Abstract
d-Galactan I is a polysaccharide with the disaccharide repeat unit structure [→3-β-d-Galf-(1→3)-α-d-Galp-(1→]. This glycan represents the lipopolysaccharide O antigen found in many Gram-negative bacteria, including several Klebsiella pneumoniae O serotypes. The polysaccharide is synthesized in the cytoplasm prior to its export via an ATP-binding cassette transporter. Sequence analysis predicts three galactosyltransferases in the d-galactan I genetic locus. They are WbbO (belonging to glycosyltransferase (GT) family 4), WbbM (GT-family 8), and WbbN (GT-family 2). The WbbO and WbbM proteins are each predicted to contain two domains, with the GT modules located toward their C termini. The N-terminal domains of WbbO and WbbM exhibit no similarity to proteins with known function. In vivo complementation assays suggest that all three glycosyltransferases are required for d-galactan I biosynthesis. Using a bacterial two-hybrid system and confirmatory co-purification strategies, evidence is provided for protein-protein interactions among the glycosyltransferases, creating a membrane-located enzyme complex dedicated to d-galactan I biosynthesis.
Keywords: ABC Transporter, Bacteria, Carbohydrate Biosynthesis, Lipopolysaccharide (LPS), Membrane Biogenesis, Membrane Function, Polysaccharide, O Antigens, Glycosyltransferases, Prokaryotic Glycobiology
Introduction
Lipopolysaccharide (LPS)3 is a major component of the outer leaflet of the Gram-negative outer membrane. In many bacteria, including members of the Enterobacteriaceae, LPS is made up of three structural domains; lipid A, the core oligosaccharide (OS), and the O antigenic polysaccharide (O-PS). Lipid-A-core OS and O-PS are synthesized by independent pathways at the cytoplasmic face of the inner membrane and are subsequently linked together in the periplasm by the O-PS ligase (reviewed in Ref. 1). Although the structure of lipid-A is highly conserved among Gram-negative bacteria, and the core OSs share structural themes, the O-PS structures are remarkably diverse (1).
The O-PS polymer known as d-galactan I contains the disaccharide repeat unit structure: [→3-β-d-Galf-(1→3)-α-d-Galp-(1→] (Fig. 1) and is found in the LPS of several Gram-negative bacteria, including some Klebsiella pneumoniae O serotypes. It is the sole O-PS in K. pneumoniae O2a (2). In other serotypes, d-galactan I can be modified by O-acetylation (3, 4) or capped by an additional polymeric domain with a different repeat unit structure (5–7). Each of these modifications confers a unique O-serospecificity. Similar structures are found in other bacterial genera, including Serratia (8, 9), and the genetic loci for d-galactan I biosynthesis are conserved in organization and content (4).
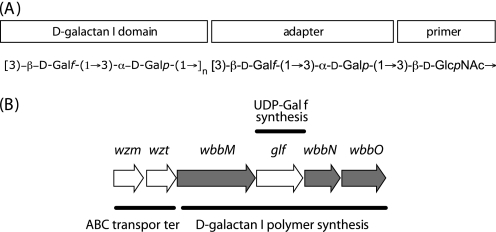
FIGURE 1.
Biosynthesis of d-galactan I from K. pneumoniae O2a. A, structure of the d-galactan I polysaccharide (11). The adaptor region consists of two galactose residues believed to be added to und-PP-GlcNAc by WbbO (14, 20). The precise glycosyltransferase activities required for extension of the d-galactan I domain remain undetermined. B, the d-galactan I biosynthesis cluster. The various roles of the proteins encoded by these genes are given. Known and predicted galactosyltransferases are highlighted (gray fill). The UDP-Galp precursor is generated by UDP-Gal-4-epimerase, a housekeeping enzyme (48). In contrast, UDP-Galf is required only for the d-galactan I biosynthesis in K. pneumoniae, so the dedicated UDP-Galp mutase enzyme (Glf) is encoded by a gene in the biosynthesis gene cluster (21).
There are two fundamentally different pathways by which most O-PSs are assembled: the Wzy-dependent pathway and the ATP-binding cassette (ABC) transporter-dependent pathway (1). d-Galactan I provides an example of ABC transporter-dependent biosynthesis. In the Enterobacteriaceae (and many other bacteria), the ABC transporter-dependent pathways begins with the synthesis of the polymer repeat units on an acceptor consisting of undecaprenol diphospho-N-acetylglucosamine (und-PP-GlcNAc). The acceptor is synthesized by the UDP-N-acetylglucosamine:Und-P GlcNAc-1-P transferase, WecA (10–13). This requirement has been established for d-galactan-I (14). O-PS is elongated on this acceptor at the cytoplasmic face of the inner membrane by dedicated O-antigen-specific glycosyltransferases. The polymer is elongated by sequential glycosyltransfer to the non-reducing terminus. Chain extension in the ABC transporter-dependent pathways is then terminated by one of two remarkably different strategies. In a model exemplified by the polymannose O-PSs from Escherichia coli O8 and O9a, a residue not found in the repeat unit structure is added to the non-reducing terminus and this serves as an export signal (15–17). The completed O-PS structure is then exported across the membrane by the ABC transporter, which is composed of two Wzm (transmembrane domain) polypeptides, and two Wzt (nucleotide-binding domain) polypeptides. In E. coli O8/O9a, Wzt contains an additional carbohydrate-binding module that recognizes the precise export signal on the nascent polymer (16, 17). In contrast, in the biosynthesis of d-galactan I in K. pneumoniae O2a, chain termination is determined by an interaction between the transporter and the glycosyltransferases (or their product) in a manner that is not yet determined (18). There is no identifiable export signal on the polymer and, unlike the O8/O9a situation, the d-galactan I Wzt protein has no specificity for a particular polysaccharide structure. Regardless of the assembly pathway, the completed O-PS is then ligated to lipid-A core at the periplasmic face of the membrane by the O-PS ligase. The mature LPS molecules are then shuttled to the outer membrane by the Lpt pathway (19).
Biosynthesis and export of the O-PS are predicted to require strict coordination of initiation, elongation, and export to maintain the specific O-PS chain length. Current models for O-PS biosynthesis invoke a coordinated multienzyme complex but experimental evidence for such complexes is generally limited. The goal of this study was to examine potential protein-protein interactions among enzymes required for d-galactan I biosynthesis. Bioinformatic analysis indicates that the K. pneumoniae d-galactan I biosynthesis gene cluster encodes three predicted galactosyltransferases, designated WbbM, WbbN, and WbbO, with confirmatory experimental evidence being available for WbbO and WbbM (20). The cluster also encodes the UDP-galactopyranose mutase (Glf) enzyme responsible for production of the UDP-Galf precursor (21) (see Fig. 1). Using a bacterial two-hybrid system and co-purification, interactions between the glycosyltransferases involved in d-galactan I biosynthesis have been confirmed, suggesting that they do indeed form a multienzyme complex.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, and Growth Conditions
The bacterial strains and plasmids used in this study are described in Table 1. Bacteria were grown at 37 or 30 °C in Luria-Bertani (LB) medium (22). Glucose (0.05–0.4% w/v), galactose (0.1% w/v), arabinose (0.002–0.2% w/v), or isopropyl 1-thio-β-d-galactopyranoside (0.5–1.0 mm) were added as required. The antibiotics ampicillin (100 μg/ml), chloramphenicol (34 μg/ml), kanamycin (50 μg/ml), and trimethoprim (100 μg/ml) were added where appropriate. For bacterial two-hybrid analyses, bromochloroindolyl galactopyranoside (X-gal; 40 μg/ml) was added to the medium.
