Abstract
Background
With upwards of 48% of human immunodeficiency virus (HIV)-infected persons having a probable psychiatric disorder, the possibility of cross-class drug interactions causing adverse effects or fatalities exists.
Aims
This report discusses an emergent case of low-flow priapism caused by an interaction between a previously prescribed combination protease inhibitor (PI) and newly added antipsychotic medications.
Methods
A 50-year-old HIV-positive man on highly active antiretroviral therapy (HAART), including the combination PI, lopinavir/ritonavir (Kaletra®), experienced an episode of priapism hours after beginning two new antipsychotic medications. Quetiapine (Seroquel®) and perphenazine (Trilafon®) were added to treat a diagnosed schizoaffective disorder.
Results
The patient presented to the emergency department complaining of a constant, painful erection lasting approximately 42 h. Treatment with intracavernous ephedrine, irrigation, and aspiration helped achieve detumescence.
Conclusion
This case displays the immediate and detrimental effects due to the addition of antipsychotic medications to previously altered cytochrome P450 (CYP450) enzyme levels. The inhibition of CYP450 enzymes 3A4 and 2D6 by the combination PI, lopinavir/ritonavir, was likely the major culprit in causing greater than expected free levels of perphenazine and quetiapine resulting in priapism.
Keywords: Drug interaction, Cytochrome P450, Antipsychotic, HIV, Priapism, Protease inhibitor
Introduction
The employment of highly active antiretroviral therapy (HAART) has raised interest and awareness in the human immunodeficiency virus (HIV) psychopharmacology arena due to potential drug-drug interactions. There is also a lack of data and documented clinical studies with regards to the wide range of adverse effects and combinations of these medications. As upwards of 48% of HIV-infected persons have a probable psychiatric disorder, the possibility of cross-class drug interactions causing adverse effects or fatalities exists [1, 2]. Undesirable symptoms or peculiar presentations may be an indication that pharmaceutical treatments may be counterproductive, requiring healthcare professionals to investigate drug therapy. The following describes a case of drug-induced low-flow priapism provoked by the addition of new antipsychotic medications to an HIV patient previously on HAART.
The cytochrome P450 (CYP450) family of enzymes has been implicated in a large number of preventable, adverse drug interactions when individually discussing HIV and psychopharmacology. HAART can pose significant side effects for patients and alter CYP450 metabolism extensively; however, when combined with antipsychotic drugs that require CYP450 enzymes for transport and metabolism, serum levels of assorted drugs can vary greatly often resulting in serious adverse affects [1–3]. Pharmacokinetic interactions among antiretroviral agents may influence the potency or toxicity of co-administered drugs, such as the addition of new antipsychotics to this patient’s regimen. This case of low-flow priapism, a urologic emergency, required immediate and aggressive management and may prove to cause lasting urologic and sexual dysfunction. Prior cases of antipsychotic-induced priapism have been reported; however, this is the first reported case of priapism associated with a likely drug interaction between antipsychotics and protease inhibitors (PIs).
Case
A 50-year-old African-American man presented to the emergency department (ED) in acute pain due to a protracted case, approximately 42 h, of untreated priapism. The patient has been HIV positive since 1996. He stated he has taken HIV medications for many years and is extremely adherent to his regimen. Current medications included a multi-drug antiretroviral regimen including the combination PI, lopinavir/ritonavir (Kaletra®, Abbott Labs) 200/50 mg two tablets twice daily and the combination reverse transcriptase inhibitor abacavir/lamivudine/zidovudine (Trizivir, GlaxoSmithKline) 300/150/300 mg two tablets daily. The patient was diagnosed with schizoaffective disorder and was started on a regimen including two antipsychotics, quetiapine (Seroquel®, AstraZeneca) 300 mg, three tablets at bedtime, and perphenazine (Trilafon®, Schering-Plough) 8 mg by mouth nightly administered two days prior. His prescribing physician also added benztropine (Cogentin®, Merck) 1 mg by mouth twice daily to abate extrapyramidal symptoms secondary to antipsychotic therapy. The patient began taking these new antipsychotic medications 48 h prior to presenting. Five to six hours after taking his new medication the patient stated the occurrence of a lasting non-stimulated erection. The patient did not attempt to self-treat, seek medical advice, or take any medications for his condition until he presented to the hospital.
