA Glc-6-PD expression on HPV. A, representative Western blot showing knockdown of Glc-6-PD (G6PD) expression by siRNA targeting Glc-6-PD but not by scrambled (NT) siRNA. Expression of α-actin, a cytoskeletal protein, was unaffected by Glc-6-PD siRNA. B, traces showing a typical response to hypoxia by PA transfected with NT (blue line) and Glc-6-PD (yellow line) siRNA. ReO, reoxygenation; WO, washout. C, group data showing the effects of NT and Glc-6-PD siRNA on HPV (n = 10). *, p < 0.05. D, Glc-6-PD activity was significantly lower in lungs from Glc-6-PDmut(−/−) mice than Glc-6-PDmut(+/−) or Glc-6-PDmut(+/+) mice (n = 6). E, hypoxia-induced PA contraction was significantly weaker in Glc-6-PDmut(−/−) than Glc-6-PDmut(+/−) mice (n = 6).
Additionally, we also studied the contractile responses of PAs obtained from a Glc-6-PD-deficient mouse model to determine whether response of PAs from these animals was similar to those of siRNA-treated cow PAs. In this Glc-6-PD-deficient model, a mutation in the 5′-promoter region of Glc-6-PD gene (Glc-6-PDmut) leads to a 50% reduction in the activity of Glc-6-PD in mice homozygous for the mutant gene (Glc-6-PDmut(−/−)) (Fig. 5D). HPV was markedly reduced in PAs isolated from Glc-6-PDmut(−/−) lungs, as compared with those harvested from Glc-6-PDmut(+/−) lungs (Fig. 5E).
To find out if knockdown of Glc-6-PD affects the activity of NADPH-dependent enzymes, we estimated GSH reductase activity in Glc-6-PD-silenced PA and -deficient lungs. Reduction in Glc-6-PD did not affect the activity of GSH reductase as compared with their respective controls (Table 2).
TABLE 2.
Glutathione reductase activity in lungs and in pulmonary arteries
| Sample | Glutathione reductase activity |
|---|---|
| nmol/min/mg protein | |
| PA untransfected | 120 ± 18 |
| PA nontargeting | 205 ± 38 |
| PA Glc-6-PD− | 178 ± 55 |
| Glc-6-PDmut(+/+) | 12 ± 2 |
| Glc-6-PDmut(+/−) | 16 ± 6 |
| Glc-6-PDmut(−/−) | 11 ± 3 |
KCl- and Serotonin-induced, Ca2+-dependent and -independent PA Contraction Are Increased by Hypoxia and Blocked by ROCK Inhibitor, Y27632, and Glc-6-PD Inhibitor, 6-Aminonicotinamide (6-AN)
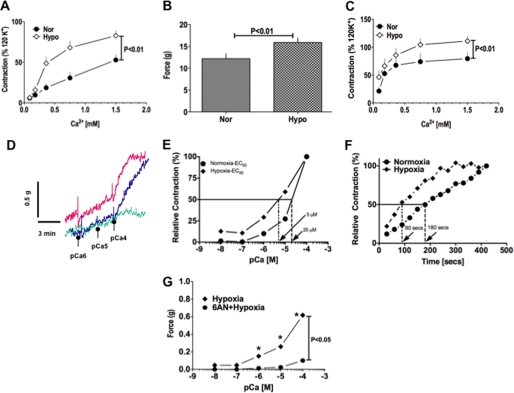
To investigate how hypoxia affects KCl-induced and receptor-mediated PA contraction, we first tested the effect of KCl (30 mmol/liter) on PA contractility in the absence of extracellular Ca2+ under normoxic and hypoxic conditions. In Ca2+-free solution, contraction of PA rings induced by KCl in normoxia and hypoxia did not differ. As the Ca2+ concentration was increased, however, PA contractions elicited under hypoxia were significantly greater than those elicited under normoxia (Fig. 6A). We next assessed the effect of hypoxia on receptor-mediated PA contraction by determining its effects on serotonin (5-HT; 10 μmol/liter)-induced PA contraction. When we applied 5-HT to PA in the absence of extracellular Ca2+, contractions elicited in hypoxia were significantly larger than those elicited in normoxia (Fig. 6B). PAs contracted in Ca2+-free solution relaxed to base-line levels in the continuous presence of 5-HT within 10–20 min. Once the PA had relaxed completely, addition of Ca2+ (0.09–1.5 mmol/liter) contracted the arteries in a concentration-dependent manner; at each concentration, moreover, the contractile responses were greater in hypoxia than normoxia (Fig. 6C). Ca2+-dependent contractions of PA evoked by KCl and 5-HT in hypoxia were reduced (p < 0.05) by Y27632 (10 μmol/liter) to 10 ± 2 and 30 ± 5%, respectively, of untreated controls, thereby suggesting activation of ROCK increased Ca2+ sensitivity to the contractile apparatus. Therefore, to determine the role of Glc-6-PD in hypoxia-induced increases in the Ca2+ sensitivity of the contractile apparatus, we used a skinned PA preparation to assess the effects of hypoxia in the absence and presence of 6-AN. PA rings were first maximally contracted with KCl (120 mmol/liter) and then skinned. The contractile responses elicited at intracellular Ca2+ concentrations ([Ca2+]i) ranging from pCa 7 to 4 were then determined under normoxia, after which the protocol was repeated under hypoxia. We found that the Ca2+ concentration-response curve was clearly shifted to the left by hypoxia, and the Ca2+ concentration required to contract PA to 50% of maximum was decreased 4-fold (Fig. 6, D and E). Additionally, the time required to contract the PA to 50% of maximum at pCa 4 was reduced by half under hypoxia (Fig. 6F). Inhibition of Glc-6-PD using 6-AN completely abolished Ca2+-induced PA contraction under hypoxia (Fig. 6G).
