Abstract
There are four isoforms of the α subunit (α1–4) and three isoforms of the β subunit (β1–3) of Na,K-ATPase, with distinct tissue-specific distribution and physiological functions. α2 is thought to play a key role in cardiac and smooth muscle contraction and be an important target of cardiac glycosides. An α2-selective cardiac glycoside could provide important insights into physiological and pharmacological properties of α2. The isoform selectivity of a large number of cardiac glycosides has been assessed utilizing α1β1, α2β1, and α3β1 isoforms of human Na,K-ATPase expressed in Pichia pastoris and the purified detergent-soluble isoform proteins. Binding affinities of the digitalis glycosides, digoxin, β-methyl digoxin, and digitoxin show moderate but highly significant selectivity (up to 4-fold) for α2/α3 over α1 (KD α1 > α2 = α3). By contrast, ouabain shows moderate selectivity (≈2.5-fold) for α1 over α2 (KD α1 ≤ α3 < α2). Binding affinities for the three isoforms of digoxigenin, digitoxigenin, and all other aglycones tested are indistinguishable (KD α1 = α3 = α2), showing that the sugar determines isoform selectivity. Selectivity patterns for inhibition of Na,K-ATPase activity of the purified isoform proteins are consistent with binding selectivities, modified somewhat by different affinities of K+ ions for antagonizing cardiac glycoside binding on the three isoforms. The mechanistic insight on the role of the sugars is strongly supported by a recent structure of Na,K-ATPase with bound ouabain, which implies that aglycones of cardiac glycosides cannot discriminate between isoforms. In conclusion, several digitalis glycosides, but not ouabain, are moderately α2-selective. This supports a major role of α2 in cardiac contraction and cardiotonic effects of digitalis glycosides.
Keywords: ATPase; Cardiac Muscle; Drug Action; Membrane Protein; Na,K-ATPase; Cardiac Glycoside; Isoform Selectivity
Introduction
For more than 200 years congestive heart failure has been treated with the plant-derived digitalis cardiac glycosides such as digoxin, which increase force of contraction of failing cardiac muscle and reduce cardiac conduction rate. However, digoxin is now less used than in the past due to the narrow therapeutic window and drug toxicity. Cardiac glycosides are produced in mammals as well as plants. As reviewed recently (1) five different cardiac glycosides have been identified in mammalian tissues including the cardenolides ouabain and digoxin and the bufadienolides marinobufagenin, telocinobufagin, and 19-norbufalin. Cardiac glycosides inhibit Na,K-ATPase and control cellular Na+ and K+ gradients and also activate cellular signaling pathways that mediate control of gene expression and tissue growth (2, 3). Thus, a multiplicity of physiological functions could be affected by the multiplicity of endogenous cardiac glycosides, but this is not well understood (1, 4, 5).
The Na,K-ATPase consists of both α and β subunits, including four isoforms of α (α1, α2, α3, α4) and three isoforms of β (β1, β2, β3) (6) and FXYD (1–7) proteins, which are accessory subunits (7). α1 is almost ubiquitously distributed, whereas other isoforms are expressed in a tissue- and development-specific fashion. α2 is found mainly in muscle (skeletal, smooth, and cardiac), α3 is found primarily in nervous tissue, and α4 is found only in testicles. Human heart expresses α1, α2, and α3 isoforms and β1 (8). In cardiac myocytes α2 is concentrated in the T-tubules adjacent to the sarcoplasmic reticulum (SR),2 whereas α1 is more evenly distributed in T-tubules and SR (9). In smooth muscle and brain astrocytes, α1 is uniformly distributed, but α2 and α3 show a punctuated distribution, overlying the SR (10).
Increased force of contraction of cardiac muscle induced by cardiac glycosides is the result of inhibition of Na,K-ATPase. Raised intracellular Na+ concentration limits Ca2+ extrusion via the 3Na+/Ca2+ exchanger, leading to enhanced Ca2+ uptake into the SR by the Ca-ATPase and increased calcium-induced calcium release during excitation-contraction coupling. Cardiac glycoside-induced toxicity is associated with excessive inhibition of Na,K-ATPase, accumulation of Ca2+ ions (i.e.“calcium overload”), and “spontaneous” SR Ca2+ release that can trigger delayed after-depolarizations and cardiac arrhythmias (11). Experiments with mice engineered to have ouabain-sensitive α1 and insensitive α2 isoforms (α1S/Sα2R/R) or ouabain-insensitive α1 and α2 (α1R/Rα2R/R) instead of the wild type with ouabain-insensitive α1 and ouabain-sensitive α2 (α1R/Rα2S/S) have shown that α2 can play a predominant role in cardiac glycoside-induced positive inotropy (12) and also that α2 carries a large fraction of the Na,K-pump current in T-tubules of cardiac myocytes (13). Very recent work shows that α2 preferentially modulates Ca2+ transients and SR Ca2+ release in cardiac myocytes compared with α1 (14). Similarly, α2 plays an important role in contractility of vascular smooth muscle and control of blood pressure (15, 16). Experiments with α1S/Sα2R/R, α1R/Rα2S/S, and α1R/Rα2R/R mice also provide strong evidence for endogenous mammalian cardiac glycosides by showing differential responses to physiological stimuli and states that alter blood pressure (12, 17). In view of the accumulated information on α1 and α2 isoforms, one could envisage that α2-selective cardiac glycosides could provide important insights into the physiological role of α2 and, in particular, complement the information obtained from genetically engineered mice. At the pharmacological level an α2-selective cardiac glycoside could induce cardiac contraction with minimal Ca2+ overload and so act as a safer cardiotonic agent than conventional cardiac glycosides.
