Abstract
Purpose
We sought to assess the impact of prostate size on operative difficulty as measured by estimated blood loss (EBL), operating room (OR) time and positive surgical margins (SM) and secondarily to assess the impact on biochemical recurrence (BCR) and the functional outcomes of potency and continence at one year following radical prostatectomy (RP) as well as postoperative bladder neck contracture (BNC).
Materials and Methods
During 1998–2007, 3067 men underwent RP by one of 5 dedicated prostate surgeons with no neoadjuvant or adjuvant therapy. Pathologic specimen weight was used as a measure of prostate size. Cox proportional hazards and logistic regression analysis was used to study the association between specimen weight and biochemical recurrence (BCR) and SM status, respectively, controlling for adverse pathologic features. Continence and potency were analyzed controlling for age, nerve-sparing status, and surgical approach.
Results
With increasing prostate size, there was increased EBL (p=0.013) and OR time (p=0.004) and a decrease in positive SM (84/632 (14%) for ≤40g, 99/862 (12%) for 41–50g, 78/842 (10%) for 51–65g, 68/731 (10%) for >65g (p<0.001)). BCR was observed in 186 of 2882 patients followed postoperatively and was not significantly associated with specimen weight (p=0.3). Complete continence was observed in 1165/1422 (82%) and potency in 425/827 (51%) at one year. Specimen weight was not significantly associated with potency (p=0.8), continence (p=0.08) or BNC (p=0.22).
Conclusions
Prostate size does not appear to affect biochemical recurrence or one-year functional results. However, EBL and OR time increased with larger prostate size and positive SM are more often observed in smaller glands.
Keywords: organ volume, outcome assessment (health care), penile erection, prostatectomy, urinary incontinence
INTRODUCTION
Localized prostate cancer (PC) can be successfully treated with RP or radiation therapy. For a majority of patients, the choice of treatment is based on personal preference. However, some considerations such as age, preoperative urinary symptoms, potency, stage and grade of the cancer may make one modality more suitable than another. Radical retropubic prostatectomy (RRP) has undergone considerable evolution over the last twenty years, and is still considered the standard to which all subsequent treatments must be compared. The likelihood of patients achieving the “trifecta” of cancer cure and full recovery of both continence and erectile function (EF) approaches 60% at 2 years.1 More recently laparoscopic prostatectomy (LP) and robotic-assisted laparoscopic prostatectomy (RLP) have been developed. While no large-scale randomized comparison of LP, RLP, and RRP has been conducted, it is generally thought that similar results can be achieved with each modality.
Each patient presents a unique set of characteristics that influence the technique of RP regardless of the approach. Prostate volume is an important consideration for surgery, particularly when patients have very large or very small glands. Smaller glands have been associated with high grade disease, seminal vesicle invasion (SVI), and positive surgical margins (SM).2–5 Furthermore, smaller prostate size has been shown to be independently associated with BCR after controlling for these adverse pathologic features. In part, this may be because there is not as much tissue for the cancer to occupy before invading the capsule, or it may reflect lower levels of testosterone, predisposing to more aggressive disease6 and increased risk for BCR.2, 7 Conversely, larger glands have been associated with lower grade and lower volume tumors, fewer positive SM, and a lower likelihood of PSA-relapse, potentially as a result of lead-time bias.2, 3, 8
Whether prostate size has an impact on functional results is more controversial. Smaller glands may allow for better visualization of the neurovascular bundle (NVB) and facilitate the apical and sphincteric dissection which could improve continence outcomes.9 Additionally, Myers has postulated that the intrafascial plane of dissection between the capsule of the prostate and the NVB is more easily developed in glands with BPH.10 However, increasing prostate size has been associated with more blood loss in RRP11 and longer operative time in LP.12, 13 Several RRP8, 11 and LP12, 14,15 series have shown no differences in postoperative continence and EF recovery according to prostate volume. However, data from the CaPSURE database as well as from a large robotic prostatectomy series indicates that there may be a modest reduction in the return of urinary continence as prostate volume increases.16,17
We hypothesize that prostate size has an impact on the overall difficulty of surgery and subsequent oncologic and functional outcomes including biochemical recurrence-free rates and recovery rates of both EF and urinary control, regardless of the surgical approach. The purpose of this study is to examine a large single center, multi-surgeon experience to determine the impact of prostate volume on surgical and clinicopathologic outcomes.
