Abstract
Myeloid cell leukemia 1 (MCL-1), an anti-apoptotic BCL-2 family member active in the preservation of mitochondrial integrity during apoptosis, has fundamental roles in development and hematopoiesis and is dysregulated in human cancers. It bears a unique, intrinsically unstructured, N-terminal sequence, which leads to its instability in cells and hinders protein production and structural characterization. Here, we present collective data from NMR spectroscopy and titration calorimetry to reveal the selectivity of MCL-1 in binding BCL-2 homology 3 (BH3) ligands of interest for mammalian biology. The N-terminal sequence weakens the BH3 interactions but does not affect selectivity. Its removal by calpain-mediated limited proteolysis results in a stable BCL-2-like core domain of MCL-1 (cMCL-1). This core is necessary and sufficient for BH3 ligand binding. Significantly, we also characterized the in vitro protein-protein interaction between cMCL-1 and activated BID by size exclusion chromatography and NMR titrations. This interaction occurs in a very slow manner in solution but is otherwise similar to the interaction between cMCL-1 and BID-BH3 peptides. We also present the solution structure of complex cMCL-1·hBID-BH3, which completes the family portrait of MCL-1 complexes and may facilitate drug discovery against human tumors.
Keywords: Apoptosis, Calpain, NMR, Protein Structure, Protein-Protein Interactions, BID, MCL-1, Complex
Introduction
The BCL-2 family of proteins plays a pivotal role in regulating apoptosis mainly by regulating the outer mitochondrial membrane integrity (1). They are classified into two functional groups: anti-apoptotic and pro-apoptotic. They each share conserved BH regions. The anti-apoptotics (BCL-2, BCL-w, BCL-XL, MCL-1, and A1) share up to four BH regions named BH1–4,4 and prevent cells from entering apoptosis. The pro-apoptotics can be further grouped into the effectors and BH3-only proteins. The effectors, BAX and BAK, contain three BH (BH1–3) regions and promote cell death by oligomerization and mitochondria outer membrane permeabilization. The BH3-only proteins share the BH3 region of sequence similarity. Members of this group include BID, BIM, BAD, BMF, BIK, PUMA, Noxa, HRK/DPS (Harakiri), NIP3, bNIP3, and MULE. The BH3 region is 16 to 25 amino acid residues long and can promote apoptosis when introduced into cells. The three groups of BCL-2 proteins form a delicately balanced network of opposing functions that regulates the fate of the cell.
MCL-1 belongs to the anti-apoptotic family but is unique due to its short half-life, the presence of an extra N-terminal sequence, and its particular selectivity in binding BH3 peptides. Its concentration in vivo is tightly regulated at multiple levels, and its N-terminal sequence presents many sites for post-translational modifications, including polyubiquitination (2, 3) at Lys136, limited proteolysis (4, 5) at Asp117, Asp127, Asp157, and phosphorylation (6, 7) of Thr64, Thr92, Thr163, and Thr159. These modifications are involved in regulating its activity in binding pro-apoptotic proteins, or in regulating its concentration by either promoting or blocking its degradation. A deregulated, high level of MCL-1 has been correlated with various hematopoietic and lymphoid cancers (8–10). It plays a major pro-survival role and is therefore an ideal therapeutic target (11). One way to develop specific human MCL-1 targeting drugs is to explore its interactions with other partners, specifically BH3-only proteins (12). In that aim, we optimized the expression and purification of various human MCL-1 constructs, and characterized their binding affinities to a panel of human BH3 peptides using a combination of structural and thermodynamic approaches.
BID, a BH3-only protein, performs its pro-apoptotic activity after it is proteolytically activated to a truncated form that is known as tBID (13, 14). It was reported that tBID could initiate BAX/BAK-dependent mitochondrial apoptosis (15), and this process could be inhibited by MCL-1 through its direct interaction with tBID (16). Here, we addressed the in vitro heterodimerization between a calpain-digested construct of each protein (cMCL-1 and cBID) by size exclusion chromatography and NMR titrations. In addition, we present the structure of cMCL-1·hBID-BH3 complex solved by NMR spectroscopy. The work reveals the structural basis of peptide recognition and insights for the rational design of drugs to specifically target MCL-1.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Constructs of human MCL-1 cDNA lacking the C-terminal transmembrane region were expressed as His-tagged fusion proteins (supplemental Fig. S2A). The complete soluble portion (ΔC23) and an N-terminal-truncated fragment (ΔN119-ΔC23) were subcloned in vector pET29b(+) with a C-terminal His tag, and ΔN151-ΔC7 was subcloned in vector pET32a with an N-terminal His tag (supplemental Fig. S2A). Escherichia coli strain BL21(DE3) containing the corresponding plasmid was grown in LB medium at 37 °C to an optical density of 0.8 at 600 nm, and then induced by 1 mm isopropyl β-d-thiogalactoside at 30 °C for 4 h. For NMR samples, cells were grown in M9 media supplemented with [15N]ammonium chloride and/or [13C]glucose to produce uniformly 15N- or 15N-,13C-labeled proteins. The soluble human MCL-1 proteins (ΔC23, ΔN119-ΔC23, and ΔN151-ΔC7) were purified through batch nickel-nitrilotriacetic acid affinity chromatography (Qiagen), Sephadex G-75 size exclusion chromatography (GE Healthcare), and Q-Sepharose anion-exchange chromatography (GE Healthcare). A calpain digestion step was further applied to ΔN119-ΔC23 or ΔN151-ΔC7 to produce cMCL-1 (ΔN162-ΔC24), and calpain was removed by Q-Sepharose anion-exchange chromatography. cBID (17), human BID-BH3 peptide (18) (residues 76–106 with four extra residues on N terminus GPLG-SESQEDIIRNIARHLAQVGDSMDRSIPPGLV), and rat μI-II calpain (19) were purified as previously described. The cMCL-1·BID-BH3 complex was prepared by mixing protein and peptide at 1:1.2 ratio, and unbound peptide was removed by Q-Sepharose chromatography. The BH3 peptides for BAD (residues 104–128 from NCBI CAG46757), BIK (residues 51–70 from NCBI CAG46681), PUMA (residues 131–150 from NCBI AAK39542), BID (residues 80–99 from NCBI NP_001187), Noxa (residues 18–38 from NCBI AAH13120), BIM (residues 82–101 from NCBI AAH33694), and MULE (residues 1969–1994 from NCBI AAY98258) were synthesized by the Sheldon Biotechnology Centre (McGill University), and purified by C18 reverse-phase chromatography. The chemically synthesized BID peptide was used for isothermal titration calorimetry (ITC). Elsewhere, BID-BH3 refers to the purified BID 35-mer produced biosynthetically. All proteins and peptides were stored in 20 mm HEPES (pH 6.5), 1 mm dithiothreitol buffer by flash freezing at −80 °C.