FIGURE 6.
Hypoxia augments Ca2+-independent and -dependent contraction of PA. A, hypoxia (Hypo) augmented PA contractions evoked by KCl (30 mmol/liter; n = 25) in Ca2+-free solution and after addition of Ca2+ to the tissue bath. Nor, normoxia. B, hypoxia enhanced contraction evoked by 5-HT (10 μmol/liter; n = 20) in Ca2+-free solution and after addition of Ca2+ to the tissue bath. C, addition of Ca2+ to the tissue bath 20 min after the initial 5-HT-evoked PA contraction in Ca2+-free buffer elicited a robust contraction in normoxia and hypoxia. D, representative concentration-response curves showing Ca2+-induced contraction of permeabilized PA. 6-AN (5 mmol/liter; green trace) significantly reduced the Ca2+ sensitivity of the myofilaments, which was otherwise increased by hypoxia (blue trace). The red trace is a normoxic control. E, hypoxia reduced the Ca2+ concentrations required to contract skinned PA (n = 5) by 4-fold, as compared with normoxia. F, time course of PA contractions (n = 5) evoked by pCa 4 were accelerated by hypoxia. G, 6-AN completely blocked contraction of skinned PA (n = 5) by decreasing the sensitization to Ca2+, which was otherwise enhanced by hypoxia.
KCl- and Serotonin-induced, Ca2+-dependent and -independent CPI-17 and MLC Phosphorylation Are Increased by Hypoxia, and Phosphorylation of MLC but Not of CPI-17 Is Blocked by 6-AN
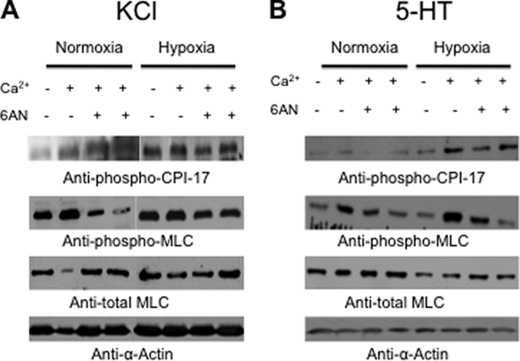
We also found that, under hypoxia, KCl significantly increased PA levels of phosphorylated (activated) CPI-17, a protein kinase C-dependent inhibitor of myosin phosphatase (30). This effect was Ca2+-independent (Fig. 7A, top blot, 1st lane), but upon addition of Ca2+ (0.375 or 0.75 mmol/liter), KCl-induced phosphorylation of CPI-17 was further enhanced under both normoxic and hypoxic conditions (Fig. 7A, top blot, 2nd and 3rd lanes). Consistent with this observation, KCl also increased phosphorylation of MLC under hypoxic conditions in the absence of Ca2+ (Fig. 7A, 2nd blot, 1st lane). This effect was enhanced by addition of 0.375 mmol/liter Ca2+, which evokes submaximal contractions in PA (see Fig. 7A), but was diminished when the Ca2+ concentration was increased to 0.75 mmol/liter. Notably, whereas pretreatment with 6-AN (1 mmol/liter), a Glc-6-PD inhibitor, had no effect on KCl-induced increases in phospho-CPI-17 (Fig. 7A, top blot, 2nd and 4th lanes), it inhibited KCl-induced increases in phospho-MLC under both normoxic and hypoxic conditions (Fig. 7A, 2nd blot, 2nd and 4th lanes). The neurotransmitter 5-HT is a potent vasoconstrictor whose levels in PA have been shown to be elevated in PH (31, 32). In the presence of extracellular Ca2+, levels of both CPI-17 and MLC phosphorylation were increased by 5-HT. As with contraction, these effects were enhanced by hypoxia (Fig. 7B, top and 2nd blot), and although pretreatment with 6-AN had no effect on phospho-CPI-17 levels, it blocked 5-HT-induced increases in phospho-MLC (Fig. 7B).
FIGURE 7.
Hypoxia augments Ca2+-independent and -dependent phosphorylation of MLC PA. A, representative Western blot of phospho-CPI-17 (top blot) and phospho-MLC (2nd blot) is shown. Levels of phospho-CPI-17 and phospho-MLC normalized by α-actin were increased (80 ± 16 and 55 ± 6%, respectively; n = 5) by hypoxia in a Ca2+-independent manner (1st lane, normoxia and hypoxia) but were further enhanced (40 ± 2% and 20 ± 1%, respectively) by addition of Ca2+ (0.375 mmol/liter; 2nd lane, normoxia and hypoxia). 6-AN (5 mmol/liter; n = 5) blocked the hypoxia-induced increase in phospho-MLC in the presence of Ca2+ by 50–60% but did not prevent the increase in phospho-CPI-17 (3rd and 4th lanes, normoxia and hypoxia). B, representative Western blot of phospho-CPI-17 (top blot) and phospho-MLC (2nd blot) is shown. Conventions are the same as in A (n = 5).
