Abstract
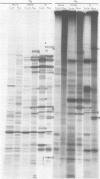
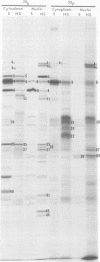
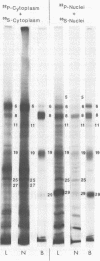
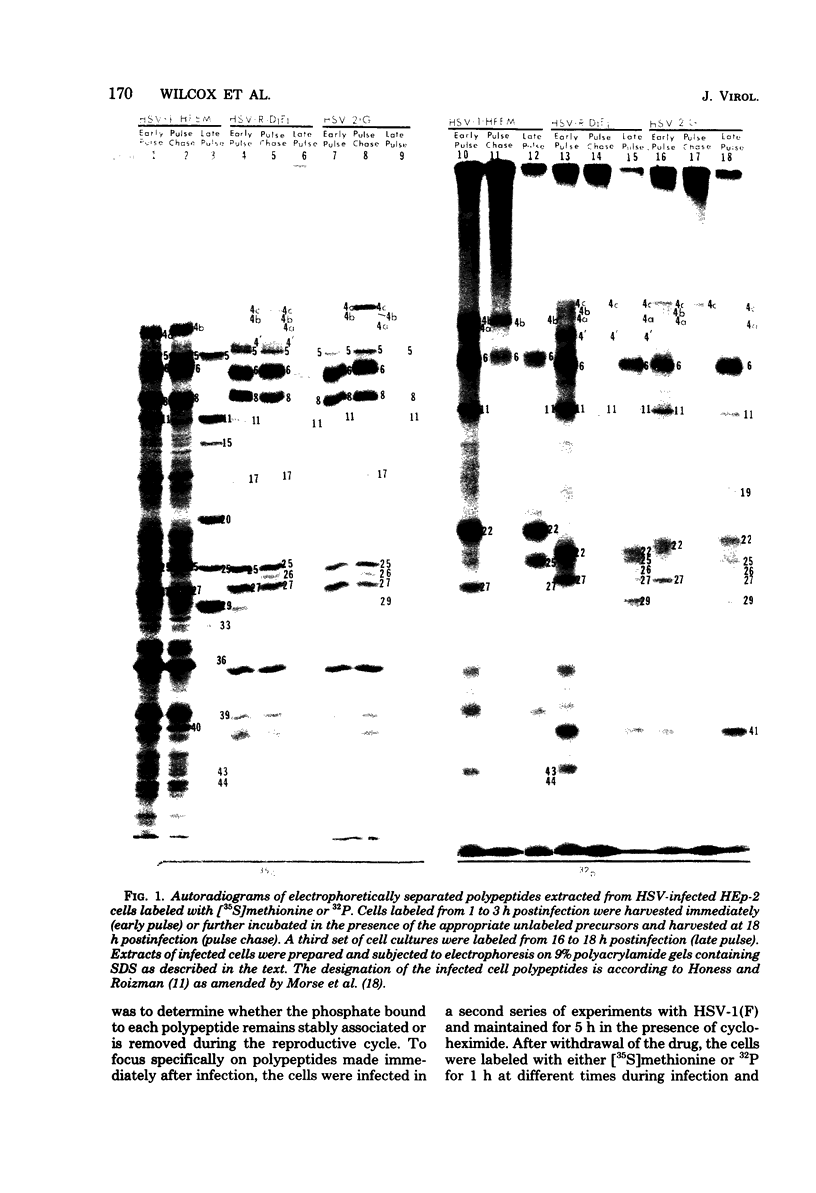
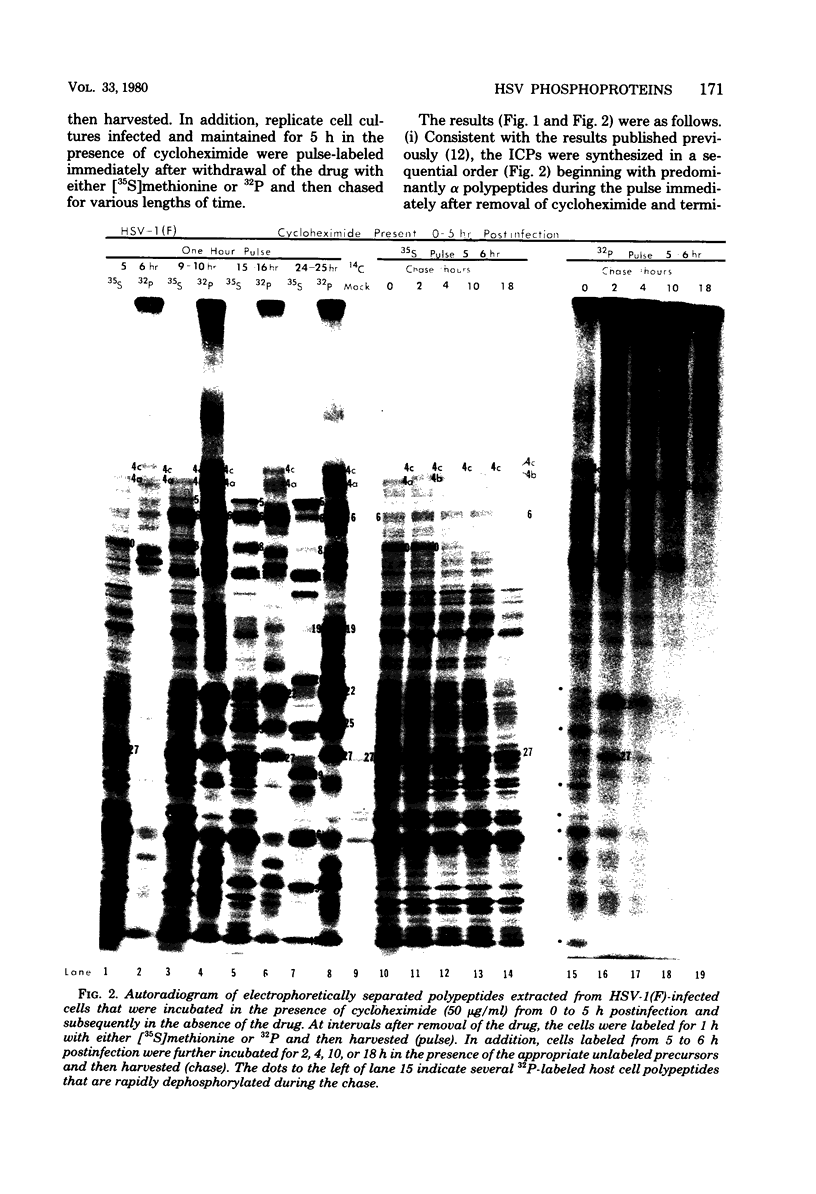
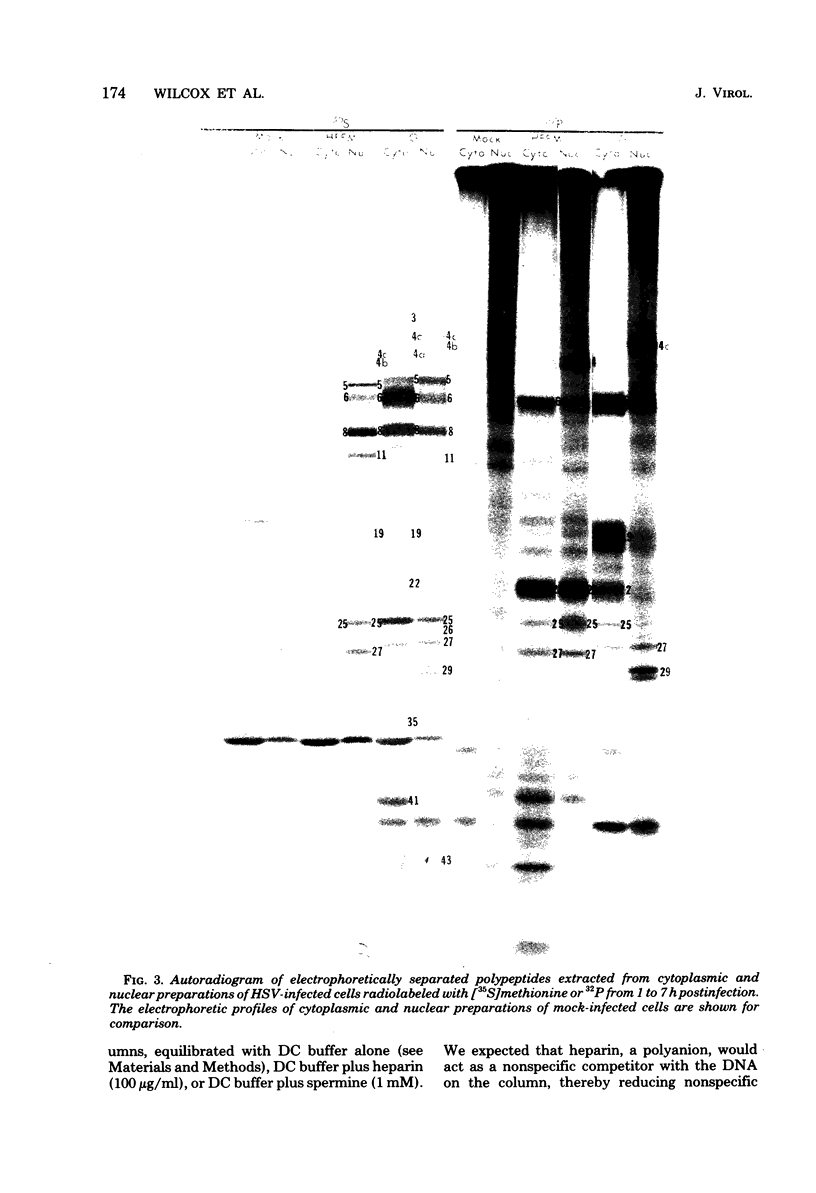
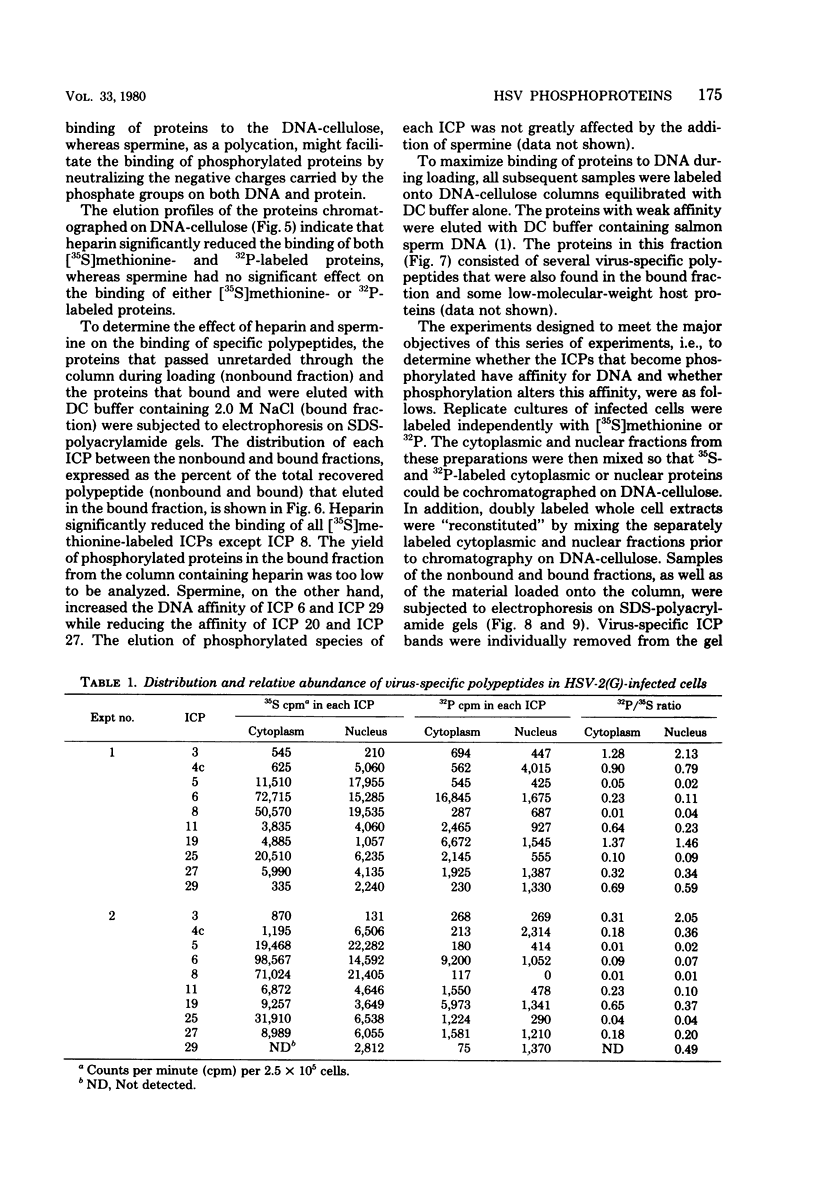
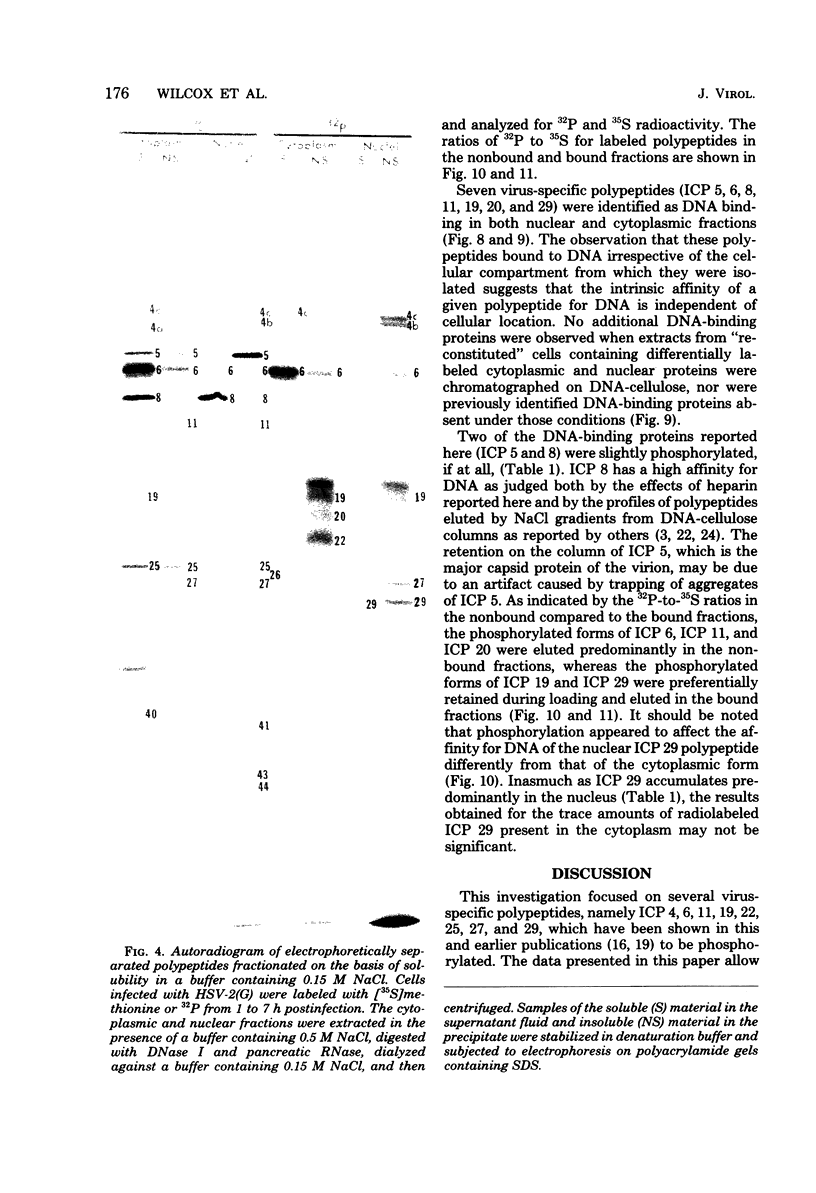
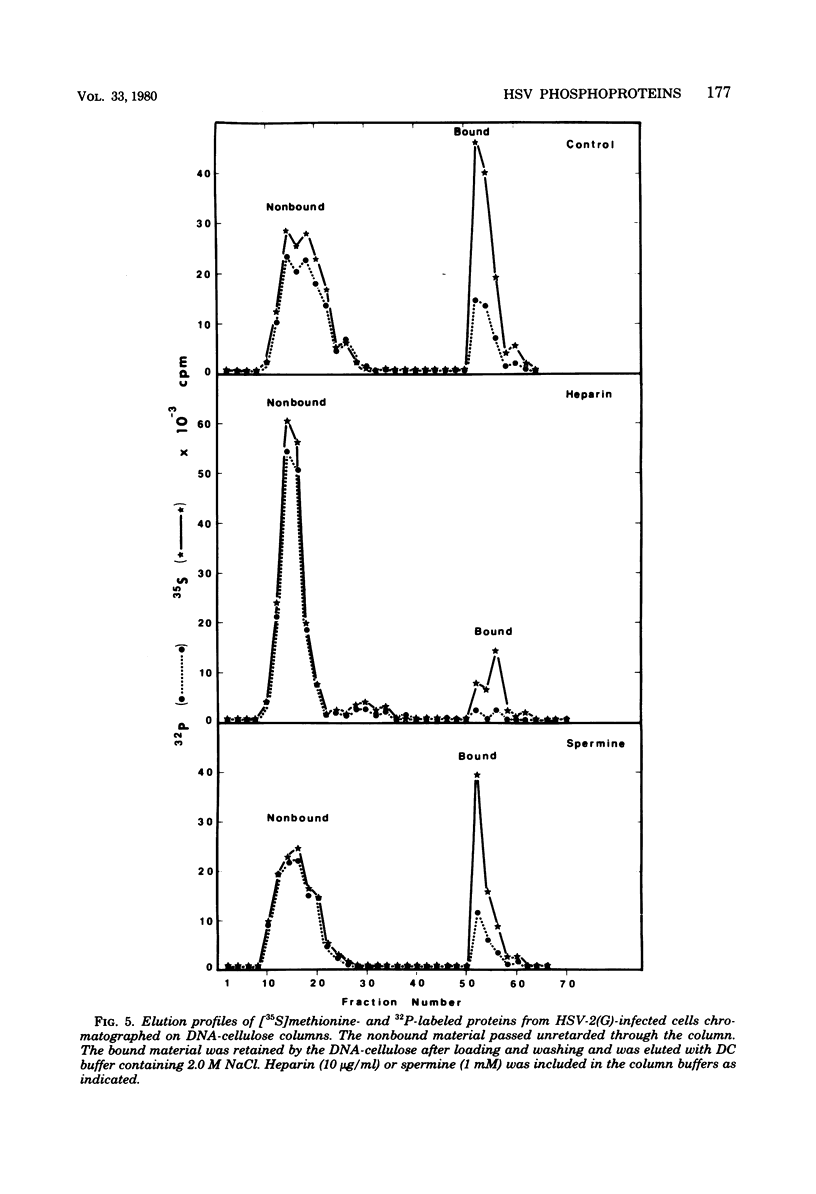
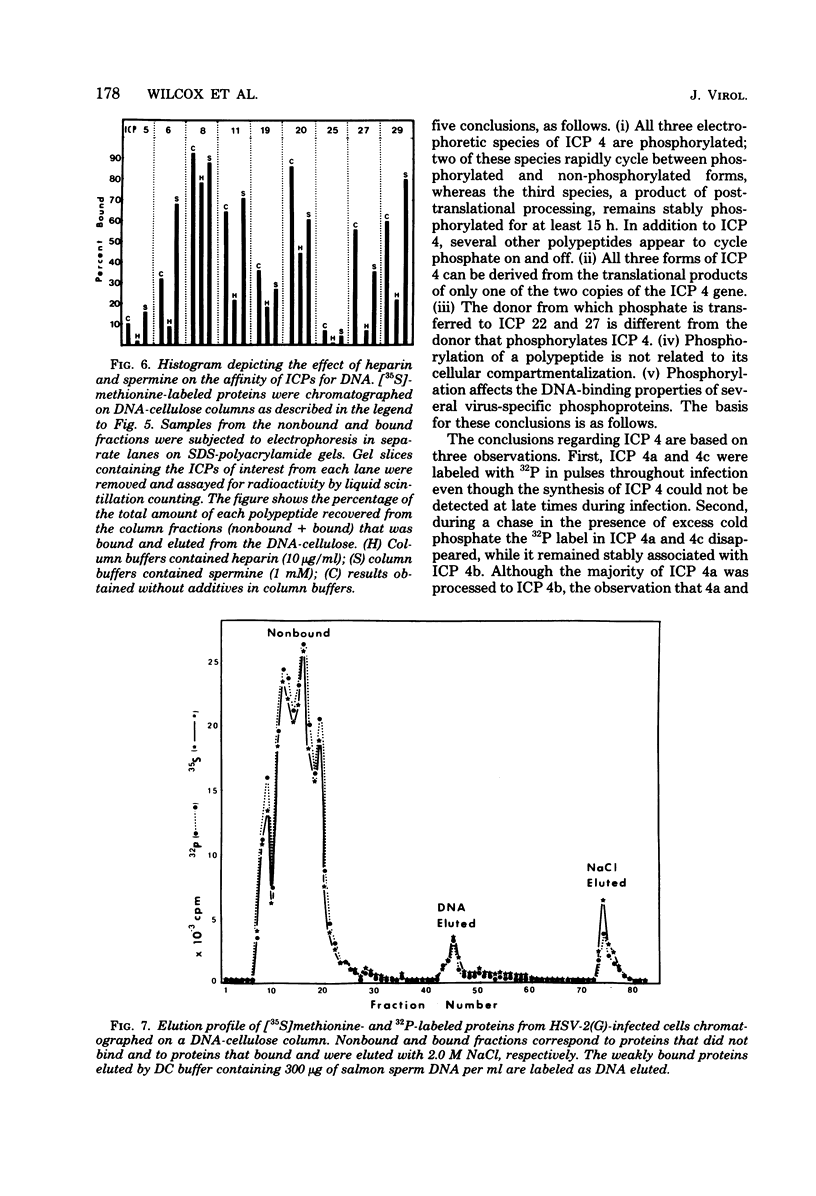
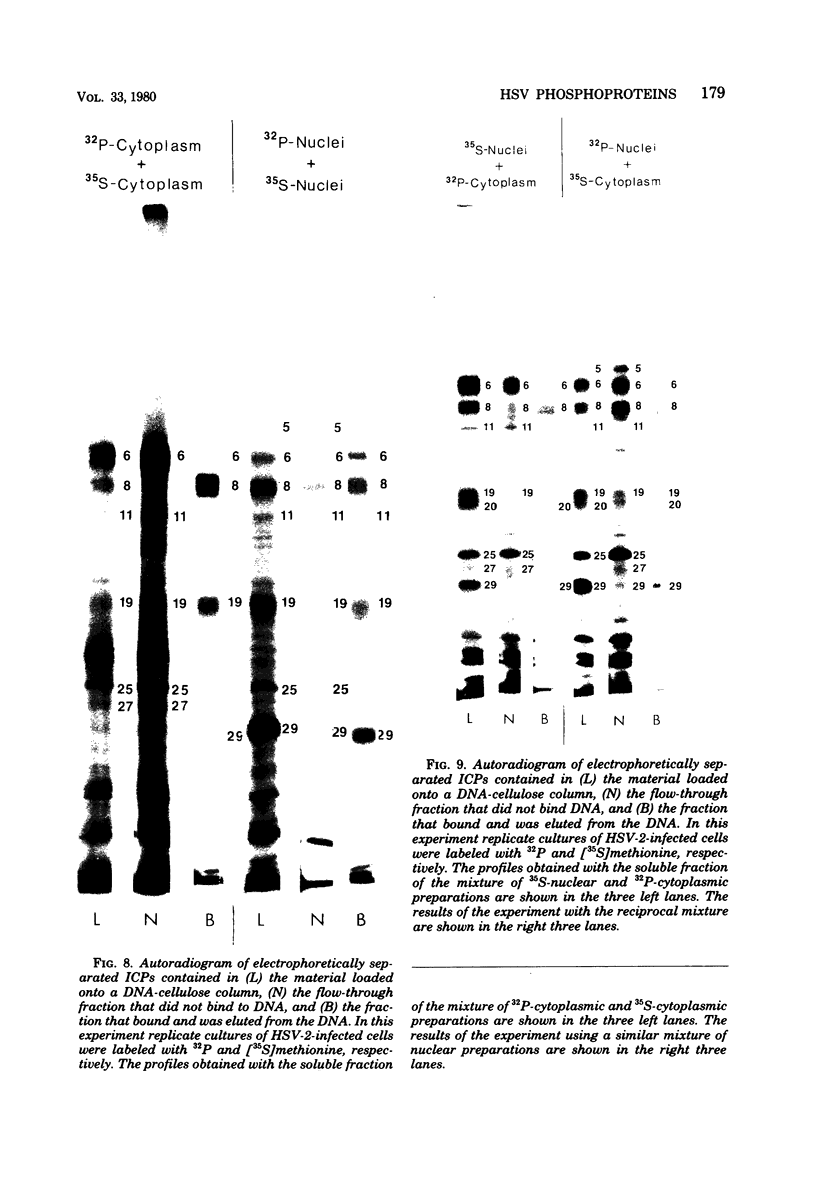
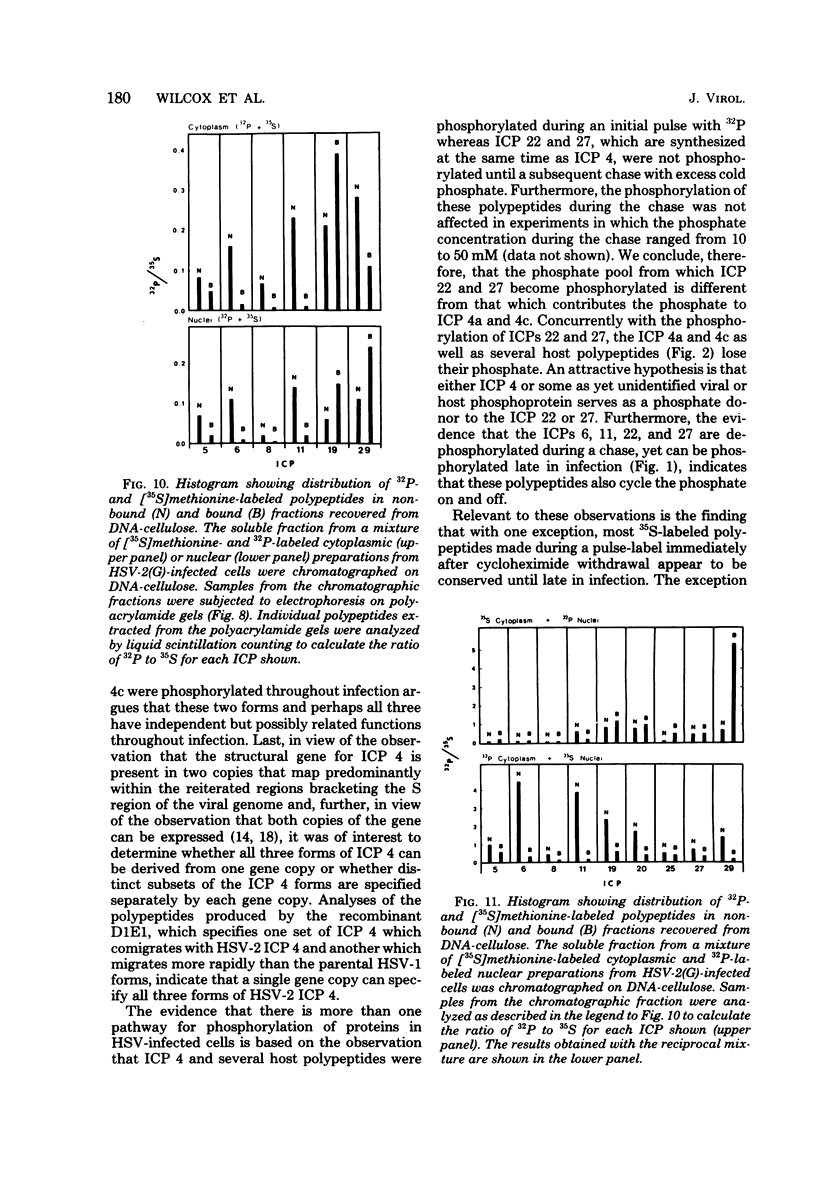
We report on phosphorylation, the stability of the bound phosphate, and the properties of several phosphorylated infected-cell polypeptides (ICPs) synthesized in cells infected with herpes simplex virus 1 and 2. Our results and conclusions are as follows. (i) Phosphorylation of ICPs occurs by at least two different pathways. Thus, the 4a and 4c electrophoretic forms of ICP 4 were labeled with 32P during a pulse concurrently with