Limited Proteolysis and N-terminal Sequencing
Analytical limited proteolysis for MCL-1 ΔN119-ΔC23 or ΔN151-ΔC7 were performed at a concentration of 5 mg/ml, in 50 mm HEPES (pH 7.6), at room temperature (22 °C), in the presence of 0.001 mg/ml of trypsin (Sigma), 0.01 mg/ml of chymotrypsin (Sigma), or 5 mg/ml of μI-II calpain (supplemented with 50 mm CaCl2) for different time intervals. Reactions were quenched by 4× SDS sample buffer and applied on an SDS-PAGE gel. Proteolysis resistant bands were blotted onto polyvinylidene difluoride membranes, and N-terminal sequenced by the Sheldon Biotechnology Center (McGill University).
Mitochondria Purification and in Vitro Cytochrome c Release
Mitochondria of KB tumor cells were purified as described previously (20). Purified proteins (cBAK and/or cMCL-1) were used at 0.05 μm (final concentration) in the cytochrome c release reactions at 37 °C for 1 h. Centrifugation at 9,000 × g (4 °C) was applied to separate mitochondria from the supernatants and to stop the reactions. The supernatants and pellets were analyzed for cytochrome c by Western blotting using antibody 556433 (BD Biosciences) and secondary anti-mouse (111-035-008) antibody (Jackson ImmunoResearch Labs).
ITC Measurements
ITC was carried out on a MicroCal VP-ITC titration calorimeter controlled by VP Viewer software (MicroCal Inc., Northampton, MA). Experiments performed in 50 mm HEPES (pH 7.0) at 23 °C, consisted of 37 injections (8 μl) of 0.1 mm BH3 peptides into 1.45 ml of 0.01 mm human MCL-1 (four different constructs) to obtain a final peptide/protein ratio of 2:1. The calorimetric data were analyzed by the ORIGIN software to determine the thermodynamic binding constants.
NMR Titrations
The backbone and side chain assignments for free cMCL-1 and free BID-BH3 were determined with 15N,13C-labeled samples and standard triple-resonance NMR experiments: HNCACB, CBCA(CO)NH, HNCA, HNCO, CCH-TOCSY, and HCCH-COSY (21). In titration studies, unlabeled ligands were added to 15N-labeled cMCL-1 or cBID until saturation. The 15N-1H HSQC spectra were recorded on a Bruker Avance DRX600 MHz spectrometer, processed by NMRPipe (22) and visualized by NmrViewJ (23). Changes in chemical shifts of amide signals were plotted as a function of the residue numbers and mapped onto the three-dimensional structure.
Analytical Size Exclusion Chromatography
cMCL-1, cBID, and cMCL-1·cBID (mixed overnight at 30 °C) were prepared at 2 mg/ml in 20 mm HEPES (pH 6.5) and 1 mm dithiothreitol. The size exclusion running buffer contained 25 mm HEPES (pH 6.5), 150 mm NaCl, and 1 mm dithiothreitol. A 100-μl sample was injected when performing analytical runs on Superdex 75 (10/30).
NMR Data Collection, Analysis, and Spectral Assignments
For structure calculation, a series of samples, 0.5 mm 13C,15N-double-labeled cMCL-1:unlabeled BID-BH3 and 0.5 mm unlabeled cMCL-1·13C,15N-double-labeled BID-BH3, were prepared in 20 mm HEPES (pH 6.5), 1 mm dithiothreitol in either 10% D2O or 100% D2O. NMR experiments were recorded at 30 °C on Varian Unity Inova 800 or 500 MHz spectrometers. 1H-Homonuclear NOEs were measured from 15N-edited NOESY and 13C-edited NOESY spectra acquired with a 90-ms mixing time (24), and the inter-molecular 1H-homonuclear NOEs were detected from 15N,13C-filtered, 13C-edited NOESY and 15N,13C-filtered, 15N-edited NOESY. Amide heteronuclear 15N{1H} NOEs were used for determination of high mobility regions of protein (25). IPAP-HSQC experiments were recorded for both an isotropic sample and a sample with 7 mg/ml of Pf1 phage to measure 15N-1H residual dipolar couplings (RDCs) (26). All spectra were processed by NMRPipe (22) and analyzed with NmrViewJ 8.0 (23). ϕ and ϕ backbone torsion angles were derived by TALOS (27).
Structure Calculation and Analysis
Typical α-helical regions were identified by chemical shift indexing of Cα and Hα, and confirmed by the identification of Hα(i) − HN (i+3) medium range NOEs. CYANA 2.1 software (28) was used to generate the initial structures of hcMCL-1·hBID-BH3 complex with optimization to obtain low target functions. The structure was refined with CNS version 1.2 (29) using the standard protocol with a total set of 3563 unambiguous constraints listed in Table 1. Ten structures were selected based on lowest overall energy and least violations to represent the final structures. These were evaluated with PROCHECK (30) to check the stereochemical geometry of the protein. The atomic coordinates of the complex have been deposited in the Protein Data Bank under accession code 2KBW. Structure figures were generated with MacPymol (31).
TABLE 1.
Structural statistics for cMCL-1·BID-BH3 complex
| Structural statistics | |
|---|---|
| Constraints used for structure calculation | |
| Intraresidue NOEs (n = 0) | 973 |
| Sequential NOEs (n = 1) | 747 |
| Medium range NOEs (n = 2,3,4) | 631 |
| Long range NOEs (n > 4) | 386 |
| Intermolecular NOEs | 197 |
| Dihedral angle constraints | 282 |
| Hydrogen bonds | 190 |
| 15N-1H residual dipolar couplings | 157 |
| Total number of constraints | 3563 |
| Average energy values (kcal mol−1)a,b | |
| Etotal | 353.97 ± 10.23 |
| Ebond | 13.40 ± 0.69 |
| Eangle | 123.19 ± 3.40 |
| Eimproper | 14.86 ± 0.87 |
| EVdW | 84.26 ± 3.97 |
| ENOE | 71.60 ± 5.03 |
| Ecdih | 0.51 ± 0.13 |
| Esani | 46.15 ± 2.92 |
| Root mean square deviation from idealized covalent geometrya,b | |
| Bonds (Å) | 0.0021 ± 0.00005 |
| Angles (°) | 0.3734 ± 0.0052 |
| Improper (°) | 0.2452 ± 0.0071 |
| Root mean square deviation from experimental restraintsa,b | |
| Distance restraints (Å) | 0.0175 ± 0.0006 |
| Dihedral angle restraints (°) | 0.1206 ± 0.0159 |
| Residual dipolar couplingsb | |
| Root mean square deviation (Hz) | 2.71 ± 0.09 |
| Rdip | 0.127 ± 0.005 |
| Average root mean square deviation to mean structurea,b (Å) | |
| Backbone atoms | 0.38 ± 0.05 |
| All heavy (non-hydrogen) atoms | 1.04 ± 0.06 |
| Average Ramachandran statisticsa,b | |
| Residues in most favored regions | 85.6% |
| Residues in additional allowed regions | 11.1% |
| Residues in generously allowed regions | 2.5% |
| Residues in disallowed regions | 0.7% |
a Residues 173–192, 204–319, and 79–99.
