Abstract
Background
To compare effects of multiple injections of small divided doses of intravitreal bevacizumab vs. a single injection using a retinal neovascular model in rabbits.
Methods
We assigned 12 pigmented rabbits to four groups of three each. All groups received an intravitreal injection of vascular endothelial growth factor (VEGF, 10 μg) on the first day. Group A received an intravitreal loading dose of bevacizumab (0.5 mg) on day 3, followed by five smaller injections (0.15 mg), one every third day. Those in group B and C received a single intravitreal injection of bevacizumab (1.25 mg) on day 3, followed by five injections of sham, one every third day in group C. Group D received only intravitreal VEGF. Follow-up examinations were performed for 26 days.
Results
In groups A and B, vascular changes associated with VEGF injection decreased substantially in the first 3 days and continued to show gradual regression during each follow-up interval. No statistically significant differences were found between the changes of mean retinal thicknesses in groups A and B at both areas. In group C, the extra sham injections did not lead to any further vascular changes. The mean retinal thickness in group B and C did not have a statistically significant difference during the follow-up period. In group D, vascular changes resolved more gradually than in other groups. The difference in retinal thickness between group D and the other groups was statistically significant on day 6 in both groups (medullary and inferior part; p=0.0003) and in medullary wing on day 12 (p=0.03).
Conclusions
Frequent smaller doses of bevacizumab can control VEGF-induced vascular changes as well as the currently utilized model of single monthly large injections. Dividing of currently used single injection (1.25 mg) of bevacizumab to multiple small doses can control VEGF-induced vascular changes as well as one large injection.
Keywords: Bevacizumab, Intravitreal, Multiple, Neovascular, Rabbit, Vascular endothelial growth factor, VEGF
1. Introduction
Angiogenesis-associated eye diseases are among the most common worldwide causes of blindness[1]. Age-related macular degeneration and proliferative diabetic retinopathy (PDR) are the most prevalent in this group [2, 3]. Increased VEGF levels in the vitreous of eyes with diabetic retinopathy, retinal vascular occlusions, and subretinal neovascularization have implicated VEGF as the major stimulus for intraocular neovascularization in these conditions [4, 5]. VEGF, as it is classically known, refers to VEGF-A, which binds to VEGF receptors (VEGFR)-1 and -2; which, in turn, are primarily involved in angiogenesis [1, 6, 7]. VEGF-A, in turn, has several isoforms, of which VEGF165 is one of the most abundant [8]. VEGF165 was used in our study to develop a retinal neovascular model in rabbits. Amrei et al have demonstrated the effects of intravitreal bevacizumab on VEGF-induced retinal neovascular model in rabbits [35]. Alikacem et al. have utilized this model to quantify retinal permeability before and after neovascularization [47]. Bevacizumab is a recombinant humanized monoclonal immunoglobulin G1 (IgG1) antibody. Bevacizumab is specific to human VEGF and blocks all of its isoforms, including VEGF165 [8, 9]. Recently, intravitreal bevacizumab has been used extensively for the treatment of macular edema and neovascularization in diseases such as diabetic retinopathy, age-related macular degeneration, retinal vein occlusion, neovascular glaucoma, etc [10-20]. It can be administered through one or more intravitreal injections monthly, or as required. However, long term control of chronic ocular pathologies such as age-related macular degeneration and diabetic retinopathy requires multiple injections at different time [21, 22]. It is inconvenient for patients to get an intravitreal injection every few weeks. Potential local side effects such as endophthalmitis, intravitreal hemorrhage, retinal detachment, and cataract present a challenge for the management of such patients [23-25]. Additionally, potential systemic adverse effects are a significant concern, as anti-VEGF drugs can enter into circulation after intravitreal injection. This can cause hypertension, non-ocular hemorrhage such as life-threatening hemorrhagic stroke [26, 27], increased cardiovascular events, proteinuria, impairment of wound healing and impairment of collateral vessels, which is particularly worrying in ischemic vasculopathies such as in diabetic patients. It can also inhibit skeletal muscle regeneration and growth of bone [28]. It is our hypothesis and that of others that ocular pathologies can be better controlled by giving frequent smaller doses of bevacizumab administered based on the prevalent clinical condition in the eye [29]. We believe this will lead to fewer cumulative adverse effects of long-term treatment. Our group has developed an ocular drug delivery device that will allow frequent administration of small doses into the eye [30]. Frequent dosing can potentially circumvent the challenges mentioned above and can maintain the efficacy of bevacizumab therapy, while reducing the potential complications associated with repeated intravitreal injections. To determine whether small frequent doses have the same effect as a single large injection, we designed our study to mimic a model for intravitreal dispensation of bevacizumab through a drug delivery device. Using this retinal neovascular model in rabbits, we compared the effects of multiple, small, divided doses of intravitreal bevacizumab with the effects of a single large injection.