their synthesis, whereas ICP 22 and ICP 27 were labeled with 32P only during a subsequent chase in the presence of unlabeled phosphate. (ii) Pulse chase studies with [35S]methionine and 32P indicate that whereas most polypeptides are stable, the bound phosphate with few exceptions cycles on and off. Of special interest is the observation that the phosphate bound to ICP 4a and 4c cycles on and off, whereas that bound to ICP 4b is stably associated. Similar cycling was observed for ICP 6, 11, 22, and 27. The observation that 4a and 4c can be phosphorylated as late as 24 h after infection, i.e., long after their synthesis ceases, suggests that all three forms may have defined functions that persist throughout the reproductive cycle. (iii) All three forms of ICP 4 can be the translational products of only one of two copies of the ICP 4 gene in the viral genome. (iv) Analyses of the distribution of the viral proteins within the cell indicate that phosphorylation is not a major determinant in the compartmentalization of most viral phosphoproteins. (v) Comparisons of the binding to DNA-cellulose of artificial mixtures of 32P- and [35S]methionine-labeled proteins from infected cells indicate that phosphorylation in some instances enhances (e.g., ICP 29) and in other instances decreases (e.g., ICP 6) binding affinity for DNA. In light of previous reports that some of the proteins identified as phosphoproteins have regulatory functions, the data suggest that phosphorylation may modify the activity of regulatory proteins in herpes simplex virus-infected cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Amodio F. J., Jenkins M., Gutmann E. D., Ferris F. L. Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:289–305. doi: 10.1101/sqb.1968.033.01.033. [DOI] [PubMed] [Google Scholar]