b 10 lowest energy conformers.
RESULTS
The Optimization of Human MCL-1 Constructs
Previous structural studies have been carried out on MCL-1 from Mus musculus (mouse) with N- and C-terminal deletions (ΔN151 and ΔC23) and on a mouse/human chimeric variant (32–34). However, the comparable construct (ΔN170 and ΔC23 from Homo sapiens (human)) MCL-1 failed to express as a soluble recombinant protein (33). The sequence alignment (supplemental Fig. S1) showed the change of Glu182 in the mouse to the corresponding Arg202 in the human MCL-1, which possibly results in a change in the electrostatic charge and could be responsible for the differences in expression. To obtain better expression, we screened different constructs of human MCL-1 as shown in supplemental Fig. S2A. A soluble fragment containing the complete N-terminal sequence, which we term ΔC23, showed degradation during purification despite the presence of protease inhibitors (supplemental Fig. S2B). Two N-terminal deletions, ΔN119-ΔC23 and ΔN151-ΔC7, could be well purified; however, they did not yield interpretable NMR spectra or diffracting crystals. We then turned to limited proteolysis of MCL-1 ΔN119-ΔC23 using trypsin, chymotrypsin, and calpain. These generated smaller protease-resistant constructs with sizes of 17, 17, and 20 kDa, respectively (supplemental Fig. S2C). We used N-terminal sequencing to determine the sites of cleavage. Both trypsin (after residue 199) and chymotrypsin (after residue 201) cleave after the first helix (supplemental Fig. S1). In contrast, calpain cuts MCL-1 before the first α-helix (after residue 162) and retains the intact eight helical bundle (supplemental Figs. S1 and S2A). This fragment, which we call cMCL-1 (ΔN162-ΔC24), produced high-resolution spectra, and could inhibit cBAK-induced cytochrome c release from purified mitochondria from KB tumor cells (supplemental Fig. S2D (17)). However, the fragment failed to express as a fusion protein bearing histidine or glutathione S-transferase tags for affinity purification when subcloned in bacterial expression vectors (data not shown), and therefore subsequent studies were carried out on samples obtained by calpain digestion of purified MCL-1 ΔN119-ΔC23 or ΔN151-ΔC7.
The Selectivity of MCL-1 in Heterodimerizing with BH3 Peptides
The anti-apoptotic activity of MCL-1 is largely represented by its binding ability to pro-apoptotic proteins. To illustrate this, we used ITC to measure the binding affinities between the BH3 peptide and MCL-1. We designed and chemically synthesized a series of BH3 peptides spanning the BH3 regions of the human BH3-only pro-apoptotic proteins, BAD, BIK, PUMA, BID, Noxa, BIM, and MULE (Fig. 1A), to examine their interactions with recombinant truncated MCL-1 constructs. Fig. 1B shows a typical titration of MCL-1 ΔN119-ΔC23 with a 20-residue BID peptide, in which negative deflections from the baseline indicate exothermic binding. Integration of the peaks and fitting of the resulting thermogram with a 1:1 binding model, yield the Kd of 0.83 μm. Binding affinities for additional MCL-1·BH3 peptide complexes were calculated similarly (Fig. 1C) and they vary from undetectable to nanomolar, demonstrating a high degree of selectivity of MCL-1 for binding BH3 peptides. Accordingly, MCL-1 did not bind BAD, and bound BIK and PUMA with micromolar affinities, and BH3 regions from BID, Noxa, BIM, and MULE with nanomolar affinities. The wide range of affinities was further confirmed by NMR titrations of 15N-labeled cMCL-1 with unlabeled BH3 peptides (Fig. 2A and data not shown).
FIGURE 1.
Binding affinities of BH3 peptides to human MCL-1. A, sequence alignment of BH3 peptides derived from human BH3-only pro-apoptotic proteins: BAD, BIK, PUMA, BID, Noxa, BIM, and MULE. The conserved hydrophobic residues are highlighted. B, representative ITC profile for MCL-1 ΔN119-ΔC23 with BID peptide (synthesized BID-BH3). The peaks shown in the thermogram were integrated and then fitted to a 1:1 binding model, with a Kd of about 0.83 μm. C, graph and table of the ITC-derived dissociation constants (Kd in μm) for BH3 peptides and recombinant MCL-1 constructs lacking the C terminus with or without engineered deletions of the N-terminal region. Binding between MCL-1 and BAD-BH3 was undetectable. D, 15N-1H HSQC spectra of human BID-BH3 peptide without MCL-1 (black), with MCL-1 ΔC23 (blue), with ΔN119-ΔC23 (red), with ΔN151-ΔC7 (green), and with cMCL-1 (yellow).
FIGURE 2.
Mapping the BH3 peptide binding groove in MCL-1 by NMR. A, slices of 15N-1H HSQC spectra of cMCL-1 without peptide (black), and with BID-BH3 (blue). The arrows show the direction of chemical shift changes upon BID-BH3 binding. B, plot of chemical shift changes (calculated as ((Δ1H ppm)2 + (0.2 × Δ15N ppm)2)0.5) of cMCL-1 amide signals upon titration with the BID-BH3 peptide as a function of residue number. C, Cα trace on free human cMCL-1 model (calculated by Modeler 9v2 (35) based on the free mmMCL-1 structure 1WSX) colored according to the chemical shift changes upon BID-BH3 binding. The more intense the blue shading, the greater the chemical shift difference that was observed upon BID-BH3 binding.