Structure-activity analyses of inhibition of Na,K-ATPase by cardiac glycosides or displacement of bound [3H]ouabain have been conducted for many years; see for example Refs. 18–22. As summarized in Ref. 23, essential structural features of active cardiac glycosides include rings A/B and C/D that are cis-fused and B/C rings that are trans-fused, a hydroxyl group at C14, and an unsaturated lactone attached at C17 of the steroid. The sugar at C3 of the steroid is not essential for inhibition but strongly affects binding affinity and rates; see Table 1B and Ref 23. Despite the great potential interest in isoform selectivity of cardiac glycosides, there is little clear-cut information. Variations in potency of digitoxigenin monoglycosides as inhibitors of kidney (α1β1) and brain (α1, α2, α3) Na,K-ATPases were attributed to isoform selectivity, and a role of the sugar was proposed (24). However, most native tissues except kidney (α1β1) contain mixtures of isoforms, and it is difficult to differentiate interactions of cardiac glycosides with the individual isoforms and exclude possible complicating factors. Because, for example, human cardiac membranes express α1, α2, and α3 isoforms (8), healthy cardiac tissue would not be useful for this purpose even if it was readily available. Individual human isoforms have been expressed in Xenopus oocytes (α1–3, β1–3) (25) and Saccharomyces cerevisiae (α1–3, β1) (26, 27) at low levels and used to characterize [3H]ouabain binding. More recently we have expressed human isoforms (α1, α2, and now also α3, with β1) at high levels in Pichia pastoris and purified the α1β1, α2β1, and α3β1 protein complexes (28–31). This system permits accurate analysis of cardiac glycoside binding and inhibition of Na,K-ATPase activity of the individual human isoforms, leading to the finding that several digitalis glycosides show moderate but highly significant selectivity for α2 (and α3) compared with α1.
TABLE 1.
Binding constants of Cardiac Glycosides to P. Pastoris membranes expressing α1β1, α2β1, and α3β1
Each value of KD ± S.E. represents the average of 3–5 separate estimates calculated as described under “Experimental Procedures.” p-Values are quoted only for KD values that are significantly different between α2 and α1 or α3 and α1 (p < 0.05).
EXPERIMENTAL PROCEDURES
Materials
Escherichia coli XL-1 blue strain was used for propagation and preparation of plasmid constructs. Yeast lytic enzyme from ICN Biomedicals Inc (catalog no. 152270) was used for transformation of P. pastoris protease-deficient strain SMD1165 (his4, prb1). n-Dodecyl-β-d-maltopyranoside (catalog no. D310) and C12E8 (25% w/w, catalog no. O330) were purchased from Anatrace. Synthetic SOPS (sodium salt)) was obtained from Avanti Polar Lipids and stored as a chloroform solution. BD Talon metal affinity resin (catalog no. 635503) was obtained from Clontech. Cholesterol and ouabain (O3125) were obtained from Sigma. [3H]Ouabain and [3H]digoxin were obtained from PerkinElmer Life Sciences. All other materials were of analytical grade.
Yeast Transformation; Expression of Human Isoforms α1β1, α2β1, and α3β1
The pHIL-D2 (α/His10-β1) vectors contained cDNAs encoding human α1, α2, and α3 (Swiss-Prot accession numbers: α1, P05023; α2, P050993; α3, p13637) and human His10-β1 (accession number P05026) were constructed by replacing the porcine α1 and His10-β1 described in Refs. 28, 30 with the appropriate human α1, α2, and α3 and β1 cDNA. Linear DNA, obtained by digestion of the pHIL-D2 with NotI, was used to transform spheroplasts of P. pastoris SMD1165, and His+Muts transformants were selected (31). Muts clones of the α1β1, α2β1, and α3β1 isoforms were grown in small-scale cultures (5 ml). Protein expression was induced with 0.5% methanol for 5 days, and membrane preparations were then screened for expression by Western blotting using anti-KETYY. Large scale growth of the optimally expressing clones was done in Bellco Spinner FlasksTM in 10-liter volumes of growth medium (28–31). Expression of the Na,K-ATPase was induced by adding 0.5% methanol daily for 5 days at 25 °C for a1β1 and a3β1 and at 20 °C for a2β1 clones (30). Cells were collected, washed, and broken with glass beads, and membranes were prepared as described (31). Membranes were stored at −80 °C in a solution containing 10 mm MOPS-Tris, pH 7.4, and 25% glycerol with protease inhibitors (1 mm phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin, chemostatin, and leupeptin). About 2 g of membrane protein was obtained per 100 g of cells.
Solubilization and Purification of Isoforms of Na,K-ATPase
Procedures for solubilization of membranes in n-dodecyl-β-d-maltopyranoside, binding to BD-Talon beads, and washing and elution of purified Na,K-ATPase have been described in detail (28–30). Purification of α3β1, which has not been described previously, follows exactly the same procedure. The three purified isoform complexes (0.3–0.5 mg/ml) were eluted from the BD-Talon beads in a solution containing 170 mm imidazole, 100 mm NaCl, 20 mm Tricine·HCl, pH 7.4, 0.1 mg/ml C12E8, 0.05 mg/ml SOPS, 0.01 mg/ml cholesterol, 25% glycerol. The proteins were stored at −80 °C. Protein concentration was determined with BCA (B9643 Sigma).
Cardiac Glycoside Binding to P. pastoris Membranes
[3H]Ouabain binding to yeast membranes (200–300 μg of protein) was assayed at 37 °C for 1 h in medium containing 10 mm MOPS-Tris, pH 7.2, 3 mm MgCl2, 1 mm vanadate-Tris, 1 mm EGTA-Tris (32). Binding of ouabain or competitive displacement by other cardiac glycosides was assessed by varying the total concentrations of ouabain or other cardiac glycosides at constant [3H]ouabain (between 1 and 2 nm; specific activity 30–40 Ci/mmol). Full curves of bound versus free ouabain were generated, and KD values were estimated using a one-site model B = Bmax [Ouf]/([Ouf] + KD), where [Ouf] refers to the concentration of free ouabain, and B and Bmax have their usual meaning. Fitted dissociation constants, KD, were obtained using Kaleidagraph. For other inhibitors K0.5 was calculated using a one-site inhibition model, B/BCG=0 = K0.5/([CG] + K0.5). B refers to the [3H]ouabain bound at a particular concentration of the cardiac glycoside (CG), and BCG=0 refers to the [3H]ouabain bound at 1–2 nm [3H]ouabain in the absence of other cardiac glycosides. The KD CG was calculated from K0.5 by taking into account ouabain-CG competition as KD CG = K0.5/(1+[Ouf]/ KD Ou). With KD Ou values α1β1 9.2 nm, α2β1 21.5 nm, and α3β1 11 nm, respectively (see Table 1), and 1 nm total ouabain, the values of 1 + [Ouf]/KD Ou were 1.06, 1.03, and 1.06, respectively. Binding of each cardiac glycoside was estimated in 3–5 separate experiments. Average KD values ± S.E. for each isoform were calculated, and statistical significance was calculated by the unpaired Student's t test. p values <0.05 were considered significant. The selectivity ratios KD α1/KD α2 and KD α1/KD α3 were calculated as the quotient of the individual KD values. The error of the ratios of KD α1/ KD α2 and KD α1/ KD α3 are calculated from the S.E. KD values using the formula
 |
Digoxin binding was measured in the same conditions as ouabain ([3H]digoxin ∼7 nm stock, specific activity 40 Ci/mmol).