- Bayliss G. J., Marsden H. S., Hay J. Herpes simplex virus proteins: DNA-binding proteins in infected cells and in the virus structure. Virology. 1975 Nov;68(1):124–134. doi: 10.1016/0042-6822(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Schaffer P. A., Powell K. L. Synthesis of virus-specific polypaptides by temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1976 Dec;75(2):306–318. doi: 10.1016/0042-6822(76)90030-1. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Erickson J. S., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. IX. Sulfated proteins. Virology. 1973 Sep;55(1):94–102. doi: 10.1016/s0042-6822(73)81011-6. [DOI] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. Inhibition of protein synthesis initiation by oxidized glutathione: activation of a protein kinase that phosphorylates the alpha subunit of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4110–4114. doi: 10.1073/pnas.75.9.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J., Petkevich J. M. On the association of virus proteins with the nuclei of cells infected with herpes simplex virus. J Gen Virol. 1978 Jun;39(3):519–529. doi: 10.1099/0022-1317-39-3-519. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Randall R. E., Killington R. A., Watson D. H. Some properties of recombinants between type 1 and type 2 herpes simplex viruses. J Gen Virol. 1977 Sep;36(3):471–484. doi: 10.1099/0022-1317-36-3-471. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Ruyechan W. T., Roizman B., Halliburton I. W. Molecular genetics of herpes simplex virus: demonstration of regions of obligatory and nonobligatory identity within diploid regions of the genome by sequence replacement and insertion. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3896–3900. doi: 10.1073/pnas.75.8.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Crombie I. K., Subak-Sharpe J. H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature-sensitive mutants of HSV strain 17. J Gen Virol. 