Abstract
Objective
This study was performed retrospectively to determine if Medicare claims data could be used to evaluate the cost-effectiveness, from a payer perspective, of different radiation treatment schedules evaluated in a national clinical trial.
Methods
Medicare costs from all providers and all places of service were obtained from the Centers for Medicare & Medicaid Services (CMS) for patients treated from 1992-1996 on Radiation Therapy Oncology Group (RTOG) 90-03, and combined with data on outcomes from the trial.
Results
Of the 1113 patients entered, Medicare cost data and clinical outcomes were available for 187 patients. Significant differences in tolerance of treatment and outcome were noted between patients with Medicare data included in the study and patients without Medicare data and non-Medicare patients excluded form it. Ninety-five percent confidence ellipses on the incremental cost-effectiveness scatterplots crossed both axes, indicating non-significant differences in cost-effectiveness between radiation treatment schedules.
Conclusions
Claims data permit estimation of cost-effectiveness, but Medicare data provide inadequate representation of results applicable to patients from the general population.
Introduction
Radiation Therapy Oncology Group (RTOG) trial 90-03 was a randomized phase III trial which evaluated four different radiotherapy fractionation schedules in the treatment of patients with locally advanced cancers of the head and neck area. Patients with locally advanced head and neck cancer treated with hyperfractionated and accelerated fractionated with concomitant boost radiotherapy on RTOG 90-03 experienced significantly improved local-regional control compared to standard fractionated radiotherapy.1 This improvement, however, was accompanied by significantly increased grade three or worse acute side effects (for example, 35% grade 3 effects for standard fractionation versus 54.5% for hyperfractionation, 50.4% for accelerated fractionation with split, and 58.8% for accelerated fractionation with concomitant boost). These results were confirmed in a recently published update of 90-03.2 The incidence of grade three or worse acute and late effects was 35% and 26.8% for standard fractionation, 54.5% and 28% for hyperfractionation, 50.4% and 27.6% for accelerated fractionation with split, and 58.8% and 37.2% for accelerated fractionation with concomitant boost. The most common sites of grade three or worse acute toxicity was in the mucous membranes and pharynx while the most common site of late toxicity was the pharynx and salivary gland.
A higher number of fractions in altered fractionated radiotherapy schedules are more costly compared to standard radiation therapy schedules because of a greater number of radiation treatments given with increased cost secondary to the technical portion of the treatment.3 Treatment of acute and late side effects would further increase the overall cost of treatment.4
The specific aim of this study was to determine if Medicare data could be used to determine if altered fraction radiotherapy (ALFX) schedules were cost-effective in the treatment of patients with locally advanced head and neck cancer treated on RTOG 90-03.
Methods
Clinical Trial and Patient Selection
Patients 18 years or older with previously untreated Stage III or IV (M0) squamous cell carcinoma of the oral cavity, oropharynx, supraglottic larynx, or Stage II-IV carcinoma of the base of tongue or hypopharynx were eligible for entry into RTOG 90-03. Karnofsky performance status ≥ 60 was required. Patients with a prior (within 5 years) or synchronous malignancy other than non-melanoma skin cancer were excluded.
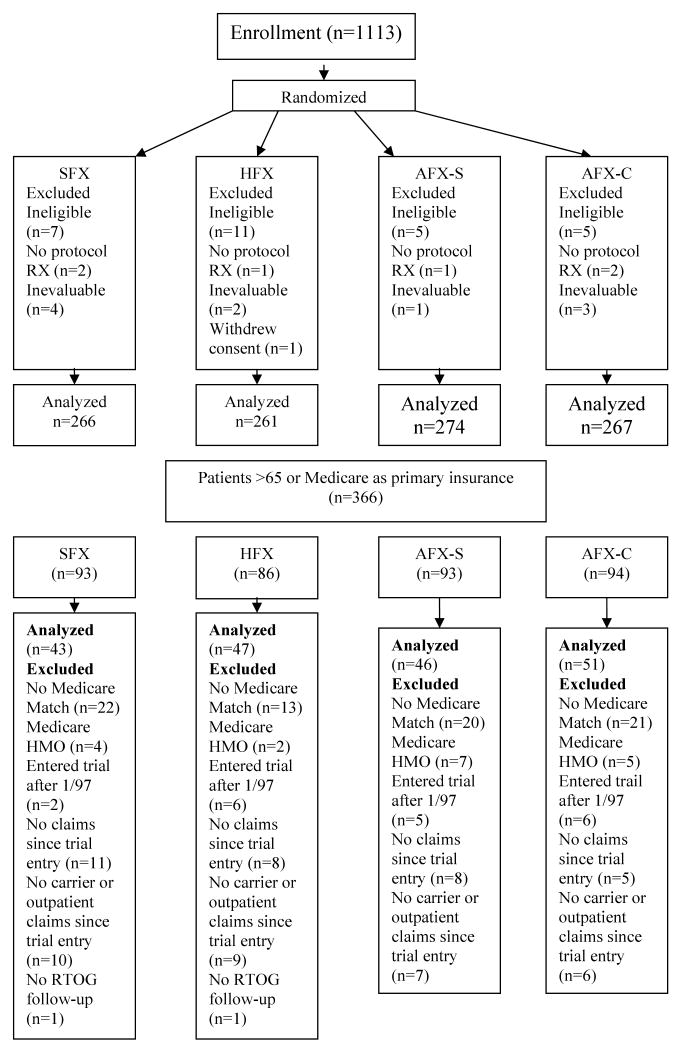