2. Material and Methods
2.1 Animals
All animal experiments were conducted in accordance with the principles of laboratory animal care (NIH publication No. 85-23, revised1985), the OPRR public health service policy on the human care and use of laboratory animals (revised 1986), the U.S animal welfare act and ARVO statement for the use of animals in ophthalmic and vision research and were approved by the Institutional animal care and use committee (IACUC) of the University of Southern California. Twelve pigmented rabbits, weighing between 2 and 3 kg, assigned to four groups (3 rabbits per group). Only the right eye of each animal was used. All animals received 10 μg of intravitreal VEGF injection diluted in 0.1 ml of balanced salt solution (BSS) (recombinant human VEGF165; Sigma-Aldrich, St. Louis, MO) on the first day of the study. Group A received six injections of bevacizumab (Avastin; Genentech Inc, San Francisco, CA). The first bevacizumab injection was 0.5 mg diluted in 0.1 ml of BSS (loading dose). The rationale for the first loading dose was to saturate all of the VEGF sites to ensure that subsequent lower dosages could be used to inhibit the presence of VEGF competitively. The five remaining injections (0.15 mg in the same volume) were administered one every third day after the first injection. Every third day was the least time interval allowed by Institutional Animal Care and Use Committee (IACUC) to perform intravitreal injection. The total amount of bevacizumab given via multiple injections in group A is equal to one single injection in group B. Group B received a single intravitreal injection of bevacizumab 3 days after the first injection of VEGF. The amount of bevacizumab injected was 1.25 mg in 0.1 ml. Group C received a single intravitreal injection of 1.25 mg bevacizumab in 0.1 ml 3 days after the first injection of VEGF followed by five injections of sham, one every third day (0.1 ml of BSS), to control for any effects secondary to the mechanical injury and inflammatory reaction from frequent intravitreal injections. Since any intraocular intervention such as an injection can potentially induce an inflammatory reaction with release of inflammatory mediators and vascular permeability factors such as TNF, IL and VEGF [31, 32], we injected the same number of shams in Group C (5 sham injections after the single injection of bevacizumab similar to 5 injections of low dose bevacizumab after injection of the loading dose in group A) as a control to demonstrate whether the inflammatory response or the volume and consistency changes in vitreous after frequent injections [33], could cause further vascular and retinal thickness changes. Group D received only intravitreal VEGF on the first day of study and was used as a control. The follow-up period was 26 days for all four groups.
2.2 Intravitreal injections
The rabbits were initially anesthetized with a subcutaneous injection of a mixture of ketamine hydrochloride (25 mg/kg) (Fort Dodge Animal Health, Fort Dodge, IA) and xylazine hydrochloride (6mg/kg) (IVX Animal Health, Inc., St. Joseph, MO). The pupils were dilated with a topical application of phenylephrine hydrochloride 2.5% (Akorn, Inc., Buffalo Grove, IL) and tropicamide 0.5% (Akorn, Inc) eye drops. The intravitreal injection was given according to the established method, taking all aseptic precautions [34]. Intravitreal injections were performed after instillation of 1-2 drops of 5% povidine iodine (Butler Animal Health Supply, Dublin, OH) in the conjunctival cul-de-sac. Topical tetracaine (Bausch & Lomb Inc, Tampa, FL) was applied for additional topical anesthesia. A lid speculum was used to keep the eyelids open, and the procedure was performed under direct visualization using a Zeiss operating microscope (Carl Zeiss Surgical Inc., Thornewood, NY) and a corneal contact lens. A 30-gauge needle attached to a 1 ml syringe was introduced transconjunctivally into the vitreous cavity in the superior temporal quadrant, 1.5 mm behind the limbus. At the end of the procedure, topical antibiotic ointment (Neo-Poly-Bac; Bausch & Lomb) was instilled in the fornix.
2.3 Follow-up examinations
Examinations included evaluation of the anterior segment by slit-lamp biomicroscopy, indirect ophthalmoscopy, color fundus photography, fluorescein angiography (FA) and optical coherence tomography (OCT). Before each follow-up examination, the rabbits were anesthetized and their pupils were dilated as described above. Baseline examinations were performed before the first intravitreal injection, and follow-up examinations were performed at days 3, 6, 12, 18, and 26 after the first injection of VEGF.
2.4 Color Fundus Photography and Fluorescein Angiography
A digital fundus camera system (Model FF450IR; Carl Zeiss, Jena, Germany) was used for both color photography and fluorescein angiography. Sequential fundus photographs were taken immediately after fluorescein injection [35]. Late photographs were taken at 3 and 5 minutes.
2.5 Optical Coherence Tomography
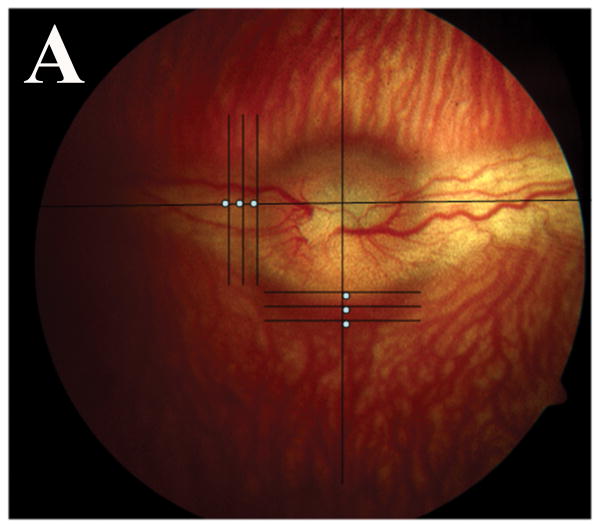
Using an OCT system (Stratus system; Ver.4.0 software; Carl Zeiss Meditec, Inc., Dublin, CA), 5 mm radial line scans were taken. Retinal thickness was measured at three different points in the medullary wings and below the optic disc; measurements were taken at the inferior and temporal margins of the optic disc and two other points, which were located 100 μm apart temporally or inferiorly, respectively (Fig. 1). The mean retinal thicknesses were calculated in each of these points, along with the total retinal thickness. The changes of mean retinal thickness in each area were compared to its counterpart respectively in each group.
Fig. 1.
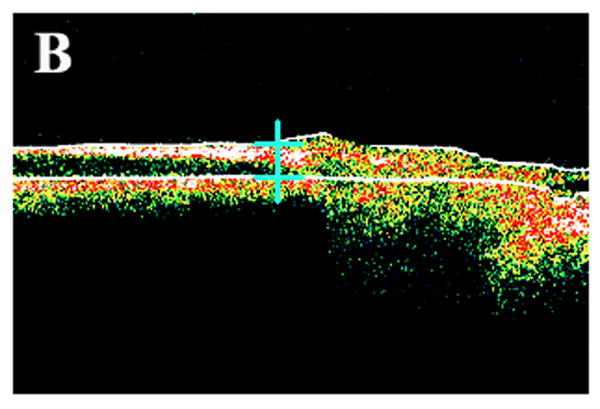
Color fundus photograph and ocular coherence tomography (OCT) of a representative rabbit. (A) Different locations at which retinal thickness was measured by OCT. Note 3 white spots in the temporal medullary wing and below the optic disc, which are located 100 μm apart. (B) Sample retinal thickness measurement below the optic disc by OCT in a representative rabbit. The scan is along a vertical line at the middle inferior edge of the optic disc.