METHODS
After receiving Institutional Review Board approval, we queried our prospectively-collected RP database for all patients treated beginning July 1998 through April 2007 by one of five dedicated prostate cancer surgeons. We excluded all patients who received adjuvant or neoadjuvant chemotherapy, hormonal or radiotherapy or cavernous nerve grafting during RP. At each postoperative visit, outcomes regarding EF were evaluated by the treating physician through patient-reported questionnaires assessing for the quality and frequency of sexual activity and graded on a 5-point rigidity scale (see Table 1).18 EF recovery was recorded if a postoperative erectile rigidity score of 1 or 2 was achieved with or without the use of PDE-5 inhibitors. Patients with erectile rigidity scores of 3, 4 or 5 or those requiring the assistance of vacuum devices, intraurethral alprostadil, or injection therapy to achieve erections suitable for sexual activity were considered impotent. Patients were classified as continent if they were pad-free. Postoperative BNC at any time point in follow-up was recorded whether or not intervention was performed. BCR was defined as a postoperative PSA value ≥0.10 ng/ml with a confirmatory rise, or a postoperative PSA ≥0.10 ng/ml where salvage treatment (e.g. radiotherapy, hormonal manipulation, chemotherapy) was delivered.
Table 1.
5-point rigidity scale
| Potency Level | Level Definition |
|---|---|
| 1 | normal, full erections |
| 2 | full, but diminished erections routinely satisfactory for sexual activity |
| 3 | partial erections occasionally satisfactory for sexual activity |
| 4 | partial erections unsatisfactory for sexual activity |
| 5 | no erection |
Specimen weight was recorded at the time of pathological examination and included the prostate, seminal vesicles, and vasa deferentia stumps. Prostate volume was determined preoperatively with endorectal coil MRI using the ellipsoid approximation. Estimated blood loss (EBL) was defined by the anesthesiologist as the amount of blood in the suction canister plus saturated laparotomy pads minus irrigation. All surgical specimens were processed with step section and a positive margin defined as tumor cells at the inked margin.
Statistical methods
We hypothesized that the most difficult prostates to remove would be the very small and very large prostates, and therefore modeled the relationship between prostate size and our dependent variables using cubic splines with knots at the tertiles to relax linearity assumptions. In the case that we found no evidence of non-linearity, we fit the models with only a linear term for pathologic weight. All variables controlled for in multivariable analyses were pre-specified before any data analysis.
For the outcomes of operative time and EBL, we used multivariable linear regression and controlled for age, NVB status (none vs unilateral vs bilateral nerve sparing), and surgical approach (LP vs RRP). Since these outcomes are known to be surgeon dependent,19 we corrected for within-surgeon clustering in these analyses.
For the outcome of positive surgical margins, we used multivariable logistic regression, controlling for preoperative PSA, surgical approach, pathologic Gleason grade (≤6 vs 7 vs ≥8), extracapsular extension (ECE), SVI, lymph node involvement (LNI). We used Cox regression to assess the impact of specimen size on BCR, controlling for pretreatment PSA, pathologic Gleason score, ECE, SVI, LNI, SM status, and surgical approach. Logistic regression was used to test for association between prostate size and pathologic Gleason score, ECE, SVI, and LNI.
Postoperative continence and EF recovery data were not collected as regularly as BCR data, resulting in a large amount of interval censoring. To handle interval censoring associated with the postoperative follow-up for continence and EF recovery, we converted these time-to-event outcomes to a binary endpoint at 1 year following surgery. For the outcome of continence, patients who recovered continence ≤ 1 year following surgery were considered to be a success; patients who recovered continence > 1 year following surgery or who were incontinent > 1 year following surgery were considered to be a failure; all others were excluded. A similar algorithm was used for the outcome of EF recovery at 1 year following surgery. Multivariable logistic regression was used for the outcomes of EF recovery and continence 1 year following surgery as well as postoperative BNC, controlling for age and NVB status, and surgical approach.
Results were repeated using MRI volume in place of pathologic weight, to check if the results are impacted by the use of pathologic weight. The agreement between MRI volume and pathologic weight was assessed using the concordance correlation coefficient All statistical analyses were conducted using Stata 9.0 (Stata Corp., College Station, TX).
RESULTS
A total of 3539 consecutive patients underwent RP with or without pelvic lymphadenectomy (PLND) by one of five dedicated surgeons during the study period. A total of 472 patients were excluded including those receiving neoadjuvant (n=191) or adjuvant (n=58) chemotherapy, hormonal ablation or radiotherapy; those undergoing cavernous nerve grafting during RP (n=175); and those missing specimen weight (n=48). Therefore, the final cohort for analysis included 3067 patients. Thirty-two percent (n=982) of patients underwent LP, including 59 patients with RLP. Bilateral PLND was performed in 2774 of 3067 men (90.4%). There was no significant difference in the incidence of PLND being performed for different specimen weights: 565/632 (89.4%) for ≤40g, 770/862 (89.3%) for 41–50g, 769/842 (91.3%) for 51–65g and 670/731 (91.7%) for >65g (p=0.26). The characteristics of the cohort are listed in Table 2. Specimen weight ranged from 15 to 389g.