Patients were randomized to receive either: 1) standard fractionation radiotherapy (SFX) at 2Gy/fraction, 5 days a week to 70 Gy in 35 fractions in 7 weeks; 2) hyperfractionation radiotherapy (HFX) at 1.2 Gy/fraction, twice daily, 6 hours apart, 5 days/week to 81.6 Gy/68 fractions; 3) accelerated fractionation radiotherapy with split (AFX-S) at 1.6 Gy/fraction, twice daily, 6 hours apart, 5 days/week, to 67.2 Gy/42 fractions/6 weeks including a two-week rest after 38.4 Gy; or 4) accelerated fractionation radiotherapy with concomitant boost (AFX-C) at 1.8 Gy/fraction/day, 5 days/week to large field + 1.5 Gy/fraction/day to boost field given 6 hours after treatment of the large field for the last 12 treatment days to a total dose of 72 Gy/42 fractions/6 weeks. Additional boost doses not exceeding 5 Gy through reduced fields to persistent primary tumor and/or clinically positive nodes was allowed with a neck dissection for neck nodes>3 cm prior to radiotherapy at the discretion of the responsible head and neck surgeon and radiation oncologists. Randomization procedures, treatment, outcome definition, and follow-up details of the trial have been previously published.1 Patient randomization, inclusion and exclusion criteria for the economic analysis are depicted in the CONSORT diagram in Figure 1.
Figure 1.
Outcomes
The clinical database from the RTOG was used to calculate the outcomes of interest: overall (or life years (LY)) and disease-free survival. The Cochran-Mantel-Haenszel test was used to determine whether the distribution of pretreatment characteristics differed between the NMD and NMA subgroups and the ICS subgroup. Overall survival was measured from the date of randomization to the date of death or last follow-up. Disease-free survival was measured from the date of randomization to the first occurrence of a local-regional failure, distant metastasis, a new primary, or death without progression. The Kaplan-Meier method was used to estimate the rates and the log-rank test was used to compare treatment arms.5,6 Statistical comparisons of association of categorical covariates with patients included in or excluded from the study were carried out using the chi-square test. For cost-effectiveness comparisons, overall and disease-free survival was discounted back to the time on entry onto the trial using an annual discount rate of 3%.
Cost Data
Medicare claims data were used to estimate Medicare and total medical costs for patients in each treatment arm. A list of patients ≥ 65 years of age or listing Medicare as their primary insurance carrier was submitted to the Centers for Medicare and Medicaid Services (CMS) to obtain cost data to link to clinical outcome. Three-hundred and sixty-six patients were ≥65 or listed Medicare as their primary insurance and had their social security number submitted to CMS for reimbursement data. Patients were excluded if there was no matching Medicare data, if they were enrolled in a Medicare Health Maintenance Organization (HMO) for the entire trial, if they entered the trial after January 1997, if they had no Medicare claims since they entered the trial, or if they did not have carrier or outpatient claims since they entered the trial. The economic analysis was performed on the remaining 187 patients.
Data requested to calculate cost of treatment included Medicare Part A and Part B claims for all providers in all care settings - inpatient, outpatient, skilled nursing facility, home health, hospice, and physicians and other Part B providers for patients treated on RTOG 90-03 from 1992-1996. Fifty-six month expected discounted costs for each arm of the trial was calculated with the Lin method.7-9 Costs were discounted back to the time of entry onto the trial, using an annual discount rate of 3%, and indexed to 1996 U.S. dollars using the Consumer Price Index (Bureau of Labor Statistics Consumer Price Index—All Urban Consumers- (CPI-U). U.S. city average. All items (1982-84=100). ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt. Accessed November 18, 3:40 pm. US Department of Labor).