The ED work-up verified the patient’s history. He had no record of prior erectile disturbances or priapism, sickle cell disease, oncologic malignancies, blood dyscrasias, or pelvic injury. Physical examination revealed an erect shaft with soft glans, indicating a low-flow priapism. The patient expressed that he had been on various medications over the years with no history of priapism. He is compliant with his current regimen and recently started new medications for his psychological issues. The patient denied use of any illicit drugs, over-the-counter (OTC) products, herbal medications, and/or vasoactive medications for erectile dysfunction. Medication history was verified by a call to the patient’s local retail pharmacy where medications, doses, and frequencies were verified. The patient verbalized that the erection began approximately 5–6 h after the initial dose of his new medications. He did not attempt to seek care or treatment until the pain became unbearable. No other antipsychotic-related side effects were present.
Initial laboratory work-up included a basic metabolic panel (BMP) and complete blood count (CBC) both of which were unremarkable. He was treated with intravenous hydromorphone (Dilaudid®) and ondansetron (Zofran®) to treat pain and nausea. He was later given oral hydrocodone with acetaminophen (Lortab®) for pain. A urology consult was ordered. Upon arrival the urologist examined the patient and determined a course of action. The corpus cavernosum was locally anesthetized with lidocaine 1% with epinephrine and irrigated with dilute ephedrine and saline. Due to the extended period of engorgement, multiple attempts at irrigation and drainage with non-heparinized saline and dilute ephedrine were made to reduce the blood volume. Eventually detumescence was achieved after aspiration of over 60 ml of blood. A post-procedure urine sample was obtained before the patient was discharged home. Urine toxicology test determined the patient was positive for opiates and tricyclic antidepressants, while negative for any recreational or drugs of abuse. Opioid metabolites were most likely from the hydromorphone and hydrocodone given for pain. Tricyclic metabolites in the urine confirm the use of quetiapine. No other records of possible medications for these positives results were obtained from the patient’s local retail pharmacy.
Discussion
There is no way to foresee which patients will develop priapism. The only predictor of it may be a history of previous prolonged painless erections, which can lead to a painful, damaging event. Priapism can occur with initial doses of antipsychotics, after years of use, or with the addition of new medications to a patient’s regimen [4]. Low-flow priapism, although rare (less then 1% occurrence), can be an adverse event of antipsychotic agents resulting from a proposed blocking action at α-adrenergic receptors. This α-blockade causes venous constriction and prevents blood return to circulation [5–12]. Previous management of patients with priapism due to antipsychotics involved reducing the dose of the offending agent or changing the medication to one with a lower α-adrenergic affinity. This management style assumes that priapism can be a dose-related function of the prescribed medication.
CYP450 enzymes are proteins produced in the liver, responsible for the oxidative metabolism of drugs. Various medications have the ability to induce or inhibit one or many of the CYP450 enzymes. This induction or inhibition can cause unforeseen variances in drug metabolism, adverse events, and therapeutic failures. Concomitant administration of inducers or inhibitors with other drugs that are metabolized by the CYP450 system can result in altered metabolism and kinetics throughout the body. Many well-known drugs alter P450 enzyme levels. Most CYP450 drug interactions can be managed through vigilance and dose adjustment.