The Effects of the N-terminal Sequence of MCL-1 on BH3 Binding
Comparison of the data for the different constructs shows that, with the intact N terminus, MCL-1 ΔC23 binds BH3 peptides with significantly lower affinities compared with truncated MCL-1 constructs (Fig. 1C). Removal of the N terminus augments the affinities between MCL-1 and BH3 peptides. On the other hand, the BH3 binding selectivity of MCL-1 was not affected by the N terminus. BAD-BH3 does not bind to any of the four constructs. The N-terminal sequence, which includes a PEST sequence that is likely unfolded, only weakens the binding affinities, but does not play a role in regulating the binding selectivity. To further test the effect of the N-terminal sequence on the ligand-binding properties of MCL-1, we carried out titrations of 15N-labeled BID-BH3 peptide with unlabeled recombinant MCL-1 (Fig. 1D). The signals in the free peptide spectrum (black) fall in the center of the spectrum, which is indicative of an unstructured conformation. When bound, most of the peaks (colored) dispersed reflecting conformational changes and folding of the peptide. Most importantly, the spectra of the peptide bound to the four MCL-1 constructs were very similar, indicating that the peptide adopted identical conformations and that the shortest and most stable construct, cMCL-1, deserves a name of core MCL-1 for providing the structural features necessary and sufficient for BH3 binding.
BH3 Binding Site on cMCL-1
We next explored the interactions of MCL-1 with the high affinity peptides from BID, BIM, Noxa, and MULE by performing NMR titrations. Chemical shift perturbations in cMCL-1 upon peptide binding were very similar (data not shown); the BID-BH3 titration spectra were selected as representative to analyze in more detail. The cross-peaks in 15N-1H HSQC spectra of cMCL-1 in both the free and bound forms were assigned, and the calculated chemical shift changes were mapped onto the free human MCL-1 structure model derived from the mouse protein (PDB code 1WSX) by Modeler 9v2 (35) (Fig. 2, B and C). Residues with the biggest chemical shift changes are mainly located in the end of helix 2 (α2), the end of α4 and the beginning of α5, and residues with smaller changes map in the middle of α5, the end of α6 and α8. These residues define a peptide binding pocket that is consistent with published structures of mouse (mm), human (hs) and chimeric mouse/human (mh) MCL-1. These are mmMCL-1·mmPUMA (PDB code 2ROC), mmMCL-1·mmNoxa_A (PDB code 2ROD), mmMCL-1·mmNoxa_B (PDB code 2JM6) mhMCL-1·mmNoxa_B (PDB code 2NLA), and mhMCL-1·hsBIM (PDB code 2NL9)(33, 34).
Calpain Cut BID (cBID) Initiates Mitochondrial Cytochrome c Release
BID initiates mitochondrial apoptosis via a proteolytically activated, truncated form termed tBID. Due to the difficulty in directly expressing tBID, we expressed the full-length protein as a glutathione S-transferase fusion, and again performed limited proteolysis. Calpain cleaves BID in the unfolded loop region following helix α2 (36), generating a truncated form of cBID (supplemental Fig. S3, A and B). As observed in caspase cleavage (37), the N- and C-terminal fragments following calpain treatment remain associated and copurify resulting in two bands on a Coomassie-stained gel (supplemental Fig. S3C). The pro-apoptotic activity of cBID was demonstrated by adding it to mitochondria purified from KB tumor cells, where it initiated the release of cytochrome c (supplemental Fig. S3D).
The Interaction between cBID and cMCL-1
MCL-1 and tBID interact both in vivo and in vitro (16). Here, we examined whether cMCL-1 interacts with cBID using NMR spectroscopy. Following addition of an excess of unlabeled cBID to 15N-labeled cMCL-1, 15N-1H HSQC spectra were recorded every 4 h. Although some specific chemical shift perturbations started to appear 4 h after the titration, the complete transition was only observed at the 16 h time point. The HSQC spectra show two sets of signals that arise from the free and bound forms of cMCL-1 (Fig. 3B). The surprisingly slow process of cBID binding is likely due to the slow dissociation of the two halves of the cleaved cBID molecule to expose the BID-BH3 region. Comparison of titrations of cMCL-1 with cBID and BID-BH3 showed similar patterns of chemical shift changes, which confirms that cBID interacts with the BH3 binding pocket of cMCL-1 (Fig. 3, C and D). To exclude the possibility of protein degradation, we performed analytical size exclusion chromatography using bovine serum albumin and ubiquitin as protein standards (Fig. 3A). Free cMCL-1 (green) and cBID (black) eluted at 9.9 and 10.3 ml, respectively, whereas the overnight mixture of cMCL-1 and cBID in 1:2 ratio (blue) gave two elution peaks: one at 10.3 ml, confirming the existence of excess, free cBID, and a second, higher molecular weight peak at 9.2 ml representing the cMCL-1·cBID complex.
FIGURE 3.
Interaction between cMCL-1 and cBID. A, size exclusion chromatography profile of bovine serum albumin (red), ubiquitin (pink), cBID (black), cMCL-1 (green) and an overnight mixture of cMCL-1 and cBID (blue). B, one-dimensional views from the 1H dimension of 15N-1H HSQC spectra, representing specific residues (Asp195, Ser202, and Phe315). The black, blue, green, purple, and red spectra were recorded, respectively, 0, 4, 8, 12, and 16 h after the addition of unlabeled cBID to 15N-labeled cMCL-1. C, 15N-1H HSQC spectra of cMCL-1 without peptide (black), with BID-BH3 (blue), and with cBID protein (red). D, plots of chemical shift changes (calculated as ((Δ1H ppm)2 + (0.2 × Δ15N ppm)2)0.5) of cMCL-1 amide signals upon titrations with cBID (red) and the differences (blue) between the spectra with BID-BH3 and cBID as a function of residue number.