TABLE 1.
Bacterial strains and plasmids
| Strain/plasmid | Description or genotype | Ref. or source |
|---|---|---|
| BTH101 | F−, cya-854, recA1,endA1, gyrA96, this1, hsdR17, spoT1, rfbD1, glnV44(AS); Nalr | 24 |
| CWG286 | K-12, lacZ, trp, Δ(sbcB-rfb), upp, rel, rpsL, galE::Tn10 | 21 |
| TOP10 | F−, mcrA, Δ (mrr-hsdRMS-mcrBC), φ80, lacZΔM15, ΔlacX74, deoR, nupG, recA1, araD139, Δ(ara-leu)7697, galU, galK, repsL(Strr), endA1 | Invitrogen |
| pBAD24 | l-Arabinose-inducible plasmid; Apr | 23 |
| pBAD33 | l-Arabinose-inducible plasmid; Cmr | 23 |
| pBAD322Tp | l-Arabinose-inducible plasmid; Tpr | 49 |
| pGEX4T-3 | Cloning vector containing GST; Apr | Amersham Biosciences |
| pKT25 | Bacterial two-hybrid vector, encodes the T25 fragment of adenylate cyclase from B. pertussis 5′ to a multiple cloning site | 24 |
| pUT18C | Bacterial two-hybrid vector, encodes the T18 fragment of adenylate cyclase from B. pertussis 5′ to a multiple cloning site | 24 |
| pUT18 | Bacterial two-hybrid vector, encodes the T18 fragment of adenylate cyclase from B. pertussis 3′ to a multiple cloning site | 24 |
| pKT25-zip | pKT25 encoding the CyaA T25 fragment fused to the leucine zipper of the yeast GCN4 protein | 24 |
| pUT18C-zip | pUT18C encoding the CyaA T18 fragment fused to the leucine zipper of the yeast GCN4 protein | 24 |
| pUT18-zip | pUT18 encoding the CyaA T18 fragment fused to the leucine zipper of the yeast GCN4 protein | This study |
| pACYC184 | Cloning vector; Cmr, Tcr | 50 |
| pWQ288 | pACYC184 derivative containing a BamHI/SmaI fragment encoding the d-galactan I cluster (wzm, wzt, wbbM, glf, wbbN, wbbO) | 18 |
| pWQ289 | pACYC184 derivative containing a BamHI/SmaI fragment encoding the d-galactan I biosynthesis genes (wbbM, glf, wbbN, and wbbO); Cmr | 18 |
| pWQ290 | pBAD24 derivative containing wzm-wzt; NcoI, XbaI | 18 |
| pWQ284 | pBAD24 derivative containing a Cmr cassette | 16 |
| pWQ516 | pACYC184 derivative containing a BamHI/SmaI fragment d-galactan I biosynthesis cluster with a wbbO deletion | This study |
| pWQ517 | pWQ288 derivative containing a BamHI/SmaI fragment d-galactan I biosynthesis cluster with a wbbM deletion | This study |
| pWQ548 | pWQ288 derivative containing a BamHI/SmaI fragment d-galactan I biosynthesis cluster with a frameshift mutation in wbbN which results in a truncated version of WbbN (only the first 79 amino acids are expressed) | This study |
| pWQ520 | pBAD24 derivative encoding His10-WbbO; Apr | This study |
| PWQ522 | pBAD24 derivative encoding His10-WbbM; Apr | This study |
| pWQ523 | pBAD24 derivative encoding His6-WbbN; Apr | This study |
| pWQ524 | pBAD24 derivative encoding WbbM; Apr | This study |
| pWQ525 | pBAD33 derivative encoding WbbM; Cmr | This study |
| pWQ526 | pWQ284 derivative encoding FLAG-WbbN; Cmr | This study |
| pWQ527 | pBAD322 Tp derivative encoding FLAG-WbbN; Tpr | This study |
| pWQ528 | pBAD24 derivative encoding His10-WbbON; residues 1–172; Apr | This study |
| pWQ529 | pBAD24 derivative encoding His10-WbbOC; residues 131–377; Apr | This study |
| pWQ530 | pBAD24 derivative encoding WbbMN-His10; residues 1–265; Apr | This study |
| pWQ531 | pBAD24 derivative encoding His10-WbbMC; residues 219–634; Apr | This study |
| pWQ532 | pBAD322Tp derivative encoding His10-WbbMC; residues 219–634;Tpr | This study |
| pWQ542 | pGEX4T-3 containing WbbO fusion; Apr | This study |
| pWQ533 | pKT25 derivative encoding T25-WbbO; Kmr | This study |
| pWQ534 | pKT25 derivative encoding T25-WbbM; Kmr | This study |
| pWQ535 | pKT25 derivative encoding T25-WbbN; Kmr | This study |
| pWQ536 | pKT25 derivative encoding T25-WbbON; Kmr | This study |
| pWQ537 | pUT18C derivative encoding T18-WbbON; Apr | This study |
| pWQ519 | pUT18 derivative encoding WbbON-T18; Apr | This study |
| pWQ539 | pUT18C derivative encoding T18-WbbO; Apr | This study |
| pWQ540 | pUT18C derivative encoding T18-WbbM; Apr | This study |
| pWQ541 | pUT18C derivative encoding T18-WbbN; Apr | This study |
| pWQ543 | pUT18 derivative encoding WbbO-T18; Apr | This study |
| pWQ544 | pUT18 derivative encoding WbbM-T18; Apr | This study |
| pWQ545 | pUT18 derivative encoding WbbN-T18; Apr | This study |
| pWQ549 | pUT18 derivative encoding WbbOC-T18; Apr | This study |
| pWQ550 | pKT25 derivative encoding WbbOC; Kmr | This study |
| pWQ551 | pUT18C derivative encoding WbbOC-T18; Apr | This study |
DNA Methods
DNAzol reagent (Invitrogen) was used to purify chromosomal DNA. DNA fragments were PCR-amplified using Pwo polymerase (Roche Applied Science) with custom oligonucleotide primers (Sigma). Where appropriate, the primers included restriction sites to facilitate cloning (supplemental Table S1). Mutagenesis was carried out using complementary oligonucleotides designed to incorporate the desired base changes (supplemental Table S1). The procedure was based upon the QuikChange method (Stratagene). Mutagenesis of the desired base pairs was confirmed by sequencing (Guelph Molecular Super Center). DNA fragments from PCR or restriction enzyme digests were purified from agarose gels using the GeneElute gel extraction kit (Sigma). Plasmid DNA was purified using the GeneElute plasmid purification kit (Sigma). Restriction enzyme digests and DNA ligation reactions were performed according to the manufacturer's instructions.