Assay of Na,K-ATPase Activity of Purified Isoform Complexes
Inhibition of Na,K-ATPase activity of the detergent-soluble α1β1, α2β1, and α3β1 complexes by cardiac glycosides was done as described (29, 30). Before assay, the proteins were incubated at 25 °C for 30 min. The inhibitors were added to the recombinant enzyme (≈0.08–0.2 μg of protein) in 400 μl of reaction medium containing 130 mm NaCl, 5 mm KCl, 3 mm MgCl2, 25 mm histidine, pH 7.4, 1 mm EGTA, 0.01 mg/ml SOPS, 0.001 mg/ml cholesterol, and 0.005 mg/ml C12E8 in 48-well plates. The reaction (37 °C for 1 h) was started by the addition of 1 mm ATP. Pi release was measured with a malachite green dye to detect the phosphomolybdate (Pi Color Lock, Innova Biosciences). The percent inhibition VCG/V0 was calculated for each cardiac glycoside concentration, and Ki values were obtained by fitting the data to the function VCG/V0 = Ki/([CG] + Ki)+ c (using Kaleidagraph). V0 and VCG represent the control rate and rate of Na,K-ATPase activity at particular concentrations of cardiac glycosides, [CG], respectively. The constant c represents a small fraction of uninhibited activity (range 0–10%). Including c improves the fit. Inhibition was estimated in 3–5 separate experiments, average Ki values ± S.E. were calculated, and the significance of differences was calculated by the unpaired Student's t test. p values <0.05 were considered significant.
Inhibitors
Most cardiac glycosides were dissolved in 70-100% ethanol. Bufalin, marinobufagenin, and gitoxin were dissolved in DMSO. Stock solutions were diluted in 50% ethanol or 50% DMSO. Inhibitors were added directly to the reaction medium containing membranes ([3H]ouabain binding) or pure enzymes (Na,K-ATPase activity), so that the final ethanol or DMSO concentration was 1%. Didigitoxosyl digoxigenin was prepared by selective degradation of digoxin as described (33). The product was analyzed by high performance liquid chromatography and shown to be close to 80% pure.
RESULTS
Expression and Purification of Human α1β1, α2β1, and α3β1 Isoforms
The three isoform complexes were expressed in P. pastoris, yeast were grown in large quantities, membranes were prepared, and the isoform proteins were purified (see “Experimental Procedures”). Ouabain binding capacities of the membranes expressing α1β1, α2β1, and α3β1 were 25–30, 20–25, and 20 pmol/mg of protein, respectively. Fig. 1 presents a gel of a typical purification experiment. The β1 subunit appears as two bands that are glycosylated to different degrees and are easily deglycosylated by endoglycosidase H to produce a single band of 37 kDa (28, 29). Between 1 and 2 mg of purified proteins were obtained per gram of membranes, with Na,K-ATPase activities of α1β1, α2β1, and α3β1, respectively, of 12–15, 6–8, and ≈10 μmol of Pi/mg/min.
FIGURE 1.
Purification of human α1β1, α2β1, and α3β1 isoform complexes. Coomassie-stained Laemmli SDS-PAGE of purified isoform complexes at 5 μg of protein per lane is shown.
Cardiac Glycoside Binding to P. pastoris Membranes Expressing α1β1, α2β1, and α3β1
The high level isoform expression permits accurate determination of [3H]ouabain binding and competitive displacement of [3H]ouabain by many cardiac glycosides (Fig. 2, Table 1).3 The ligand conditions (3 mm with MgCl2 and 1 mm vanadate-Tris) are optimal for high affinity ouabain binding to an E2-vanadate conformation (32), vanadate serving as a transition-state analogue of inorganic phosphate. Full binding curves of [3H]ouabain yielded dissociation constants KD (see Table 1, line 1) with values in the tens of nm range and order α1 < α3 < α2. These values are consistent with previous observations on [3H]ouabain binding to human isoforms expressed in S. cerevisiae (26, 27) or Xenopus oocytes (25). Fig. 2 depicts typical displacement curves for three digitalis glycosides, digoxin, digitoxin, and a semisynthetic derivative, β-methyl digoxin as well as three aglycones, digoxigenin, digitoxigenin, and bufalin, a bufadienolide (see Table 1 for structures). For digoxin, digitoxin, and β-methyl digoxin, the curves for α2 and α3 are shifted to the left of the curves for α1, with little or no difference between α2 and α3. By contrast, for all three aglycones the curves are superimposable. A single-site displacement model was used to calculate values of KD, compiled in Table 1. Digoxin, β-methyl digoxin, and digitoxin indeed show a moderate but highly significant selectivity for both α2 and α3 over α1, up to 4-fold (see also Table 1, Group B). The three aglycones digoxigenin, digitoxigenin (see also Table 1, Group B), and bufalin (see also Table 1, Group F) show essentially identical affinity for all three isoforms, i.e. they show no isoform selectivity. Experiments like those in Table 1 were carried out on four different preparations of membranes expressing the different isoforms, with essentially similar results.
FIGURE 2.
Competitive displacement of [3H]ouabain by cardiac glycosides and aglycones. Representative experiments for displacement of [3H]ouabain by digoxin (upper left), digitoxin (upper middle), β-methyl digoxin (upper right), digoxigenin (lower left), digitoxigenin (lower middle), and bufalin (lower right) are shown. ●, α1; ▴, α2; ■, α3. The concentration of [3H]ouabain was 1 nm. Solid lines are the fitted curves for a one-site competitive displacement model (see “Experimental Procedures”).