Solution Structure of cMCL-1·BID-BH3 Complex
Using a variety of NMR spectra, over 95% of backbone and side chain resonances were determined for the 1H, 15N, and 13C atoms in both cMCL-1 and BID-BH3 in the complex. The secondary structure chemical shift indexes based on Cα and Hα, and the analysis of the sequential and medium range NOE connectivities were used to determine the secondary structure. In the complex, cMCL-1 is composed of 8 helices (α1, 173–192; α2, 204–225; α3, 226–236; α4, 239–255; α5, 260–281; α6, 286–302; α7, 303–309; α8, 312–319). The BID-BH3 peptide forms one α-helix (α1′, residues 79–99). The limits of the folded regions of the structure were verified by the measurement of 15N{1H} heteronuclear NOEs values greater than 0.6 (supplemental Fig. S4A, bottom). By comparing the regular NOESY spectra with 15N,13C-filtered, 13C-edited NOESY, and 15N,13C-filtered, 15N-edited NOESY, respectively, we were able to assign 197 inter-molecular NOEs (Fig. 4A) between protons from the two different molecules (Fig. 4B). In the final structure calculation, 3563 constraints were invoked using standard molecular dynamics protocols (Table 1). The final ensemble (Fig. 4C) of structures with the lowest energies and the fewest violations had a mean root mean square deviation from the average of 0.38 Å for backbone atoms, excluding the residues at the termini (residues 167–172 and 320–326 in cMCL-1; residues 76–78 and 100–106 in BID-BH3) and the ones in the disordered loop between helices α1 and α2 (cMCL-1 residues 193–203). A schematic representation of the structure closest to the average structure is presented in Fig. 4D.
FIGURE 4.
Solution structure of the cMCL-1:BID-BH3 complex. A, determination of inter-molecular NOEs in the complex. Slices of 1H-1H planes are compared between a three-dimensional 13C-edited NOESY (green background) and a 15N,13C-filtered, 13C-edited NOESY (red background) spectrum. The first pair of strips (green frame) show NOEs for Val253 HG11 from cMCL-1. The following pairs (red frames) show NOEs for Ala87 HB1 and Leu90 HD11 from the BID-BH3 peptide. The inter-molecular NOEs are indicated by blue and red ovals and dashed lines. B, detail of the structure showing residues responsible for the NOEs identified in panel A. cMCL-1 is in green and BID-BH3 is in magenta. C, ribbon diagram of the 10-structure ensemble of the complex cMCL-1·BID-BH3, with cMcl-1 in blue and BID-BH3 peptide in magenta. D, schematic representation of the complex. The BH1, BH2, and BH3 regions in cMcl-1 are colored green, beige, and blue, respectively. The peptide is colored magenta. N, C, N′, and C′ are used to label the termini of the molecules.
As part of structure determination, a set of 182 15N-1H RDCs were measured on two complexes with 15N labeling of either cMCL-1 or the BID-BH3 peptide in Pf1 phage (supplemental Fig. S4A, top). For proline residues and residues with significant overlap (Pro198, Pro289, Ile182, and Arg207 in cMCL-1; Pro102 and Pro103 in BID-BH3), the RDC values were not obtained. Normally, RDCs cannot be used to refine the mobile regions (38) including the N- and C-terminal regions and the unstructured loop region between helix α1 and α2 in this case. However, the RDCs for the residues in the partially disordered loop fit the structure well, indicating that the loop following helix α1 of cMCL-1 is fixed to some degree. Thus, 157 RDCs including those for that loop were included in the final refinement, which decreased the backbone root mean square deviation from 0.52 to 0.38 Å, and dropped the RDC Rdip from 0.55 to 0.127 (supplemental Fig. S4B).
The structure of cMCL-1·BID-BH3 (PDB code 2KBW) shows a very similar topology to other BCL-2 complexes. The central helix α5 is surrounded by other α-helices, and the hydrophobic groove defined by α2–α5 and α8 is occupied by the α-helix of the BID-BH3 peptide. This is consistent with the BH3-binding site as detected by NMR titrations. By aligning our structure with other MCL-1 complexes using the DALI program (Fig. 5A), the pairwise Cα atomic coordinate root mean square deviations for the secondary structure elements range from 1.4 to 1.6 Å, and the DALI Z factors range from 16.6 to 18.0 (Fig. 5B). In the cMCL-1 structure, the major difference is that helix α4 is shorter comprising residues 239–255 instead of 239–257. The side chains of the residues in the hydrophobic peptide binding pocket adopt similar conformations across the family of structures but with minor differences due to variation in the sequences of the bound peptides (Fig. 5C). The hydrophilic interactions between BID-BH3 and cMCL-1 are mainly present at both ends of the peptide (Fig. 5D). At the peptide N terminus, Gln79 interacts with the side chain of Asp242 in cMCL-1. At the peptide C terminus, the backbone amides of Ala91 and Gly94 interact with the side chains of Arg263 and Thr266 in cMCL-1. The side chain of one of the last ordered BID-BH3 residues, Asp98, makes hydrogen bonds with the side chain of Asn260 and the backbone amide of Gly262.
FIGURE 5.
Comparison of cMCL-1·BID-BH3 with other BH3 complexes of MCL-1. A, superimposition of cMCL-1·BID-BH3 (green, this work, PDB code 2KBW) and MCL-1·BIM-BH3 (purple, PDB code 2PQK). The complex is shown in the same orientation as in Fig. 4D. B, table listing the root mean square deviations and Z-factors for the alignment by the program DALI of hs-cMCL-1·BID-BH3 with other MCL-1 complexes. Protein/BH3 peptide names are prefaced by their sequence origin: mm, mouse; hs, human; mh, chimeric mouse/human. C, aligned key hydrophobic residues in helices α2/α3 of MCL-1 and the BH3 peptides from the complexes: cMCL-1·BID-BH3 (blue, this work), hsMCL-1·hsBIM-BH3 (magenta, 2PQK), and mhMCL-1·mmNoxa-B (yellow, 2NLA). Selected amino acids side chains are shown and labeled in black for MCL-1 and color-coded for the three different BH3 peptides. D, detail of the hydrophilic interactions between the BID-BH3 peptide and the peptide-binding groove of cMCL-1.
DISCUSSION
MCL-1 plays a notable pro-survival role in vivo and its elimination in response to cytotoxic signals is critical in promoting cell death (39–41). Accordingly, it has become an important target in the search for new anti-cancer drugs. Although development of ABT-737 and its derivatives have validated the approach of using BH3 mimetics for treating tumors (42), their low affinities for MCL-1 precludes their use in combating malignancies driven by high MCL-1 levels (43–45). Multiple structures of MCL-1 in complex with BH3 peptides are crucial for the development of small molecule inhibitors specifically targeting MCL-1 or capable of broadly interacting with all anti-apoptotic BCL-2 proteins.
The first obstacle to structural studies of MCL-1 is the difficulty in producing stable protein due to heterogeneity brought about by degradation during bacterial expression and subsequent purification. The problem of degradation was solved by the deletion of the N-terminal sequence (32–34); however, the mouse/human chimera construct failed to produce high-resolution NMR spectra (33). These are a requirement not only for solving the solution structure, but also for drug screening by NMR. In this study, we report the use of limited proteolysis to produce a calpain cleavage product (cMCL-1) that is the first human construct suitable for NMR studies. In its free form, 95% of the cMCL-1 backbone signals could be assigned and this will enable future screening for compounds that specifically target MCL-1.