Separation of Cellular Fractions
Cultures were grown in 250 ml of LB medium at 37 °C. Genes encoding proteins of interest were cloned behind arabinose-inducible promoters in pBAD vectors (23). Protein expression was induced at mid-exponential growth phase (A600 = 0.6) by the addition of 0.02% arabinose and the cultures were grown for an additional 2 h. Cells were collected by centrifugation and resuspended in 25 ml of buffer A (50 mm HEPES, 100 mm NaCl, pH 7.5), prior to lysis by passage through a French pressure cell. A cleared cell-free lysate was obtained by centrifugation at 4,000 × g for 10 min. This was followed by a second centrifugation step at 12,000 × g for 15 min. Centrifugation of the lysate at 100,000 × g for 60 min separated the membrane fraction (P100) from the soluble fraction (S100).
SDS-PAGE and Western Immunoblot Analysis of Proteins
Proteins were analyzed by SDS-PAGE using 12% resolving gels and visualized by Simply Blue stain (Invitrogen). Samples containing FLAG-, polyhistidine-, or glutathionine S-transferase (GST)-tagged proteins were transferred to nitrocellulose membranes (PerkinElmer Life Science). Immunoblots were probed with a 1:2,000 dilution of anti-FLAG antibody (Sigma), anti-His5 antibody (Qiagen), or anti-GST antibody (Novagen). Nitrocellulose membranes were then washed three times for 5 min in phosphate-buffered saline. This was followed by incubation for 1 h in a 1:3,000 dilution of horseradish peroxidase-conjugated goat anti-mouse antibody (Jackson ImmunoResearch Laboratories). Detection was carried out according to instructions supplied with the Western Lightning reagent (PerkinElmer).
Preparation of WbbM-specific Antibody
His10-WbbM protein was purified from E. coli Top10 containing plasmid pWQ522 and protein expression was induced as described above using 0.2% arabinose. The cell pellet was then stored at −20 °C until required. Cell pellets were resuspended in buffer B (50 mm sodium phosphate, 500 mm NaCl, pH 7.5) containing the dissolved protease inhibitor tablets (Roche) and lysed by sonication. The lysate was cleared by centrifugation steps at 4,000 × g for 10 min and 12,000 × g for 15 min. The soluble fraction was then collected by further centrifugation at 100,000 × g for 1 h and His10-WbbM was purified using His-Select Nickel Affinity Gel (Sigma). After sequential washes with buffer B and buffer B containing 10 mm imidazole and 25 mm imidazole, the protein was eluted from the column in buffer B containing 250 mm imidazole. The eluate containing His10-WbbM protein was exchanged into buffer C (25 mm Tris-HCl, 100 mm NaCl, pH 7.5), using a PD-10 column (GE Healthcare) and concentrated using a Microcon YM-10 column (Millipore). Purified protein was mixed with Freund's Incomplete Adjuvant (Sigma) in a 1:1 ratio prior to inoculation into New Zealand White rabbits at the Central Animal Facility at the University of Guelph. The antibody preparation was partially purified over a DEAE Affi-Gel Blue column (Bio-Rad) and nonspecific antibody was then removed by adsorption against whole nitrocellulose membrane-immobilized cell lysate of E. coli Top10. For Western blot analysis, anti-WbbM was used at a 1:2,000 dilution and detected with a 1:3,000 dilution of goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories).
Co-purification Strategies to Detect Interacting Proteins
Combinations of the putative galactosyltransferases bearing various epitope and purification tags were coexpressed in E. coli Top10. Protein expression was induced by the addition of 0.2% arabinose or 1 mm isopropyl 1-thio-β-d-galactopyranoside as described above. Cell pellets were collected and stored at −20 °C overnight. Pellets were resuspended in 25 ml of buffer D (20 mm sodium phosphate buffer, 750 mm NaCl, 10% (v/v) glycerol, pH 7.5) and lysed by sonication. Triton X-100 (0.1% final concentration) was added to the lysate to release the typically membrane-associated glycosyltransferases into the soluble fraction. After centrifugation at 12,000 × g for 15 min, the supernatant was collected and mixed with the appropriate affinity resin. Mixing was performed for either 1 h at 4 °C for TALON metal affinity His-select resin (BD Biosciences), or overnight at 4 °C for GST affinity resin (Novagen). After binding, the resin was collected by centrifugation for 5 min at 800 × g and 10 volumes of buffer D containing 0.1% Triton X-100 was added and mixed at room temperature for 10 min. This wash step was repeated before the resin was loaded into a column. The relevant protein was then eluted using buffer D containing either 300 mm imidazole or 10 mm reduced glutathione. Eluates were collected by removing the resin in a final centrifugation step at 800 × g. Samples were collected and analyzed by SDS-PAGE and Western immunoblotting.
Bacterial Two-hybrid Analysis
The d-galactan I-biosynthesis enzymes were fused to the catalytic domains of adenylate cyclase from Bordetella pertussis via cloning into the BTH vectors: pKT25, pUT18C, and pUT18 (24). Bait and prey plasmids were co-transformed into chemically competent BTH101 E. coli cells (24) and plated on LB plates containing the appropriate antibiotic, isopropyl 1-thio-β-d-galactopyranoside, and X-gal. Plates were incubated at 30 °C for 48 h, at which time the colonies expressing the leucine zipper (positive control) became dark blue in color, due to the degradation of the chromogenic substrate X-gal. Initial scoring of potential interactions was achieved based on the plate assay. For a quantitative measurement, broth cultures were grown at 30 °C to mid-exponential phase (A660 ∼ 0.5–0.8) and the β-galactosidase activity of each sample was measured using a commercial assay kit following the manufacturer's instructions (Pierce).
Detection of d-Galactan I Biosynthesis
Cultures of E. coli CWG286 containing the appropriate plasmids were grown overnight in antibiotic-supplemented LB broth. Protein expression was induced by the addition of arabinose. The galE::Tn10 insertion in CWG286 makes d-galactan I production conditional on addition of galactose to the growth medium (18). After arabinose (varying concentrations) and galactose (0.1%) were added to the medium, cultures were inoculated and then grown for ∼2.5 h. Immunofluorescence microscopy of intact cells was performed as described previously, using d-galactan I-specific rabbit polyclonal antibodies (18). Detection was performed using rhodamine red-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories Inc.). Visualization was carried out using a Zeiss Axiovert 200 microscope using a ×100 objective lens, and the images were processed using Openlab software (Improvision).
LPS samples were prepared using the whole cell lysate method of Hitchcock and Brown (25). Samples were separated using 15% polyacrylamide gels and visualized by silver staining, or by immunoblot analysis after transfer to nitrocellulose membrane (PerkinElmer Life Sciences). Immunoblots were probed with absorbed polyclonal rabbit anti-d-galactan I antibody, as previously described (18). The secondary antibody was alkaline phosphatase-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories).
Computer Analysis
Secondary structure predictions of protein sequences were performed using the programs Prof, PredictProtein, PSIpred, and Jpred (26–29). A domain linker program using a supported vector machine (SVM) was used to determine ideal locations to make domain constructs of the protein.