The observation that the aglycones do not discriminate between the isoforms implies that α2/α3:α1 selectivity depends on the sugar residues. To test this hypothesis more systematically as well as the effect of the number and nature of the sugar substituents and the possible influence of the steroid and lactone moieties, we have performed similar experiments on a large number of glycosides and aglycones. These results are collected together in Table 1 into five main groups, B, C, D, E, and F. Group B consists of the digoxigenin and digitoxigenin glycosides with one, two, three, or four digitoxose substituents (see also Fig. 3). The addition of a single digitoxose to digoxigenin strongly increases the affinity for all isoforms but even more for α2 and α3 compared with α1, leading to a significant α2/α3:α1 selectivity (∼2-fold). The addition of the two and then three sugars to digoxigenin increases α2/α3:α1 selectivity further, to more than 3-fold in the case of digoxin, whereas the addition of a fourth sugar slightly reduces the affinity for all isoforms but does not affect the α2/α3:α1 selectivity. Fig. 3 illustrates these features and confirms that α2 and α3 show the same profile compared with α1. In the case of digitoxigenin glycosides the addition of either two or three digitoxoses to digitoxigenin has a similar effect on affinity and α2/a3:α1-selectivity. Group C consists of nine synthetic monoglycosyl derivatives of digitoxigenin (20). Several of these derivatives show a higher affinity for all three isoforms compared with the parent digitoxigenin, but only the β-l-rhamnosyl derivative, evomonoside, seems to show significant selectivity, in this case for α3:α1. Group D consists of natural or semisynthetic 16-OH steroid and formylated or acetylated derivatives of gitoxin (19) and oleandrin. The aglycone gitoxigenin has a low affinity, whereas the tridigitoxosyl gitoxigenin (gitoxin) binds much better to all isoforms, but in neither case is there significant isoform selectivity. The 16-formylated gitoxigenin has a much higher affinity compared with the 16-OH gitoxigenin, but no isoform selectivity. By contrast, the 3β-digitoxosyl derivatives of 16-formyl and 16-acetyl gitoxigenin both show significant α2/α3:α1 selectivity. Similarly, oleandrin, with a 16-acetyl modification and a single oleandrose sugar, shows very high affinity and significant α2/α3:α1-selectivity. Group E consists of uzarin derivatives, which are rare cardenolides with planar steroid moieties. The aglycone shows low affinity and no selectivity, whereas the uzarigenin diglucoside shows even lower affinity and significant selectivity, at least for α2:α1. The rhamnoside shows a much a higher affinity for all three isoforms but no selectivity. Group F consists of two bufadienolide aglycones, marinobufagenin and bufalin. Bufalin was discussed above. Marinobufagenin, which is reported to have α1 selectivity in some systems (for review, see Ref. 34), shows low affinity in this assay and, like bufalin, shows no isoform selectivity. These data confirm the importance of the sugars in α2/α3:α1 selectivity and provide several other conclusions on structural requirements of the sugar and steroid moieties (Fig. 3 and “Discussion”).
FIGURE 3.
Dependence of isoform selectivity on the number of digitoxose residues. The ratios of KD ±S.E. for α1/α2 and α1/α3 are taken from Table 1 (Group B).
We have also looked directly at [3H]digoxin binding to the three isoforms. Estimates of the KD values based on full binding curves up to μm concentrations are unsatisfactory due to a high background count (about 10-fold higher for [3H]digoxin compared with [3H]ouabain). At low concentrations of [3H]digoxin (nm), specific binding is easily detected and has shown two interesting features (Fig. 4). Fig. 4A depicts the well known [3H]digoxin-K+ antagonism at increasing concentrations of K+. The striking point is that K0.5K+ for displacing [3H]digoxin is 5–6-fold higher for α2 compared with α1, whereas that for α3 is closer to that for α1 (see Fig. 4A). Fig. 4B shows the rate of dissociation of [3H]digoxin. The rate is about 5-fold faster for α2 compared with α1, and that for α3 is closer to α2. The order of the isoform-dependent differences in K0.5 K+ ions and dissociation rates is similar to properties of [3H]ouabain binding to human isoforms expressed in Xenopus oocytes (25). Thus, these features are intrinsic properties of the isoforms, and all other cardiac glycosides should show the same features.
FIGURE 4.
[3H]Digoxin-K+ antagonism (A) and [3H]digoxin dissociation rates (B). ●, α1; ▴, α2; ■, α3. A, [3H]digoxin binding in the presence of 0–10 mm K+ is shown. Average K0.5K values based on single site inhibition for two separate experiments are shown. B, digoxin 1 mm was added to the membranes pre-bound with [3H]digoxin, and the remaining bound [3H]digoxin was measured at 37 °C at the indicated times.
Inhibition of Na,K-ATPase Activity of Purified α1β1, α2β1, and α3β1 Complexes
As an independent test of isoform selectivity, we have compared inhibition by the different cardiac glycosides of Na,K-ATPase activities of the three purified detergent-soluble isoform complexes.4 Representative inhibition experiments are shown in Fig. 5 for digoxin, digoxigenin, and β-methyl digoxin, and fitted Ki values for a selection of glycosides and aglycones are presented in Table 2. An obvious feature in Fig. 5 is that the curves for inhibition of α2 by digoxin and β-methyl digoxin lies to the left of that for α1, and those for α3 lie between those for α1 and α2. The curves for inhibition by digoxigenin are similar for all three isoforms. The fitted Ki values with statistical tests for significance in Table 2 confirm these observations and also illustrate an important additional property. The ratio of Ki for α1/α2 is 3–5-fold in the case of digoxin, digitoxin, β-methyl digoxin, oleandrin, and 16-formate 3-β-digitoxose gitoxigenin. By contrast, the ratio Ki α1/α3 for all these glycosides is lower than Ki α1/α2, and in most cases it is nearer to 1. Again, ouabain is different in that it is the only glycoside for which Ki α1 ≈ α2 ≈ α3. For all the aglycones, ouabagenin, digoxigenin, and digitoxigenin, there are no or only minor differences between the isoforms. By comparison with KD values for cardiac glycoside binding in Table 1, the Ki values in Table 2 are all higher, which is expected on account of the presence of K+ and the K+-cardiac glycoside antagonism. Because, however, K0.5 K+ values for antagonism of digoxin binding lie on the order α2 > α3 > α1 (Fig. 4A), CG binding could be antagonized by the K+ to different extents in the order α1 > α3 > α2. Differential K+-cardiac glycoside antagonism on α1, α3, and α2, respectively, could explain the observed order of inhibitory potency of β-methyl digoxin or digoxin (Ki α2 < α3 < α1) and also the finding that for ouabain Ki α1 ≈ α2 ≈ α3 (Table 2). To test this notion we have looked at the inhibition by β-methyl digoxin at 20 mm K+ rather than the usual 5 mm K+. Under this condition, the Ki for α1 and α3 was substantially raised, whereas the Ki for α2 was raised only slightly (see Fig. 5 and Table 2). As a result the apparent selectivity of α2:α1 increased further to 7.3-fold, compared with 4.5-fold at 5 mm K+, and the order is clear-cut, Ki α2 < α3 ≤ α1. Experiments like those in Fig. 5 were carried on three different preparations of the purified isoform complexes and also complexes of α1β1 purified from membranes prepared from yeast grown at 20 °C as well as 25 °C, with essentially similar results. Furthermore, reconstitution of α1β1 and α2β1 with FXYD1 (see Ref. 30) does not significantly affect the Ki values for inhibition by digoxin or the α1/α2 selectivity ratio (unpublished result, to be presented in detail in a separate paper).