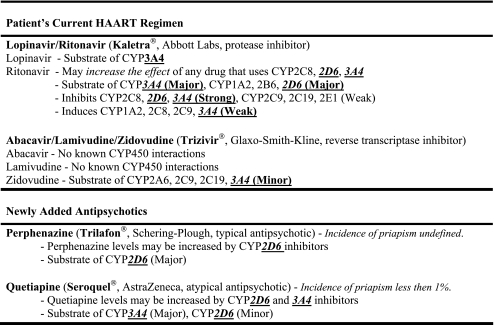
In examining the PIs affinity for CYP450 enzymes, lopinavir is a substrate of the enzyme CYP3A4, while ritonavir is a substrate of CYP1A2, 2B6, 2D6, and 3A4. Ritonavir also is a strong inhibitor of CYP2C8, 2D6, and 3A4. Due to the fact that perphenazine is a major substrate of CYP2D6, levels can be increased by 2D6 inhibitors, such as ritonavir. Quetiapine binds to CYP3A4 and 2D6 as a substrate; the PIs inhibit and also bind to 3A4 and 2D6 creating a larger amount of active quetiapine in the serum. The combination of these drugs increases the free amount of both perphenazine and quetiapine in the blood, predisposing the patient to possible toxicity and unwanted side effects, such as priapism (see Table 1) [12].
Table 1.
CYP450 enzyme characteristics by medication [12]
Harrison et al. encountered a case of priapism in an HIV patient due to quetiapine and reported the possibility of a drug interaction due to illicit amphetamine use [7]. Their case made no mention of any antiretroviral medication or PI their patient used, so a correlation between PI use and quetiapine could not be made. The current patient did not have any amphetamines in his system, as verified by a post-procedure urine toxicology screen. A review conducted by Sood et al. states that priapism, although rare, can occur with all atypical-antipsychotic medications [4]. As stated above, priapism can occur with initial doses, after years of use, or with the addition of new medications to a regimen. In reviewing 50 publications and cases regarding priapism and drug-drug reactions, there is no mention of priapism due to the combination of HAART medications and antipsychotics. Although the possibility exists that the patient would have developed priapism using one or both of the antipsychotics as a single agent, the immediate onset of this situation was enhanced and is most likely fully to blame upon previously altered CYP450 enzyme levels due to the HAART, specifically the patient’s PI.
The Naranjo algorithm is a questionnaire designed to determine the likelihood of whether an adverse drug reaction is actually due to the drug rather than the result of other factors. It is the most widely used algorithm to assess such events and has been tested for internal validity with between-rater reliability testing, and its probability scale has consensual, content, and concurrent validity as well as ease of use [13, 14]. The Naranjo algorithm rates this drug interaction as “probable.”
Conclusion
Though a paucity of definitive data from controlled studies regarding interactions between HAART medication and antipsychotics exists, CYP450 enzyme disturbances are documented within each drug class. This case demonstrates the immediate and detrimental effects due to the addition of antipsychotic medications to previously altered CYP450 enzyme levels. The CYP450 inhibition of enzymes 3A4 and 2D6 by the combination PI, lopinavir/ritonavir, is likely the major culprit in causing increased free levels of perphenazine and quetiapine. The immediate high level of free drug caused an α-blockade resulting in priapism shortly after ingesting the new antipsychotics. Clinicians should be aware of this rare but potentially serious adverse event. Further investigation is warranted regarding potential interactions between HAART and antipsychotic medications to elucidate potential mechanisms of adverse effects.
Author’s contributions
All authors contributed substantially to the report and its revision.
Conflict of interest None.
Funding No grants, financial support, or compensation has been provided for this work.
Biographies
Matthew J. Geraci is a Clinical Pharmacist in the Emergency Department at Baptist Medical Center Downtown in Jacksonville, FL and a Clinical Assistant Professor of Pharmacy Practice with the University of Florida. He completed his postgraduate residency at the Mayo Clinic, Jacksonville, FL. He received his PharmD from the University of Utah and a BS in Physics/Nuclear Engineering from West Point.
Stacey L. McCoy is a Clinical Pharmacist in the Emergency Department at Baptist Medical Center Downtown in Jacksonville, FL and a Clinical Assistant Professor of Pharmacy Practice with the University of Florida. She completed her postgraduate residency at St. Joseph’s/Candler Hospital, Savannah, GA. She received her PharmD from Mercer University and a BS/MS from Clark Atlanta University. She is a Board Certified Pharmacotherapy Specialist.