Table 2.
Patient characteristics for all patients (N=3067 unless otherwise noted).
| Median (IQR) or Frequency (Proportion) | Number without missing data | |
|---|---|---|
| Age at surgery (years) | 59 (55, 64) | 3064 |
| PSA (ng/ml) | 5.3 (4, 7.3) | 2995 |
| Operating Room Time (minutes) | 190 (180, 225) | 2185 |
| Estimated Blood Loss (cc) | 800 (350, 1500) | 2482 |
| Pathological Weight (grams) | 51 (42, 65) | |
| MRI volume (g) | 31 (24, 44) | 2295 |
| Clinical Stage ≥T2 | 391 (15%) | 2680 |
| Biopsy Gleason Grade | 2706 | |
| ≤6 | 1655 (54%) | |
| =7 | 895 (29%) | |
| ≥8 | 156 (5%) | |
| Positive surgical margins | 329 (11%) | 2981 |
| ECE | 761 (26%) | 2941 |
| SVI | 139 (5%) | 2980 |
| Lymph node status | ||
| Negative | 2459 (80%) | |
| Positive | 105 (3%) | |
| Not done | 503 (16%) | |
| Nerve Sparing | 2831 | |
| None | 317 (11%) | |
| Unilateral | 534 (19%) | |
| Bilateral | 1980 (70%) | |
| Received Open Surgery (vs. Lap) | 2085 (68%) | |
| Continent at 1 year following surgery* | 1165 (82%) | 1422 |
| EF recovery at 1 year following surgery** | 425 (51%) | 827 |
| Postoperative bladder neck contracture | 74 (2.4%) |
Excludes patients who were incontinent preoperatively or who were not followed postoperatively for continence
Excludes patients who were impotent preoperatively or who were not followed postoperatively for potency
EBL, Allogenic Blood Transfusion and Operative Room Time
The median EBL for patients receiving LP was 250cc (n=801; interquartile range (IQR) 200, 400cc), compared to 1200cc (n=1681; IQR 800, 1700cc) for patients receiving RRP. Allogenic blood transfusion was given in 365 of 3026 (12.1%) patients in whom transfusion data were available: 37/982 (3.8%) for LP compared to 328/2044 (16.0%) for RRP (p<0.001). On multivariable analysis, pathologic weight was significantly associated with EBL (p=0.013), allogenic blood transfusion (p<0.001) and operative time (p=0.004), such that patients with larger prostates had higher EBL, greater incidence of having an allogenic blood transfusion and longer operative times (Table 3). There was no association of specimen weight with the incidence of allogenic blood transfusion for LP: 10/218 (4.6%) for ≤40g, 6/302 (2.0%) for 41–50g, 10/251 (4.0%) for 51–65g, and 11/211 (5.2%) for >65g (p=0.23). However, there was an increasing incidence of allogenic blood transfusion with increasing specimen weight for RRP: 54/400 (13.5%) for ≤40g, 69/555 (12.4%) for 41–50g, 91/580 (15.7%) for 51–65g, and 114/509 (22.4%) for >65g (p<0.001).
Table 3.
Intraoperative and postoperative outcomes, stratified by specimen weight groups. Groupings were based roughly on the quartiles of specimen weight and are for illustrative purposes only.