2.6 Histopathology
At the end of the last follow-up examination in each group, the rabbits were euthanized by intracardiac injection of 2 ml of pentobarbital (Beuthanasia-D; Schering Plough Animal Health, Omaha, NE). The eyes were enucleated and immersed in Davidson's fixative solution for 16 to 24 hours and then dehydrated in a series of graded alcohol solutions over the next 24 to 48 hours before paraffin embedding [36]. Sections 5 μm thick were obtained with a microtome, stained with hematoxylin and eosin and examined by light microscopy.
3 Results
Examination by slit-lamp biomicroscopy and funduscopy did not demonstrate any increased inflammation between the groups regardless of the number of intravitreal injections or the dose of bevacizumab.
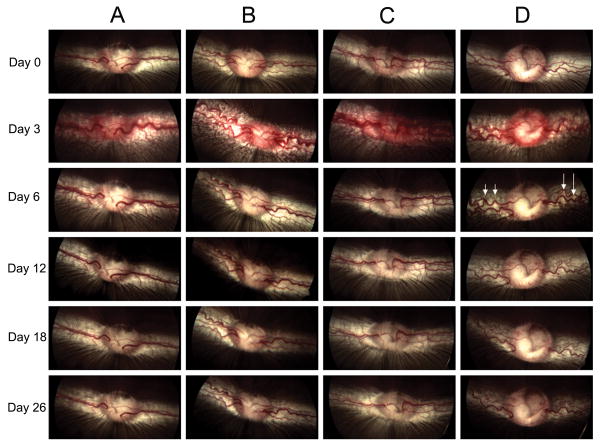
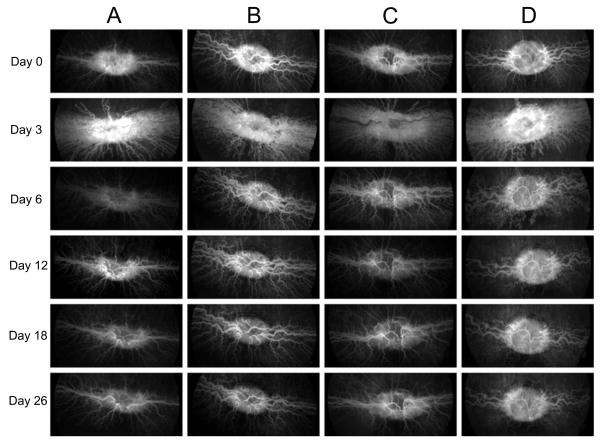
The rabbits were followed on days 3, 6, 12, 18, and 26 after the first injection of VEGF. Color fundus photographs and fluorescein angiographs from all groups were evaluated by two masked retina specialists. Intravitreal injection of VEGF resulted in disc hyperemia, vascular dilatation, tortuosity, and fluorescein leakage at the optic disc and medullary wings at day 3 in all groups (Figs. 2, 3).
Fig. 2.
Color fundus images in groups A to D. Images in each column show changes in fundus appearance of a representative rabbit in each group over time. All groups received VEGF injection on day 0. A: multiple small bevacizumab injections from day 3 to day 18 (one every third day). B: single bevacizumab injection on day 3. C: single bevacizumab injection on day 3 and five sham injections from day 6 to day 18 (one every third day). D: only VEGF injection on day 0. Arrows show marked vascular dilatation and tortuosity at day 6 in group D versus other groups. These rabbits continued to show vascular dilatation and tortuosity until the last day of study.
Fig. 3.
Fluorescein angiography (FA) images in groups A to D. Each column shows changes in FA of a representative rabbit in each group over time. All groups received VEGF injection on day 0. A: multiple small bevacizumab injections from day 3 to day 18 (one every third day). B: single bevacizumab injection on day 3. C: single bevacizumab injection on day 3 and five sham injections from day 6 to day 18 (one every third day). D: only VEGF injection on day 0.
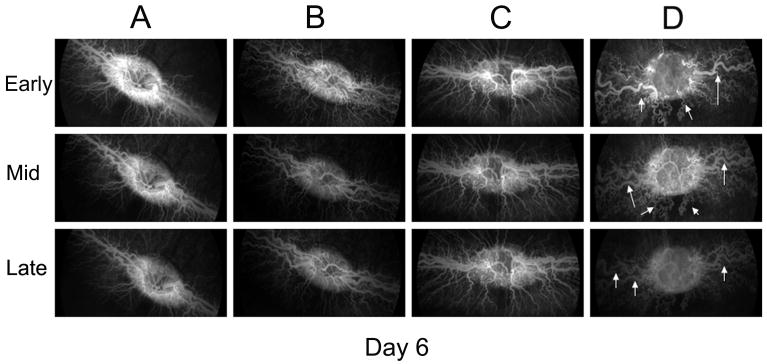
In group A and B, disc hyperemia, vascular dilatation, tortuosity and fluorescein leakage decreased significantly through the first 3 days after the first loading dose or a single injection of bevacizumab, respectively (Figs. 2, 3). Later, vascular dilatation and tortuosity decreased gradually in both groups. In group C, the extra sham injections did not cause any further changes in disc hyperemia, vascular dilation, tortuosity and fluorescein leakage. Vascular dilatation and tortuosity were noted to decrease comparably in all three groups (A, B & C) after bevacizumab injection. In group D, disc vascular changes decreased more gradually than in other groups (Figs. 2, 3). There is a considerable difference between dilatation and tortuosity of vessels in this group in comparison to other groups at day 6 in the early, middle and late phases of fluorescein angiography and color fundus photography (Figs. 2, 4). The animals in this group continued to show vascular dilatation and tortuosity until the last day of study (Figs. 2, 3).
Fig. 4.