1976 Jun;31(3):347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Buchman T. G., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus DNA. IX. Apparent exclusion of some parental DNA arrangements in the generation of intertypic (HSV-1 X HSV-2) recombinants. J Virol. 1977 Oct;24(1):231–248. doi: 10.1128/jvi.24.1.231-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Poonian M. S., Schlabach A. J., Weissbach A. Covalent attachment of nucleic acids to agarose for affinity chromatography. Biochemistry. 1971 Feb 2;10(3):424–427. doi: 10.1021/bi00779a011. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. DNA-binding proteins of cells infected by herpes simplex virus type 1 and type 2. Intervirology. 1976;7(4-5):225–239. doi: 10.1159/000149955. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Davison A. J., Marsden H. S., Timbury M. C., Subak-Sharpe J. H., Wilkie N. M. Recombinants between herpes simplex virus types 1 and 2: analyses of genome structures and expression of immediate early polypeptides. J Virol. 1978 Nov;28(2):499–517. doi: 10.1128/jvi.28.2.499-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. DNA-binding proteins induced by herpes simplex virus type 2 in HEp-2 cells. J Virol. 1976 Aug;19(2):717–731. doi: 10.1128/jvi.19.2.717-731.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Hayward G., Jacob R., Wadsworth S., Frenkel N., Honess R. W., Kozak M. Human herpersviruses I: a model for molecular organization and regulation of herpesviruses-a review. IARC Sci Publ. 1975;(11 Pt 1):3–38. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Aurelian L. Proteins of herpesvirus type 2: I. Virion, nonvirion, and antigenic polypeptides in infected cells. Virology. 1976 Feb;69(2):438–452. doi: 10.1016/0042-6822(76)90475-x. [DOI] [PubMed] [Google Scholar]
- Taborsky G. Phosphoproteins. Adv Protein Chem. 1974;28:1–210. doi: 10.1016/s0065-3233(08)60230-2. [DOI] [PubMed] [Google Scholar]
- Tjian R., Robbins A. Enzymatic activities associated with a purified simian virus 40 T antigen-related protein. Proc Natl Acad Sci U S A. 1979 Feb;76(2):610–614. doi: 10.1073/pnas.76.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox K. W., Smith H. O. Binding of the ATP-dependent DNase from Hemophilus influenzae to duplex DNA molecules. J Biol Chem. 1976 Oct 10;251(19):6122–6126. [PubMed] [Google Scholar]
- Zillig W., Fujiki H., Blum W., Janeković D., Schweiger M., Rahmsdorf H., Ponta H., Hirsch-Kauffmann M. In vivo and in vitro phosphorylation of DNA-dependent RNA polymerase of Escherichia coli by bacteriophage-T7-induced protein kinase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2506–2510. doi: 10.1073/pnas.72.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]