RESULTS
Localization and Domain Analysis of WbbO
The first dedicated step in d-galactan I biosynthesis is catalyzed by WbbO. From both in vivo and in vitro experiments, WbbO is proposed to add the first two sugars (Galp-Galf) to the und-PP-GlcNAc acceptor (14, 20), forming the adaptor region of the O-PS (Fig. 1). Based on its sequence, WbbO belongs to the CAZy glycosyltransferase family 4 (GT-4) (30). The predicted WbbO polypeptide contains 377 residues (42.6 kDa) and SDS-PAGE of His10-WbbO reveals a polypeptide of the expected size (Fig. 2). In E. coli cells, the majority of the overexpressed WbbO protein is located in the membrane (P100) fraction (Fig. 2). WbbO is not predicted to have any membrane-spanning regions (according to DAS, HHMTOP 2.0; TMpred, TopPred II). The protein sequence predicts one short hydrophobic segment (residues 108–120). Although this is not sufficient to span the membrane, this region could potentially intercalate into the membrane (31). WbbO does contain a number of additional helices (particularly the segment between residues 326 and 342), which contain hydrophobic residues and could also participate in its interaction with the membrane; there is precedent for this in other glycosyltransferases (32, 33).
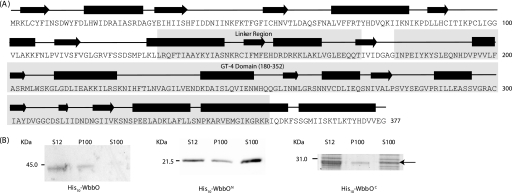
FIGURE 2.
Secondary structure prediction and localization of His10-WbbO. A, sequence and predicted secondary structure of WbbO outlining the β-sheets (black arrows) and α-helices (black boxes). The GT-4 domain (residues 131–377) is identified, as is a putative linker region that may separate two independent domains (WbbON and WbbOC). B, cellular localization of the His10-WbbO protein (pWQ528) and its two domains, His10-WbbON (pWQ528) and His10-WbbOC (pWQ529), expressed in E. coli CWG286. Cell-free lysates (S12) were separated into soluble (S100) and membrane-containing (P100) fractions and the His10-tagged proteins were detected using mouse anti-His5 monoclonal antibody. His10-WbbOC could not be detected using anti-His5 antibodies, so the SDS-PAGE Simply Blue-stained gel showing the complete localization is illustrated. The arrow indicates a band present in the whole cell lysate and subsequent fractions, which is found only in cells containing pWQ529; the band corresponds to the predicted size of His10-WbbOC (29.0 kDa). Sample loading was standardized to the original cell suspension (prior to lysis), allowing direct visual assessment of the cellular distribution of His10-WbbO.
The predicted WbbO protein sequence was analyzed using a domain linker prediction program using a SVM. The results identified a putative linker, suggesting that WbbO may contain two potential independently folded domains, with the galactosyltransferase activity predicted to be contained within the C-terminal domain (residues 170–365; WbbOC) (Fig. 2). The two putative domains were cloned separately into pBAD vectors. Although the majority of His10-WbbO was confined to the membrane fraction, consistent with its predicted structural features, the separated polypeptide domains were found in both the soluble and membrane fractions with the majority in the soluble fraction. These results suggest that membrane association of full-length WbbO is directed by regions throughout the protein and not solely by the predicted N-terminal hydrophobic region (residues 108–120).
Mutant complementation experiments established that full-length His10-WbbO could restore d-galactan I biosynthesis in E. coli CWG286 cells harboring the ΔwbbO construct. This was evident in SDS-PAGE, which revealed long-chain “smooth” LPS (S-LPS) molecules missing in the mutant (Fig. 3). The addition of higher levels of arabinose inducer yielded a S-LPS profile with similar size distribution and staining intensity to the parent. When expressed alone, the galactosyltransferase-containing WbbOC domain (plasmid pWQ529) was sufficient to complement the biosynthesis defect in the ΔwbbO mutant, at least in an overexpression situation. Based on the silver-stained gel, the efficiency of complementation was less than that observed with the full-length WbbO protein, particularly with lower amounts of arabinose, but the size distribution was comparable at higher levels of arabinose. Given the nature of the system, it is difficult to monitor expression of properly folded and localized enzyme, so the significance (if any) of the reduced efficiency is impossible to interpret. In all cases, the identity of the S-LPS was verified by immunoblotting with anti-d-galactan I antibodies (data not shown). Immunofluorescence was used to verify that the S-LPS was present on the cell surface (Fig. 3B). The predicted N-terminal domain (WbbON) could not complement the ΔwbbO mutation as expected (data not shown). Therefore, when expressed from a plasmid, WbbO does not require the N terminus for galactosyltransferase function and the reported α-galactopyranosyl- and β-galactofuranosyltransferase activity (14, 20) of the enzyme is confined to the C-terminal domain of WbbO.
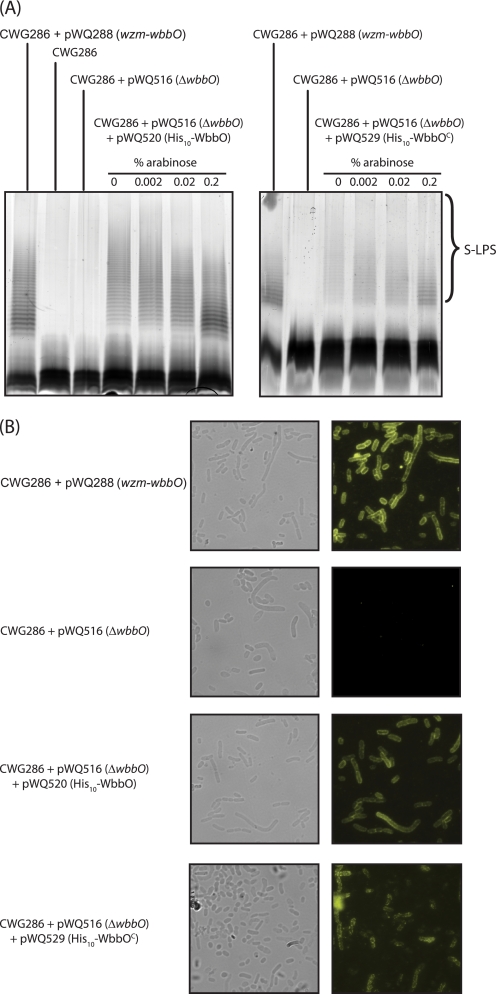
FIGURE 3.
Complementation of wbbO deletion requires only the C-terminal domain of WbbO. A, LPS samples from whole cell lysates were analyzed by SDS-PAGE and visualized by silver staining. The LPS of CWG286 containing pWQ288 provides the positive control. Cells expressing pWQ516 (ΔwbbO) are devoid of O-PS. Full-length His10-WbbO (pWQ520), as well as the separated C-terminal domain (WbbOC; pWQ529) both restore O-PS biosynthesis. B, immunofluorescence microscopy confirming surface expression of d-galactan I in cells harboring the same constructs.