Incremental cost-effectiveness estimations
The cost-effectiveness analysis was performed from a payer's perspective, i.e. Medicare. Incremental cost-effective ratios were calculated comparing ALFX schedules to SFX. Non-parametric bootstrap analysis was used to generate 95% confidence intervals on the cost-effectiveness plane.10,11
Validation and Sensitivity Analysis
These 187 patients in the cost study (In Cost Study group, or ICS) were compared with patients who were eligible for Medicare data but on whom no Medicare claims data were available (No Medicare Data group, or NMD) and with patients who were too young to be eligible for Medicare (Non Medicare Age group, or NMA), to determine if results from this study could be generalized to the overall patient population.
Sensitivity analysis was performed evaluating the effect on treatment cost of using only ICD-9 codes associated with head and neck cancer and the effect of age at the time of diagnosis, <65 compared to >65, on expected mean costs. For the sensitivity analysis restricted to claims related to the condition, claims with the following ICD-9 codes were used: 141, 142, 143, 145, 146, 147, 148 160, 161, 170, 171. Medical costs were also calculated for all services provided through Medicare – including costs to beneficiaries and other primary payers. Medicare claims document these payments.
This study was reviewed and approved by the Institutional Review Boards of the American College of Radiology and Fox Chase Cancer Center. The manuscript was also reviewed by CMS in accordance with our Data Use Agreement to ensure patients participating in the study could not be identified.
Results
Comparison of outcomes for patients in the cost-effectiveness study
No significant difference is found in survival across treatment arms (p=0.23, log-rank test), or in disease-free survival across treatment (p=0.34, log-rank test) among patients included in the study. There was no difference in patient disease stage between the treatment groups in the ICS group.
Across all patients in the clinical trial (in cost study, non-Medicare age, and patients with no Medicare claims data), MST was highest for the hyperfractionated radiotherapy treatment arm (median 2.1 years (95% CI 1.4-3.7)) and lowest for the accelerated fractionated radiotherapy with concomitant boost treatment arm (median 1.3 years (95% CI 1.0-2.3))