Fluorescein angiogram in groups A to D at day 6. Each column shows changes in early, middle and late FA of a representative rabbit in each group on day 6. Arrows show marked vascular dilatation and tortuosity at day 6 in group D versus other groups.
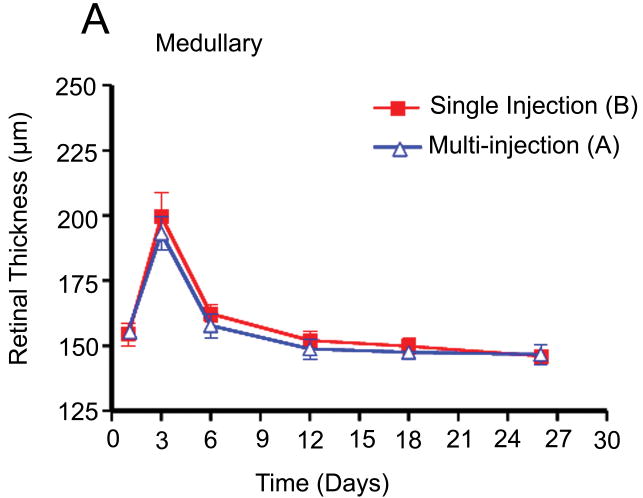
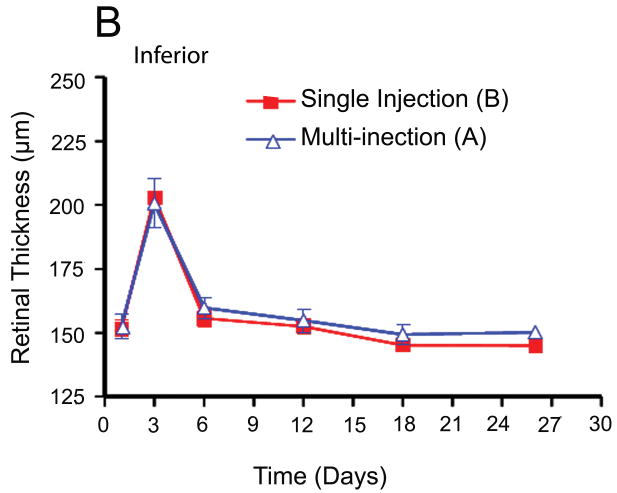
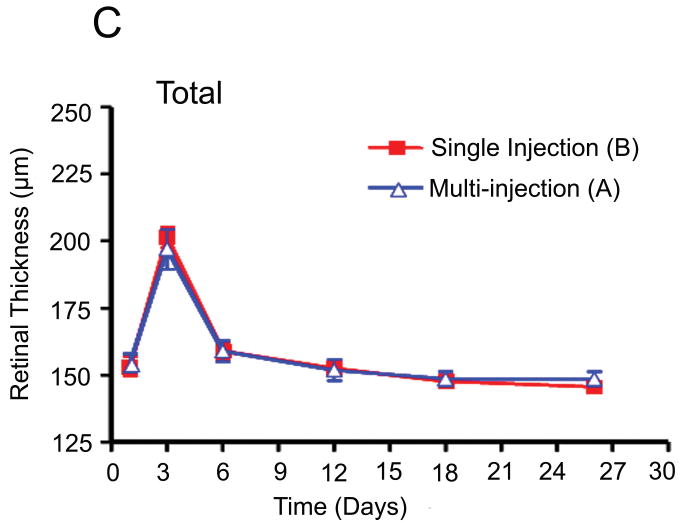
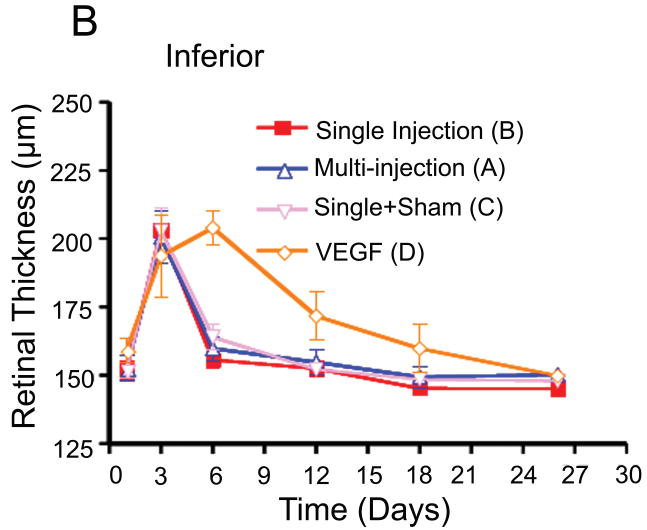
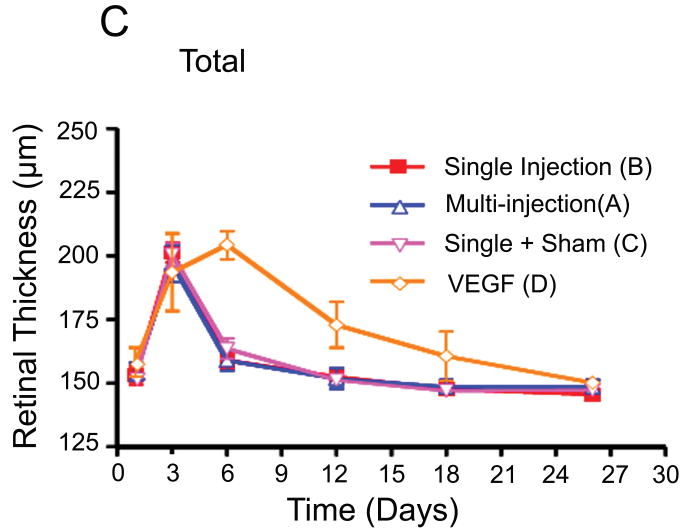
Retinal thickness was measured at three points in the temporal medullary wings and below the optic disc to evaluate retinal edema and retinal thickness changes in areas with different vascular densities. The mean retinal thickness was calculated in each area and compared in the different groups using an independent sample t-test and analysis of variance (ANOVA). There were no statistically significant differences between the mean retinal thickness changes in groups A and B at the medullary wings and below the optic disc, respectively (p =0.46; p =0.56) during the follow-up period. In other words, both groups had a similar mean retinal thickness increase at day 3 after VEGF injection and a similar decrease in retinal edema and mean retinal thicknesses in both areas on the days following the single or multiple injections of bevacizumab (Fig. 5).