FIGURE 5.
Inhibition of Na,K-ATPase activity of purified isoform complexes. Shown are representative experiments for inhibition of Na,K-ATPase activity by digoxin (upper left), digoxigenin (upper right), and β-methyl digoxin (lower left, 5 mm K+; lower right, 20 mm K+). ●, α1; ▴, α2■, α3. Solid lines are the fitted curves for a one-site inhibition model (see “Experimental Procedures”).
TABLE 2.
Inhibition of Na,K-ATPase activity of α1β1, α2β1, and α3β1 complexes
Each value of Ki ± S.E. represents the average of 3–5 separate estimates calculated as described under “Experimental Procedures.” p values are quoted only for Ki values that are significantly different between α2 and α1 or α3 and α1 (p < 0.05).
| CG |
Ki ± S.E., p value |
Ratio of Ki values ± S.E. |
|||
|---|---|---|---|---|---|
| α1 | α2 | α3 | α1/α2 | α1/α3 | |
| nm | |||||
| Ouabain | 97 ± 4.6 | 94 ± 16 | 97 ± 13 | 1.03 ± 0.18 | 1.0 ± 01.4 |
| Ouabagenin | 721 ± 70 | 739 ± 89 | 592 ± 3 | 0.97 ± 0.15 | 1.21 ± 0.11 |
| Digitoxin | 72 ± 8 | 23 ± 4, p = 0.006 | 52 ± 11 | 3.13 ± 0.64 | 1.38 ± 0.33 |
| Digitoxigenin | 76 ± 18 | 45 ± 6.7 | 72.7 ± 7 | 1.69 ± 0.47 | 1.04 ± 0.26 |
| Digoxin | 250 ± 44 | 63 ± 10, p = 0.003 | 136 ± 24 | 3.96 ± 0.93 | 1.83 ± 0.45 |
| β-Methyl digoxin, 5 mm K+ | 253 ± 57 | 56 ± 13, p = 0.015 | 151 ± 24 | 4.51 ± 1.4 | 1.67 ± 0.46 |
| β-Methyl digoxin, 20 mm K+ | 655 ± 110 | 89 ± 27, p = 0.008 | 540 ± 14 | 7.36 ± 2.55 | 1.21 ± 0.20 |
| Digoxigenin | 139 ± 17 | 130 ± 13.5 | 144 ± 15 | 1.07 ± 0.17 | 0.96 ± 0.15 |
| Oleandrin | 58 ± 4 | 20 ± 3, p = 0.0028 | 46 ± 1 | 2.90 ± 0.47 | 1.26 ± 0.09 |
| 16-Formate 3β-digitoxin gitoxigenin | 80 ± 2 | 19.5 ± 1.1, p = 0.001 | 73 ± 6 | 4.10 ± 0.25 | 1.09 ± 0.09 |
DISCUSSION
Isoform Selectivity and Structures of Cardiac Glycosides
The central finding in this work is that digoxin, digitoxin, β-methyl digoxin, and some other glycosides show a partial but highly significant selectivity for binding to α2 and α3 compared with α1. By contrast, α2/α3:α1 selectivity was not detected for any aglycone tested, indicating the essential role of the sugar. Ouabain behaves differently to the digitalis glycosides, showing some selectivity for α1:α2 and little difference between α1 and α3, confirming the findings in Refs. 25–27. The pattern of inhibition of Na,K-ATPase activity by the digitalis glycosides is consistent with the binding data but is modified by a large difference in apparent affinity of K+ for antagonizing cardiac glycoside binding. As a result, the order of potency of the digitalis glycosides as inhibitors of Na,K-ATPase is Ki α2 < α3 ≤ α1. Again, ouabain stands out in that Ki α2 ≈ α3 ≈ α1. It is of course significant that the selectivities observed for cardiac glycoside binding and inhibition of Na,K-ATPase activities are mutually consistent. Furthermore, similar results were obtained on several independent membrane preparations and purified α1β1, α2β1, and α3β1 complexes and for the purified α1β1 prepared from cells grown at 20 °C as well as the usual 25 °C and also for the α1β1FXYD1 and α2β1FXYD1 complexes. These findings all suggest that the moderate α2 selectivity of the digitalis glycosides is a robust result and cannot be attributed to the type and conditions of the assays, the state of the protein (intact membranes versus detergent-soluble proteins), type of the purified complex, or mere chance.
As mentioned in the Introduction, extensive structure-activity relationships for binding of many cardiac glycosides and inhibition of Na,K-ATPase activities, utilizing native enzyme preparations, have been published previously (see for example Refs. 18–22 and 35). These have established the basic structural features necessary for cardiac glycoside action. Overly detailed comparisons between the different studies or with the current study using recombinant human proteins are not worthwhile because of wide differences in experimental conditions, species differences, and enzyme sources with mixed isoform content. Results for the recombinant human α1β1 and native sheep and pig kidney Na,K-ATPase (α1β1) (19, 20, 22), the only native source of the enzyme with the defined isoform content, do appear in general to be consistent despite the species difference.