Cost
Mean costs per patient to Medicare for each of the treatment arms is listed in Table 1. Average costs were highest for HFX (58,124; 95% CI (34,777; 74,729)) and lowest for AFX-C (37,946; 95% CI (23,635; 55,507))
Table 1. Incremental cost-effectiveness ratios (ICER) from Medicare perspective.
| Expected 56-month discounted Medicare costs, 1996 U.S. dollars (95% CI) | Discounted Survival (Years) | Discounted Disease-free Survival (Years) | Survival ICER | DFS ICER | |
|---|---|---|---|---|---|
| SFX, N = 43 | $47,719 (29,672; 69,015) |
3.0 | 2.1 | ||
| HFX, N = 47 | $58,124 (34,777; 74,729) |
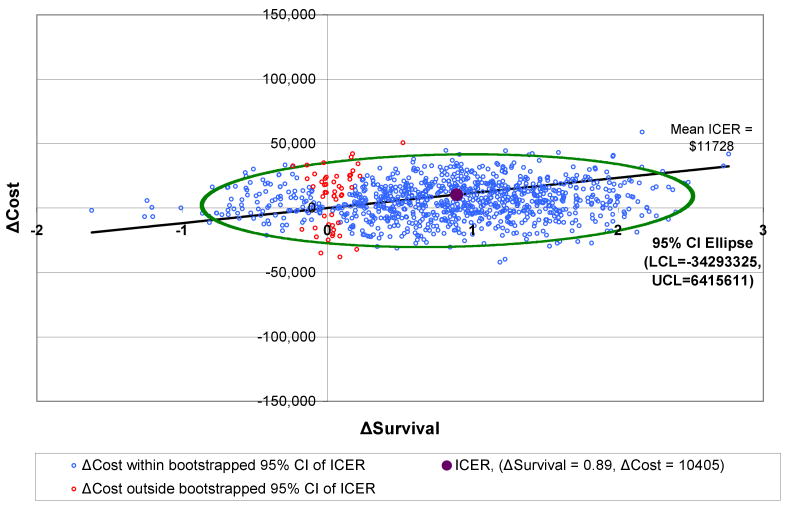
3.9 | 2.9 | $11,728 | $11,832 |
| AHFX-S, N = 46 | $52,488 (35,017; 72,988) |
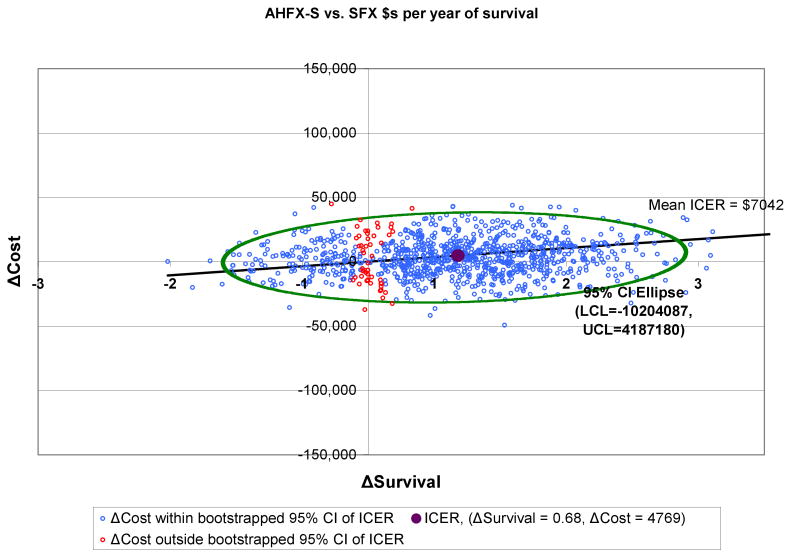
3.7 | 2.8 | $7,042 | $6,384 |
| AFX-C, N = 51 | $37,946 (23,635; 55,507) |
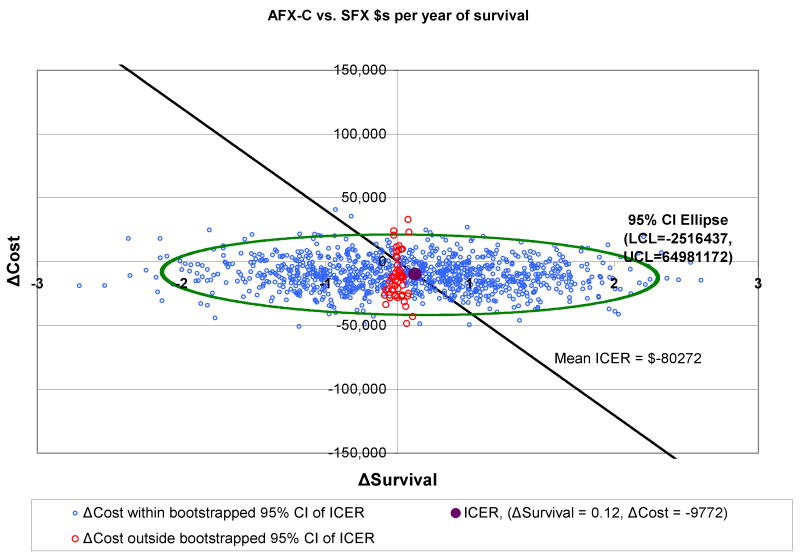
3.2 | 2.3 | -$80,272 | -$44,605 |
SFX-Standard fraction radiotherapy; HFX-Hyperfractionated radiotherapy; AHFX-Accelerated Hyperfractionated radiotherapy with split; AFX-C-Accelerated Fractionated radiotherapy with concomitant boost
Incremental cost-effectiveness ratios (ICER) (Table 1)
Outcomes for AFX-C were better than outcomes for SFX, and the costs were lower. Therefore, the ICERs for AFX-C are negative in comparison to SFX; in other words, AFX-C dominates SFX. HFX has an ICER of $11,728 per life year, and AHFX-S has an ICER of $7,042 per life year.
Figures 2-4 show the 95% confidence intervals on the incremental cost-effectiveness scatterplots comparing the altered fractionated radiotherapy schedules to SFX.
Figure 2.
Plot of 95% Confidence Intervals comparing HFX to SFX
Figure 4.
Plot of 95% Confidence Intervals Comparing AFX-C to SFX
Representativeness of patients in the study
Table 2 shows comparisons of disease stage between patients in the study and patients excluded because of Medicare ineligibility or lack of claims data. The distributions of AJCC stage were not the same between the ICS and the NMD (p= 0.04). Furthermore, there are significant difference in the distribution of AJCC Stage between the ICS and the NMA (p <0.0001 respectively).
