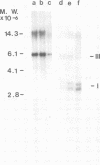
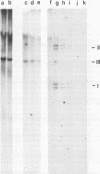
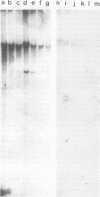
Abstract
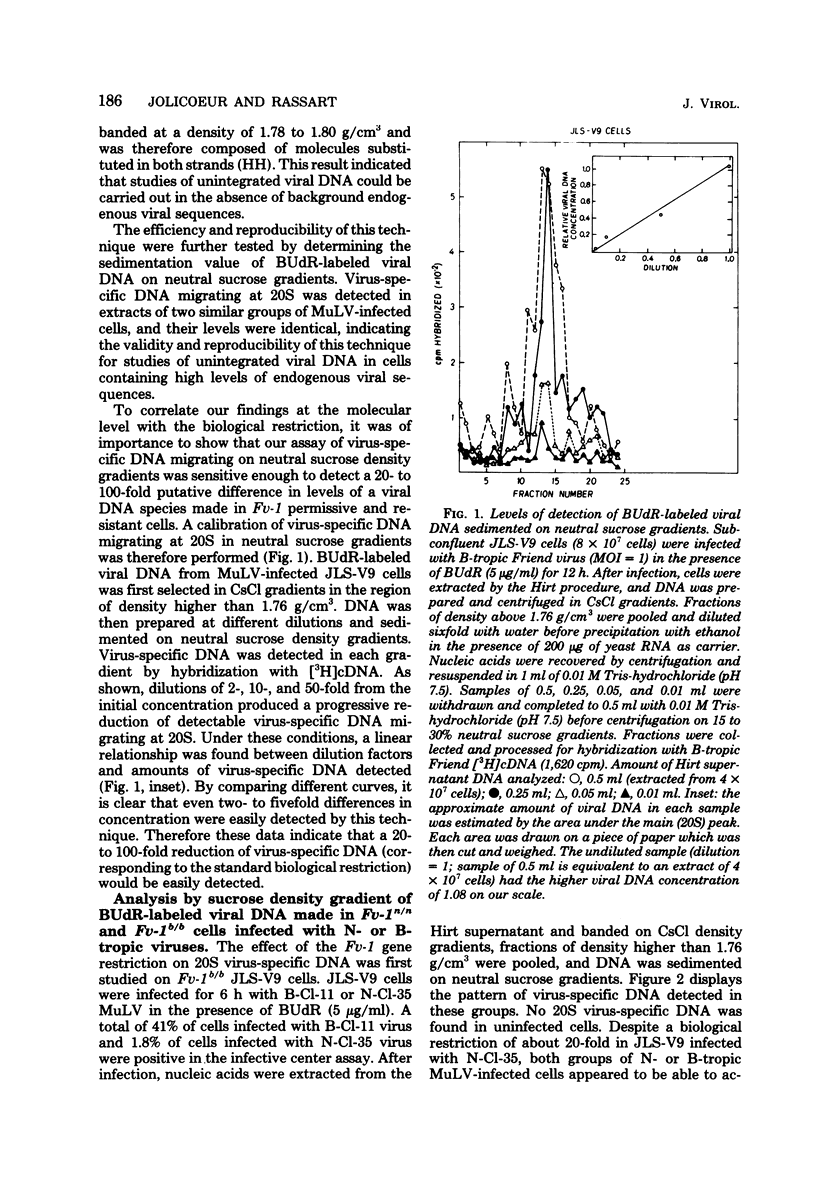
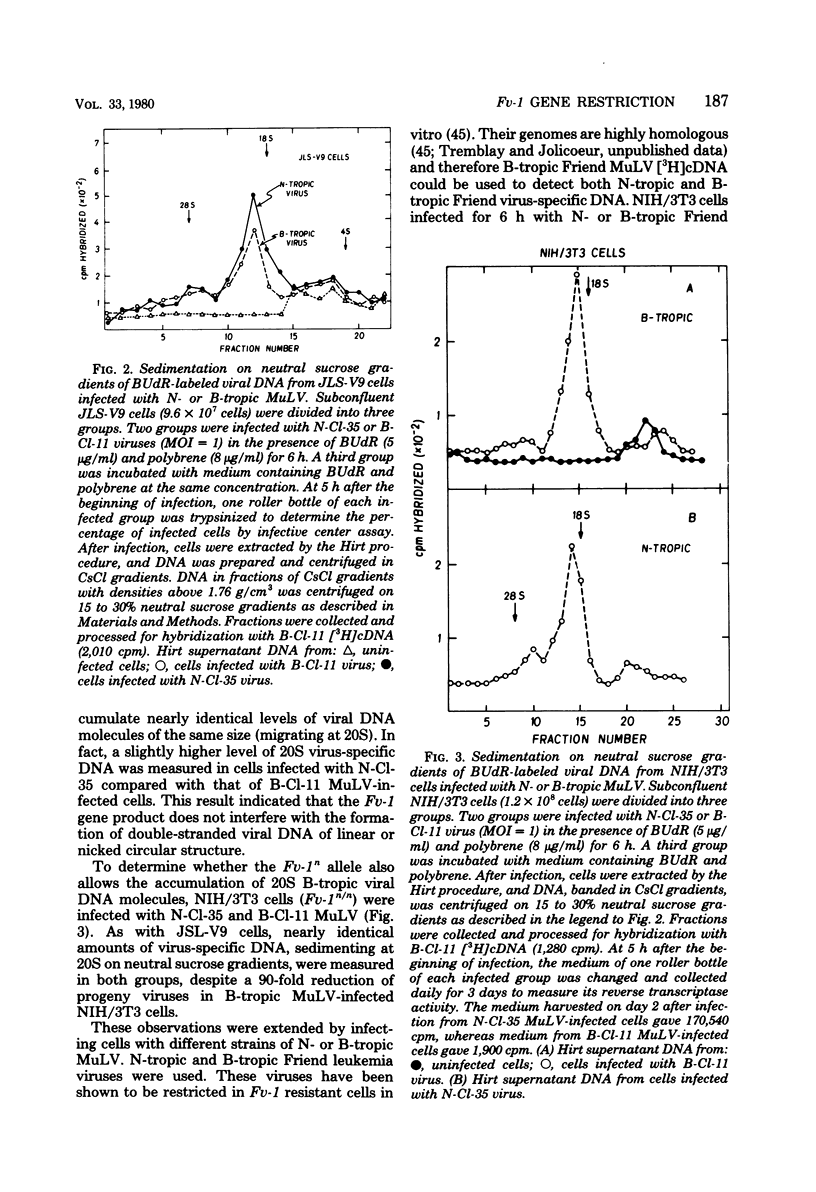
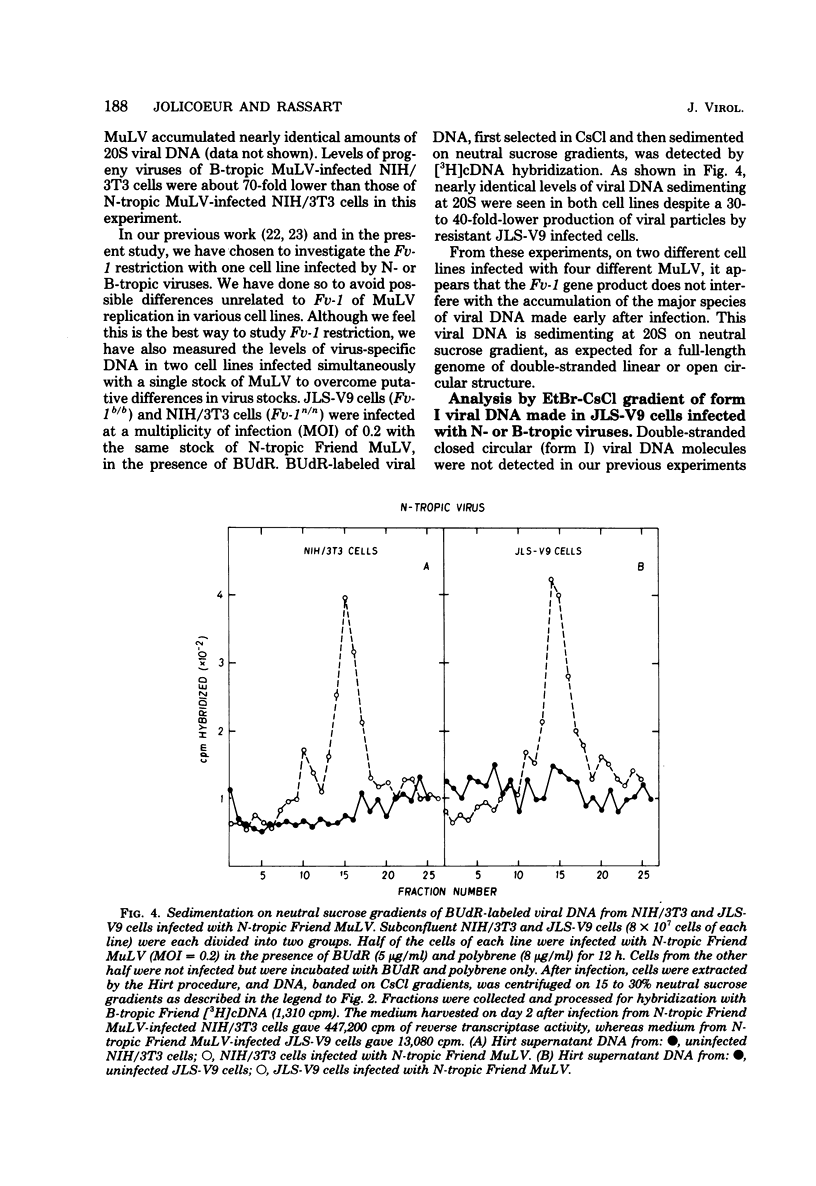
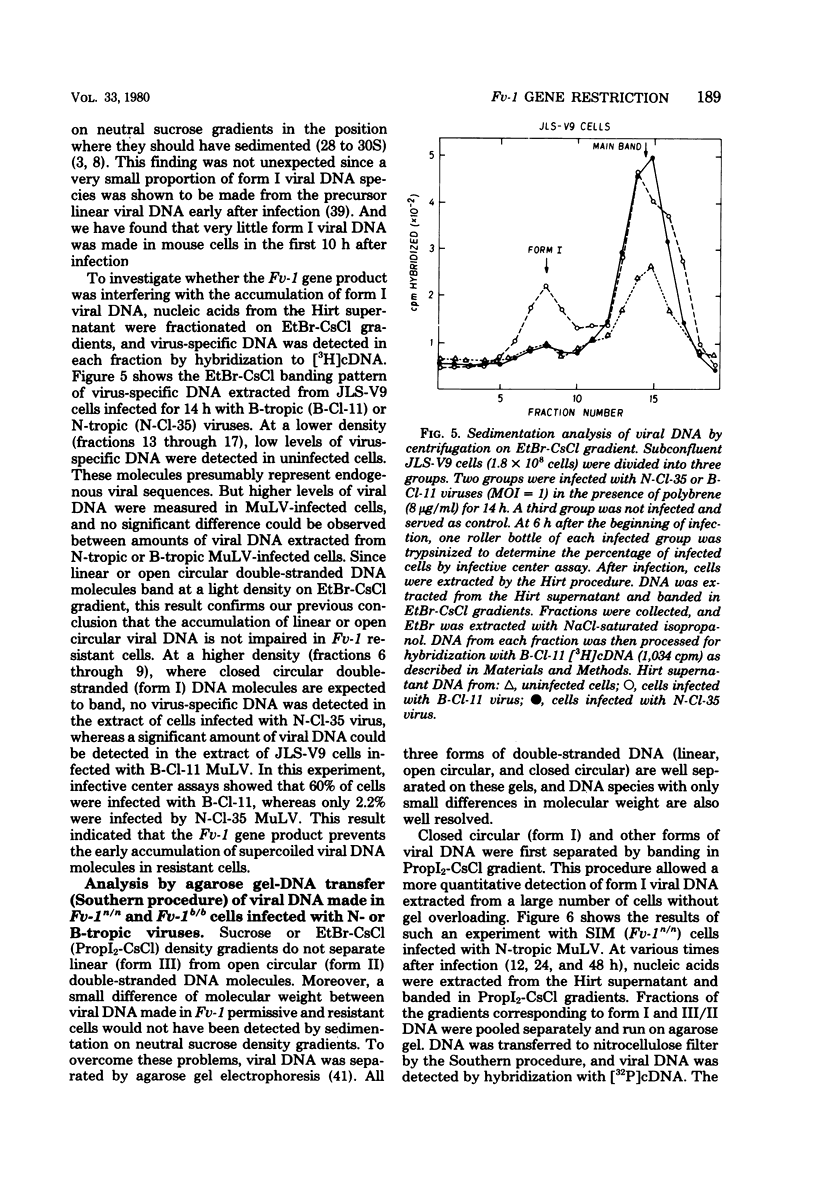
Levels of unintegrated viral DNA made in Fv-1b/b (SIM.R, JLS-V9) and Fv-1n/n (NIH/3T3) cell lines after infection with N- or B-tropic murine leukemia virus (MuLV) have been measured. Different forms of viral DNA were sedimented on neutral sucrose or ethidium bromide-cesium chloride density gradients and detected by hybridization with complementary DNA. It was found that the major viral DNA species made in Fv-1 permissive or resistant cells was sedimenting at 20S on neutral sucrose gradient. Levels of this 20S viral DNA species were not significantly different in both systems. However levels of closed circular (form I) viral DNA separated on ethidium bromide-cesium chloride gradients were found to be decreased in Fv-1 resistant cells. Various species of viral DNA were also analyzed by the agarose gel-DNA transfer procedure of Southern. The major viral DNA species was found to migrate as a double-stranded linear DNA of 5.7 x 10(6) daltons. The molecular weight of linear viral DNA molecules extracted from Fv-1 permissive or resistant cells appeared to be the same. Levels of this linear viral DNA were almost identical in both systems except in B-tropic MuLV-infected resistant NIH/3T3 cells in which a moderate decrease has been measured. Two closed circular viral DNA species were observed by this technique. Their levels were markedly decreased in Fv-1 resistant cells. Our results indicate that the Fv-1 restriction does not grossly affect the formation of linear double-stranded viral DNA, but prevents the accumulation of closed circular viral DNA. Therefore the Fv-1 gene product could allow the synthesis of