Localization and Domain Analysis of WbbM
WbbM is classified in GT family 8 and in vitro data shows that it adds galactose residues to elongate the product of WbbO (20). However, the precise structure of the residue(s) added has not been established. WbbM is the largest galactosyltransferase involved in the synthesis of d-galactan I at 634 amino acids and 73.3 kDa (Fig. 4). His10-WbbM (74.6 kDa) is localized predominantly to the membrane fraction (Fig. 4), despite the absence of any identifiable membrane-spanning regions. Like WbbO, WbbM potentially contains two independently folding domains (WbbMN and WbbMC) with the galactosyltransferase sequence occupying the C-terminal domain. WbbMN appears to be predominantly soluble (S100), whereas WbbMC is membrane-associated (P100) (Fig. 4). This would suggest that localization of the enzyme is dictated by the C-terminal domain. However, when WbbMC was expressed in cells containing pWQ517 (ΔwbbM), no restoration of the d-galactan I biosynthesis defect was achieved (Fig. 5). In contrast, when the separated WbbMN and WbbMC domains were co-expressed as independent polypeptides in the same background, S-LPS biosynthesis was restored on the surface of the cell. These results indicate that the GT domain can fold independently into a functional peptide (at least when co-expressed with the N-terminal domain). Furthermore, both domains are required for restoration of WbbM activity in d-galactan I biosynthesis. As was the case with the ΔwbbO complementation experiments, the amount of S-LPS formed was higher when the full-length WbbM protein was used to complement the corresponding defect.
FIGURE 4.
Secondary structure predictions and localization of His10-WbbM. A, sequence and predicted secondary structure of WbbM outlining β-sheets (black arrows) and α-helices (black boxes). The GT-8 region (residues 284–549) is identified, as is a putative linked region that may separate two independent domains (WbbMN and WbbMC). B, localization of His10-WbbM (expressed from pWQ522 in E. coli CWG286), His10-WbbMN (pWQ530), and His10-WbbMC (pWQ531). Cell-free lysates (S12) were separated into soluble (S100) and membrane-containing (P100) fractions and the His-tagged proteins were detected in Western immunoblots using mouse anti-His5 monoclonal antibody followed by goat anti-mouse horseradish peroxidase-conjugated antibody. Loading was standardized to the original cell suspension (prior to lysis), allowing direct visual assessment of the cellular distribution of His10-WbbM.
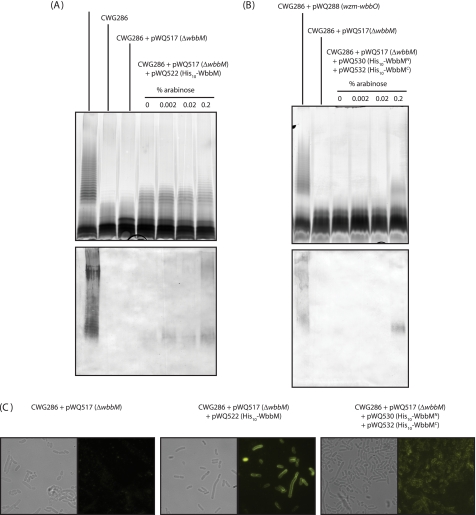
FIGURE 5.
Complementation of wbbM deletion requires both domains of WbbM. LPS samples from whole cell lysate were analyzed by SDS-PAGE and visualized by silver staining (upper panels). The LPS of CWG286 containing pWQ288 provides the positive control. The CWG286+pWQ517 (ΔwbbM) is devoid of O-PS but does make a modified lipid A-core species. A, full-length WbbM (pWQ522) restores O-PS biosynthesis. The corresponding immunoblot (lower panel) illustrates that in the absence of WbbM, no high molecular weight polymer is made. B, both domains are required for WbbM to functionally restore O-PS biosynthesis as illustrated by the silver stain (upper panel). The corresponding immunoblot is shown (lower panel). C, immunofluorescence microscopy confirms the surface expression of O-PS. See Fig. 3 for control immunofluorescence images.
Analysis of the Putative Galactosyltransferase WbbN
BLAST analysis identifies WbbN as a third putative galactosyltransferase. WbbN belongs to GT-2, one of the largest families within the GT-A superfamily (34, 35). Unlike WbbO and WbbM, WbbN (290 residues, 33.2 kDa) is predicted to contain a single domain (Fig. 6). An N-terminal FLAG epitope-tagged protein (FLAG-WbbN) migrated in SDS-PAGE consistent with its expected size (34.2 kDa) (Fig. 6). This protein was localized predominantly to the membrane fraction.
FIGURE 6.
Secondary structure predictions and localization of WbbN. A, sequence and predicted secondary structure of WbbN outlining β-sheets (black arrows) and α-helices (black boxes). The GT-2 domain is located within residues 24–144. B, localization of WbbN in CWG286 overexpressing FLAG-WbbN (pWQ526). Cell-free lysates (S12) were separated into soluble (S100) and membrane-containing (P100) fractions and FLAG-WbbN was detected in Western immunoblots using an anti-FLAG monoclonal. Loading was standardized to the original cell suspension (prior to lysis), allowing direct visual assessment of the cellular distribution of FLAG-WbbN.
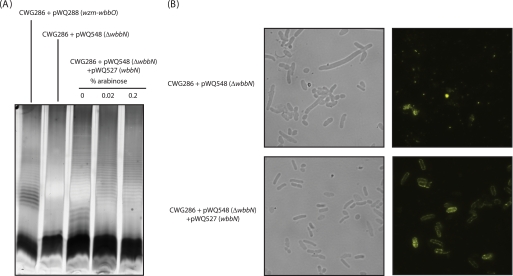
Previous in vitro work suggested that WbbO and WbbM could together form a galactose-containing polymer (20). Although the structure (and size) of the product was not established, these results raised questions about whether WbbN participates in d-galactan I biosynthesis. To address this question, the requirement for WbbN was assessed in vivo. Plasmid pWQ548 is a derivative of pWQ288 that contains a frameshift mutation in wbbN. This is predicted to result in a truncated peptide terminating at residue 79 and therefore lacking a large portion of the GT-2 domain. This mutant is unable to produce S-LPS containing d-galactan I (Fig. 7). Introduction of plasmid pWQ527 (carrying wbbN) restored d-galactan I biosynthesis and S-LPS production. The results suggest that WbbN is indeed a galactosyltransferase and furthermore, that this enzyme is required for the synthesis of the d-galactan I polymer.
FIGURE 7.
WbbN is required for d-galactan I polymer synthesis. A, LPS samples from whole cell lysates were analyzed by SDS-PAGE and visualized by silver staining. The LPS of CWG286 containing pWQ288 provides a positive control. Plasmid pWQ548 is a derivative of pWQ288 but contains a frameshift mutation in wbbN. This eliminates all synthesis of d-galactan I. Reconstitution of the system is achieved by cotransforming plasmids pWQ527 (encoding WbbN) and pWQ548, resulting in the formation of S-LPS containing d-galactan I. B, immunofluorescence microscopy confirmed surface expression of d-galactan I in CWG286 containing both pWQ527 and pWQ548.
The wbbN mutant cells were pleomorphic (Fig. 7). In the absence of an ABC transporter, the four d-galactan I biosynthesis genes (in pWQ289) direct the formation of intracellular polymer (18). When a ΔwbbN version of the pWQ289 construct was examined, the cells lacked d-galactan I but had a normal cell size and shape (data not shown). Transport defects in the E. coli O9a O-PS assembly system also give rise to pleomorphic cells, which lose viability (15, 17). Although the underlying mechanisms may differ, both instances could reflect either some toxicity resulting from accumulation of d-galactan I intermediates, or depletion of the cellular pool of the essential und-P carrier, which participates in peptidoglycan biosynthesis (36).