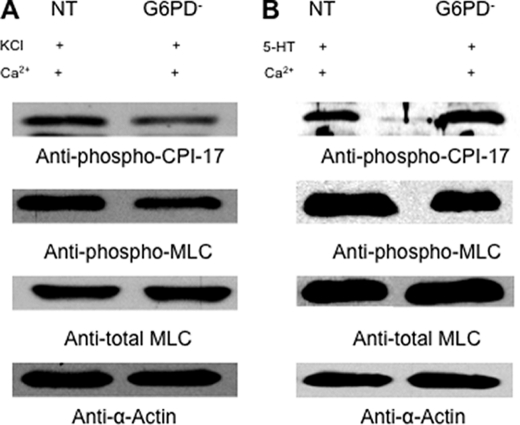
Knocking Down Glc-6-PD Expression Blocks Hypoxia-induced Increases in KCl- and 5-HT-evoked PA Contractions and MLC Phosphorylation
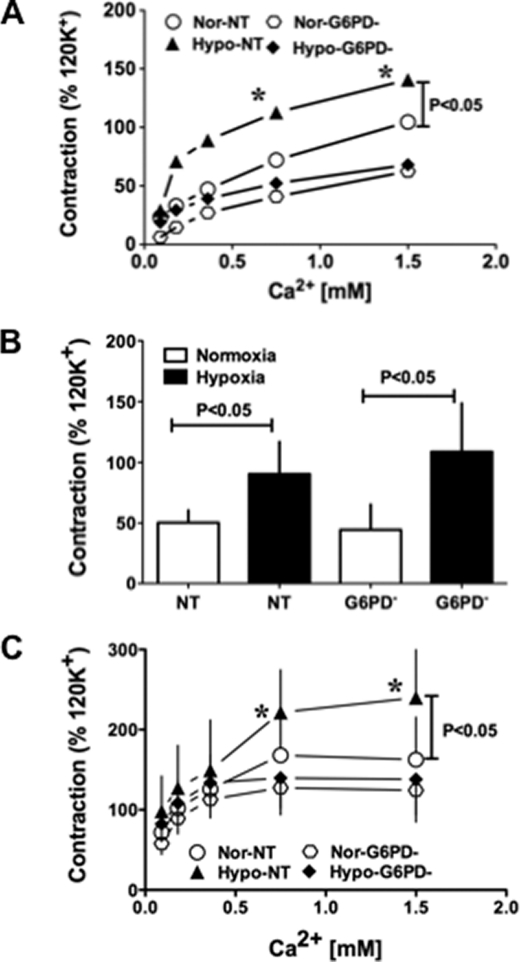
To further clarify the role of Glc-6-PD in activation of Ca2+-dependent PA contraction under hypoxic conditions, we assessed the effects of KCl and 5-HT on PA transfected with NT or Glc-6-PD siRNA. KCl did not evoke contraction of PA in Ca2+-free solution, but addition of Ca2+ (0.09–1.5 mmol/liter) increased KCl-induced PA contractions in a concentration-dependent manner. These contractions were significantly enhanced by hypoxia in NT-transfected PA (Fig. 8A). Knocking down Glc-6-PD expression reduced the contractile response of PA at all Ca2+ concentrations examined and abolished the hypoxia-induced increase in PA contraction. Moreover, CPI-17 and MLC phosphorylation stimulated by KCl in the presence of Ca2+ (0.375 mmol/liter) and hypoxic contraction was reduced in PA transfected with Glc-6-PD siRNA (Fig. 9A).
FIGURE 8.
Hypoxia-induced, Ca2+-independent and -dependent PA contraction is dependent on Glc-6-PD. A, KCl (30 mmol/liter)-induced contraction of PA treated with scrambled siRNA (NT; n = 8) or Glc-6-PD siRNA (Glc-6-PD−; n = 8) after addition of Ca2+ to the tissue bath under normoxic (Nor) and hypoxic (Hypo) conditions. Glc-6-PD (G6PD) knockdown completely abolished the hypoxia-induced augmentation of contractions at all concentrations of Ca2+. B, 5-HT-induced contraction of PA treated with NT (n = 6) or Glc-6-PD (n = 6) siRNA in the absence of extracellular Ca2+ was significantly increased by hypoxia. C, knocking down Glc-6-PD blocked hypoxia-induced augmentation of PA contractions elicited by addition of extracellular Ca2+. *, p < 0.05.
FIGURE 9.
Hypoxia-induced, Ca2+-independent and -dependent phosphorylation of MLC in PA is dependent on Glc-6-PD. A, representative Western blot showing that knocking down Glc-6-PD (G6PD) significantly reduced levels (normalized by α-actin) of KCl (30 mmol/liter)-induced phospho-CPI-17 (70 ± 12% of NT) and phospho-MLC (48 ± 6% of NT) in PA exposed to hypoxia. B, representative Western blot showing that knocking down Glc-6-PD significantly reduced (56 ± 3% of NT) levels of 5-HT (10 μmol/liter)-induced phospho-MLC in PAs exposed to hypoxia. The experiment was repeated four times with similar results.