An attempt to measure competition of various cardiac glycosides with [3H]ouabain for binding to human α1, α2, and α3 expressed in S. cerevisiae was reported recently (36). With the exception of [3H]ouabain binding data, the results for other cardiac glycosides were quite different from the present findings. In particular, in comparable conditions of high affinity binding, no difference was detected for digoxin or digitoxin binding to α1, α2, and α3, and β-methyl digoxin was stated to be partially selective for α1 over α2 and α3. We believe that the most likely explanation of the inconsistency is that very low levels of expression of the isoforms in S. cerevisiae (α1, 4.95; α2, 1.32; α3, 4.51 pmol/mg) compared with P. pastoris (α1, 25–30; α2, 20–25; α3, 20 pmol/mg) compromised the accuracy of the measurements and the ability to detect moderate isoform-dependent differences.5
The data in Table 1 provide several additional conclusions on the role of the number and nature of the sugars. First, compared with digoxigenin and digitoxigenin, the addition of only one digitoxose residue raises affinity for all isoforms and produces some α2/α3:α1 selectivity, whereas two or more digitoxoses added to digoxigenin or digitoxigenin induce the maximal affinity and α2/α3:α1 selectivity ratio (see also Fig. 3). Second, only cardiac glycosides with sugars in β-glycosidic links display α2/α3:α1 selectivity. Third, only sugars having 5-methyl groups (digitoxose, oleandrose and to some extent l-rhamnose) display α2/α3:α1 selectivity. Fourth, the nature of the steroid is crucial as well as the sugar (as seen for groups D (gitoxin) and E (uzarin) derivatives in Table 1). The latter observations make it less surprising that ouabain behaves differently to digoxin and digitoxin. Presumably the hydrophilic steroid and α-l-rhamnoside of ouabain interact better with α1 than α2 compared with the hydrophobic steroid and β-digitoxose in digoxin and digitoxin, which bind better to α2 and α3 than to α1.
Compatibility with the Structure of Na,K-ATPase with Bound Ouabain
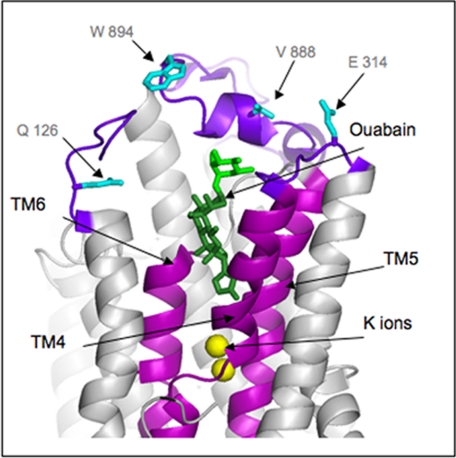
The structures of shark rectal gland Na,K-ATPase (37), especially a structure with bound ouabain (38), provide an interesting insight into the proposed role of the sugars in isoform selectivity. Fig. 6 shows a detail of the structure of Na,K-ATPase with two occluded K+ ions and ouabain bound in a deep cavity facing the external surface. Because the digitalis glycoside binding affinities are the same and higher for both human α2 and α3 compared with α1, one could assume that the higher affinity is due to interactions with residues that are the same in α2 and α3 but differ in α1. All such residues at the extracellular surface and trans-membrane segments have been mapped onto the structure (α1 residues). The interesting feature is that all these residues are located in the extracellular loops in proximity to the rhamnose moiety of ouabain, but within the steroid-lactone binding cavity there are no such isoform-specific differences. This structure is not an ideal model because ouabain is bound with low affinity due to the presence of occluded Rb+, but as discussed in Ref. 38, it explains most effects of mutations on high affinity ouabain binding. In addition, the high affinity state was modeled by movement of trans-membrane segments TM1/TM2 to close off the binding cavity, with ouabain bound in an almost identical position (38). With this caveat, the structure predicts quite naturally that no aglycones can show isoform selectivity, because the differences between the isoforms are found only in proximity to the sugar. This prediction is exactly consistent with all our observations. Conversely, our observations imply that the steroid-lactone moieties of all cardiac glycosides should be bound similarly to that of ouabain. By comparison with this structure, prior models of cardiac glycoside binding have assumed that the cardiac glycoside molecule lies more or less horizontally across the extracellular surface and do not explain our results. Of course, the structure cannot explain why ouabain somewhat favors α1 or why the digitalis glycosides somewhat favor α2/α3, but it confirms that the key lies in interactions of the sugars near the extracellular loops. This structural insight fits very well with a two-step binding model proposed by Yoda (39) to explain the fact that the sugar is not essential for inhibition but strongly affects the binding affinity and rates (23). On the Yoda model (39), the steroid-lactone and sugar moieties occupy specific subsites. An independent kinetic contribution of the sugar to binding is consistent with the proposed role in isoform selectivity, assuming that only the sugar subsite differs in α1 compared with α2/α3 isoforms.
FIGURE 6.
Structure of Na,K-ATPase with bound ouabain. The figure shows the Protein Data Bank codes 3A3Y structure (38). Residues identical in human α2 and α3 and different in α1 are shown in cyan. Residues and numbering are those for human α1. Dark green, ouabain, steroid-lactone; light green, rhamnose. The figure was drawn by PYMOL.
Pharmacological and Physiological Implications
Of all the cardiac glycosides we have tested, those that show the greatest, even if partial selectivity for α2:α1, digoxin, and β-methyl digoxin are the ones in most extensive clinical use. This supports the evidence mentioned in the Introduction that α2 is the major player in cardiac contractility and primary target for cardiac glycosides such as digoxin. By contrast, ouabain, which shows no selectivity for α2:α1, is not used clinically. Although in a comprehensive clinical trial (DIG trial), digoxin did not reduce overall mortality (40), a subsequent post hoc analysis found that a lower serum concentration (0.5–0.9 compared with >1 ng·ml) has overall benefit and reduces mortality (41). This could fit a hypothesis that therapeutic (α2?) and toxic (α1?) effects are separable, and in principle, a more α2-selective cardiac glycoside may increase the therapeutic window and reduce toxicity. Expression of Na,K-ATPase in human cardiac muscle is lowered up to 40% in congestive heart failure, especially α1, whereas α3 or α2 levels may be unchanged or lower depending on the region of the heart (42). Therefore, a more α2-selective cardiac glycoside than digoxin could be considered to be tailored to the failing heart.