Interaction Network within the d-Galactan I Biosynthetic Machinery
It has been assumed that the glycosyltransferases that synthesize O-PS polymer form a membrane-associated multienzyme complex but data to support this contention has been lacking for all but a very few systems. Potential binary interactions between all of the proteins involved in d-galactan I synthesis were therefore examined using both co-purification methods and a bacterial two-hybrid approach (24).
For the bacterial two-hybrid assay, the T25 and T18 fragments of the catalytic domain of the B. pertussis adenylate cyclase were fused to full-length copies of each of the d-galactan I proteins. One potential advantage of this system over other two-hybrid approaches is that it does not require the interaction partners to be associated with the chromosomal DNA for the interaction to be reported. It is therefore more suited for the study of membrane (or membrane-associated) proteins (24). All of the N-terminal fusion constructs (pKT25 and pUT18C) were functional, based on complementation (restoration of d-galactan I expression) in the respective deletion mutants (data not shown).
A positive interaction was considered valid when the β-galactosidase activity measured was at least two times that of the background levels measured for the vector controls (Table 2). Using these criteria, strong homotypic interactions were observed in WbbO and WbbM, but not in WbbN. These results suggest that WbbO and WbbM may form dimers (or higher order multimers) in the final complex. WbbM, WbbN, and WbbO all gave positive interaction signals with one another. Importantly, reciprocal interactions were detected when the various bait and prey combinations were reversed. The strongest inter-enzyme interaction occurred between WbbO and WbbM (Table 2).
TABLE 2.
In vivo interactions between the putative galactosyltransferases involved in d-galactan I synthesis
| N-terminal T25 | N- or C-terminal T18 derivative | β-Galactosidase activity Miller unitsa |
||
|---|---|---|---|---|
| N-terminal T18 | C-terminal T18 | |||
| Positive control | Leucine zipper | Leucine zipper | 700 ± 70 | 880 ± 200 |
| Negative control | 190 ± 40 | 160 ± 30 | ||
| Homotypic interactions | WbbO | WbbO | 1010 ± 20 | 1360 ± 130 |
| WbbM | WbbM | 1940 ± 200 | 1480 ± 80 | |
| WbbN | WbbN | 100 ± 30 | 170 ± 10 | |
| Heterotypic interactions | WbbO | WbbN | 880 ± 50 | 140 ± 10 |
| WbbO | WbbM | 1560 ± 250 | 1420 ± 70 | |
| WbbN | WbbO | 1010 ± 20 | 540 ± 140 | |
| WbbN | WbbM | 1160 ± 20 | 920 ± 70 | |
| WbbM | WbbO | 1640 ± 40 | 1700 ± 70 | |
| WbbM | WbbN | 1270 ± 110 | 130 ± 20 | |
| WbbO domain interactions | WbbON | WbbO | 180 ± 30 | 510 ± 90 |
| WbbO | WbbON | 760 ± 20 | 120 ± 10 | |
| WbbON | WbbM | 960 ± 110 | 800 ± 50 | |
| WbbM | WbbON | 790 ± 90 | 550 ± 50 | |
| WbbON | WbbN | 450 ± 140 | 620 ± 110 | |
| WbbN | WbbON | 990 ± 90 | 1100 ± 280 | |
| WbbOC | WbbO | 230 ± 60 | 120 ± 20 | |
| WbbO | WbbOC | 150 ± 30 | 180 ± 20 | |
| WbbOC | WbbM | 110 ± 40 | 100 ± 30 | |
| WbbM | WbbOC | 90 ± 30 | 160 ± 40 | |
| WbbOC | WbbN | 110 ± 20 | 160 ± 30 | |
| WbbN | WbbOC | 140 ± 20 | 110 ± 30 | |
a Data were obtained from triplicate samples each done in triplicate experiments.
As a further control, we investigated a second set of constructs where the T18 fragment was located at the C terminus of the galactosyltransferases and the interactions with the T25 N-terminal-tagged constructs tested (Table 2). Of these, only the WbbO-T18 construct was inactive in mutant complementation analyses (data not shown). In most cases, the results obtained were qualitatively consistent with the N-terminal-tagged derivatives. The exceptions were negative results for C-terminal-tagged WbbO-WbbN and WbbM-WbbN pairs. In each case, a positive result was obtained from the reciprocal C-terminal-tagged pair, suggesting that steric hindrance may be a factor in certain combinations. As might be expected, the absolute β-galactosidase activities varied.
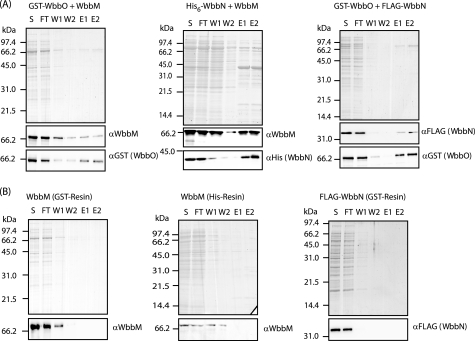
To support this data, pairwise combinations of galactosyltransferases were assessed for interaction by a co-purification strategy. One or both partners were epitope-tagged for detection and purification of the interacting proteins. Examination of proteins present in the bound fractions from the affinity columns (E1, E2) indicated that transferases WbbM, WbbN, and WbbO all interact with one another (Fig. 8). Thus the same result for protein-protein interactions was obtained by two radically different experimental strategies.
FIGURE 8.
Co-purification demonstrates interactions between WbbM, WbbN, and WbbO. Co-purification of pairwise combinations of the synthesis components of d-galactan I O-PS biosynthesis was examined in lysates from E. coli containing two plasmids expressing combinations of biosynthesis components. The protein of interest was bound to TALON metal affinity resin via a polyhistidine tag on one of the partner proteins (His6-WbbN), or to GST affinity resin via a glutathione tag (GST-WbbO). Washes were completed before buffer containing imidazole (300 mm) or glutathionine (10 mm) was added to elute the appropriately tagged protein and any interacting proteins from the resin. S, lysate added to the resin; FT, flow through; W1, wash one (10 resin volumes); W2, wash two (10 resin volumes); E1, elution one (1 resin volume); E2, elution two (1 resin volume). A, samples of 5 μl of each fraction were separated by SDS-PAGE and visualized with Simply Blue stain. Corresponding Western blots for each partner pair are shown below the corresponding gels. B, negative controls verifying that purification of each partner protein is dependent on its interaction with His- or GST-tagged protein.