The effect of knocking down Glc-6-PD on 5-HT-induced PA contraction in the absence and presence of extracellular Ca2+ was also tested. The results shown in Fig. 8B indicate that PA contractions evoked by 5-HT in the absence of extracellular Ca2+ were increased by hypoxia in PA treated with either NT or Glc-6-PD siRNA. On the other hand, Glc-6-PD knockdown abolished the hypoxia-induced enhancement of 5-HT-evoked PA contraction in the presence of Ca2+ (Fig. 8C). Western blot analysis of phospho-CPI and -MLC levels in PA (Fig. 9B) showed that, under hypoxia, levels of phospho-CPI-17 (Fig. 9B, top blot) were unaffected by Glc-6-PD knockdown, but phospho-MLC levels (Fig. 9B, 2nd blot) were reduced in PA exposed to KCl plus Ca2+ (0.375 mmol/liter).
HPV Is Positively Correlated with Glc-6-PD Activity in PA Prior to Exposure to Hypoxia
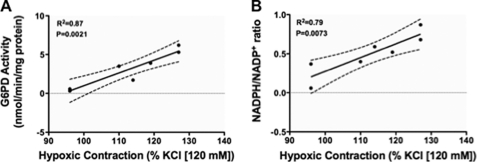
After dividing PA segments into three parts, Glc-6-PD activity and NADPH-to-NADP+ ratios were determined in two parts, respectively, and the third part was contracted with KCl (30 mmol/liter) under hypoxia. Subsequent linear regression analysis showed a positive correlation between Glc-6-PD activity and HPV (Fig. 10A), and a similar relationship was found between the NADPH-to-NADP+ ratio and HPV (Fig. 10B).
FIGURE 10.
Glc-6-PD activity is positively correlated with HPV. A and B, regression analysis relating Glc-6-PD (G6PD) activity (A) and NADPH-to-NADP+ ratios (B) to peak contractions elicited by exposure to hypoxia for 10 min.
DISCUSSION
PA contraction in response to airway hypoxia is an immediate physiological response to redirect blood flow from poorly ventilated regions of the lungs to well oxygenated regions. Although it is now clear that both the O2 sensor and the effector molecules mediating this response reside in the PASMCs, the O2 sensor has not yet been identified nor has the mechanism responsible for initiating HPV. In this study, we provide novel evidence that activation of Glc-6-PD evokes HPV, and we suggest that Glc-6-PD is a link between hypoxia-induced inhibition of glycolysis and mitochondrial ETC and the development of HPV.
In PA smooth muscle, intracellular glucose is metabolized through glycolysis (12). Incubation of PA in glucose-free solution for 30 min almost completely (∼95–98%) depleted the artery of NADPH and reduced NADH by 50%. At the same time, there was a significant reduction in the peak and sustained phases of hypoxia-induced PA contraction. This suggests that HPV is signaled, at least in part, by a change in glycolysis and PPP activity. Previously, Leach et al. (11), reported that depletion of glucose or inhibition of glycolysis abolishes the sustained phase (phase 2) of HPV in rat small intrapulmonary arteries, and they concluded that inhibition of glycolysis and mitochondrial ETC is responsible for O2 sensing and initiating HPV. Interestingly, our estimation of pyridine nucleotide revealed that a transient decrease in NADP+ levels coincided with the initiation of HPV and that a decrease in NADP+ and a corresponding increase in NADPH were sustained throughout the period of hypoxia. Consistent with that finding, decreases in pO2 reportedly lead to increases in the NADPH-to-NADP+ ratio in isolated rat PA and lungs (13, 14). Moreover, the PPP plays a key role in vascular smooth muscle metabolism (33), and increases in Glc-6-PD expression and activity in bovine PA smooth muscle promotes  production (23). Our present finding (Fig. 1C) that NADPH levels are depleted in the absence of glucose is consistent with earlier reports that inhibition of Glc-6-PD reduces HPV in rat lungs (13, 14), and activation of Glc-6-PD by hypoxia (Fig. 2, C and D) suggests Glc-6-PD and the PPP play key roles in initiating and maintaining HPV.
production (23). Our present finding (Fig. 1C) that NADPH levels are depleted in the absence of glucose is consistent with earlier reports that inhibition of Glc-6-PD reduces HPV in rat lungs (13, 14), and activation of Glc-6-PD by hypoxia (Fig. 2, C and D) suggests Glc-6-PD and the PPP play key roles in initiating and maintaining HPV.