| Specimen Weight (grams) | Adjusted analysis | |||||
|---|---|---|---|---|---|---|
| ≤40 N=632 | 41–50 N=862 | 51–65 N=842 | >65 N=731 | Number of patients included | P Value* | |
| Median estimated blood loss1 (IQR), cc | 750 (300, 1250) | 750 (300, 1300) | 900 (400, 1500) | 1000 (450, 1600) | 2362 | 0.013 |
| Allogenic blood transfusion1 | 64 (10.4%) | 75 (8.7%) | 101 (12.2%) | 125 (17.4%) | 2789 | <0.001 |
| Median operating room time1 (IQR), min | 180 (165, 220) | 190 (175, 225) | 195 (180, 230) | 199 (180, 240) | 2098 | 0.004 |
| Number of positive surgical margins2 (%) | 84 (14%) | 99 (12%) | 78 (10%) | 68 (10%) | 2526 | <0.001 |
| Number continent at 1 year post-op1 (%) | 249 (80%) | 323 (83%) | 319 (83%) | 274 (81%) | 1333 | 0.08 |
| Number potent at 1 year post-op1 (%) | 102 (52%) | 134 (55%) | 104 (47%) | 85 (50%) | 755 | 0.8 |
| Number with postoperative bladder neck contracture1 | 18 (2.8%) | 13 (1.5%) | 19 (2.3%) | 24 (3.3%) | 2169 | 0.22 |
| 2-year probability of freedom from biochemical recurrence3(95% confidence interval), % | 94 (92, 96) | 96 (95, 98) | 94 (92, 96) | 93 (91, 95) | 2393 | 0.3 |
IQR: interquartile range
Adjusted P-value when specimen weight is entered as continuous
P-value is with adjustment for age, nerve sparing, and surgical approach
P-value is with adjustment for PSA, pathologic Gleason grade, ECE, SVI, LNI, and surgical approach
P-value is with adjustment for PSA, pathologic Gleason grade, ECE, SVI, LNI, SMS, and surgical approach
Biochemical Recurrence
Postoperative follow up for BCR was available for 2882 patients (94%). In total, 186 patients experienced BCR, with a median follow up for recurrence-free patients of 2.2 years. On multivariable analysis, there was no significant association between specimen weight and BCR (p=0.3). On univariate analysis, we found that as specimen weight decreased, the incidence of specimen Gleason score>6 and ECE increased (p<0.001 and p=0.01, respectively), while the incidence of LNI decreased (p=0.007). These associations may be confounded by PSA, since patients with larger glands tend to have higher baseline PSA levels and recommendation for biopsy is based on PSA. When controlling for PSA, these associations remained, although the relationship with LNI was weakened (p=0.11). There was no significant association between specimen weight and SVI (p=0.9).
Margin Status
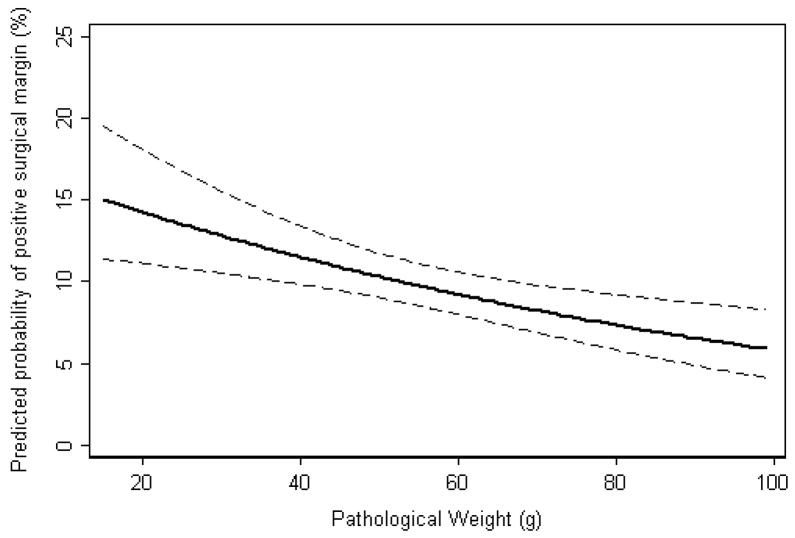
SM status was evaluated for 2981 patients (97%), of which 329 (11%) had a positive SM. Pathologic weight was significantly associated with SM status (p<0.001) on multivariable analysis (Table 3, Figure 1). It is plausible that the increased incidence in positive SM with smaller prostates is due to difficulty in pathologic evaluation of the prostate, and not surgical difficulty. We tested formally for this type of effect using an interaction analysis with the outcome of biochemical recurrence: if there are false positive SM in small glands, then the effect of positive SM on BCR will be smaller in these glands. With adjustment for PSA, stage, and grade, the interaction term was not statistically significant (p=0.4), suggesting that the lower higher incidence in positive SM was mainly explained by surgical difficulty.
Figure 1.
Predicted probability of positive surgical margins with increasing pathological weight, controlling for PSA, pathologic Gleason grade, ECE, SVI, LNI, and surgical approach (laparoscopic vs. open). Dashed lines are 95% confidence intervals.