Attempts were made to investigate the roles of the different domains of WbbO in protein-protein interactions, using the two-hybrid approach (Table 2). The outcome of these experiments was more complex. Some evidence was found for the interaction of WbbON with full-length WbbO, WbbM, and WbbN. In contrast, the WbbOC domain was unable to mediate any interactions sufficient for a positive result in the two-hybrid system. The simplest explanation is that WbbO contains one domain involved in catalysis (WbbOC) and another mediating both homotypic and heterotypic protein-protein interactions (WbbON). However, the negative results should be interpreted with caution because functionality in the T25-WbbOC and T18-WbbOC constructs could not be confirmed in complementation experiments (data not shown). There is no means to eliminate the possibility that these constructs are subject to improper expression and/or folding. In the case of WbbM, T18- or T25-tagged derivatives of WbbMN and WbbMC were non-functional, and these were not pursued further. It appears that maintenance of efficient expression, folding, catalysis, and interactions is too much to demand from this system when the domains are artificially separated and expressed with a substantial fusion partner.
DISCUSSION
In the biosynthesis and export of d-galactan I, polymer chain length is regulated by the coordination of the ABC transporter components with the galactosyltransferases (18). The coupling of O-PS synthesis and export may be extended to other bacteria whose O antigens lack an obvious non-reducing terminal export signal, for example, Yersinia enterocolitica O:3 (37) and Vibrio cholerae O1 (38). The central requirement for coordination could be accommodated by organization of the key enzymes into a multienzyme complex. As a first step in understanding the underlying mechanism, we have identified a membrane-associated complex of galactosyltransferases involved in d-galactan I biosynthesis.
In the biosynthesis of d-galactan I, WbbO, a GT-4 enzyme commits und-PP-GlcNAc to d-galactan I synthesis. It is responsible for forming the adaptor region (14, 20). Its activity is followed by WbbM (20), implicating this GT-8 enzyme as an α1,3-Galp-transferase. If correct, the GT-2 WbbN enzyme presumably completes the synthesis by providing the α1,3-Galf-transferase activity. However, there is some inconsistency between the limited in vitro available data and the essential role of WbbN unequivocally established in these in vivo studies. We are unable to explain the basis for the different results. The precise catalytic activities for these enzymes can only be established by examining each purified enzyme with the appropriate synthetic acceptor, reagents that are not currently available. However, such information is not essential for the current investigation.
Although none of the three galactosyltransferases are integral membrane proteins, all target independently to the membrane. This potentially puts those enzymes in proximity to the und-PP-GlcNAc acceptor and the polymer export apparatus. The galactosyltransferases all interact with one another based on two independent experimental strategies: two-hybrid and co-purification methods. In the case of WbbO and WbbM a functional complex may contain more than one copy as these proteins form homotypic interactions. Interestingly, these interactions do not extend to Glf, the only dedicated protein involved in producing the UDP-linked precursors. No interactions were detected between Glf and WbbO, WbbM, and WbbN in two-hybrid experiments (data not shown). Logically, such interactions would not be essential given that the Glf product, UDP-Galf, is soluble and may diffuse to the site of synthesis.
WbbO has been shown to have two separable domains, with only WbbOC being essential for the glycosyltransferase activity. However, the activity of this construct was low relative to the full-length protein and it was only detected in the presence of high amounts of arabinose inducer, presumably reflecting substantial overexpression. Consistent with the activity, a significant amount of WbbOC targets to the membrane independent of WbbON. WbbON apparently participates in interactions between WbbO and the other galactosyltransferases. It is tempting to speculate that this identifies a clear separation in domain functions. However, we cannot currently rule out additional interactions mediated by WbbOC. Although all of the two-hybrid experiments involving WbbOC gave negative results, the key fusion-protein constructs were functionally inactive and must be interpreted with caution.
WbbM, also has two separable domains but, unlike WbbO, both domains are essential for its participation in d-galactan I biosynthesis. The CAZy GT-8 family has a relatively strong foundation of structure-function data. WbbM contains the characteristic fold and motif within the C-terminal domain. In contrast, WbbMN has no motif that would identify a catalytic activity. Interestingly, the two-domain organization and conserved N-terminal domain sequence is found in other putative GT-8 glycosyltransferases. A BLAST search identifies putative glycosyltransferases from Bifidobacterium longum sp. infantis ATCC15697 (ACJ53443.1; 32% identity 50% similarity), Lactococcus lactis subsp. cremoris SK11 (ABJ72535.1; 33% identity 54% similarity), Campylobacter jejuni (CAI38725.1; 29% identity, 48% similarity), and Burkholderia multivorans (EED99048.1; 34% identity, 52% similarity). Conservation of the N-terminal domain is consistent with its essential role in the glycosyltransferase activity of WbbM. Its function warrants further investigation given the surprising observation that trans-expression of the two WbbM domains reconstitutes WbbM (albeit with low efficiency). WbbMN is not required for membrane association of WbbMC. Unfortunately, no active fusion proteins were obtained to give insight into its possible role in protein-protein interactions.
WbbN was initially assigned as a galactosyltransferase based on its Rossman-like fold, characteristic of the GT-A fold (34, 39). The frameshift mutation of wbbN in the d-galactan I cluster eliminated formation of d-galactan I, a result entirely consistent with WbbN being a galactosyltransferase involved in the overall chain extension process. In this family of GT enzymes, a DXD motif binds a catalytically important divalent metal ion (40, 41). Site-directed changes of the identified DXD motif (in WbbN this is DDD) to AAA eliminate its activity as expected (data not shown).
There is growing evidence supporting multienzyme complexes in the biosynthesis of cell-surface polymers. Protein interactions have been examined by a two-hybrid approach in the group 2 capsule system of E. coli K1, which also involves export of the polymer via an ABC transporter (42). Several of the biosynthesis proteins were found to interact. In the related E. coli K5 system, recent studies of glycosyltransferases involved in the synthesis of the K5 capsular polysaccharide found that protein-protein interactions were essential for activity. The glucuronyltransferase activity of KfiC is dependent on physical association with the N-acetylglucosaminyltransferase, KfiA (43). These proteins may be part of a larger trans-envelope complex (44, 45). Interactions have also been observed between glycosyltransferases involved in wall teichoic synthesis in Bacillus subtilis 168 (46).
In the only other O-PS biosynthesis system where interactions between the components have been investigated to date, E. coli O9a, three glycosyltransferases are required for O-PS biosynthesis. Two of these target to the membrane independently, whereas the third is absolutely dependent on interaction with a membrane-bound chain terminating protein (47). In the O9a system, there are no detectable inter-glycosyltransferase interactions. Comparison with the K. pneumoniae d-galactan I system suggests that membrane association is a conserved theme, as might be expected, but the manner by which this is achieved and the extent of protein-protein interactions differs significantly between systems. Notably, the O9a and d-galactan I-assembly systems also couple biosynthesis, chain termination, and export in different ways and this may be reflected in the surprisingly different interaction strategies (16–18). The next challenge for this research field will be to provide a structural context to describe the molecular architecture of model multienzyme complexes. The characterization of these prototype systems represents the essential first step.
Supplementary Material
Acknowledgments
We thank D. E. Taylor and E. Vimr for providing vector plasmids. We also thank C. Bouwman for construction of pUT18-zip.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
- LPS
- lipopolysaccharide
- OS
- oligosaccharide
- O-PS
- O antigen polysaccharide
- ABC
- ATP-binding cassette
- GST
- glutathione S-transferase
- X-gal
- bromochloroindolyl galactopyranoside
- SVM
- supported vector machine
- GT-4
- galactosyltransferase family 4.