Fig. 5.
Comparison of retinal thickness changes between single-dose and multi-dose bevacizumab groups (A & B). (A) Medullary wing area. (B) Inferior (below to optic disc) area. (C) Total retina (A+B).
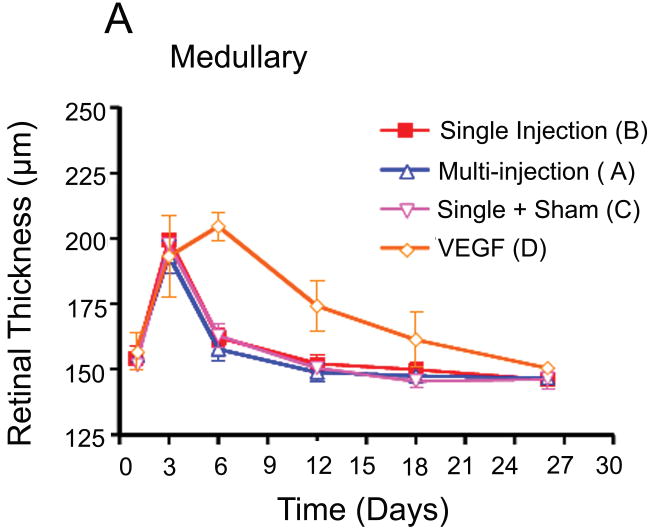
The mean retinal thicknesses in groups B and C did not have statistically significant differences on days 3, 6, 12, 18, and 26 after VEGF injection in both medullary wings and below the optic disc, respectively (p =0.44;p =0.34). These findings demonstrate that extra sham injections did not cause significant breakthrough in the blood retinal barrier and inflammatory reactions that cause retinal edema. In group D, retinal thickness increased during the first week after VEGF injections in both areas, before gradually decreasing until the end of the study period (Fig. 6).
Fig. 6.
Comparison of retinal thickness changes between group D (VEGF only injection) and other groups. (A) Medullary wing area. (B) Inferior (below to optic disc) area. (C)Total retina (A+B).
The differences in retinal thickness between group D and the other groups were statistically significant on day 6 in both areas (medullary and inferior part; p = 0.0003) and in the medullary wings on day 12 (p =0.03) (Fig. 6). After day 12, the retinal thickness did not differ significantly in the three groups (A, B & C). In group D, retinal thicknesses decreased slowly in both areas after day 12.
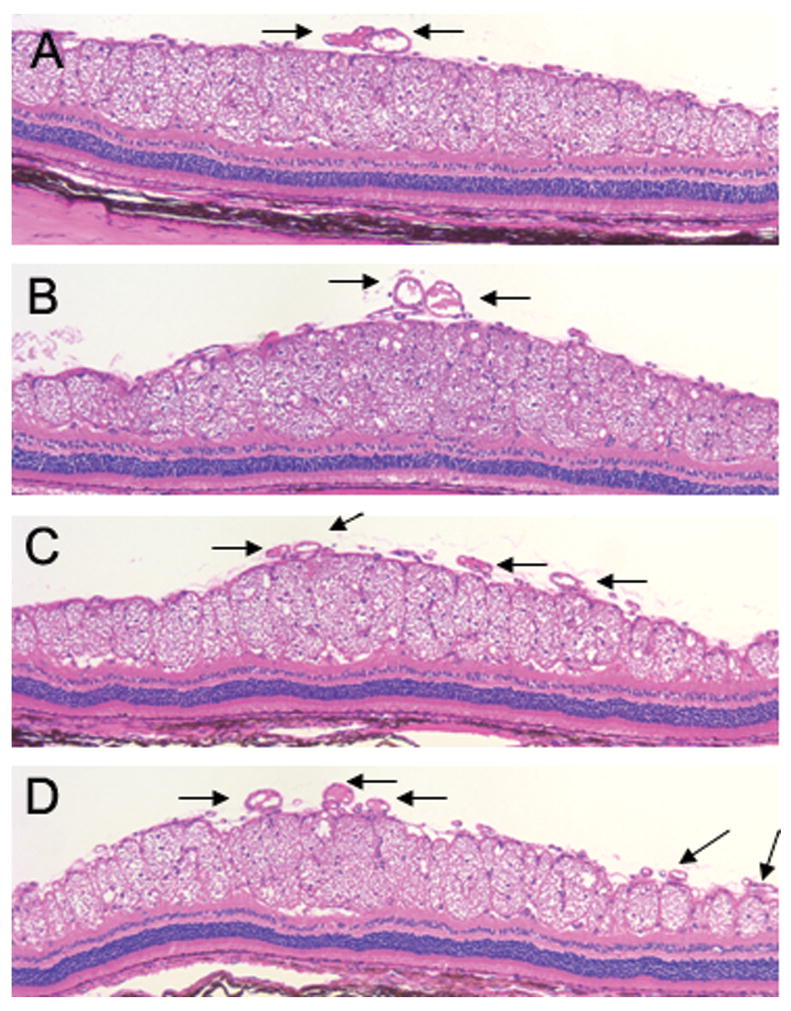
Cross sections of the medullary wings near the optic disc in the histology slides were evaluated by a masked pathologist. Groups A, B, and C had few vessels in cross sections in comparison with group D as control, which had more crowded vessels (Fig. 7).
Fig. 7.
Hematoxylin and eosin (magnification 10×) staining of cross sections of the medullary wings near the optic disc, comparing all four groups. Arrows show more crowded vessels in group D in comparison with other groups.