a normal linear viral DNA but interfere with the formation of supercoiled viral DNA. Alternatively, it could promote the synthesis of a faulty linear viral DNA whose defect (yet undetected) would prevent its circularization. In any case, the Fv-1 restriction mechanism appears to occur before the integration event itself.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Gerwin B. I., Duran-Troise G., Gisselbrecht S., Rein A. Murine sarcoma virus pseudotypes acquire a determinant specifying N or B tropism from leukaemia virus during rescue. Nature. 1975 Jul 17;256(5514):223–225. doi: 10.1038/256223a0. [DOI] [PubMed] [Google Scholar]
- Böttger M., Bierwolf D., Wunderlich V., Graffi A. New calibration correlations for molecular weights of circular DNA: the molecular weight of the DNA of an oncogenic papova virus of the Syrian hamster. Biochim Biophys Acta. 1971 Feb 25;232(1):21–31. doi: 10.1016/0005-2787(71)90487-4. [DOI] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Lieber M. M., Todaro G. J. Nucleic acid homology of murine type-C viral genes. J Virol. 1974 Dec;14(6):1394–1403. doi: 10.1128/jvi.14.6.1394-1403.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Niwa O., Kojola J., Kaplan H. S. New gene locus modifying susceptibility to certain B-tropic murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Feb;73(2):585–590. doi: 10.1073/pnas.73.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Duran-Troise G., Bassin R. H., Rein A., Gerwin B. I. Loss of Fv-1 restriction in Balb/3T3 cells following infection with a single N tropic murine leukemia virus particle. Cell. 1977 Mar;10(3):479–488. doi: 10.1016/0092-8674(77)90035-6. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. Replication of bacteriophage PM2 deoxyribonucleic acid: a closed circular double-stranded molecule. J Mol Biol. 1971 Mar 28;56(3):597–621. doi: 10.1016/0022-2836(71)90404-9. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Schindler J., Jensen F. C., Lerner R. A. Structural markers on core protein p30 of murine leukemia virus: functional correlation with Fv-1 tropism. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4170–4174. doi: 10.1073/pnas.75.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Hynes R. Six-NB-tropic murine leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J Virol. 1977 Jan;21(1):309–318. doi: 10.1128/jvi.21.1.309-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. C., Yang W. K. DNA transfection of ecotropic murine leukemia viruses in mouse cell cultures. Cancer Res. 1977 Jun;37(6):1709–1714. [PubMed] [Google Scholar]
- Hsu I. C., Yang W. K., Tennant R. W., Brown A. Transfection of Fv-1 permissive and restrictive mouse cells with integrated DNA of murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1451–1455. doi: 10.1073/pnas.75.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto A., Hartley J. W., Rowe W. P. Fv-1 restriction of xenotropic and amphotropic murine leukemia virus genomes phenotypically mixed with ecotropic virus. Virology. 1979 Feb;93(1):215–225. doi: 10.1016/0042-6822(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on synthesis of N-tropic and B-tropic murine leukemia viral RNA. Cell. 1976 Jan;7(1):33–39. doi: 10.1016/0092-8674(76)90252-x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of the Fv-1 locus on the titration of murine leukemia viruses. J Virol. 1975 Dec;16(6):1593–1598. doi: 10.1128/jvi.16.6.1593-1598.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr Top Microbiol Immunol. 1979;86:67–122. doi: 10.1007/978-3-642-67341-2_3. [DOI] [PubMed] [Google Scholar]
