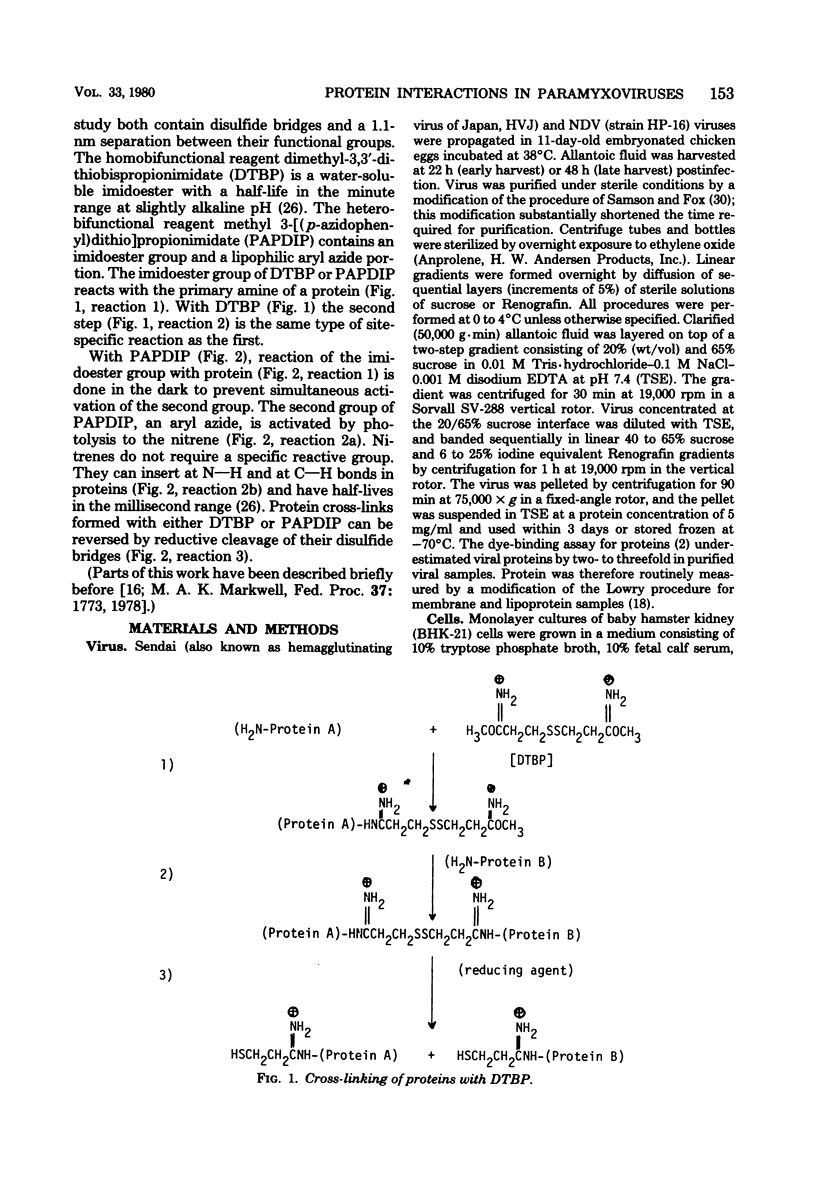
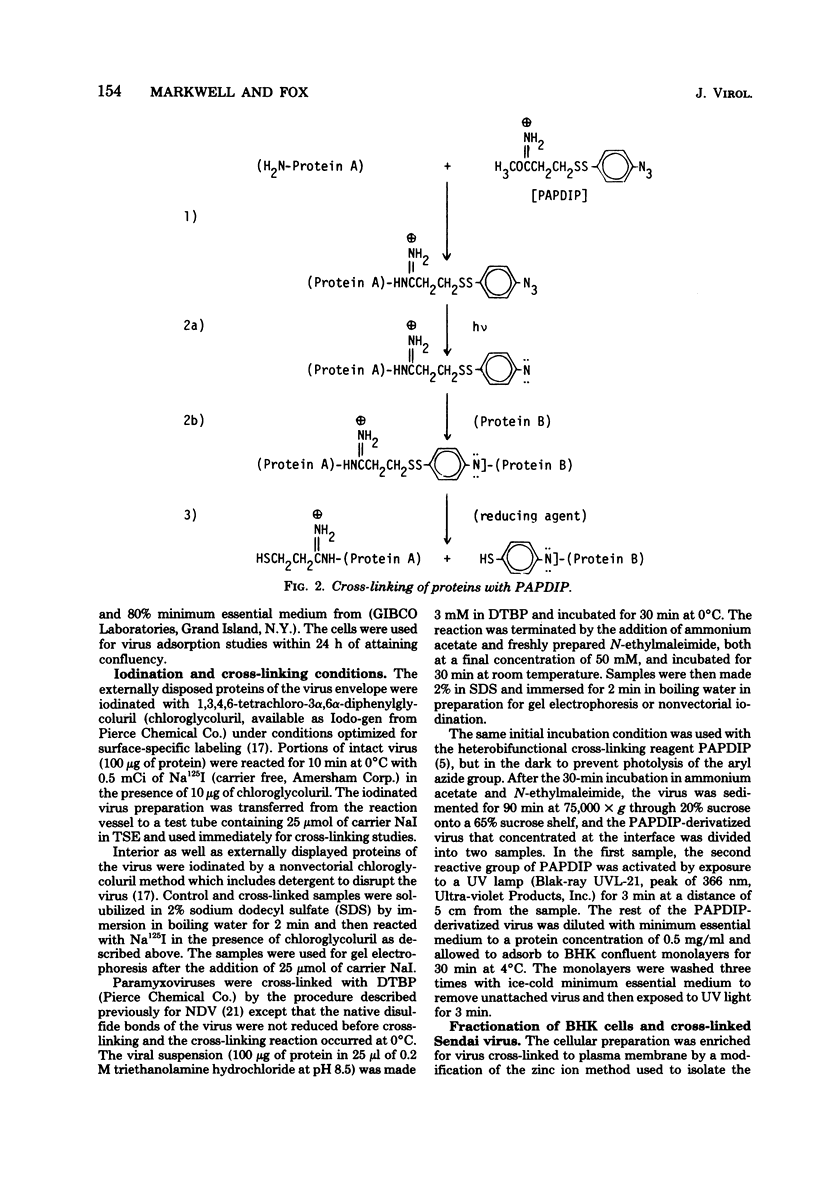
Abstract
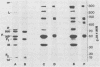
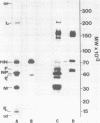
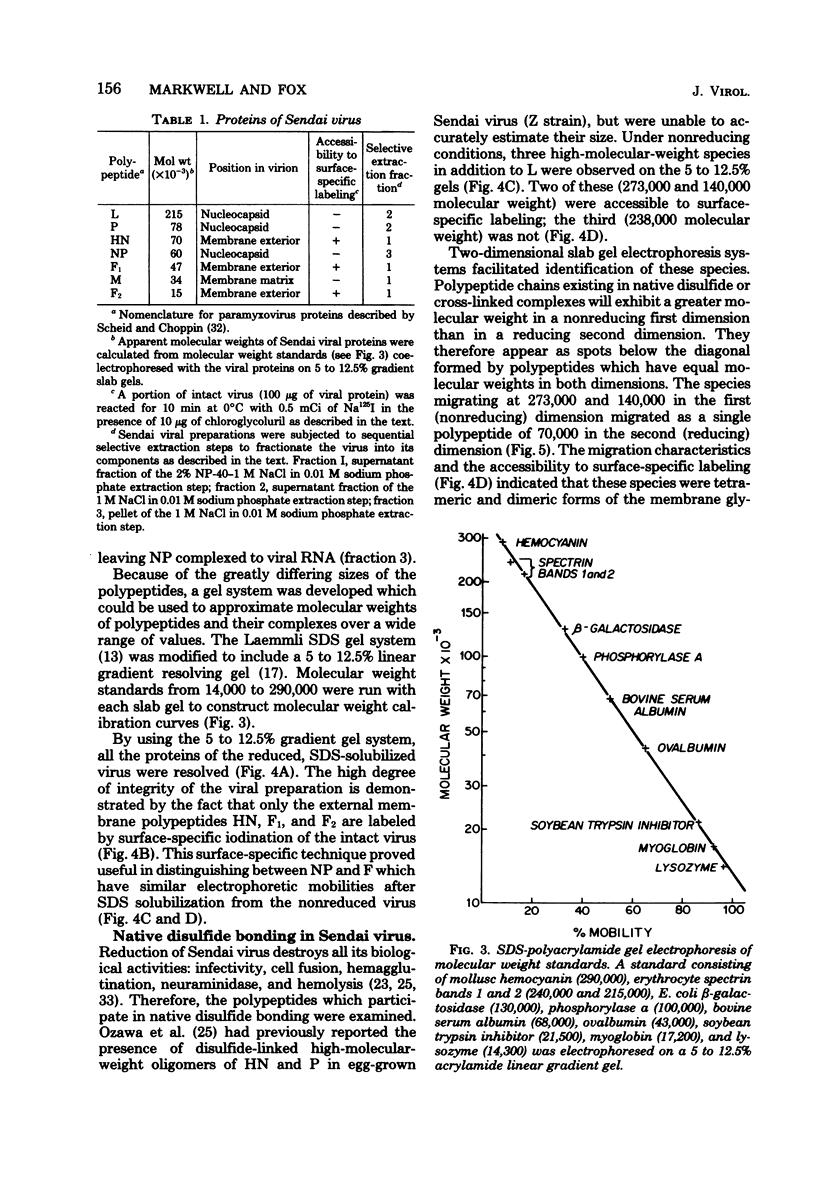
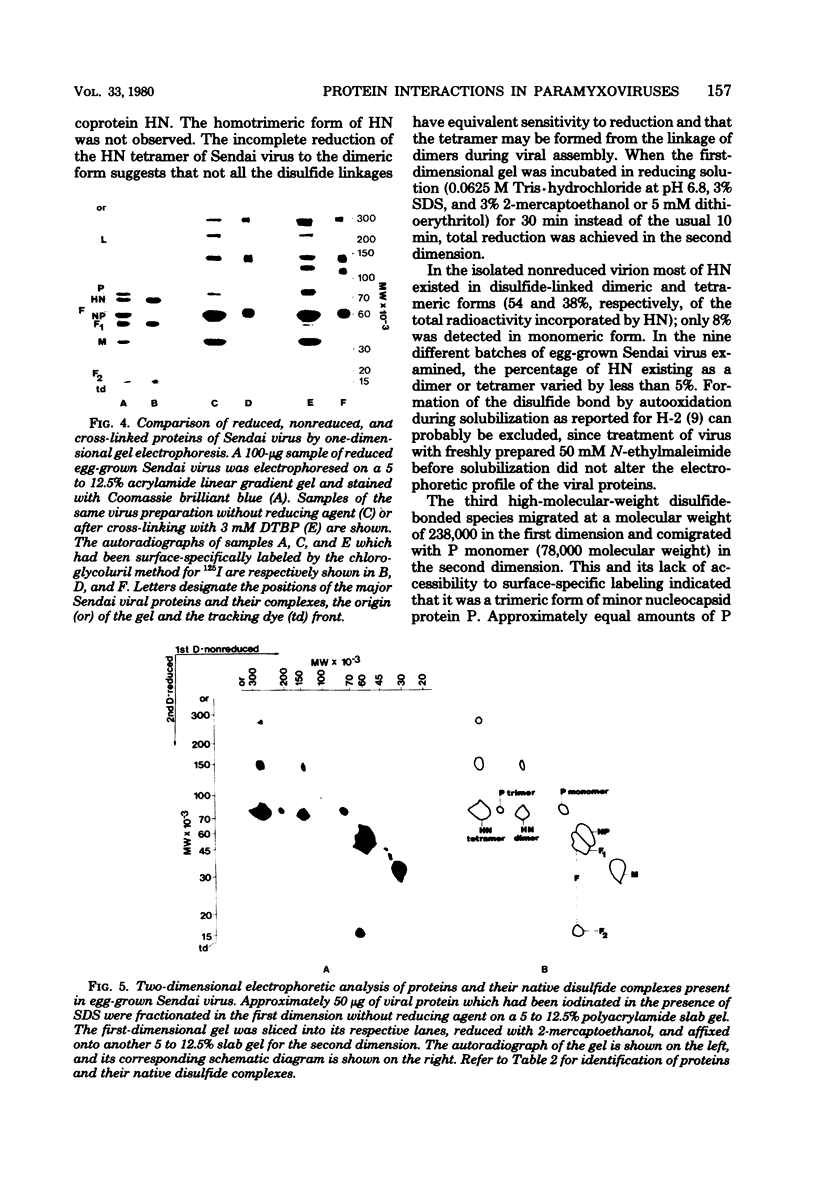
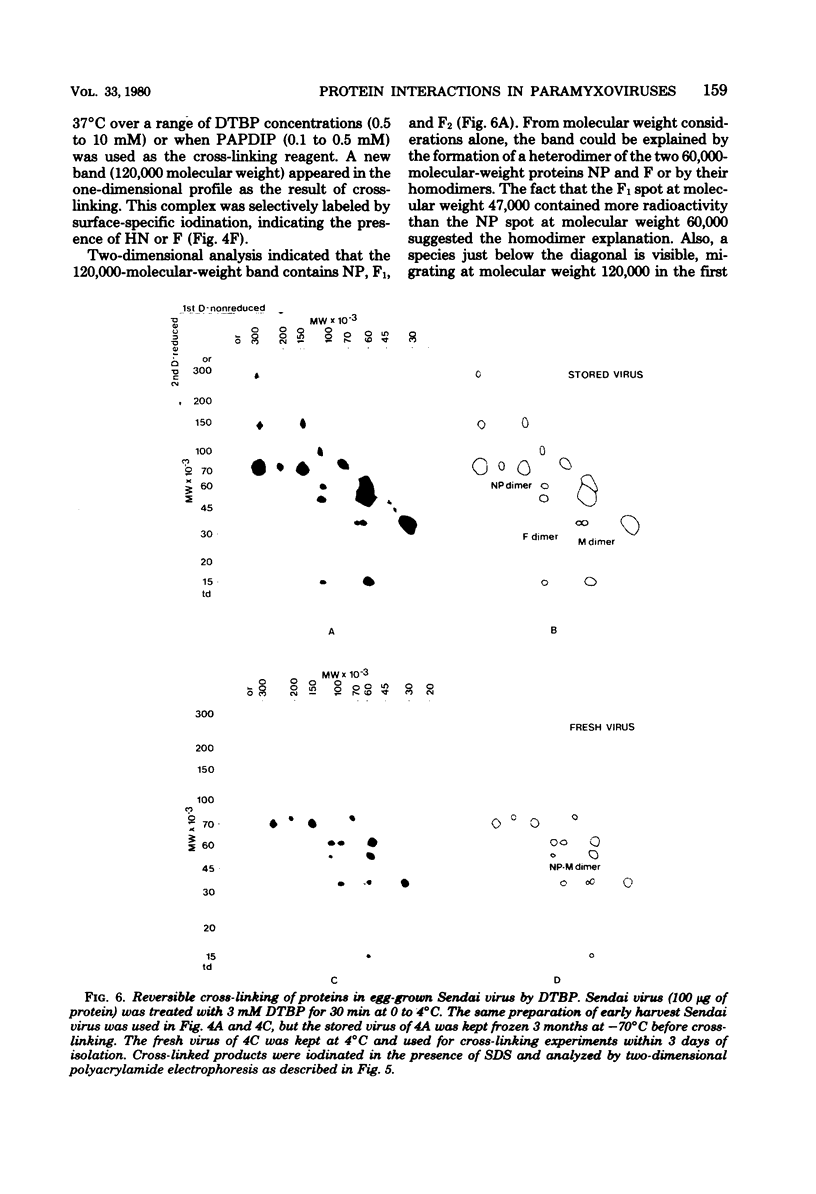
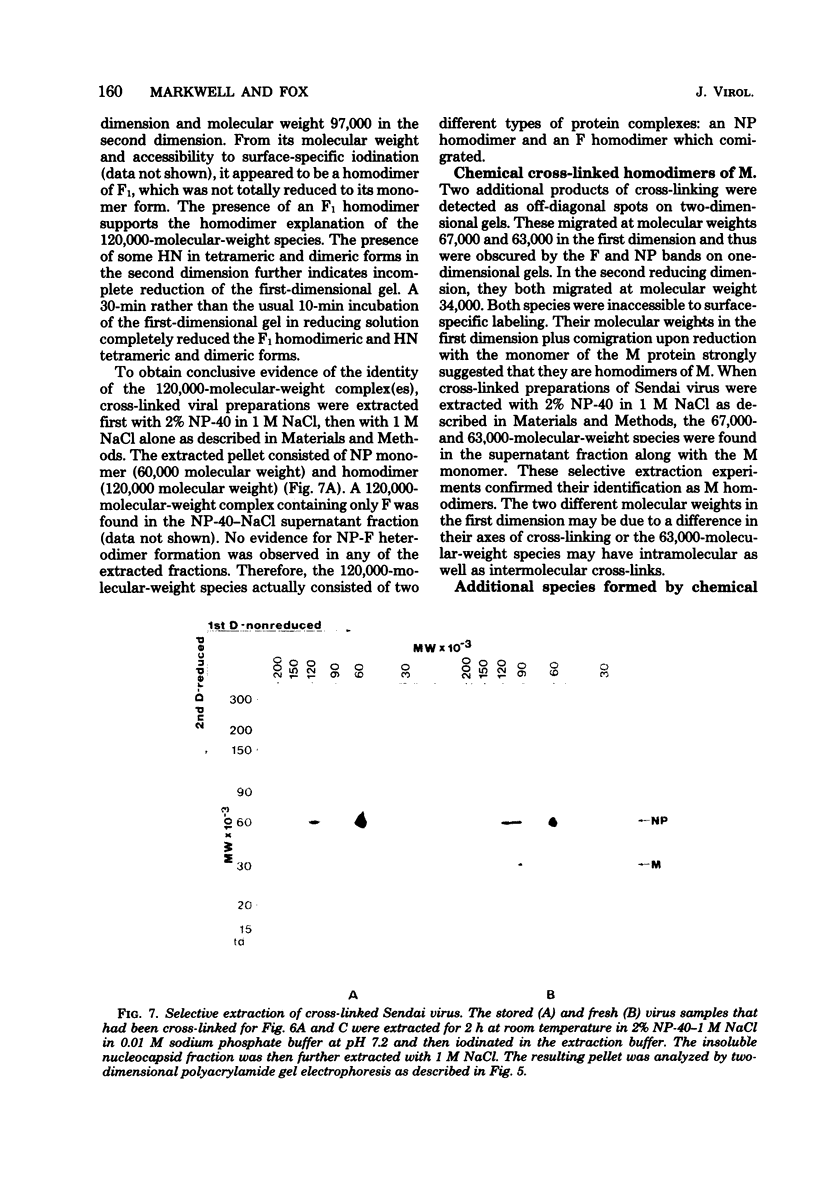
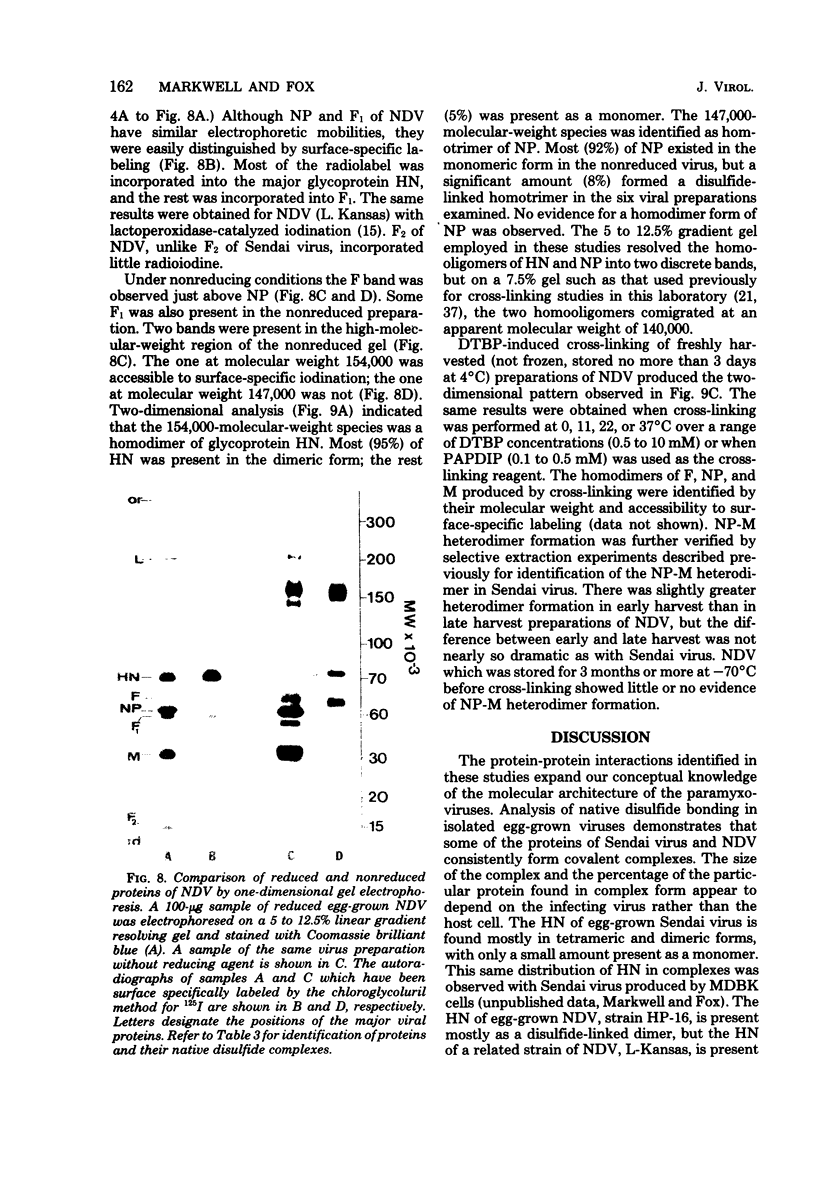
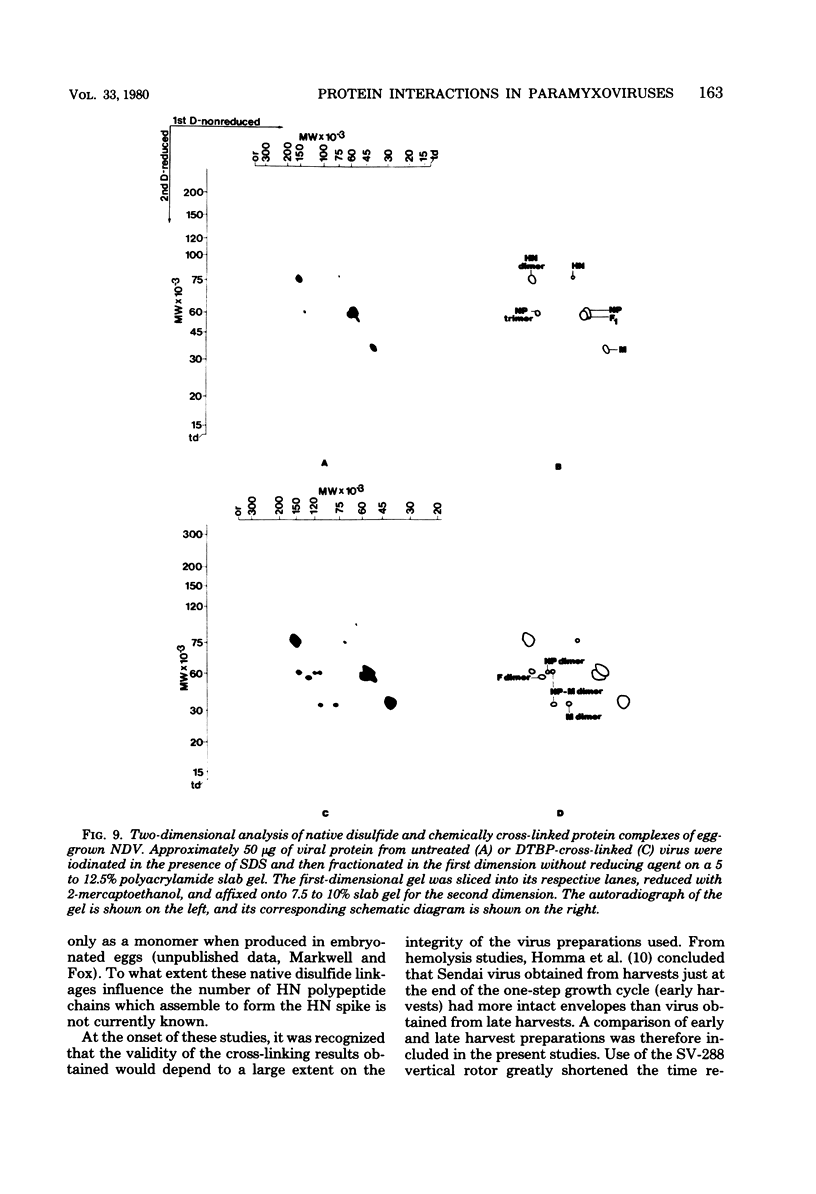
Analysis of native disulfide-bonded protein oligomers in paramyxoviruses showed that some viral proteins are consistently present as covalent complexes. In isolated Sendai virus the hemagglutinating protein HN is present in homodimeric and homotetrameric forms, and the minor nucleocapsid protein P exists partly as a monomer and partly as a disulfide-linked homotrimer. Similar disulfide-linked complexes were observed in Newcastle disease virus (strain HP-16), in which HN exists as a homodimer and some