Functional Outcomes
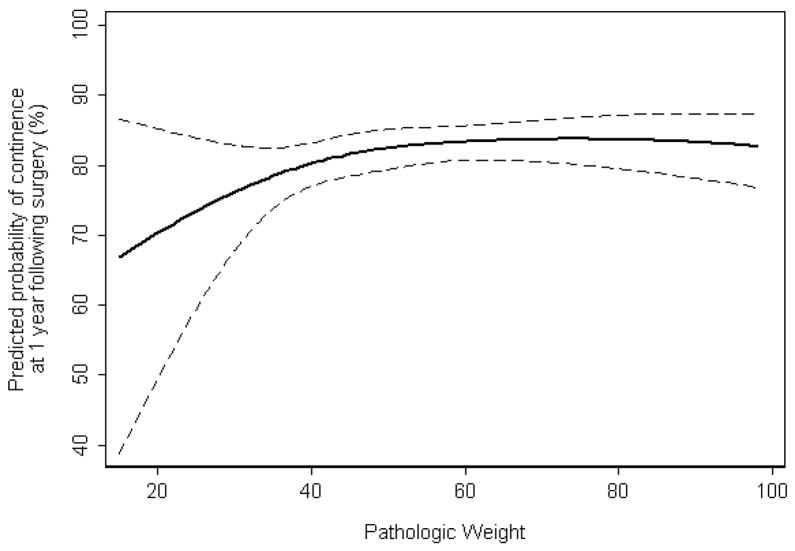
There were 1422 preoperatively continent patients who had sufficient follow-up for the analysis of postoperative continence. Of the 1422 included patients, 1165 (82%) were continent at 1 year following surgery. There was a small, but non-statistically significant association between pathologic weight and postoperative continence at 1 year (p=0.08) on multivariable analysis (Figure 2). On multivariate analysis, specimen weight was not significantly associated with the development of postoperative BNC (odds ratio 0.99, p=0.22). There were 827 preoperatively potent patients who had sufficient follow-up for the analysis of postoperative potency. Of the 827 included patients, 425 (51%) were potent at 1 year following surgery. The median age of postoperatively potent and impotent patients was 56 (IQR 51, 60) and 60 (IQR 55, 63), respectively. On multivariable analysis, pathologic weight was not significantly associated with EF recovery (p=0.8, table 3).
Figure 2.
Predicted probability of continence at 1 year following surgery, with increasing pathologic weight, controlling for age, nerve sparing (none, unilateral, bilateral), and surgical approach (laparoscopic vs. open). Dashed lines are 95% confidence intervals.
Type of Surgery
One-third of the patients in our cohort had a laparoscopic procedure (n=982, 32%). To formally test whether the association between prostate size and outcome varied depending on type of surgery, we performed interaction analyses for each outcome. We observed statistically significant interactions for the outcomes of surgical margin status, continence, blood loss, and operative time (all p<0.04). However, the interaction effects were very small except for the outcome of surgical margin status: the increased risk in positive surgical margins as a result of lower specimen weight was larger in patients receiving laparoscopic surgery. For example, the proportion of laparoscopic patients with a positive surgical margin was 14% for specimen weight <50g and 9% for >50 (absolute risk difference 5%, relative risk 1.6). In comparison, the proportion of open patients with a positive surgical margin was 12% for specimen weight <50g and 10% for >50g (absolute risk difference 2%, relative risk 1.2).
MRI Correlation
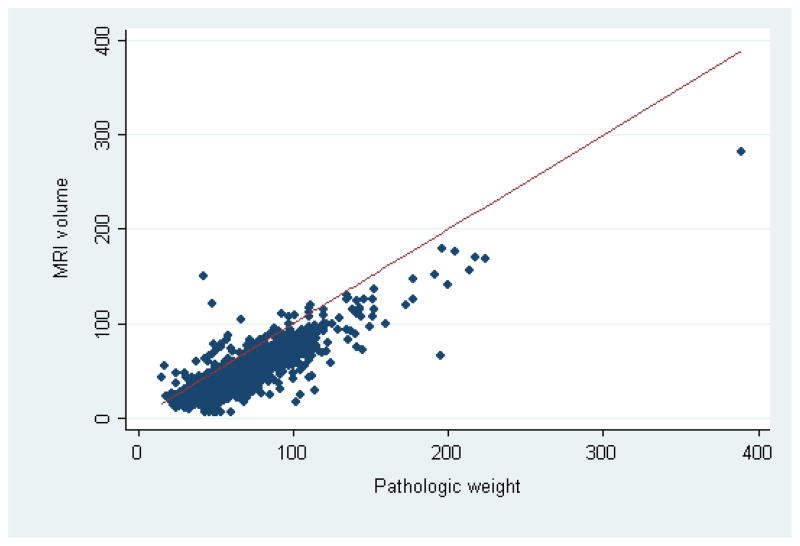
MRI volume was available for 2295 patients (75%). Specimen weight was highly correlated with MRI volume (concordance and Spearman correlation coefficients 0.61 and 0.78, respectively), but MRI volume tended to underestimate prostate size as measured by specimen weight (Figure 3). To check whether our results were affected by our use of specimen weight, we substituted MRI volume for pathologic weight in all models. None of the results were significantly changed (data not shown).
Figure 3.
Scatter plot of MRI volume by pathologic weight, with the line of equality. The concordance and Spearman correlation coefficients were 0.61 and 0.78, respectively.