The different selectivity of digoxin and ouabain for human α1 and α2 at the protein level was unexpected and may be relevant to differences between ouabain and digoxin observed in vivo. For example, injection of rats with low doses of ouabain over several days raise BP, but paradoxically, digoxin does not have this effect and even protects against the ouabain (43). The effect of ouabain derivatives, modified in the lactone ring, on BP does not correlate directly with inhibition of dog kidney Na,K-ATPase (α1β1) (44). Thus, the mechanism, especially the difference between ouabain and digoxin, is not well understood. The inability of digoxin to raise BP suggests that inhibition of α2 may not be involved. Furthermore, digoxin is not known to raise BP in man. Both ouabain and digoxin have been detected in mammalian tissues and fluids (1). Taken together, the selectivity differences of ouabain and digoxin and their different effects in vivo support the notion that these two cardiac glycosides have specific but distinct physiological roles (for reviews, see also Refs. 1 and 5). For example, digoxin might affect primarily α2-dependent and ouabain primarily α1-dependent functions. Furthermore, ouabain induces Ca2+ oscillations (45), and long-term effects of ouabain on cell Ca2+ and BP might be mediated via α1 and the cellular signaling pathways, as suggested in Ref. 2. The first step in ouabain-mediated signaling in vivo is tyrosine phosphorylation of the soluble tyrosine kinase, Src (3). In this respect, it is of interest that, unlike ouabain, digoxin does not activate tyrosine phosphorylation of Src in an in vitro assay.6
Perspective
The observations that several digital glycosides are partially selective for α2:α1 and the mechanistic insight that aglycones show no isoform selectivity suggest that improved α2 selectivity might be achieved by modifying the digitoxose. A more α2-selective cardiac glycoside could have important applications as a tool to analyze toxicity of cardiac glycosides, physiological functions of α2, and different endogenous cardiac glycosides and possibly as a safer cardiotonic agent compared with digoxin. Isoform-selective cardiac glycosides could also be important in recently discussed applications of cardiac glycosides such as treatment of cancer (46).
Acknowledgments
We are greatly indebted to Dr. W. Schoner (University of Giessen) for providing the cardiac glycosides in groups B and E, Drs. D. Fullerton (University of Washington), K. Ahmed (University of Minnesota), Toshihiro Hashimoto (University of Tokushima), and Kouichi Youhioka for the cardiac glycosides in Group C and D, and Dr. A. Bagrov (National Institutes of Health) for marinobufagenin in Group F of Table 1. We are also grateful to Dr. Grazia Tripodi (Prassis- Sigma-Tau) for providing the human α3 cDNA. We thank Drs. Patrizia Ferrari, Mara Ferrandi, and Giuseppe Bianchi (Prassis- Sigma-Tau) and Dr. Chikashi Toyoshima (University of Tokyo) for valuable comments on this work. We also thank Alon Lam for devoted technical assistance.
This work was supported by the Weizmann Institute Renal Research Fund and the Mauerberger Foundation, South Africa.
The accuracy of these measurements crucially depends on two factors. The first is a high signal to background ratio. At 1 nm [3H]ouabain, 50–70% of the total radioactivity is bound (about 10,000 cpm per sample) and is displaced by increasing concentrations of other cardiac glycosides to a very low background level (about 200 cpm per sample), i.e. roughly a 50-fold ratio. Second, at 1 nm ouabain, corrections of the K0.5 for [3H]ouabain-CG competition to calculate KD values are very small.
An important methodological consideration is that the reaction time must be long enough to ensure full equilibration of all cardiac glycosides with all three isoforms throughout the assay. It is known, for example, that binding of ouabain to α1 is much slower than to α2 and α3 (25). In practice, for assay times ≥40 min at 37 °C, the Ki values for inhibition of all three isoforms were constant. For reaction times that are too short (e.g. 5–10 min), Ki values for α1, but not for α2, were significantly raised, strongly distorting the selectivity patterns.
Low expression levels in S. cerevisiae necessitate use of much higher [3H]ouabain concentrations (20 nm) compared to the 1–2 nm used with P. pastoris membranes. This reduces signal to background ratio and also entails large corrections for ouabain-CG competition to convert the K0.5 to KD values. Both factors must decrease the accuracy of the experiments (see also footnote 3).
M. Ferrandi, personal communication.
- SR
- sarcoplasmic reticulum
- C12E8
- octaethylene glycerol mondodecyl ether
- SOPS
- 1-stearoyl-2-oleoyl-sn-glycero-3-[phosphor-l-serine]
- CG
- cardiac glycoside
- BP
- blood pressure
- MOPS
- 4-morpholinepropanesulfonic acid
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1.Schoner W., Scheiner-Bobis G. (2007) Am. J. Physiol. Cell Physiol. 293, C509–C536 [DOI] [PubMed] [Google Scholar]
- 2.Xie Z., Askari A. (2002) Eur. J. Biochem. 269, 2434–2439 [DOI] [PubMed] [Google Scholar]
- 3.Li Z., Xie Z. (2009) Pflugers Arch. 457, 635–644 [DOI] [PubMed] [Google Scholar]
- 4.Blaustein M. P., Zhang J., Chen L., Song H., Raina H., Kinsey S. P., Izuka M., Iwamoto T., Kotlikoff M. I., Lingrel J. B., Philipson K. D., Wier W. G., Hamlyn J. M. (2009) Hypertension 53, 291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvela M., Rosen H., Feldmann T., Nesher M., Lichtstein D. (2007) Pathophysiology 14, 159–166 [DOI] [PubMed] [Google Scholar]