We took advantage of the high quality NMR spectra to study ligand binding by cMCL-1. All tested peptides that bound MCL-1 with micromolar to nanomolar affinities perturbed the NMR signals of cMCL-1 similarly (data not shown), which indicates that they induce the same conformational change in cMCL-1 upon binding. Detailed analysis of the chemical shift changes showed that the cMCL-1 residues with the largest changes are mainly located in the hydrophobic groove of cMCL-1 formed by the BH1–3 regions (Fig. 2, B and C). This represents a universal feature of all pro-survival BCL-2 complexes. We determined the selectivity of MCL-1 for BH3 ligands by comparing the binding affinities measured by ITC (Fig. 1, B and C). The failure of MCL-1 to bind BAD-BH3 and its strong preference for BIM-BH3, Noxa-BH3, and MULE-BH3 are consistent with results reported by other groups, although the values vary somewhat due to differences in the methods and peptides used (34, 46, 47). For example, the shorter PUMA-BH3 (residues 131–150, our work) has a much lower affinity to human MCL-1 than reported for a longer peptide (residues 130–155) binding to mouse MCL-1 (34).
The higher affinity of longer peptides may be due to their greater helical propensity or the presence of binding elements outside of the consensus BH3 region. In the case of BID-BH3 binding to human MCL-1, we observed almost 10-fold higher affinity for a longer biosynthetically prepared peptide comprising residues 76–106 than for the chemically synthesized peptide comprising residues 80–99 (Fig. 1C). ITC measurements with the longer BID-BH3 peptide yielded affinities of 85 nm for MCL-1-ΔN119-ΔC23 and 40 nm for MCL-1-ΔN151-ΔC7. These are similar to the values reported for a 34-residue mouse BID peptide binding to mouse MCL-1 (34).
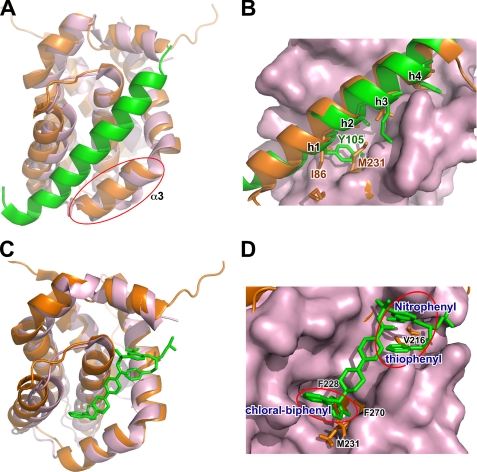
A detailed examination of the cMCL-1·BID-BH3 structure shows that there is an extra hydrophilic interaction between the side chains of Gln79 in the N terminus of the peptide and Asp242 in the binding pocket of cMCL-1 (Fig. 5D). This interaction may contribute to the higher affinity observed for the longer BID-BH3 peptide. The structure also explains the low affinities of MCL-1 in binding BAD-BH3 and ABT-737, the hotly studied BCL-2 inhibitor. The overlay of our structure and the crystal structure of BCL-XL·BAD-BH3 (2BZW) reveals that the biggest conformational difference between MCL-1 and BCL-XL lies in helix α3 (Fig. 6A). The better formation of helix α3 in MCL-1 leads to its restriction in peptide binding (33). Fig. 6B presents that the conformations of the first hydrophobic residues (h1) in BH3 peptides varies largest, and Met231 in MCL-1 helix α3 prevents the engagement of Tyr105 in BAD-BH3 by spatial confliction. Superimposing our structure with the crystal structure of BCL-XL·ABT-737 complex (Fig. 6C) clearly shows that the hydrophobic groove in BCL-XL is narrow and deep, whereas the one in cMCL-1 is wider and shallower. As a result, the pocket formed by Phe228, Met231, and Phe270, which accommodates the binding of Leu90 in BID-BH3, results in spatial restriction that blocks binding of the chloral-biphenyl group in ABT-737 (Fig. 6D). Similarly, the side chain of Val216 hinders the engagement of the nitrophenyl and the thiophenyl groups in ABT-737 spatially.
FIGURE 6.
The BH3 binding groove of cMCL-1 poorly accommodates BAD-BH3 and ABT-737. A and B, overlay of the structures of cMCL-1·BID-BH3 (orange, this work, BID-BH3 is hidden for clarity in A) and BCL-XL·BAD-BH3 (pink, BCL-XL; green, BAD-BH3, 2BZW) with the only involvement of the hydrophobic grooves of cMCL-1 and BCL-XL for alignment. The significant difference in α3 is circled in A, and the hydrophobic residues in peptides (h1–4) are labeled in B. C and D, overlay of the structures of cMCL-1·BID-BH3 (orange, this work, BID-BH3 is hidden for clarity) and BCL-XL·ABT-737 (pink, BCL-XL; green, ABT-737, 2YXJ) with the only involvement of the hydrophobic grooves of cMCL-1 and BCL-XL for alignment. MCL-1 residues that appear to hinder sterically ABT-737 binding are labeled in black, and the chloral-biphenyl, nitrophenyl, and the thiophenyl groups of ABT-737 are labeled and circled.
The N-terminal sequence of MCL-1 has always been deleted in structural studies due to its lack of structure; however, its regulatory functions have garnered attention recently. With two PEST regions, it not only plays a vital regulating role in the subcellular localization of MCL-1 (48), but also provides critical sites for polyubiquitination (2, 3), limited proteolysis (4, 5), and phosphorylation (7) that modulate the degradation and turnover of MCL-1. It has been reported that calpain inhibitors can prevent the degradation of MCL-1, indicating that calpain digestion of MCL-1 could possibly play a physiological role. Here, using four human MCL-1 constructs with different N-terminal deletions, we further explored the effects of this intrinsically unstructured sequence, unique to MCL-1, on BH3-peptide binding. HSQC spectra of the labeled BID-BH3 peptide bound to different unlabeled MCL-1 constructs were very similar suggesting that the N-terminal sequence does not influence the structure of the bound peptide (Fig. 1D). In addition, whereas the N-terminal sequence does not alter the binding preference of MCL-1; its deletion always led to higher binding affinity. This may be due to nonspecific steric crowding from the N terminus or it may arise from low affinity binding of the N terminus to the MCL-1 hydrophobic pocket. Whatever the mechanism, proteolytic processing of MCL-1 by caspase, granzyme, and other proteases may be expected to increase not only the stability but also the affinity of MCL-1 binding of the BH3 regions of pro-apoptotic proteins.