DISCUSSION
While prostate size is an important consideration in treatment planning and execution of RP, the present study demonstrates that, at least for experienced prostate surgeons, prostate size does not seem to impact biochemical recurrence or functional outcomes, regardless of the operative approach. However, larger glands are associated with greater EBL, longer operative time, and higher allogenic transfusion rates, the latter primarily in patients undergoing open RRP. The longer operative time in patients with larger prostates is not because of a greater proportion of these patients receiving PLND in this series. Further, we have reaffirmed that smaller prostates are at higher risk for positive SM, even after accounting for tumor grade, stage and preoperative PSA.
Similar findings have been reported by others. One of the earlier comprehensive investigations of the impact of prostate volume on operative time, EBL, transfusion rates, positive SM rates as well as continence and potency was the study of Hsu et al.11 In that study, Hsu and associates reported a significant association of prostate volume with EBL (p=0.021) and allogenic transfusion (p=0.011), but not operative time (p=0.121), continence (p=0.227) or potency (p=0.900). A significant inverse relation between prostate volume and positive SM was identified (p=0.03). In a series of 802 consecutive patients undergoing LP, Levinson et al reported that on multivariate analysis controlling for patient age, BMI, performance of PLND, PSA, pathologic Gleason score and pathologic stage, increasing prostate size was associated with longer operative time, higher EBL, longer length of stay and more perioperative complications but no difference in blood transfusions.20 Smaller glands had a trend toward higher positive SM rates (p=0.07).20 In a large study of 1847 consecutive patients undergoing RLP, Link et al reported that patients with a prostate gland >70g had longer operative time, higher EBL, and longer hospital stay.17 No difference in blood transfusion rates was reported across the varying prostate sizes. Patients with smaller glands had a higher positive SM rate: 34.8% for <30g, 27.9% for 30–49g, 17.8% for 50–69g, and 21.2% for ≥70g (p<0.0001). These investigators reported lower 1-year continence in men with the largest glands: 93.1% for <30g, 93.6% for 30–49g, 92.8% for 50–69g, and 88.4% for ≥70g (p=0.001).17 Our results showing no association between prostate size and allogenic blood transfusion for LP patients are consistent with these LP20 and RLP17 series. The increased incidence of allogenic blood transfusion in RRP patients with larger prostates would argue that LP or RLP may be safer for patients with large prostate glands with respect to EBL and avoiding blood transfusion.
In the current study, we found prostate size was not significantly associated with BCR when controlling for PSA and pathologic stage and grade. Our results are consistent with the results of Link et al who found no association between prostate volume and 1-year BCR rates.17 Our results contrast with the findings of Freedland et al21, who reported an independent association with BCR, even after adjusting for these adverse pathologic factors. The reason for the discrepancy may be related to methodology. Only prostate-dedicated surgeons were included in our study, we had a larger cohort, and analyzed specimen weight as a continuous variable. However, had our follow up been longer, we likely would have observed more BCR events and the association may have been clearer. Patient selection may also account for some differences since the patients in this cohort are from a large tertiary care center whereas those in the Freedland study were from several VA/military hospitals.
Our results on potency and continence are similar to that presented in other series. Hsu et al studied 1024 men who underwent RRP, reporting no significant association of prostate volume with continence or potency when prostate volume was stratified into equal quartiles.11 Frota et al14 found no effect on potency and continence in 193 LP patients, categorizing specimen weight into 30g, 30–75g, and >75g. Foley and coworkers8 reported no difference in potency or continence in 450 RRP patients when they categorized specimen weight as <75g vs. >75g. In that series, they found inferior biochemical recurrence rates among those with prostates <75g but no difference in positive surgical margins. Zorn et al categorized prostate weight based on quartiles in 375 RLP patients and again found no association with inferior functional results.12 None of these studies provided controlled for age or nerve-sparing status. However, a recent study by Levinson et al on 729 consecutive patients undergoing LP reported no significant association of prostate size on postoperative urinary health related quality of life (HRQOL) as assessed by the EPIC questionnaire, controlling for age, preoperative urinary function and number of nerves preserved.15 In their study, patients with prostates >70g were older, had longer operative times and more blood loss. Despite worse baseline urinary function scores in men with larger prostates, all prostate size groups approached similar urinary HRQOL outcomes at all time points postoperatively. The absence of an association between prostate size and continence as well as between prostate size and BNC in our series is consistent with the results of Levinson et al15 showing no significant impact of prostate size on urinary HRQOL. Recent data from the CaPSURE database16 indicate a modest reduction in continence return among patients with larger prostates based on preoperative ultrasound volume controlling for age but not nerve-sparing status. A similar modest reduction in continence for larger prostates was reported by Link et al17 in a large robotic prostatectomy series but this study did only univariate analyses. While from figure 2 in our study, it would appear that small prostate size (<40g) is associated with worse urinary continence at 1 year, caution must be exercised to not misinterpret this figure. The 95% confidence intervals are wide for specimen weight <40g with no statistically significant difference in 1 year continence over varying prostate sizes. As presented in table 3, the percentage of continent men at 1 year for specimen weight ≤40g (80%) is similar to the 81–83% rates for the other three higher quartiles of specimen weight.