of the major nucleocapsid protein NP exists as a homotrimer. Noncovalent intermolecular interactions between proteins were studied with the reversible chemical cross-linkers dimethyl-3,3'-dithiobispropionimidate and methyl 3-[(p-azidophenyl)dithio]propionimidate, which contain disulfide bridges and a 1.1-nm separation between their functional groups. The same results were achieved with both reagents. The conditions of preparation, isolation, and storage of the viruses affected the protein-protein interactions observed upon cross-linking. Homooligomers of the glycoprotein F, the matrix protein M, and the major nucleocapsid protein NP were produced in both Sendai and Newcastle disease viruses after mild cross-linking of all viral preparations examined, but NP-M heterodimer formation in both viruses was most prevalent in early harvest preparations that were cross-linked soon after isolation. The ability of NP and M to form a heterodimer upon cross-linking indicates that the matrix protein layer lies in close proximity (within 1.1 nm) to the nucleocapsid in the newly formed virion. Some noncovalent intermolecular protein interactions in Sendai and Newcastle disease viruses, i.e., those leading to the formation of F, NP, and M homooliogmers upon cross-linking, are more stable to virus storage than others, i.e., those leading to the formation of an NP-M heterodimer upon cross-linking. The storage-induced loss of the ability of NP and M to form a heterodimer is not accompanied by any apparent loss of infectivity. This indicates that some spacial relationships which form during virus assembly can alter after particle formation and are not essential for the ensuing stages of the infectious process.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley H., Knowles J. R. Photogenerated reagents for membrane labeling. 