BID, the only pro-apoptotic BH3-only protein known to assume a globular-fold, has been widely studied in research on the direct activation of BAX and BAK; however, its regulation by anti-apoptotic proteins is always carried out using the BID-BH3 peptide, and whether the protein will behave similarly to the peptide has remained an open question. In 2006, Brady and colleagues (16) reported that MCL-1 directly interacts with tBID in vivo to inhibit the release of cytochrome c. In this study using a calpain-activated form, we were able to detect the direct interaction between cBID and cMCL-1 in vitro. This interaction is very slow in solution and requires 16 h for completion as detected by NMR. This is most likely due to the kinetic barrier for the dissociation of the N- and C-terminal fragments, which is postulated to increase the accessibility of the BH3 region. In the undissociated complex, the cBID BH3 region is not available for cMCL-1 binding. The interaction of cBID and cMCL-1 can be accelerated by conditions that destabilize cBID. Addition of 0.1% of the detergent IGEPAL decreased the interaction time to less than 1 h (data not shown). The HSQC NMR spectra of cMCL-1 (Fig. 3B) suggests that cBID binds cMCL-1 very tightly. The strong similarity of cMCL-1 spectra in complex with BID-BH3 or cBID (Fig. 3, C and D) confirms that cBID forms a stable heterodimer by engaging its BH3 region in the hydrophobic pocket of cMCL-1. This result is consistent with the recently reported direct interaction between tBID and BCL-XL (49).
In conclusion, we report a human MCL-1 construct (cMCL-1) suitable for NMR studies, the solution structure of a human MCL-1·BH3 complex, and critical features of the highly anticipated MCL-1·activated BID complex. By NMR and ITC, we show that the N-terminal sequence of MCL-1 affects its binding affinity but not selectivity. Together these results will be valuable for future drug screening and design of compounds that specifically target human MCL-1.
Supplementary Material
Acknowledgment
We acknowledge GeminX for providing the human MCL-1 expressing plasmids and the cells for mitochondria import assays.
This work was supported by Canadian Institute of Health Research Grant MOP-81277 (to K. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
The atomic coordinates and structure factors (code 2KBW) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- BH
- BCL-2 homology
- ITC
- isothermal titration calorimetry
- RDC
- residual dipolar coupling
- MCL-1
- myeloid cell leukemia 1
- cBID
- calpain cut BID
- cMCL-1
- core domain of MCL-1
- PDB
- Protein Data Bank
- HSQC
- heteronuclear single quantrum coherence.
REFERENCES
- 1.Chipuk J. E., Moldoveanu T., Llambi F., Parsons M. J., Green D. R. (2010) Mol. Cell 37, 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warr M. R., Acoca S., Liu Z., Germain M., Watson M., Blanchette M., Wing S. S., Shore G. C. (2005) FEBS Lett. 579, 5603–5608 [DOI] [PubMed] [Google Scholar]
- 3.Zhong Q., Gao W., Du F., Wang X. (2005) Cell 121, 1085–1095 [DOI] [PubMed] [Google Scholar]
- 4.Han J., Goldstein L. A., Gastman B. R., Rabinovitz A., Rabinowich H. (2005) J. Biol. Chem. 280, 16383–16392 [DOI] [PubMed] [Google Scholar]
- 5.Weng C., Li Y., Xu D., Shi Y., Tang H. (2005) J. Biol. Chem. 280, 10491–10500 [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S., Lee S. H., Meng X. W., Mott J. L., Bronk S. F., Werneburg N. W., Craig R. W., Kaufmann S. H., Gores G. J. (2007) J. Biol. Chem. 282, 18407–18417 [DOI] [PubMed] [Google Scholar]
- 7.Ding Q., Huo L., Yang J. Y., Xia W., Wei Y., Liao Y., Chang C. J., Yang Y., Lai C. C., Lee D. F., Yen C. J., Chen Y. J., Hsu J. M., Kuo H. P., Lin C. Y., Tsai F. J., Li L. Y., Tsai C. H., Hung M. C. (2008) Cancer Res. 68, 6109–6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derenne S., Monia B., Dean N. M., Taylor J. K., Rapp M. J., Harousseau J. L., Bataille R., Amiot M. (2002) Blood 100, 194–199 [DOI] [PubMed] [Google Scholar]
- 9.Alvi A. J., Austen B., Weston V. J., Fegan C., MacCallum D., Gianella-Borradori A., Lane D. P., Hubank M., Powell J. E., Wei W., Taylor A. M., Moss P. A., Stankovic T. (2005) Blood 105, 4484–4491 [DOI] [PubMed] [Google Scholar]
- 10.Cavarretta I. T., Neuwirt H., Untergasser G., Moser P. L., Zaki M. H., Steiner H., Rumpold H., Fuchs D., Hobisch A., Nemeth J. A., Culig Z. (2007) Oncogene 26, 2822–2832 [DOI] [PubMed] [Google Scholar]
- 11.Sieghart W., Losert D., Strommer S., Cejka D., Schmid K., Rasoul-Rockenschaub S., Bodingbauer M., Crevenna R., Monia B. P., Peck-Radosavljevic M., Wacheck V. (2006) J. Hepatol. 44, 151–157 [DOI] [PubMed] [Google Scholar]
- 12.Fesik S. W. (2005) Nat. Rev. Cancer 5, 876–885 [DOI] [PubMed] [Google Scholar]
- 13.Li H., Zhu H., Xu C. J., Yuan J. (1998) Cell 94, 491–501 [DOI] [PubMed] [Google Scholar]
- 14.Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. (1998) Cell 94, 481–490 [DOI] [PubMed] [Google Scholar]
- 15.Wei M. C., Lindsten T., Mootha V. K., Weiler S., Gross A., Ashiya M., Thompson C. B., Korsmeyer S. J. (2000) Genes Dev. 14, 2060–2071 [PMC free article] [PubMed] [Google Scholar]