- Kashmiri S. V., Rein A., Bassin R. H., Gerwin B. I., Gisselbrecht S. Donation of N- or B-tropic phenotype to NB-tropic murine leukemia virus during mixed infections. J Virol. 1977 Jun;22(3):626–633. doi: 10.1128/jvi.22.3.626-633.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Soeiro R., Fields B. N. Host restriction of Friend leukemia virus. Role of the viral outer coat. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2549–2553. doi: 10.1073/pnas.70.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F., Steeves R. A. B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV). Virology. 1973 Oct;55(2):363–370. doi: 10.1016/0042-6822(73)90176-1. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., McCarthy B. J. The interaction of oligodeoxynucleotides with denatured DNA. Biochim Biophys Acta. 1967 Nov 21;149(1):180–189. doi: 10.1016/0005-2787(67)90700-9. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Deitch C. J., Pincus T. Multiplicity-dependent kinetics and murine leukemia virus infection in Fv-1-sensitive and Fv-1-resistant cells. Virology. 1976 Aug;73(1):23–35. doi: 10.1016/0042-6822(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Otten J. A., Myer F. E., Tennant R. W., Brown A. Effect of the Fv-1 locus in vivo: host range pseudotypes of murine sarcoma virus. J Natl Cancer Inst. 1978 Apr;60(4):875–880. doi: 10.1093/jnci/60.4.875. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology. 1975 Jun;65(2):333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Rein A., Kashmiri S. V., Bassin R. H., Gerwin B. L., Duran-Troise G. Phenotypic mixing between N- and B-tropic murine leukemia viruses: infectious particles with dual sensitivity to Fv-1 restriction. Cell. 1976 Mar;7(3):373–379. doi: 10.1016/0092-8674(76)90166-5. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Cabradilla C. D., Stephenson J. R., Aaronson S. A. Segregation of genetic information for a B-tropic leukemia virus with the structural locus for BALB:virus-1. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2953–2957. doi: 10.1073/pnas.74.7.