4. Discussion
The same pattern of vascular changes and no significant statistical difference in retinal thicknesses in groups A and B demonstrated that frequent smaller doses of bevacizumab can control VEGF-induced vascular changes as well as the currently utilized model of single monthly large injections. Vascular changes and retinal thickness in both single and multiple injection groups (A & B) decreased significantly within 3 days of the first loading dose or a single large injection of bevacizumab, respectively, and continued to decrease gradually thereafter. These findings are supported by the findings in another study in the primate eye, which demonstrated the most significant reduction of choriocapillaris endothelial cell fenestrations on day 4 after 1.25 mg injection of bevacizumab [37]. Fenestrations increased again from days 7 to 14 but were still significantly lower than in the control. In groups B and C, retinal thickness decreased considerably during the first 3 days after bevacizumab injection (days 3 to 6) followed by gradual reduction until the study period. This can be described by monoexponential decline of intravitreal bevacizumab with a half life of 4.32 days in rabbits and following concentrations of >10 μg/ml of bevacizumab in the vitreous humor for 30 days [38]. Intravitreal bevacizumab has shorter half life in rabbits compared to human (4.32 vs. 6.7 days). The vitreous volume in animal models such as rabbits and monkeys is about 1.5 ml. The difference here is not significant since the intravitreal volumes in rabbits and human is approximately one third. Smaller vitreous volume may imply a higher concentration of bevacizumab with shorter time interval required for distribution and elimination, which can explain the differences in the estimated pharmacokinetic parameters [38-40]. The molecular concentration of the drugs can be determined by Molar Concentration (μM) = drug dosage (gram/liter)/Volume (liter) × Molecular weight (Dalton).
Zhu et al. measured free VEGF-A levels in the vitreous of neovascular AMD patients, which ranged from 0.2 to 33.9 pg/ml, and showed a negative correlation with the bevacizumab concentration. This confirmed the in vivo binding affinity of Bevacizumab to VEGF-A [40]. Since it is not known how much of the injected bevacizumab could bind to VEGF and its clearance is faster in rabbits, we applied high loading dose of Bevacizumab (0.5 mg ∼ 3.35 μM) in group A to ensure providing excessive molar concentration (molar concentration of Bevacizumab is 3.35 μM vs. 2.6 μM in VEGF) to saturate all VEGF sites (10μg ∼ 2.6 μM). Later on, the subsequent lower dose of Bevacizumab (0.15 mg ∼ 1.00 μM) was injected every third day to inhibit the presence of VEGF competitively (competitive inhibition).
Bevacizumab has been administered intravitreally in VEGF-mediated diseases such as choroidal neovascularization, central retinal vein occlusion, and PDR, with encouraging results [10-20]. With the increasing use of bevacizumab for VEGF-mediated retinal diseases in patients, its tissue distribution and clearance have been demonstrated after 1.25 mg intravitreal injection in a rabbit model [38]. Several safety studies have reported the lack of toxicity of intravitreal bevacizumab in rabbits [41-44]. Although previous studies demonstrated normal retinal function and structure after bevacizumab injection by electrophysiology and light microscopy, respectively, some toxicity effects were detected by electron microscopy, specially in doses of 1.25 mg and higher [45, 46]. This suspicion of toxicity in higher doses does not contradict our hypothesis and rationale of the study, as we intend to dispense very small daily doses of bevacizumab through our drug delivery device. Several investigators have created retinal neovascularization in rabbits using sustained-release pellets loaded with VEGF, with or without basic fibroblast growth factor (bFGF) [47-49]. In our study, we used a single intravitreal injection of VEGF to generate our retinal neovascular model. The VEGF dose of 10 μg was chosen because it was consistent with a study showing that 10 μg VEGF causes neovascular membrane in rabbits at week one [35]. Some investigators have speculated that the leakage persisted and a well-developed neovascular membrane was visible on color fundus photography and fluorescein angiography 1 week after VEGF injection in rabbits. There was no leakage or neovascular membrane in this group at week 2 [35]. Based on this study we tried to inject the multiple doses of bevacizumab in almost 2 weeks, although we knew that a single injection of VEGF may not be an ideal model in our study. Since it is not possible to mimic the amount of VEGF that is produced in the disease model, the use of intermittent dosing is adequate because there is no continuous production of VEGF. The proof of concept was to evaluate whether lower doses spread over the same period can have the similar effect. The intermittent dosing used to mimic continuous infusion model since the dosing frequency is shorter than the half life.
In group D, disc hyperemia, vascular dilatation, tortuosity and retinal edema increased during the first week after the VEGF injection. This can explain the statistically significant differences in retinal thicknesses between group D and the other groups in both areas on day 6 (medullary and inferior part; p = 0.0003). After the first week, vascular changes decreased gradually; however, the difference in retinal thickness remained statistically significant between group D and other groups in medullary wings until day 12. In the other groups, injection of bevacizumab on day 3 (loading or large dose in group A or (B&C) respectively), and additional small doses in group A explained the statistically significant differences of retinal thicknesses in the medullary wing between the control group (group D) and other groups through the second week (day 12). fThe differences of retinal thicknesses between group D and other groups were insignificant after day 12 because of the gradual regression of the neovascular membrane and decrease of retinal edema in group D in comparison with other groups with almost the same retinal thicknesses after day 12.
Bevacizumab has been found in small amounts in the fellow uninjected eye (0.35 ng/ml at 1 day to 11.17 ng/ml at 4 weeks) after 1.25 mg intravitreal injection in one eye [38]. Several patients with PDR underwent intravitreal injections of lower doses of bevacizumab (6.2, 12.5, 62, 125, 625 μg) after it was reported that some patients with bilateral PDR experienced regression of neovascularization in both eyes when injected with 1.25 mg of bevacizumab in only one eye [29]. Biologic effects were noted at all doses, but the durability of the effect was unknown. In our study, we were not only establishing that intermittent dosing is as efficacious as single dosing, but also support their findings as a smaller dose of bevacizumab (0.5mg) was effective in reducing VEGF-induced vascular changes as well as a large single injection (1.25 mg) for a short time after injection. However, it appears that additional smaller doses would be required over time for continued effectiveness on the retinal vasculature. Further studies with various small doses in a larger group and over a longer period of follow-up are required to find out the possible lowest continuous dose with comparable results and duration of effect as single injection.