There are no established criteria for what constitutes small, large, and huge prostates. Additionally, there are different ways of measuring or estimating size which are not perfectly equal. Thus, studying prostate size as a categorical variable may lead to misleading results. To avoid these pitfalls, we analyzed size as a continuous variable and used two different measures of size, MRI volume and specimen weight. Further, increasing age is associated with higher specimen weights,8 and age is known to impact EF and continence recovery and should be accounted for.1, 22
Continence and EF recovery rates in this study are slightly lower than those reported elsewhere. The explanation likely is in the definition. We used the strictest definition of continence (no pads), which was reported by patients on a self-administered questionnaire in clinic. EF recovery is also evaluated by a self-administered questionnaire that has close association with the IIEF instrument.18
For this study, we used pathologic specimen weight as a measure of prostate size because it was available for all patients and it is an exact measurement, whereas MRI volume was not available for all patients and is only an estimate. Not surprisingly, there was a difference between the specimen weight and the volume calculated by MRI. This can be accounted for by several mechanisms. The specimen contains the prostate, overlying fat and dorsal venous complex, prostatic fascia as well as the vasa deferentia and seminal vesicles. MRI volume is calculated based on the assumption that the prostate gland is ellipsoid and is subject to interobserver variation in measuring the dimensions of the prostate. Thus while information on prostate size could be useful in preoperative counseling, it is important to bear in mind that prostate size as measured by transrectal ultrasound or MRI is only an estimation of the prostate size as transrectal ultrasound is known to underestimate prostate size by 10% or more in 80% of cases23 and MRI can underestimate prostate size by approximately 10% as shown in figure 3. Nevertheless, the correlation between MRI volume and specimen weight was high (Spearman correlation coefficient 0.78).
There are limitations to this study. Although the data was collected prospectively, it was not collected to address this particular question. Therefore, this study is retrospective in nature and subject to selection and referral bias. In addition, there were multiple surgeons contributing to this dataset accounting for possible heterogeneity in results.
CONCLUSIONS
Prostate size influences operative difficulty as measured by EBL and OR time, but the increased difficulty does not seem to translate into worse functional results. However, positive SM are observed more often in smaller glands. Further research should explore the degree to which the increased rate of positive SM can be explained by operative difficulty, as opposed to pathologic evaluation of smaller glands.
Acknowledgments
Supported by: The Sidney Kimmel Center for Prostate and Urologic Cancers and SPORE grant
ABBREVIATIONS AND ACRONYMS
- BCR
biochemical recurrence
- BNC
bladder neck contracture
- BPH
benign prostatic hypertrophy
- EBL
estimated blood loss
- ECE
extracapsular extension
- EF
erectile function
- IQR
interquartile range
- LNI
lymph node involvement
- LP
laparoscopic prostatectomy
- MRI
magnetic resonance imaging
- NVB
neurovascular bundle
- PC
prostate cancer
- PDE-5
5-phosphodiesterase inhibitor
- PLND
pelvic lymphadenectomy
- PSA
prostate specific antigen
- RLP
robotic-assisted laparoscopic prostatectomy
- RP
radical prostatectomy
- RRP
radical retropubic prostatectomy
- SM
surgical margin
- SVI
seminal vesicle invasion
References
- 1.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66:83. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 2.Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005;23:7546. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 3.Hong SK, Yu JH, Han BK, Chang IH, Jeong SJ, Byun SS, et al. Association of prostate size and tumor grade in Korean men with clinically localized prostate cancer. Urology. 2007;70:91. doi: 10.1016/j.urology.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Kassouf W, Nakanishi H, Ochiai A, Babaian KN, Troncoso P, Babaian RJ. Effect of prostate volume on tumor grade in patients undergoing radical prostatectomy in the era of extended prostatic biopsies. J Urol. 2007;178:111. doi: 10.1016/j.juro.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Bianco FJ, Jr, Mallah KN, Korets R, Hricak H, Scardino PT, Kattan MW. Prostate volume measured preoperatively predicts for organ-confined disease in men with clinically localized prostate cancer. Urology. 