REFERENCES
- 1.Raetz C. R., Whitfield C. (2002) Annu. Rev. Biochem. 71, 635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitfield C., Perry M. B., MacLean L. L., Yu S. H. (1992) J. Bacteriol. 174, 4913–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly R. F., Severn W. B., Richards J. C., Perry M. B., MacLean L. L., Tomás J. M., Merino S., Whitfield C. (1993) Mol. Microbiol. 10, 615–625 [DOI] [PubMed] [Google Scholar]
- 4.Kelly R. F., Whitfield C. (1996) J. Bacteriol. 178, 5205–5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kol O., Wieruszeski J. M., Strecker G., Fournet B., Zalisz R., Smets P. (1992) Carbohydr. Res. 236, 339–344 [DOI] [PubMed] [Google Scholar]
- 6.Kol O., Wieruszeski J. M., Strecker G., Montreuil J., Fournet B., Zalisz R., Smets P. (1991) Carbohydr. Res. 217, 117–125 [DOI] [PubMed] [Google Scholar]
- 7.Whitfield C., Richards J. C., Perry M. B., Clarke B. R., MacLean L. L. (1991) J. Bacteriol. 173, 1420–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabo M., Bronner D., Whitfield C. (1995) J. Bacteriol. 177, 1544–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxley D., Wilkinson S. G. (1989) Carbohydr. Res. 193, 241–248 [DOI] [PubMed] [Google Scholar]
- 10.Alexander D. C., Valvano M. A. (1994) J. Bacteriol. 176, 7079–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier-Dieter U., Barr K., Starman R., Hatch L., Rick P. D. (1992) J. Biol. Chem. 267, 746–753 [PubMed] [Google Scholar]
- 12.Rick P. D., Hubbard G. L., Barr K. (1994) J. Bacteriol. 176, 2877–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jann K., Goldemann G., Weisgerber C., Wolf-Ullisch C., Kanegasaki S. (1982) Eur. J. Biochem. 127, 157–164 [DOI] [PubMed] [Google Scholar]
- 14.Clarke B. R., Bronner D., Keenleyside W. J., Severn W. B., Richards J. C., Whitfield C. (1995) J. Bacteriol. 177, 5411–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke B. R., Cuthbertson L., Whitfield C. (2004) J. Biol. Chem. 279, 35709–35718 [DOI] [PubMed] [Google Scholar]
- 16.Cuthbertson L., Kimber M. S., Whitfield C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19529–19534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuthbertson L., Powers J., Whitfield C. (2005) J. Biol. Chem. 280, 30310–30319 [DOI] [PubMed] [Google Scholar]
- 18.Kos V., Cuthbertson L., Whitfield C. (2009) J. Biol. Chem. 284, 2947–2956 [DOI] [PubMed] [Google Scholar]
- 19.Ruiz N., Kahne D., Silhavy T. J. (2009) Nat. Rev. Microbiol. 7, 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan S., Clarke A. J., Whitfield C. (2001) J. Bacteriol. 183, 3318–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köplin R., Brisson J. R., Whitfield C. (1997) J. Biol. Chem. 272, 4121–4128 [DOI] [PubMed] [Google Scholar]
- 22.Miller J. H. (1972) Experiments in Molecular Genetics, Cold Spring Harbor, Cold Spring Harbor, NY [Google Scholar]
- 23.Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) J. Bacteriol. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karimova G., Pidoux J., Ullmann A., Ladant D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hitchcock P. J., Brown T. M. (1983) J. Bacteriol. 154, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouali M., King R. D. (2000) Protein Sci. 9, 1162–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rost B., Yachdav G., Liu J. (2004) Nucleic Acids Res. 32, W321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryson K., McGuffin L. J., Marsden R. L., Ward J. J., Sodhi J. S., Jones D. T. (2005) Nucleic Acids Res. 33, W36–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole C., Barber J. D., Barton G. J. (2008) Nucleic Acids Res. 36, W197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantarel B. L., Coutinho P. M., Rancurel C., Bernard T., Lombard V., Henrissat B. (2009) Nucleic Acids Res. 37, D233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Heijne G. (2006) Nat. Rev. Mol. Cell Biol. 7, 909–918 [DOI] [PubMed] [Google Scholar]
- 32.Brockhausen I., Hu B., Liu B., Lau K., Szarek W. A., Wang L., Feng L. (2008) J. Bacteriol. 190, 4922–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leipold M. D., Kaniuk N. A., Whitfield C. (2007) J. Biol. Chem. 282, 1257–1264 [DOI] [PubMed] [Google Scholar]
- 34.Breton C., Snajdrová L., Jeanneau C., Koca J., Imberty A. (2006) Glycobiology 16, 29R–37R [DOI] [PubMed] [Google Scholar]
- 35.Lairson L. L., Henrissat B., Davies G. J., Withers S. G. (2008) Annu. Rev. Biochem. 77, 521–555 [DOI] [PubMed] [Google Scholar]
- 36.van Heijenoort J. (2007) Microbiol. Mol. Biol. Rev. 71, 620–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L., al-Hendy A., Toivanen P., Skurnik M. (1993) Mol. Microbiol. 9, 309–321 [DOI] [PubMed] [Google Scholar]
- 38.Stroeher U. H., Karageorgos L. E., Morona R., Manning P. A. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 2566–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lairson L. L., Chiu C. P., Ly H. D., He S., Wakarchuk W. W., Strynadka N. C., Withers S. G. (2004) J. Biol. Chem. 279, 28339–28344 [DOI] [PubMed] [Google Scholar]
- 40.Breton C., Bettler E., Joziasse D. H., Geremia R. A., Imberty A. (1998) J. Biochem. 123, 1000–1009 [DOI] [PubMed] [Google Scholar]
- 41.Wiggins C. A., Munro S. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 7945–7950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steenbergen S. M., Vimr E. R. (2008) Mol. Microbiol. 68, 1252–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiura N., Baba Y., Kawaguchi Y., Iwatani T., Suzuki K., Kusakabe T., Yamagishi K., Kimata K., Kakuta Y., Watanabe H. (2010) J. Biol. Chem. 285, 1597–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rigg G. P., Barrett B., Roberts I. S. (1998) Microbiology 1442905–2914 [DOI] [PubMed] [Google Scholar]
- 45.McNulty C., Thompson J., Barrett B., Lord L., Andersen C., Roberts I. S. (2006) Mol. Microbiol. 59, 907–922 [DOI] [PubMed] [Google Scholar]
- 46.Formstone A., Carballido-López R., Noirot P., Errington J., Scheffers D. J. (2008) J. Bacteriol. 190, 1812–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke B. R., Greenfield L. K., Bouwman C., Whitfield C. (2009) J. Biol. Chem. 284, 30662–30672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng H. L., Fu T. F., Liu S. F., Chang H. Y. (1992) J. Biochem. 112, 604–608 [DOI] [PubMed] [Google Scholar]
- 49.Cronan J. E. (2006) Plasmid 55, 152–157 [DOI] [PubMed] [Google Scholar]
- 50.Chang A. C., Cohen S. N. (1978) J. Bacteriol. 134, 1141–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.