Since the actual required dose of bevacizumab in some patients could be less than the current dosage (1.25 mg) [29], the use of frequent smaller doses can help us to start with initial smaller dose at the first time and continue only in those cases, who require more treatment with the same or different doses. This can also, prevent from unnecessary amount of bevacizumab that would increase systemic and cumulative adverse side effects in long term therapy [25].
Small daily doses can be dispensed through a drug delivery device that our group has developed [30]. It provides a potential model for the release of small daily doses of bevacizumab into the vitreous to control chronic VEGF-induced vascular changes over time. Delivery of small daily doses through a drug delivery device will increase patient comfort. It promises controlled and constant drug availability with fewer potential complications of intravitreal injections such as intraocular pressure elevation, cataract formation, retinal detachment, vitreous hemorrhage, and endophthalmitis [23-25].
In conclusion, despite the shortcomings of this study (small number of animals in each group; angiogenesis model with only a single injection of VEGF; use of 0.5 mg of bevacizumab as minimum loading dose which may far exceed the actual required dose; absence of analysis of vitreous for measurement of free VEGF or Bevacizumab, and limited follow-up) the study outcome demonstrates that dividing of current utilized single injection (1.25 mg) of bevacizumab to multiple smaller doses has the same effect in a VEGF-induced neovascular model as a single large injection. Thus, it is probable that a novel, refillable drug delivery device could provide a way to dispense small daily doses of bevacizumab into the vitreous to control VEGF-induced pathologies with fewer potential side effects. Such a drug delivery device would allow for long-term treatment of chronic, VEGF-induced ocular disease without the burden of repeated intravitreal injections. Frequent (or even daily) dispensation of bevacizumab may offer many advantages over existing delivery methods, such as an increase in drug bioavailability, constant and sustained drug release, achievement of elevated local concentrations of drugs, less ocular and cumulative systemic side effects, and reduced frequency of intraocular injections. These advantages can increase the comfort level, acceptance, and compliance in patients and reduce the complications associated with intraocular injections. Furthermore, on a purely economic sense, decreasing the frequency and dose of injection will likely reduce the total cost for treatment to each patient. Further studies with different small doses in larger groups with a longer follow-up are required to study the chronic effect.
Acknowledgments
This study was supported by Biomimetic Micro-Electronic Systems-Engineering Research Center, National Science Foundation EEC-0310723 and also in part by NIH core grant EY03040 and an unrestricted grant by Research to Prevent Blindness.
Footnotes
Disclosure: None of the authors have any proprietary interest in the work presented. All the authors have full control of all primary data and they agree to allow Graefes Archive for Clinical and Experimental Ophthalmology to review their data upon request.
References
- 1.Rahimi N. Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials. Exp Eye Res. 2006;83:1005–1016. doi: 10.1016/j.exer.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Kempen JH, O'Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, Taylor HR, Hamman RF. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 5.Wells JA, Murthy R, Chibber R, Nunn A, Molinatti PA, Kohner EM, Gregor ZJ. Levels of vascular endothelial growth factor are elevated in the vitreous of patients with subretinal neovascularisation. Br J Ophthalmol. 1996;80:363–366. doi: 10.1136/bjo.80.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25:111–118. doi: 10.1097/00006982-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 8.Bhisitkul RB. Vascular endothelial growth factor biology: clinical implications for ocular treatments. Br J Ophthalmol. 2006;90:1542–1547. doi: 10.1136/bjo.2006.098426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuh G, Wu P, Liang WC, Ultsch M, Lee CV, Moffat B, Wiesmann C. Structure-function studies of two synthetic anti-vascular endothelial growth factor Fabs and comparison with the Avastin Fab. J Biol Chem. 2006;625:6281–6631. doi: 10.1074/jbc.M507783200. [DOI] [PubMed] [Google Scholar]
- 10.Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:352–354. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Cordero Coma M, Sobrin L, Onal S, Christen W, Foster CS. Intravitreal bevacizumab for treatment of uveitic macular edema. Ophthalmology. 2007;114:1574–1579. e1571. doi: 10.1016/j.ophtha.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (Avastin) injection. Retina. 2006;26:354–356. doi: 10.1097/00006982-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Finger PT. Anti-VEGF bevacizumab (Avastin) for radiation optic neuropathy. Am J Ophthalmol. 2007;143:335–338. doi: 10.1016/j.ajo.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R, Gandorfer A, Ulbig M, Kampik A. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 15.Jorge R, Costa RA, Calucci D, Scott IU. Intravitreal bevacizumab (Avastin) associated with the regression of subretinal neovascularization in idiopathic juxtafoveolar retinal telangiectasis. Graefes Arch Clin Exp Ophthalmol. 2007;245:1045–1048. doi: 10.1007/s00417-006-0468-2. [DOI] [PubMed] [Google Scholar]
- 16.Mason JO, 3rd, Albert MA, Jr, Vail R. Intravitreal bevacizumab (Avastin) for refractory pseudophakic cystoid macular edema. Retina. 2006;26:356–357. doi: 10.1097/00006982-200603000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336–339. [PubMed] [Google Scholar]
- 18.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–335. [PubMed] [Google Scholar]