PASMCs derive energy from metabolism of glucose through aerobic glycolytic and glycogenolytic pathways (34, 35), and it is likely that aerobic glycolysis would be the first reaction to stop in PASMCs under hypoxic conditions. Consistent with that idea, we found that NAD+ accumulated and the ATP-to-ADP ratio significantly declined within 5 min after exposing PA to hypoxia. Although it has been reported that ATP levels are unaffected by acute short term hypoxia, recent studies have shown that AMP levels are elevated in rat PA under hypoxia (36), and our present results indicate there is indeed a drop in the ATP-to-ADP ratio. The following were also shown: 1) HPV is accentuated by inhibition of glycolysis in isolated perfused rat lungs (5); 2) high glucose levels potentiate HPV in perfused ferret lungs (37); and 3) the sustained late phase constriction (phase 2) of isolated PA is dependent on ATP derived from glycolysis (38). On the other hand, even though initiation of HPV is not dependent on oxidative phosphorylation, it has been proposed that mitochondrial ETC could be involved in O2 sensing (8, 9, 11). In that regard, Bell et al. (10) provided genetic and pharmacological evidence that the Qo site of complex III mediates transduction of the hypoxic signal via release of ROS. They observed that cells deficient in the complex III subunit cytochrome b, and therefore respiration-incompetent, do not show a hypoxia-induced increase in ROS. On the other hand, inhibition of complex I with rotenone, or complex III with antimycin or myxothiazol, reportedly augments ROS production (particularly  ), raises [Ca2+]i, and initiates HPV (8, 9, 11).
), raises [Ca2+]i, and initiates HPV (8, 9, 11).
Glc-6-PD is known to be activated by ROS (39) and by GSSG, which exerts a fine control of the PPP by counteracting the NADPH inhibition of Glc-6-PD (40). Thus, mitochondrial ROS generation associated with inhibition of glycolysis and mitochondrial ETC would likely increase cytosolic Glc-6-PD activity. Although it is accepted that mitochondrial ROS are involved in mediating HPV, whether their levels increase or decrease is somewhat controversial (3, 6, 7). Also, there is disagreement about the source of ROS in hypoxic PA, as studies have shown that both mitochondrial and NADPH oxidase-derived ROS may be involved in hypoxic PA contraction (3). To address that issue, we used DHE and MitoSOX, respectively, to assess extramitochondrial and mitochondrial  formation in hypoxic PA. Our findings suggest that cytosolic but not mitochondrial ROS increase under hypoxia in PA. We make this suggestion with caution because in addition to oxidation by
formation in hypoxic PA. Our findings suggest that cytosolic but not mitochondrial ROS increase under hypoxia in PA. We make this suggestion with caution because in addition to oxidation by  , DHE and MitoSOX are also oxidized by cytochrome c3+, independently of mitochondrial
, DHE and MitoSOX are also oxidized by cytochrome c3+, independently of mitochondrial  (41). We therefore cannot rule out the possibility that the observed decrease in MitoSOX fluorescence in hypoxic PA was a reflection of attenuated MitoSOX oxidation by cytochrome c3+ (42, 43). Nonetheless, the observed increase in DHE fluorescence supports the hypothesis that
(41). We therefore cannot rule out the possibility that the observed decrease in MitoSOX fluorescence in hypoxic PA was a reflection of attenuated MitoSOX oxidation by cytochrome c3+ (42, 43). Nonetheless, the observed increase in DHE fluorescence supports the hypothesis that  generation is elevated in hypoxic PA (9, 44) and is strikingly opposite the observations that extramitochondrial
generation is elevated in hypoxic PA (9, 44) and is strikingly opposite the observations that extramitochondrial  levels are decreased in hypoxic coronary artery (45). Because it is Glc-6-PD that supplies the NADPH to NADPH oxidases (23), ROS-induced activation of Glc-6-PD should feed back positively to further amplify ROS generation in hypoxic PA, which is consistent with the idea that ROS generation in hypoxic PA is mediated, at least in part, by Glc-6-PD.
levels are decreased in hypoxic coronary artery (45). Because it is Glc-6-PD that supplies the NADPH to NADPH oxidases (23), ROS-induced activation of Glc-6-PD should feed back positively to further amplify ROS generation in hypoxic PA, which is consistent with the idea that ROS generation in hypoxic PA is mediated, at least in part, by Glc-6-PD.
To clarify further the role of Glc-6-PD in HPV, we knocked down Glc-6-PD expression using siRNA in cultured PA. Glc-6-PD expression was reduced by 70–80% in fourth-order PA rings (0.1 mm in diameter and 1 mm in length) transfected with Glc-6-PD siRNA. In these arteries, HPV was not only inhibited but in some cases the KCl-precontracted vessels actually dilated in response to hypoxia. Glc-6-PD expression and activity were similarly reduced in Glc-6-PDmut(−/−) mice, and there was a corresponding inhibition of HPV. Silencing or deficiency of Glc-6-PD could induce compensatory changes in NADPH-dependent enzymes or other redox-sensitive pathways and affect PA function. Hence, we estimated GSH reductase activity in Glc-6-PD-deficient PA and lungs. We found that Glc-6-PD silencing or deficiency did not alter GSH reductase activity in PA and in lungs (Table 2). However, compensation in and their role in HPV and PH remain to be explored. Taken together, these results further confirm that hypoxia-induced activation of Glc-6-PD is one of the signals through which the O2 sensor communicates with the effectors that ultimately evoke the HPV response.