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Schindler J., Hynes R., Hopkins N. Evidence for recombination between N- and B-tropic murine leukemia viruses: analysis of three virion proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1977 Sep;23(3):700–700. doi: 10.1128/jvi.23.3.700-.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Varmus H. E. Virus-specific DNA in the cytoplasm of avian sarcoma virus-infected cells is a precursor to covalently closed circular viral DNA in the nucleus. J Virol. 1978 Jan;25(1):104–104. doi: 10.1128/jvi.25.1.104-104.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Yoshimura F. K., Weinberg R. A. Infectious, linear, unintegrated DNA of Moloney murine leukemia virus. J Virol. 1976 Dec;20(3):621–626. doi: 10.1128/jvi.20.3.621-626.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sveda M. M., Soeiro R. Host restriction of Friend leukemia virus: synthesis and integration of the provirus. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2356–2360. doi: 10.1073/pnas.73.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Schluter B., Yang W., Brown A. Reciprocal inhibition of mouse leukemia virus infection by Fv-1 allele cell extracts. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4241–4245. doi: 10.1073/pnas.71.10.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Deng C. T., Bishop J. M. Synthesis, structure and function of avian sarcoma virus-specific DNA in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):987–996. doi: 10.1101/sqb.1974.039.01.113. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Structure of the intermediates leading to the integrated provirus. Biochim Biophys Acta. 1977 Mar 21;473(1):39–55. doi: 10.1016/0304-419x(77)90006-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura F. K., Weinberg R. A. Restriction endonuclease cleavage of linear and closed circular murine leukemia viral DNAs: discovery of a smaller circular form. Cell. 1979 Feb;16(2):323–332. doi: 10.1016/0092-8674(79)90009-6. [DOI] [PubMed] [Google Scholar]