Paul M. Crum is a Consulting Urologist at McIver Urological Clinic, Riverside Office, in Jacksonville, FL. He completed his postgraduate residency at the U.S. Naval Hospital in Philadelphia, PA and attended medical school at the University of Pennsylvania. He is a Fellow of the American College of Surgeons, Board Certified in Urology, and member of the Society of Pediatric Urologists.
Rajnikant A. Patel is an Attending Physician with the Emergency Resources Group at Baptist Medical Center in Jacksonville, FL. He completed his postgraduate residency at King Edward VII Memorial Hospital in Bombay, India; Albert Dock Hospital in London, UK; and at the University Hospital of Jacksonville in FL. He attended medical school at G.S. Medical College, University of Bombay in India and is a Fellow of the American College of Emergency Physicians.
Footnotes
The views expressed in this paper are those of the author(s) and not those of the editors, editorial board or publisher.
References
- 1.Repetto MJ, Petitto JM. Psychopharmacology in HIV-infected patients. Psychosom Med. 2008;70(5):585–592. doi: 10.1097/PSY.0b013e3181777190. [DOI] [PubMed] [Google Scholar]
- 2.Ferrando SJ, Wapenyi K. Psychopharmacological treatment of patients with HIV and AIDS. Psychiatr Q. 2002;73(1):33–49. doi: 10.1023/A:1012840717735. [DOI] [PubMed] [Google Scholar]
- 3.Sikka R, Magauran B, Ulrich A, Shannon M. Bench to bedside: pharmacogenomics, adverse drug interactions, and the cytochrome P450 system. Acad Emerg Med. 2005;12(12):1227–1235. doi: 10.1111/j.1553-2712.2005.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 4.Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9–17. doi: 10.1097/YIC.0b013e3282f1c1ef. [DOI] [PubMed] [Google Scholar]
- 5.Casiano H, Globerman D, Enns MW. Recurrent priapism during treatment with clozapine, quetiapine and haloperidol. J Psychopharmacol. 2007;21(8):898–899. doi: 10.1177/0269881107077372. [DOI] [PubMed] [Google Scholar]
- 6.Davol P, Rukstalis D. Priapism associated with routine use of quetiapine: case report and review of the literature. Urology. 2005;66(4):880. doi: 10.1016/j.urology.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 7.Harrison G, Dilley JW, Loeb L, Nelson K. Priapism and quetiapine in an HIV-positive male. J Clin Psychopharmacol. 2006;26(1):100–101. doi: 10.1097/01.jcp.0000196115.01001.04. [DOI] [PubMed] [Google Scholar]
- 8.Pais VM, Ayvazian PJ. Priapism from quetiapine overdose: first report and proposal of mechanism. Urology. 2001;58(3):462. doi: 10.1016/S0090-4295(01)01208-0. [DOI] [PubMed] [Google Scholar]
- 9.Prado MJA, Formoso MV. Priapism associated with quetiapine in an elderly patient. Actas Esp Psiquiatr. 2006;34(3):209–210. [PubMed] [Google Scholar]
- 10.Chan J, Alldredge BK, Baskin LS. Perphenazine-induced priapism. DICP. 1990;24(3):246–249. doi: 10.1177/106002809002400306. [DOI] [PubMed] [Google Scholar]
- 11.Tejera CA, Ramos-Lorenzi JR. Priapism in a patient receiving perphenazine. J Clin Psychopharmacol. 1992;12(6):448–449. doi: 10.1097/00004714-199212000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Lacy C, Armstrong L, Goldman M, Lance L (eds) (2008) Lexi-Comp drug database drug information handbook, 17th edn. Lexi-Comp Information Management Service (LIMS), Hudson, pp 15–16, 939–942, 1227, 1332–1335
- 13.Kelly WN (2008) How can I recognize an adverse drug event? MedscapeCME Pharmacists
- 14.Naranjo C, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]