Hypoxia-induced activation of Ca2+ entry through voltage-gated and store-operated Ca2+ channels (6, 7) and increased myofilament Ca2+ sensitivity are thought to be the major mechanisms by which HPV and PH are induced (7). With respect to the increased Ca2+ sensitivity, hypoxia reportedly increases phosphorylation of CPI-17 (30) and activates RhoA-ROCK, which phosphorylates MYPT1 (46), thereby inactivating myosin phosphatase. This reduces MLC dephosphorylation, increasing the Ca2+ sensitivity of the contractile apparatus and enhancing force generation. We found that hypoxia augmented PA contraction at all Ca2+ concentrations and shifted the concentration-response curve to the left. Similarly, hypoxia augmented contractions evoked by 5-HT, which initially stimulates Ca2+ release from internal stores via activation of 5-HT1, 5-HT2, and 5-HT3 receptors and then stimulates Ca2+ influx through voltage-gated cation channels or perhaps through store-operated Ca2+ entry channels (47, 48). In addition, both KCl and 5-HT have been reported to increase the Ca2+ sensitivity of myofilaments through activation of CPI-17-dependent and ROCK-dependent pathways (49–51). Our present findings indicate that hypoxia increases phosphorylation of CPI-17 and MLC stimulated by KCl in the absence of Ca2+, and these increases were further enhanced by addition of Ca2+. Although hypoxia enhanced CPI-17 phosphorylation elicited by 5-HT, it did not affect the phosphorylation status of MLC in the absence of Ca2+. From this we infer that there are differences in the phosphorylation of MLC by KCl and 5-HT under hypoxia, which we speculate reflects a difference in their abilities to activate RhoA-ROCK. Nonetheless, our results suggest that both Ca2+-independent and -dependent pathways are involved in mediating HPV.
We also found that inhibition of Glc-6-PD had no effect on KCl- or 5-HT-induced phosphorylation of CPI-17 evoked under normoxia or hypoxia. More importantly, we found that pretreating PA with a Glc-6-PD inhibitor (6-AN) reduced Ca2+-dependent increases in MLC phosphorylation evoked by KCl and 5-HT under hypoxia or normoxia. In another series of experiments in which we clamped [Ca2+]i, we observed that the time required to elicit half-maximal PA contraction at a fixed [Ca2+]i was accelerated by hypoxia, and the Ca2+ concentration-response curve was significantly shifted to the left. Notably, this hypoxia-induced Ca2+ sensitization was significantly inhibited by inhibition of Glc-6-PD. Moreover, knocking down Glc-6-PD expression using siRNA blocked the hypoxia-induced increase in Ca2+-dependent MLC phosphorylation and PA contraction. Neither the hypoxia-induced increase of 5-HT-evoked PA contraction in Ca2+-free solution nor hypoxia-induced CPI-17 phosphorylation was affected by Glc-6-PD knockdown. Thus, Glc-6-PD appears to be involved in increasing both Ca2+ entry and Ca2+ sensitization of the myofilaments under hypoxic conditions. That Glc-6-PD activity and the NADPH-to-NADP+ ratio in PA were positively correlated with peak HPV suggests that up-regulated Glc-6-PD expression likely contributes significantly to chronic HPV and, in turn, PH.
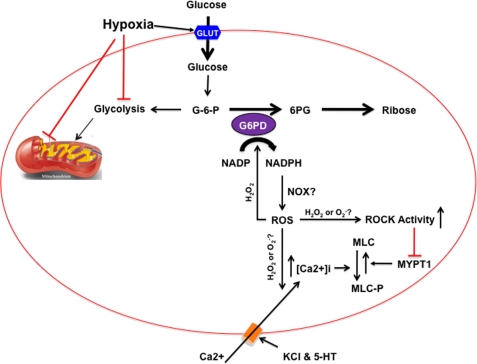
In conclusion, as shown in the schematic diagram in Fig. 11, we provide the following novel evidence: 1) Glc-6-PD is a potential link between the O2 sensor and effectors responsible for HPV; 2) inhibition of Glc-6-PD activity or expression decreases the hypoxia-induced increase in the Ca2+ sensitivity of the myofilaments by attenuating MLC phosphorylation mediated via a CPI-17-independent, ROCK-dependent pathway; and 3) hypoxia-induced Glc-6-PD activation plays a significant role in HPV by activating extracellular Ca2+-dependent contraction. We therefore propose that Glc-6-PD and NADPH redox are crucially involved in the mechanism of HPV and, in turn, may play a key role in the development of PH.
FIGURE 11.
Schematic diagram illustrating the potential role played by Glc-6-PD during the development of HPV. Hypoxia increases glucose uptake (via glucose transporter (GLUT)) and inhibits glycolysis and mitochondrial ETC. As a result, glucose 6-phosphate (G-6-P) is shunted into the PPP, due to simultaneous activation of glucose-6-phosphate dehydrogenase (G6PD) and inhibition of e− flow-through the mitochondrial ETC complex III, in the PASMC. Increase in Glc-6-PD activity elevates intracellular NADPH, which is a reducing co-factor for numerous enzymes, including NADPH oxidases (NOX). NADPH enhances reactive oxygen species (ROS) generation. Elevated hydrogen peroxide (H2O2) stimulates Glc-6-PD in a feed-forward reaction. Superoxide ( ) anion and/or H2O2 activates RhoA-ROCK and inhibits myosin light chain phosphate (MYPT1), enhances intracellular [Ca2+]i via influx and release, and increases myosin light chain phosphorylation (MLC-P). Increase in MLC phosphorylation causes contraction of PASMC and promotes hypoxic pulmonary vasoconstriction.