Table 2. Patient Stage by Group.
| Stage II | Stage III | Stage IV | |
|---|---|---|---|
| ICS | |||
| SFX | 4 (9%) | 18 (42%) | 21 (49%) |
| HFX | 2 (4%) | 24 (51%) | 21 (45%) |
| AHFX-S | 1 (2%) | 17 (37%) | 28 (61%) |
| AFX-C | 2 (4%) | 17 (33%) | 32 (63%) |
| NMD | |||
| SFX | 2 (4%) | 12 (25%) | 35 (71%) |
| HFX | 2 (5%) | 10 (26%) | 26 (68%) |
| AHFX-S | 4 (8%) | 15 (32%) | 28 (60%) |
| AFX-C | 2 (5%) | 14 (33%) | 27 (63%) |
| NMA | |||
| SFX | 5 (3%) | 50 (29%) | 119 (68%) |
| HFX | 6 (3%) | 44 (25%) | 126 (72%) |
| AHFX-S | 2 (1%) | 42 (23%) | 137 (76%) |
| AFX-C | 4 (2%) | 39 (23%) | 129 (75%) |
SFX-Standard fraction radiotherapy; HFX-Hyperfractionated radiotherapy; AHFX-Accelerated Hyperfractionated radiotherapy with split; AFX-C-Accelerated Fractionated radiotherapy with concomitant boost; ICS- In cost study; NMD- No Medicare Data; NMA- Non-Medicare Age
Outcomes were different for patients included in the cost study than for patients excluded from the cost study. For example, patients receiving SFX in the ICS group had the lowest percentage of grade three or higher toxicities (28%) occurring within 180 days of the start of RT compared to patients treated on the SFX arms in the NMD and NMA groups, (55% and 37% respectively). The mean survival time (MST) was lower in the NMD subgroup compared to the ICS subgroup for all treatment arms.
Sensitivity Analysis
A sensitivity analysis was performed evaluating the cost-effectiveness of altered fractionation schedules if total expected 56- month reimbursement, Medicare + Primary (non-military primary payer data available in Medicare claims) + Beneficiary, was considered. The expected 56- month mean cost of SFX, HFX, AHFX-S, and AFX-C increased to $53,101, $65,819, $58,290, and $43,348 respectively. The ICER of HFX, AHFX-S and AFX-C compared to SFX were $14,334/LY, $7,662/LY, and -$80,118/LY respectively. Sensitivity analysis was also performed only using claims with a cancer diagnosis. The expected mean Medicare reimbursement for SFX, HFX, AHFX-S, and AFX-C was $17,270, $30,784, $28,246, and $17,447 respectively resulting in an ICER of HFX, AHFX-S and AFX-C compared to SFX of $8,228/LY, $8,688/LY, and $268/LY. The expected mean cost of patients <65, n=16, was lower across all treatment groups (SFX=$28,985, HFX=$32,171, AHFX-S=$14,634, and AFX-C=$23,626) compared to patients ≥65 (SFX=$86,026, HFX=$66,081, AHFX-S=$93,145, AFX-C=$72,731).
The small number of patients in each arm available for analysis resulted in wide 95% confidence intervals and the 95% confidence ellipses crossed both the x and y axes. This trial was not powered for an economic analysis. A significantly greater number of patients would have to have been entered into the study to show cost-effectiveness.23,26,27 Given the clinical and economic results of RTOG 90-03, a total of 384 patients would have been needed in each arm of HFX and SFX if $500/LY was the minimum meaningful acceptable ICER and $50,000/LY was the maximum acceptable ICER to show statistical significance compared to 47 and 43 patients in the HFX and SFX arms respectively. In addition, we would have required 650 patients in each arm to compare and determine cost-effectiveness of AHFX-S to SFX while we only had 46 and 43 patients respectively in each arm. Finally, 494 patients would have been required in each arm given the clinical and economic results to determine a significant difference in cost-effectiveness comparing AFX-C to SFX when we only had 51 and 43 patients respectively.
Discussion
The cost-effectiveness of altered fractionated radiotherapy schedules was evaluated in a patient population covered by Medicare insurance. There have been few articles addressing cost-effectiveness in cancer therapies; fifteen percent (170/1164) of articles listed in the CEA Registry from Tufts Medical Center involve malignant neoplasms. (https://research.tuftsnemc.org/cear/default.aspx). Some of the studies have measured resource consumption multiplied by a unit cost to calculate the cost estimate12-16, while others have measured only costs attributable to the cancer or treatment.17,18 while another collected charges from selected institutions participating in the study.19 This study measures all costs from a payer perspective, and uses a national sample of patients.