1. Phenylnitrene formed within the lipid bilayer. Biochemistry. 1978 Jun 13;17(12):2414–2419. doi: 10.1021/bi00605a025. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crain R. C., Marinetti G. V. Cross-linking of membrane phospholipid and protein using putative monofunctional imidoesters. Chem Phys Lipids. 1978 Jun;21(3):195–204. doi: 10.1016/0009-3084(78)90067-1. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Chemical cross-linking in biology. Annu Rev Biophys Bioeng. 1979;8:165–193. doi: 10.1146/annurev.bb.08.060179.001121. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Miyakawa T., Fox C. F., Pruss R. M., Aharonov A., Herschman H. R. Specific radiolabeling of a cell surface receptor for epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2790–2794. doi: 10.1073/pnas.74.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3988–3992. doi: 10.1073/pnas.71.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning R., Milner R. J., Reske K., Cunningham B. A., Edelman G. M. Subunit structure, cell surface orientation, and partial amino-acid sequences of murine histocompatibility antigens. Proc Natl Acad Sci U S A. 1976 Jan;73(1):118–122. doi: 10.1073/pnas.73.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Shimizu K., Shimizu Y. K., Ishida N. On the study of Sendai virus hemolysis. I. Complete Sendai virus lacking in hemolytic activity. Virology. 1976 May;71(1):41–47. doi: 10.1016/0042-6822(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Ji T. H. The application of chemical crosslinking for studies on cell membranes and the identification of surface reporters. Biochim Biophys Acta. 1979 Apr 23;559(1):39–69. doi: 10.1016/0304-4157(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Kim J., Hama K., Miyake Y., Okada Y. Transformation of intramembrane particles of HVJ (Sendai virus) envelopes from an invisible to visible form on aging of virions. Virology. 