) anion and/or H2O2 activates RhoA-ROCK and inhibits myosin light chain phosphate (MYPT1), enhances intracellular [Ca2+]i via influx and release, and increases myosin light chain phosphorylation (MLC-P). Increase in MLC phosphorylation causes contraction of PASMC and promotes hypoxic pulmonary vasoconstriction.
Acknowledgments
We thank Drs. Jane Leopold and Joseph Loscalzo, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, for their generous supply of Glc-6-PD-deficient mice.
This work was supported, in whole or in part, by National Institutes of Health Grants HL085352, HL31069, HL43023, and HL66331.
- PH
- pulmonary hypertension
- HPLC
- high pressure liquid chromatography
- HPV
- hypoxic pulmonary vasoconstriction
- PA
- pulmonary artery
- siRNA
- small interfering RNA
- ETC
- electron transport chain
- Glc-6-PD
- glucose 6-phosphate dehydrogenase
- ROS
- reactive oxygen species
- MLC
- myosin light chain
- PASMC
- pulmonary artery smooth muscle cell
- 6-AN
- 6-aminonicotinamide
- 5-HT
- serotonin
- PPP
- pentose phosphate pathway
- NT
- nontargeting
- DHE
- dihydroethidium.
REFERENCES
- 1.Sommer N., Dietrich A., Schermuly R. T., Ghofrani H. A., Gudermann T., Schulz R., Seeger W., Grimminger F., Weissmann N. (2008) Eur. Respir. J. 32, 1639–1651 [DOI] [PubMed] [Google Scholar]
- 2.Stenmark K. R., Fagan K. A., Frid M. G. (2006) Circ. Res. 99, 675–691 [DOI] [PubMed] [Google Scholar]
- 3.Gupte S. A., Wolin M. S. (2008) Antioxid. Redox. Signal. 10, 1137–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMurtry I. F., Rounds S., Stanbrook H. S. (1982) Adv. Shock Res. 8, 21–33 [PubMed] [Google Scholar]
- 5.Stanbrook H. S., McMurtry I. F. (1983) J. Appl. Physiol. 55, 1467–1473 [DOI] [PubMed] [Google Scholar]
- 6.Archer S. L., Gomberg-Maitland M., Maitland M. L., Rich S., Garcia J. G., Weir E. K. (2008) Am. J. Physiol. Heart. Circ. Physiol. 294, H570–H578 [DOI] [PubMed] [Google Scholar]
- 7.Ward J. P., McMurtry I. F. (2009) Curr. Opin. Pharmacol. 9, 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waypa G. B., Marks J. D., Mack M. M., Boriboun C., Mungai P. T., Schumacker P. T. (2002) Circ. Res. 91, 719–726 [DOI] [PubMed] [Google Scholar]
- 9.Waypa G. B., Guzy R., Mungai P. T., Mack M. M., Marks J. D., Roe M. W., Schumacker P. T. (2006) Circ. Res. 99, 970–978 [DOI] [PubMed] [Google Scholar]
- 10.Bell E. L., Klimova T. A., Eisenbart J., Moraes C. T., Murphy M. P., Budinger G. R., Chandel N. S. (2007) J. Cell Biol. 177, 1029–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leach R. M., Hill H. M., Snetkov V. A., Robertson T. P., Ward J. P. (2001) J. Physiol. 536, 211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leach R. M., Hill H. S., Snetkov V. A., Ward J. P. (2002) Respir. Physiol. Neurobiol. 132, 55–67 [DOI] [PubMed] [Google Scholar]
- 13.Gupte S. A., Li K. X., Okada T., Sato K., Oka M. (2002) J. Pharmacol. Exp. Ther. 301, 299–305 [DOI] [PubMed] [Google Scholar]
- 14.Gupte S. A., Okada T., McMurtry I. F., Oka M. (2006) Pulm. Pharmacol. Ther. 19, 303–309 [DOI] [PubMed] [Google Scholar]
- 15.Lee S., Park M., So I., Earm Y. E. (1994) Pflugers Arch. 427, 378–380 [DOI] [PubMed] [Google Scholar]
- 16.Park M. K., Bae Y. M., Lee S. H., Ho W. K., Earm Y. E. (1997) Pflugers Arch. 434, 764–771 [DOI] [PubMed] [Google Scholar]
- 17.Peri R., Wible B. A., Brown A. M. (2001) J. Biol. Chem. 276, 738–741 [DOI] [PubMed] [Google Scholar]
- 18.Tipparaju S. M., Barski O. A., Srivastava S., Bhatnagar A. (2008) Biochemistry 47, 8840–8854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Post J. M., Hume J. R., Archer S. L., Weir E. K. (1992) Am. J. Physiol. 262, C882–C890 [DOI] [PubMed] [Google Scholar]
- 20.Homma N., Nagaoka T., Karoor V., Imamura M., Taraseviciene-Stewart L., Walker L. A., Fagan K. A., McMurtry I. F., Oka M. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 295, L71–L78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oka M., Karoor V., Homma N., Nagaoka T., Sakao E., Golembeski S. M., Limbird J., Imamura M., Gebb S. A., Fagan K. A., McMurtry I. F. (2007) Cardiovasc. Res. 74, 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet S., Dumas-de-La-Roque E., Bégueret H., Marthan R., Fayon M., Dos Santos P., Savineau J. P., Baulieu E. E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9488–9493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupte S. A., Kaminski P. M., Floyd B., Agarwal R., Ali N., Ahmad M., Edwards J., Wolin M. S. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H13–H21 [DOI] [PubMed] [Google Scholar]