2007;69:343. doi: 10.1016/j.urology.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Massengill JC, Sun L, Moul JW, Wu H, McLeod DG, Amling C, et al. Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2003;169:1670. doi: 10.1097/01.ju.0000062674.43964.d0. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S, Yonese J, Kawakami S, Ohkubo Y, Tatokoro M, Komai Y, et al. Preoperative serum testosterone level as an independent predictor of treatment failure following radical prostatectomy. Eur Urol. 2007;52:696. doi: 10.1016/j.eururo.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 8.Foley CL, Bott SR, Thomas K, Parkinson MC, Kirby RS. A large prostate at radical retropubic prostatectomy does not adversely affect cancer control, continence or potency rates. BJU Int. 2003;92:370. doi: 10.1046/j.1464-410x.2003.04361.x. [DOI] [PubMed] [Google Scholar]
- 9.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 10.Myers RP. Practical surgical anatomy for radical prostatectomy. Urol Clin North Am. 2001;28:473. doi: 10.1016/s0094-0143(05)70156-7. [DOI] [PubMed] [Google Scholar]
- 11.Hsu EI, Hong EK, Lepor H. Influence of body weight and prostate volume on intraoperative, perioperative, and postoperative outcomes after radical retropubic prostatectomy. Urology. 2003;61:601. doi: 10.1016/s0090-4295(02)02422-6. [DOI] [PubMed] [Google Scholar]
- 12.Zorn KC, Orvieto MA, Mikhail AA, Gofrit ON, Lin S, Schaeffer AJ, et al. Effect of prostate weight on operative and postoperative outcomes of robotic-assisted laparoscopic prostatectomy. Urology. 2007;69:300. doi: 10.1016/j.urology.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Chang CM, Moon D, Gianduzzo TR, Eden CG. The impact of prostate size in laparoscopic radical prostatectomy. Eur Urol. 2005;48:285. doi: 10.1016/j.eururo.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Frota R, Turna B, Santos BM, Lin YC, Gill IS, Aron M. The effect of prostate weight on the outcomes of laparoscopic radical prostatectomy. BJU Int. 2008;101:589. doi: 10.1111/j.1464-410X.2007.07263.x. [DOI] [PubMed] [Google Scholar]
- 15.Levinson AW, Bagga HS, Pavlovich CP, Mettee LZ, Ward NT, Link RE, et al. The impact of prostate size on urinary quality of life indexes following laparoscopic radical prostatectomy. J Urol. 2008;179:1818. doi: 10.1016/j.juro.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 16.Konety BR, Sadetsky N, Carroll PR CaPSURE Investigator. Recovery of urinary continence following radical prostatectomy: the impact of prostate volume--analysis of data from the CaPSURE Database. J Urol. 2007;177:1423. doi: 10.1016/j.juro.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 17.Link BA, Nelson R, Josephson DY, Yoshida JS, Crocitto LE, Kawachi MH, et al. The impact of prostate gland weight in robot assisted laparoscopic radical prostatectomy. J Urol. 2008;180:928. doi: 10.1016/j.juro.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Secin FP, Koppie TM, Scardino PT, Eastham JA, Patel M, Bianco FJ, et al. Bilateral cavernous nerve interposition grafting during radical retropubic prostatectomy: Memorial Sloan-Kettering Cancer Center experience. J Urol. 2007;177:664. doi: 10.1016/j.juro.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Bianco FJ, Jr, Riedel ER, Begg CB, Kattan MW, Scardino PT. Variations among high volume surgeons in the rate of complications after radical prostatectomy: further evidence that technique matters. J Urol. 2005;173:2099. doi: 10.1097/01.ju.0000158163.21079.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levinson AW, Ward NT, Sulman A, Mettee LZ, Link RE, Su L-M, et al. The impact of prostate size on perioperative outcomes in a large laparoscopic radical prostatectomy series. J Endourol. 2009;23:147. doi: 10.1089/end.2008.0366. [DOI] [PubMed] [Google Scholar]
- 21.Freedland SJ, Aronson W, Presti JC, Jr, Kane CJ, Terris MK, Elashoff D, et al. Should a positive surgical margin following radical prostatectomy be pathological stage T2 or T3? Results from the SEARCH database. J Urol. 2003;169:2142. doi: 10.1097/01.ju.0000061760.23169.be. [DOI] [PubMed] [Google Scholar]
- 22.Eastham JA, Kattan MW, Rogers E, Goad JR, Ohori M, Boone TB, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156:1707. [PubMed] [Google Scholar]
- 23.Rodriguez E, Jr, Skarecky D, Narula N, Ahlering TE. Prostate Volume Estimation Using the Ellipsoid Formula Consistently Underestimates Actual Gland Size. J Urol. 2008;179:501. doi: 10.1016/j.juro.2007.09.083. [DOI] [PubMed] [Google Scholar]