- 19.Siqueira RC, Costa RA, Scott IU, Cintra LP, Jorge R. Intravitreal bevacizumab (Avastin) injection associated with regression of retinal neovascularization caused by sickle cell retinopathy. Acta Ophthalmol Scand. 2006;84:834–835. doi: 10.1111/j.1600-0420.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- 20.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–278. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Algvere PV, Steen B, Seregard S, Kvanta A. A prospective study on intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration of different durations. Acta Ophthalmol. 2007 doi: 10.1111/j.1600-0420.2007.01113.x. DOI AOS1113 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, Berrocal MH, Farah ME, Maia M, Roca JA, Rodriguez FJ. Twelve-month safety of intravitreal injections of bevacizumab (Avastin(R)): results of the Pan-American Collaborative Retina Study Group (PACORES) Graefes Arch Clin Exp Ophthalmol. 2008;246:81–87. doi: 10.1007/s00417-007-0660-z. [DOI] [PubMed] [Google Scholar]
- 23.Arevalo JF, Maia M, Flynn HW, Jr, Saravia M, Avery RL, Wu L, Eid Farah M, Pieramici DJ, Berrocal MH, Sanchez JG. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:213–216. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- 24.Meyer CH, Mennel S, Eter N. Incidence of endophthalmitis after intravitreal Avastin injection with and without postoperative topical antibiotic application. Ophthalmologe. 2007;104:952–957. doi: 10.1007/s00347-007-1634-6. [DOI] [PubMed] [Google Scholar]
- 25.Shima C, Sakaguchi H, Gomi F, Kamei M, Ikuno Y, Oshima Y, Sawa M, Tsujikawa M, Kusaka S, Tano Y. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmol. 2008;86:372–376. doi: 10.1111/j.1600-0420.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ophthalmology. Vol. 116. 2009. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study; pp. 57–65.pp. e55 [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 28.Simo R, Hernandez C. Intravitreous anti-VEGF for diabetic retinopathy: hopes and fears for a new therapeutic strategy. Diabetologia. 2008;51:1574–1580. doi: 10.1007/s00125-008-0989-9. [DOI] [PubMed] [Google Scholar]
- 29.Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695, e1691–1615. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 30.Li PY, Shih J, Lo R, Saati S, Agrawal R, Humayun MS, Tai YC, Meng E. An electrochemical intraocular drug delivery device. Sensors and Actuators, A: Physical. 2008;143:41–48. [Google Scholar]
- 31.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19:442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 32.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Maurice DM. Flow of water between aqueous and vitreous compartments in the rabbit eye. Am J Physiol. 1987;252:F104–108. doi: 10.1152/ajprenal.1987.252.1.F104. [DOI] [PubMed] [Google Scholar]
- 34.Aiello LP, Brucker AJ, Chang S, Cunningham ET, Jr, D'Amico DJ, Flynn HW, Jr, Grillone LR, Hutcherson S, Liebmann JM, O'Brien TP, Scott IU, Spaide RF, Ta C, Trese MT. Evolving guidelines for intravitreous injections. Retina. 2004;24:S3–19. doi: 10.1097/00006982-200410001-00002. [DOI] [PubMed] [Google Scholar]
- 35.Ameri H, Chader GJ, Kim JG, Sadda SR, Rao NA, Humayun MS. The effects of intravitreous bevacizumab on retinal neovascular membrane and normal capillaries in rabbits. Invest Ophthalmol Vis Sci. 2007;48:5708–5715. doi: 10.1167/iovs.07-0731. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal RN, He S, Spee C, Cui JZ, Ryan SJ, Hinton DR. In vivo models of proliferative vitreoretinopathy. Nat Protoc. 2007;2:67–77. doi: 10.1038/nprot.2007.4. [DOI] [PubMed] [Google Scholar]
- 37.Peters S, Heiduschka P, Julien S, Ziemssen F, Fietz H, Bartz-Schmidt KU, Schraermeyer U. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007;143:995–1002. doi: 10.1016/j.ajo.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114:855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Mintz-Hittner HA, Kuffel RR., Jr Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008;28:831–838. doi: 10.1097/IAE.0b013e318177f934. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Q, Ziemssen F, Henke-Fahle S, Tatar O, Szurman P, Aisenbrey S, Schneiderhan-Marra N, Xu X, Grisanti S. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology. 2008;115:1750–1755. 1755, e1751. doi: 10.1016/j.ophtha.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Bakri SJ, Pulido JS, Mukherjee P, Marler RJ, Mukhopadhyay D. Absence of histologic retinal toxicity of intravitreal nanogold in a rabbit model. Retina. 2008;28:147–149. doi: 10.1097/IAE.0b013e3180dc9360. [DOI] [PubMed] [Google Scholar]
- 42.Feiner L, Barr EE, Shui YB, Holekamp NM, Brantley MA., Jr Safety of intravitreal injection of bevacizumab in rabbit eyes. Retina. 2006;26:882–888. doi: 10.1097/01.iae.0000230717.85319.f5. [DOI] [PubMed] [Google Scholar]
- 43.Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26:257–261. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP, Johnson PT, Fisher SK, Perlman I, Loewenstein A. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin) Retina. 2006;26:262–269. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Avci B, Avci R, Inan UU, Kaderli B. Comparative Evaluation of Apoptotic Activity in Photoreceptor Cells After Intravitreal Injection of Bevacizumab and Pegaptanib Sodium in Rabbits. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-2871. DOI iovs.08-2871 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Inan UU, Avci B, Kusbeci T, Kaderli B, Avci R, Temel SG. Preclinical safety evaluation of intravitreal injection of full-length humanized vascular endothelial growth factor antibody in rabbit eyes. Invest Ophthalmol Vis Sci. 2007;48:1773–1781. doi: 10.1167/iovs.06-0828. [DOI] [PubMed] [Google Scholar]
- 47.Alikacem N, Yoshizawa T, Nelson KD, Wilson CA. Quantitative MR imaging study of intravitreal sustained release of VEGF in rabbits. Invest Ophthalmol Vis Sci. 2000;41:1561–1569. [PubMed] [Google Scholar]
- 48.Ozaki H, Hayashi H, Vinores SA, Moromizato Y, Campochiaro PA, Oshima K. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res. 1997;64:505–517. doi: 10.1006/exer.1996.0239. [DOI] [PubMed] [Google Scholar]
- 49.Wong CG, Rich KA, Liaw LH, Hsu HT, Berns MW. Intravitreal VEGF and bFGF produce florid retinal neovascularization and hemorrhage in the rabbit. Curr Eye Res. 2001;22:140–147. doi: 10.1076/ceyr.22.2.140.5528. [DOI] [PubMed] [Google Scholar]