- 24.Ozaki H., Gerthoffer W. T., Publicover N. G., Fusetani N., Sanders K. M. (1991) J. Physiol. 440, 207–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauffman F. C., Brown J. G., Passonneau J. V., Lowry O. H. (1969) J. Biol. Chem. 244, 3647–3653 [PubMed] [Google Scholar]
- 26.Gupte S. A., Arshad M., Viola S., Kaminski P. M., Ungvari Z., Rabbani G., Koller A., Wolin M. S. (2003) Am. J. Physiol. Heart Circ. Physiol. 285, H2316–H2326 [DOI] [PubMed] [Google Scholar]
- 27.Wardle R. L., Gu M., Ishida Y., Paul R. J. (2006) J. Physiol. 572, 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato K., Leposavic R., Publicover N. G., Sanders K. M., Gerthoffer W. T. (1994) J. Physiol. 481, 677–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohazzab-H K. M., Agarwal R., Wolin M. S. (1999) Am. J. Physiol. 276, H235–H241 [DOI] [PubMed] [Google Scholar]
- 30.Fagan K. A., Oka M., Bauer N. R., Gebb S. A., Ivy D. D., Morris K. G., McMurtry I. F. (2004) Am. J. Physiol. Lung Cell Mol. Physiol. 287, L656–L664 [DOI] [PubMed] [Google Scholar]
- 31.Esteve J. M., Launay J. M., Kellermann O., Maroteaux L. (2007) Cell Biochem. Biophys. 47, 33–44 [DOI] [PubMed] [Google Scholar]
- 32.Eddahibi S., Morrell N., d'Ortho M. P., Naeije R., Adnot S. (2002) Eur. Respir. J. 20, 1559–1572 [DOI] [PubMed] [Google Scholar]
- 33.Barron J. T., Glonek T., Messer J. V. (1986) Atherosclerosis 59, 57–62 [DOI] [PubMed] [Google Scholar]
- 34.Lynch R. M., Paul R. J. (1983) Science 222, 1344–1346 [DOI] [PubMed] [Google Scholar]
- 35.Paul R. J., Bauer M., Pease W. (1979) Science 206, 1414–1416 [DOI] [PubMed] [Google Scholar]
- 36.Evans A. M., Mustard K. J., Wyatt C. N., Peers C., Dipp M., Kumar P., Kinnear N. P., Hardie D. G. (2005) J. Biol. Chem. 280, 41504–41511 [DOI] [PubMed] [Google Scholar]
- 37.Wiener C. M., Sylvester J. T. (1991) J. Appl. Physiol. 70, 439–446 [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y., Packer C. S., Rhoades R. A. (1996) Exp. Lung Res. 22, 51–63 [DOI] [PubMed] [Google Scholar]
- 39.Pandolfi P. P., Sonati F., Rivi R., Mason P., Grosveld F., Luzzatto L. (1995) EMBO J. 14, 5209–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eggleston L. V., Krebs H. A. (1974) Biochem. J. 138, 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zielonka J., Srinivasan S., Hardy M., Ouari O., Lopez M., Vasquez-Vivar J., Avadhani N. G., Kalyanaraman B. (2008) Free Radic. Biol. Med. 44, 835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palacios-Callender M., Hollis V., Mitchison M., Frakich N., Unitt D., Moncada S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18508–18513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaManna J. C., Saive J. J., Snow T. R. (1980) Circ. Res. 46, 755–763 [DOI] [PubMed] [Google Scholar]
- 44.Ward J. P., Snetkov V. A., Aaronson P. I. (2004) Cell Calcium 36, 209–220 [DOI] [PubMed] [Google Scholar]
- 45.Gao Q., Wolin M. S. (2008) Am. J. Physiol. Heart Circ. Physiol. 295, H978–H989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oka M., Fagan K. A., Jones P. L., McMurtry I. F. (2008) Br. J. Pharmacol. 155, 444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin G. R. (1994) Pharmacol. Ther. 62, 283–324 [DOI] [PubMed] [Google Scholar]
- 48.Perez J. F., Sanderson M. J. (2005) J. Gen. Physiol. 125, 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Somlyo A. P., Somlyo A. V. (2000) J. Physiol. 522, 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Yoshioka K., Azam M. A., Takuwa N., Sakurada S., Kayaba Y., Sugimoto N., Inoki I., Kimura T., Kuwaki T., Takuwa Y. (2006) Biochem. J. 394, 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakurada S., Takuwa N., Sugimoto N., Wang Y., Seto M., Sasaki Y., Takuwa Y. (2003) Circ. Res. 93, 548–556 [DOI] [PubMed] [Google Scholar]