1979 Jun;95(2):523–535. doi: 10.1016/0042-6822(79)90506-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenard J. Virus envelopes and plasma membranes. Annu Rev Biophys Bioeng. 1978;7:139–165. doi: 10.1146/annurev.bb.07.060178.001035. [DOI] [PubMed] [Google Scholar]
- Li J. K., Fox C. F. Radioiodination of the envelope proteins of Newcastle disease virus. J Supramol Struct. 1975;3(1):51–60. doi: 10.1002/jss.400030106. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Marx P. A., Portner A., Kingsbury D. W. Sendai virion transcriptase complex: polyeptide composition and inhibition by virion envelope proteins. J Virol. 1974 Jan;13(1):107–112. doi: 10.1128/jvi.13.1.107-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Yoshida T., Hamaguchi M., Iinuma M., Maeno K., Matsumoto T. Cross-linking of Newcastle disease virus (NDV) proteins. Arch Virol. 1978;58(1):15–28. doi: 10.1007/BF01315531. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Vernon S. K., Hartzell R. W., Rubin B. A. Virocidal cleavage of disulphide bonds within structural proteins of Sendai virus. J Gen Virol. 1973 Apr;19(1):9–20. doi: 10.1099/0022-1317-19-1-9. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Asano A., Okada Y. Importance of interpeptide disulfide bond in a viral glycoprotein with hemagglutination and neuraminidase activities. FEBS Lett. 1976 Nov;70(1):145–149. doi: 10.1016/0014-5793(76)80745-4. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Raghow R., Kingsbury D. W., Portner A., George S. Topography of a flexible ribonucleoprotein helix: protein-protein contacts in Sendai virus nucleocapsids. J Virol. 1979 Jun;30(3):701–710. doi: 10.1128/jvi.30.3.701-710.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson A. C., Fox C. F. Precursor protein for Newcastle disease virus. J Virol. 1973 Sep;12(3):579–587. doi: 10.1128/jvi.12.3.579-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Isida N. The smallest protein of Sendi virus: its candidate function of binding nucleocaspsid to envelope. Virology. 1975 Oct;67(2):427–437. [PubMed] [Google Scholar]
- Shimizu K., Shimizu Y. K., Koama T., Ishida N. Isolation and characterization of two distinct types of HVJ (Sendai virus) spikes. Virology. 1974 Nov;62(1):90–101. doi: 10.1016/0042-6822(74)90305-5. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J. Crosslinking and modification of Na,K-ATPase by ethyl acetimidate. Biochem Biophys Res Commun. 1977 Oct 10;78(3):962–969. doi: 10.1016/0006-291x(77)90515-0. [DOI] [PubMed] [Google Scholar]
- Takemoto L. J., Miyakawa T., Fox C. F. Analysis of membrane protein topography of Newcastle disease virus and cultured mammalian fibroblasts. Prog Clin Biol Res. 1977;17:605–614. [PubMed] [Google Scholar]