The results of the analyses were not sensitive to the definition of “cost” as was shown in the sensitivity analysis. The expected mean reimbursement was higher when considering all sources compared to when only a cancer diagnosis was present, which resulted in the lowest expected mean reimbursement. It is difficult to know with certainty the exact proportion of reimbursement that was a result of cancer care as opposed to general medical care. A review of each individual claim could help to determine if the claim was related to the cancer. The SEER-Medicare database has a random 5% sample of Medicare enrollees from SEER areas without cancer to compare phase-specific costs of cancer care to.8,9 The control cases can help researchers determine the relative proportion of cancer related reimbursement. We did not have a comparable control sample of patients of a similar age without head and neck cancer for comparison.
As our analysis has shown, an underestimation of reimbursement could have occurred if only claims with a cancer diagnosis were used. As mentioned previously cost accounting could give the most accurate representation of cost of treatment but cost accounting has its limitations as well. Patients may forget to document procedures or medication used resulting in an underestimation of the cost. In addition the variable cost of assigning overhead could fluctuate between centers in a cooperative group study. Capturing indirect costs related to treatment could be difficult but would not impact an analysis from a payor perspective.
The RTOG has performed economic analyses of clinical trials using Markov models but has never before performed an economic analysis using health insurance claims.20,21 Performing economic analyses alongside clinical trials is challenging since clinical outcomes and priorities may differ from economic outcomes.22-24
This economic analysis fulfilled the majority of criteria supporting an economic analysis of a clinical trial outlined by Hillner.25 Factors supporting an economic analysis alongside a clinical trial include: sizeable amounts of resources are at stake (common disease, therapy is readily transferable to marketplace, therapy will supersede, not supplement, other interventions) and alternative strategies differ substantially in cost and/or morbidity (costs, intervention, settings, acute mortality and chronic morbidity). The therapy studied in this case will not supersede other interventions given the move towards targeted therapies. But can these results be translated to the general population as a whole? Were the characteristics of the study population representative of the entire trial population?
Our analysis was restricted to a small percentage of patients, 17%, who were older than most of the participants in the trial. We have no data documenting the general overall health of the ICS subgroup compared to the NMD or NMA subgroups. Older patients may have poorer overall health and may not be able to tolerate aggressive treatment compared to younger patients and therefore may experience higher healthcare costs in treating both the acute and late toxicities associated with protocol related treatment. This is confirmed in this study since the distribution of severe acute and late toxicities differed among the three subgroups. In addition, overall survival was lower in the NMD group compared to the ICS group although the age in both groups was similar and theoretically they should have had similar co-morbidities. Patients ≥ 65 in this analysis had higher cost of care compared to younger patients but the small numbers of patients <65 makes further analysis problematic. It is not known whether the lower cost was due to less healthcare resource utilization or healthcare reimbursement by other payers in addition to Medicare.
In addition, transportation costs were not included in the analysis because a payer's perspective was taken for the analysis. Including transportation and/or housing costs would have increased the “cost” of care for patients in the altered fractionation treatment groups. A limit was placed on the number of years of CMS data we were allowed to acquire at one time. Including 19 patients with Medicare data entered after 1997 in addition to adding longer follow-up costs may further strengthen the analysis.
Furthermore, the current standard of care treating patients with locally advanced head and neck cancer has evolved to incorporate systemic chemotherapy and with targeted agents such as C225, the epidermal growth factor receptor antibody, currently being investigated in clinical trials. The importance of this study may not be in the actual results finding more aggressive therapies are cost-effective, but in the experience in linking Medicare claims to clinical data for future economic analyses. This will enhance our ability to perform economic analysis of these more expensive therapies in the future.
As more expensive targeted therapies are introduced into clinical practice, secondary analyses of clinical trials will be important. Although technically achievable, the use of Medicare data to inform the cost component of an economic analysis may result in outcomes that are not translatable to the general population as a whole because of small patient numbers and non-random missing data (as evident from the differences in patient characteristics between patients in the study, younger patients, and Medicate eligible patients for whom we did not have claims data (Table 2).
Limitations of this study included differences between the three groups of patients in this study that could have influenced the outcome of the analysis. Because of the small number of patients in each group we could not draw conclusions concerning the cost-effectiveness of the different radiotherapy fractionation schedules. However, with the availability of claims data for a more representative population, such as claims from the national system in Canada, or claims from a commercial health plan, can be used for economic analysis.