- 16.Clohessy J. G., Zhuang J., de Boer J., Gil-Gómez G., Brady H. J. (2006) J. Biol. Chem. 281, 5750–5759 [DOI] [PubMed] [Google Scholar]
- 17.Moldoveanu T., Liu Q., Tocilj A., Watson M., Shore G., Gehring K. (2006) Mol. Cell 24, 677–688 [DOI] [PubMed] [Google Scholar]
- 18.Denisov A. Y., Chen G., Sprules T., Moldoveanu T., Beauparlant P., Gehring K. (2006) Biochemistry 45, 2250–2256 [DOI] [PubMed] [Google Scholar]
- 19.Moldoveanu T., Hosfield C. M., Lim D., Elce J. S., Jia Z., Davies P. L. (2002) Cell 108, 649–660 [DOI] [PubMed] [Google Scholar]
- 20.Goping I. S., Gross A., Lavoie J. N., Nguyen M., Jemmerson R., Roth K., Korsmeyer S. J., Shore G. C. (1998) J. Cell Biol. 143, 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavanagh J. (1996) Protein NMR Spectroscopy: Principles and Practice, Academic Press, San Diego [Google Scholar]
- 22.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 23.Johnson B. A., Blevins R. A. (1994) J. Biomol. NMR 4, 603–614 [DOI] [PubMed] [Google Scholar]
- 24.Clore G. M., Gronenborn A. M. (1994) Methods Enzymol. 239, 349–363 [DOI] [PubMed] [Google Scholar]
- 25.Peng J. W., Wagner G. (1994) Methods Enzymol. 239, 563–596 [DOI] [PubMed] [Google Scholar]
- 26.Ottiger M., Delaglio F., Bax A. (1998) J. Magn. Reson. 131, 373–378 [DOI] [PubMed] [Google Scholar]
- 27.Cornilescu G., Delaglio F., Bax A. (1999) J. Biomol. NMR 13, 289–302 [DOI] [PubMed] [Google Scholar]
- 28.Güntert P. (2004) Methods Mol. Biol. 278, 353–378 [DOI] [PubMed] [Google Scholar]
- 29.Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 30.Laskowski R. A., Rullmannn J. A., MacArthur M. W., Kaptein R., Thornton J. M. (1996) J. Biomol. NMR 8, 477–486 [DOI] [PubMed] [Google Scholar]
- 31.Delano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific, San Carlos, CA [Google Scholar]
- 32.Day C. L., Chen L., Richardson S. J., Harrison P. J., Huang D. C., Hinds M. G. (2005) J. Biol. Chem. 280, 4738–4744 [DOI] [PubMed] [Google Scholar]
- 33.Czabotar P. E., Lee E. F., van Delft M. F., Day C. L., Smith B. J., Huang D. C., Fairlie W. D., Hinds M. G., Colman P. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6217–6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day C. L., Smits C., Fan F. C., Lee E. F., Fairlie W. D., Hinds M. G. (2008) J. Mol. Biol. 380, 958–971 [DOI] [PubMed] [Google Scholar]
- 35.Sali A., Blundell T. L. (1993) J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 36.Mandic A., Viktorsson K., Strandberg L., Heiden T., Hansson J., Linder S., Shoshan M. C. (2002) Mol. Cell Biol. 22, 3003–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou J. J., Li H., Salvesen G. S., Yuan J., Wagner G. (1999) Cell 96, 615–624 [DOI] [PubMed] [Google Scholar]
- 38.Meiler J., Prompers J. J., Peti W., Griesinger C., Brüschweiler R. (2001) J. Am. Chem. Soc. 123, 6098–6107 [DOI] [PubMed] [Google Scholar]
- 39.Craig R. W. (2002) Leukemia 16, 444–454 [DOI] [PubMed] [Google Scholar]
- 40.Willis S. N., Chen L., Dewson G., Wei A., Naik E., Fletcher J. I., Adams J. M., Huang D. C. (2005) Genes Dev. 19, 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunelle J. K., Shroff E. H., Perlman H., Strasser A., Moraes C. T., Flavell R. A., Danial N. N., Keith B., Thompson C. B., Chandel N. S. (2007) Mol. Cell. Biol. 27, 1222–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oltersdorf T., Elmore S. W., Shoemaker A. R., Armstrong R. C., Augeri D. J., Belli B. A., Bruncko M., Deckwerth T. L., Dinges J., Hajduk P. J., Joseph M. K., Kitada S., Korsmeyer S. J., Kunzer A. R., Letai A., Li C., Mitten M. J., Nettesheim D. G., Ng S., Nimmer P. M., O'Connor J. M., Oleksijew A., Petros A. M., Reed J. C., Shen W., Tahir S. K., Thompson C. B., Tomaselli K. J., Wang B., Wendt M. D., Zhang H., Fesik S. W., Rosenberg S. H. (2005) Nature 435, 677–681 [DOI] [PubMed] [Google Scholar]
- 43.Konopleva M., Contractor R., Tsao T., Samudio I., Ruvolo P. P., Kitada S., Deng X., Zhai D., Shi Y. X., Sneed T., Verhaegen M., Soengas M., Ruvolo V. R., McQueen T., Schober W. D., Watt J. C., Jiffar T., Ling X., Marini F. C., Harris D., Dietrich M., Estrov Z., McCubrey J., May W. S., Reed J. C., Andreeff M. (2006) Cancer Cell 10, 375–388 [DOI] [PubMed] [Google Scholar]
- 44.Chauhan D., Velankar M., Brahmandam M., Hideshima T., Podar K., Richardson P., Schlossman R., Ghobrial I., Raje N., Munshi N., Anderson K. C. (2007) Oncogene 26, 2374–2380 [DOI] [PubMed] [Google Scholar]
- 45.Trudel S., Stewart A. K., Li Z., Shu Y., Liang S. B., Trieu Y., Reece D., Paterson J., Wang D., Wen X. Y. (2007) Clin. Cancer Res. 13, 621–629 [DOI] [PubMed] [Google Scholar]
- 46.Chen L., Willis S. N., Wei A., Smith B. J., Fletcher J. I., Hinds M. G., Colman P. M., Day C. L., Adams J. M., Huang D. C. (2005) Mol. Cell 17, 393–403 [DOI] [PubMed] [Google Scholar]
- 47.Certo M., Del Gaizo Moore V., Nishino M., Wei G., Korsmeyer S., Armstrong S. A., Letai A. (2006) Cancer Cell 9, 351–365 [DOI] [PubMed] [Google Scholar]
- 48.Germain M., Duronio V. (2007) J. Biol. Chem. 282, 32233–32242 [DOI] [PubMed] [Google Scholar]
- 49.Yao Y., Bobkov A. A., Plesniak L. A., Marassi F. M. (2009) Biochemistry 48, 8704–8711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.