- 6.Blanco G., Mercer R. W. (1998) Am. J. Physiol. 275, F633–F650 [DOI] [PubMed] [Google Scholar]
- 7.Garty H., Karlish S. J. (2006) Annu. Rev. Physiol. 68, 431–459 [DOI] [PubMed] [Google Scholar]
- 8.Sweadner K. J., Herrera V. L., Amato S., Moellmann A., Gibbons D. K., Repke K. R. (1994) Circ. Res. 74, 669–678 [DOI] [PubMed] [Google Scholar]
- 9.Berry R. G., Despa S., Fuller W., Bers D. M., Shattock M. J. (2007) Cardiovasc. Res. 73, 92–100 [DOI] [PubMed] [Google Scholar]
- 10.Juhaszova M., Blaustein M. P. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 1800–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bers D. M. (2008) Annu. Rev. Physiol. 70, 23–49 [DOI] [PubMed] [Google Scholar]
- 12.Dostanic-Larson I., Lorenz J. N., Van Huysse J. W., Neumann J. C., Moseley A. E., Lingrel J. B. (2006) Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R524–R528 [DOI] [PubMed] [Google Scholar]
- 13.Despa S., Bers D. M. (2007) Am. J. Physiol. Cell Physiol. 293, C321–C327 [DOI] [PubMed] [Google Scholar]
- 14.Despa S., Wu Y., Lingrel J. B., Stefani E., Bers D. M. (2010) Biophys. J. 98, [Google Scholar]
- 15.Blaustein M. P., Zhang J., Chen L., Hamilton B. P. (2006) Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R514–R523 [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Lee M. Y., Cavalli M., Chen L., Berra-Romani R., Balke C. W., Bianchi G., Ferrari P., Hamlyn J. M., Iwamoto T., Lingrel J. B., Matteson D. R., Wier W. G., Blaustein M. P. (2005) J. Physiol. 569, 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingrel J. B. (2010) Annu. Rev. Physiol. 72, 395–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schönfeld W., Weiland J., Lindig C., Masnyk M., Kabat M. M., Kurek A., Wicha J., Repke K. R. (1985) Naunyn-Schmiedebergs Arch. Pharmacol. 329, 414–426 [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T., Rathore H., Satoh D., Hong G., Griffin J. F., From A. H., Ahmed K., Fullerton D. S. (1986) J. Med. Chem. 29, 997–1003 [DOI] [PubMed] [Google Scholar]
- 20.Rathore H., From A. H., Ahmed K., Fullerton D. S. (1986) J. Med. Chem. 29, 1945–1952 [DOI] [PubMed] [Google Scholar]
- 21.Schön R., Weiland J., Megges R., Repke K. R. (1995) Naunyn Schmiedebergs Arch. Pharmacol. 351, 282–292 [DOI] [PubMed] [Google Scholar]
- 22.Paula S., Tabet M. R., Ball W. J., Jr. (2005) Biochemistry 44, 498–510 [DOI] [PubMed] [Google Scholar]
- 23.Forbush B. (1983) Curr. Top. Membr. Transp. 19, 167–201 [Google Scholar]
- 24.From A. H., Fullerton D. S., Ahmed K. (1990) Mol. Cell. Biochem. 94, 157–165 [DOI] [PubMed] [Google Scholar]
- 25.Crambert G., Hasler U., Beggah A. T., Yu C., Modyanov N. N., Horisberger J. D., Lelièvre L., Geering K. (2000) J. Biol. Chem. 275, 1976–1986 [DOI] [PubMed] [Google Scholar]
- 26.Wang J., Velotta J. B., McDonough A. A., Farley R. A. (2001) Am. J. Physiol. Cell Physiol. 281, C1336–C1343 [DOI] [PubMed] [Google Scholar]
- 27.Müller-Ehmsen J., Juvvadi P., Thompson C. B., Tumyan L., Croyle M., Lingrel J. B., Schwinger R. H., McDonough A. A., Farley R. A. (2001) Am. J. Physiol. Cell Physiol. 281, C1355–1364 [DOI] [PubMed] [Google Scholar]
- 28.Cohen E., Goldshleger R., Shainskaya A., Tal D. M., Ebel C., le Maire M., Karlish S. J. (2005) J. Biol. Chem. 280, 16610–16618 [DOI] [PubMed] [Google Scholar]
- 29.Haviv H., Cohen E., Lifshitz Y., Tal D. M., Goldshleger R., Karlish S. J. (2007) Biochemistry 46, 12855–12867 [DOI] [PubMed] [Google Scholar]
- 30.Lifshitz Y., Petrovich E., Haviv H., Goldshleger R., Tal D. M., Garty H., Karlish S. J. (2007) Biochemistry 46, 14937–14950 [DOI] [PubMed] [Google Scholar]
- 31.Strugatsky D., Gottschalk K. E., Goldshleger R., Bibi E., Karlish S. J. (2003) J. Biol. Chem. 278, 46064–46073 [DOI] [PubMed] [Google Scholar]
- 32.Pedersen P. A., Rasmussen J. H., Jørgensen P. L. (1996) Biochemistry 35, 16085–16093 [DOI] [PubMed] [Google Scholar]
- 33.Satoh D., Aoyama K. (1970) Chem. Pharm. Bull. 18, 94–99 [Google Scholar]
- 34.Bagrov A. Y., Shapiro J. I., Fedorova O. V. (2009) Pharmacol. Rev. 61, 9–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed K., Rohrer D. C., Fullerton D. S., Deffo T., Kitatsuji E., From A. H. (1983) J. Biol. Chem. 258, 8092–8097 [PubMed] [Google Scholar]
- 36.Hauck C., Potter T., Bartz M., Wittwer T., Wahlers T., Mehlhorn U., Scheiner-Bobis G., McDonough A. A., Bloch W., Schwinger R. H., Müller-Ehmsen J. (2009) Eur. J. Pharmacol. 622, 7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shinoda T., Ogawa H., Cornelius F., Toyoshima C. (2009) Nature 459, 446–450 [DOI] [PubMed] [Google Scholar]
- 38.Ogawa H., Shinoda T., Cornelius F., Toyoshima C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 13742–13747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoda A. (1973) Mol. Pharmacol. 9, 51–60 [PubMed] [Google Scholar]
- 40.(1997) N. Engl. J. Med. 336, 525–533 [DOI] [PubMed] [Google Scholar]
- 41.Ahmed A., Rich M. W., Love T. E., Lloyd-Jones D. M., Aban I. B., Colucci W. S., Adams K. F., Gheorghiade M. (2006) Eur. Heart J. 27, 178–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller-Ehmsen J., Wang J., Schwinger R. H., McDonough A. A. (2001) Cell. Mol. Biol. 47, 373–381 [PubMed] [Google Scholar]
- 43.Manunta P., Rogowski A. C., Hamilton B. P., Hamlyn J. M. (1994) J. Hypertens. 12, 549–560 [PubMed] [Google Scholar]
- 44.Manunta P., Hamilton B. P., Hamlyn J. M. (2001) Hypertension 37, 472–477 [DOI] [PubMed] [Google Scholar]
- 45.Aizman O., Uhlén P., Lal M., Brismar H., Aperia A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 13420–13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mijatovic T., Ingrassia L., Facchini V., Kiss R. (2008) Expe. Opin. Ther. Targets 12, 1403–1417 [DOI] [PubMed] [Google Scholar]