Conclusion
Due to the cost and compliance barriers of prospective collection of resource use in large national multi-site cooperative group trials, use of Medicare or other health insurance data-bases for cost-effectiveness analyses may still be useful under specific circumstances. Economic analysis using Medicare data would be useful if the study included sufficient patients in the Medicare eligible age group (age 65 and older) to determine statistical difference between the groups, or if the Medicare cohort and general study populations were similar.
Figure 3.
Plot of 95% Confidence Intervals Comparing AHFX-S to SFX
Acknowledgments
This work was supported by the Pennsylvania Commonwealth Universal Research Enhancement (C.U.R.E.) Program ME-02-149 grant
Abbreviations
- ALFX
Altered Fraction Radiotherapy
- SFX
Standard Fractionated Radiotherapy
- HFX
Hyperfractionation Radiotherapy
- AFX-S
Accelerated Fractionation Radiotherapy
- AFX-C
Accelerated Fractionation Radiotherapy with Concomitant Boost
- CMS
Centers for Medicare and Medicaid Services (CMS)
- ICD
International Statistical Classification of Diseases and Health Related Problems
- CONSORT
Consolidated Standards of Reporting Trials
- NMD
No Medicare Data
- ICS
In Cost Study
- NMA
Non-Medicare Age
- RT
Radiation Therapy
- MST
Mean Survival Time
- DFS
Disease-Free Survival
- OS
Overall Survival
- ICER
Incremental Cost-Effective Ratio
- LY
Life Year
- RTOG
Radiation Therapy Oncology Group
- RVU
Relative Value Units
Footnotes
Presented at the 42nd Annual Meeting, ASCO June 2-6, 2006
References
- 1.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 2.Trotti A, Fu KK, Pajak TJ, Jones CU, Spencer SA, Phillips TL, Garden AS, Ridge JA, Cooper JS, Ang KK. Long term outcomes of RTOG 90-03: A comparison of hyperfractionation and two variants of accelerted fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;63(Suppl 1):S70–S71. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 3.Owen JB, Grigsby PW, Caldwell TM, et al. Can costs be measured and predicted by modeling within a cooperative clinical trials group? Economic methodologic pilot studies of the radiation therapy oncology group (RTOG) studies 90-03 and 91-04. Int J Radiat Oncol Biol Phys. 2001;49:633–9. doi: 10.1016/s0360-3016(00)00770-7. [DOI] [PubMed] [Google Scholar]
- 4.Konski AA, Bhargavan M, Owen J, Paulus R, Cooper J, Forastiere A, Goepfert H, Ang KK, Watkins-Bruner D. The Price of Failure: Economic Analysis of Rrecurrence and Complications fo Patients Treated on RTOG 91-11. Proceedings American Radium Society Annual Meeting; 2007. [Google Scholar]
- 5.Kaplan EaM P. Nonparametric estimation from incomplete observations. J Am Stats Assoc. 1958;53:447–457. [Google Scholar]
- 6.Cox DR. Regression models and life tables. J Roy Stats Soc. 1972;34:187–220. [Google Scholar]
- 7.Lin DY, Feuer EJ, Etzioni R, et al. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53:419–34. [PubMed] [Google Scholar]
- 8.Brown ML, Riley GF, Schussler N, et al. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV104–17. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 9.Etzioni R, Riley GF, Ramsey SD, et al. Measuring costs: administrative claims data, clinical trials, and beyond. Med Care. 2002;40:III63–72. [PubMed] [Google Scholar]
- 10.Warren JL, Brown ML, Fay MP, et al. Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol. 2002;20:307–16. doi: 10.1200/JCO.2002.20.1.307. [DOI] [PubMed] [Google Scholar]
- 11.Manning WG, Fryback DG, Weinstein MC. Reflecting uncertainty in cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. pp. 247–275. [Google Scholar]
- 12.Bloomfield DJ, Krahn MD, Neogi T, et al. Economic evaluation of chemotherapy with mitoxantrone plus prednisone for symptomatic hormone-resistant prostate cancer: based on a Canadian randomized trial with palliative end points. J Clin Oncol. 1998;16:2272–9. doi: 10.1200/JCO.1998.16.6.2272. [DOI] [PubMed] [Google Scholar]
- 13.Novello S, Kielhorn A, Stynes G, et al. Cost-minimisation analysis comparing gemcitabine/cisplatin, paclitaxel/carboplatin and vinorelbine/cisplatin in the treatment of advanced non-small cell lung cancer in Italy. Lung Cancer. 2005;48:379–87. doi: 10.1016/j.lungcan.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Mauriac L, Welnicka-Jaskiewicz M, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study. J Clin Oncol. 2003;21:843–50. doi: 10.1200/JCO.2003.05.135. [DOI] [PubMed] [Google Scholar]
- 15.van Agthoven M, Segeren CM, Buijt I, et al. A cost-utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma; a prospective randomised phase III study. Eur J Cancer. 2004;40:1159–69. doi: 10.1016/j.ejca.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Verma S, Ilersich AL. Population-based pharmacoeconomic model for adopting capecitabine/docetaxel combination treatment for anthracycline-pretreated metastatic breast cancer. Oncologist. 2003;8:232–40. doi: 10.1634/theoncologist.8-3-232. [DOI] [PubMed] [Google Scholar]
- 17.Sacristan JA, Kennedy-Martin T, Rosell R, et al. Economic evaluation in a randomized phase III clinical trial comparing gemcitabine/cisplatin and etoposide/cisplatin in non-small cell lung cancer. Lung Cancer. 2000;28:97–107. doi: 10.1016/s0169-5002(99)00120-8. [DOI] [PubMed] [Google Scholar]
- 18.Kosmidis P, Mylonakis N, Nicolaides C, et al. Paclitaxel plus carboplatin versus gemcitabine plus paclitaxel in advanced non-small-cell lung cancer: a phase III randomized trial. J Clin Oncol. 2002;20:3578–85. doi: 10.1200/JCO.2002.12.112. [DOI] [PubMed] [Google Scholar]
- 19.Bennett CL, Stinson TJ, Tallman MS, et al. Economic analysis of a randomized placebo-controlled phase III study of granulocyte macrophage colony stimulating factor in adult patients (> 55 to 70 years of age) with acute myelogenous leukemia. Eastern Cooperative Oncology Group (E1490) Ann Oncol. 1999;10:177–82. doi: 10.1023/a:1008318930947. [DOI] [PubMed] [Google Scholar]
- 20.Konski A, Sherman E, Krahn M, et al. Economic analysis of a phase III clinical trial evaluating the addition of total androgen suppression to radiation versus radiation alone for locally advanced prostate cancer (Radiation Therapy Oncology Group protocol 86-10) Int J Radiat Oncol Biol Phys. 2005;63:788–94. doi: 10.1016/j.ijrobp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Konski A, Watkins-Bruner D, Brereton H, et al. Long-term hormone therapy and radiation is cost-effective for patients with locally advanced prostate carcinoma. Cancer. 2006;106:51–7. doi: 10.1002/cncr.21575. [DOI] [PubMed] [Google Scholar]
- 22.Mauskopf J, Schulman K, Bell L, et al. A strategy for collecting pharmacoeconomic data during phase II/III clinical trials. Pharmacoeconomics. 1996;9:264–77. doi: 10.2165/00019053-199609030-00007. [DOI] [PubMed] [Google Scholar]
- 23.O'Sullivan AK, Thompson D, Drummond MF. Collection of health-economic data alongside clinical trials: is there a future for piggyback evaluations? Value Health. 2005;8:67–79. doi: 10.1111/j.1524-4733.2005.03065.x. [DOI] [PubMed] [Google Scholar]
- 24.Bennett CL, Westerman IL. Economic analysis during phase III clinical trials: who, what, when, where, and why? Oncology (Williston Park) 1995;9:169–75. [PubMed] [Google Scholar]
- 25.Hillner BE. Potential evaluation of the incremental cost-effectiveness of paclitaxel in advanced non-small-cell lung cancer (Eastern Cooperative Oncology Group 5592) J Natl Cancer Inst Monogr. 1995:65–7. [PubMed] [Google Scholar]
- 26.Drummond MF, Davies L. Economic analysis alongside clinical trials. Revisiting the methodological issues. Int J Technol Assess Health Care. 1991;7:561–73. doi: 10.1017/s0266462300007121. [DOI] [PubMed] [Google Scholar]
- 27.Willan AR, O'Brien BJ. Sample size and power issues in estimating incremental cost-effectiveness ratios from clinical trials data. Health Econ. 1999;8:203–11. doi: 10.1002/(sici)1099-1050(199905)8:3<203::aid-hec413>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]