Abstract
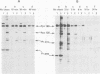
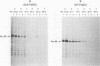
The McDonough (SM), Gardner-Arnstein (GA), and Snyder-Theilen (ST) strains of feline sarcoma virus (FeSV) code for high-molecular-weight polyproteins that contain varying amounts of the amino-terminal region of the FeLV gag gene-coded precursor protein and a polypeptide(s) of an as yet undetermined nature. The SM-FeSV primary translational product is a 180,000-dalton polyprotein which is immediately processed into a highly unstable 60,000-dalton molecule containing the p15-p12-p30 fragment of the FeLV gag gene-coded precursor protein and a 120,000-dalton FeSV-specific polypeptide. The GA- and ST-FeSV genomes code for polyproteins of 95,000 and 85,000 daltons, respectively, which in addition to the amino-terminal moiety (p15-12 and a portion of p30) of the FeLV gag gene-coded precursor protein also contain FeSV-specific polypeptides. However, the GA- and ST-FeSV polyproteins appear to be relatively stable molecules (half-lives of around 16 h) and are not significantly processed into smaller polypeptides. Immunological and biochemical analysis of each of the above FeSV translational products revealed that the sarcoma-specific regions of the GA- and ST-FeSV polyproteins are antigenically cross-reactive and exhibit common methionine-containing peptides. These findings favor the concept that these sarcoma-specific polypeptides are coded for by the similar subsets of cellular sequences incorporated into the GA- and ST-FeSV genomes during the generation of these transforming agents.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Barbacid M. Origin and biological properties of a new BALB/c mouse sarcoma virus. J Virol. 1978 Aug;27(2):366–373. doi: 10.1128/jvi.27.2.366-373.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Bassin R. H., Weaver C. Comparison of murine sarcoma viruses in nonproducer and S + L - -transformed cells. J Virol. 1972 Apr;9(4):701–704. doi: 10.1128/jvi.9.4.701-704.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Stephenson J. R., Hino S., Tronick S. R. Differential expression of helper viral structural polypeptides in cells transformed by clonal isolates of woolly monkey sarcoma virus. J Virol. 1975 Nov;16(5):1117–1123. doi: 10.1128/jvi.16.5.1117-1123.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson P., Goldfarb M. P., Weinberg R. A. A defined subgenomic fragment of in vitro synthesized Moloney sarcoma virus DNA can induce cell transformation upon transfection. Cell. 1979 Jan;16(1):63–75. doi: 10.1016/0092-8674(79)90188-0. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Stephenson J. R., Aaronson S. A. Structural polypeptides of mammalian type C RNA viruses. Isolation and immunologic characterization of a low molecular weight polypeptide, p10. J Biol Chem. 1976 Aug 25;251(16):4859–4866. [PubMed] [Google Scholar]
- Barbacid M., Stephenson J. R., Aaronson S. A. gag Gene of mammalian type-C RNA tumour viruses. Nature. 1976 Aug 12;262(5569):554–559. doi: 10.1038/262554a0. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Troxler D. H., Scolnick E. M., Aaronson S. A. Analysis of translational products of Friend strain of spleen focus-forming virus. J Virol. 1978 Sep;27(3):826–830. doi: 10.1128/jvi.27.3.826-830.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Dina D., Beemon K., Duesberg P. The 30S Moloney sarcoma virus RNA contains leukemia virus nucleotide sequences. Cell. 1976 Oct;9(2):299–309. doi: 10.1016/0092-8674(76)90120-3. [DOI] [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Gilbert J. H., Porzig K. J., Scolnick E. M., Aaronson S. A. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. doi: 10.1128/jvi.30.3.821-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Neubauer R. L., Fischinger P. J. Fractionation of DNA nucleotide transcripts from Moloney sarcoma virus and isolation of sarcoma virus-specific complementary DNA. J Virol. 1976 May;18(2):481–490. doi: 10.1128/jvi.18.2.481-490.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Officer J. E., Rongey R. W., Parker J. C., Oliver C., Estes J. D., Huebner R. J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973 Oct;51(4):1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Vogt P. K. Avian oncovirus MH2 is defective in Gag, Pol, and Env. Virology. 1979 Jan 30;92(2):278–284. doi: 10.1016/0042-6822(79)90132-6. [DOI] [PubMed] [Google Scholar]
- Hu S., Davidson N. A heteroduplex study of the sequence relationships between the RNAs of M-MSV and M-MLV. Cell. 1977 Mar;10(3):469–477. doi: 10.1016/0092-8674(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Stephenson J. R. Feline leukemia virus: biochemical and immunological characterization of gag gene-coded structural proteins. J Virol. 1977 Sep;23(3):599–607. doi: 10.1128/jvi.23.3.599-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakower J. M., Barbacid M., Aaronson S. A. Differential synthesis of mammalian type C viral gene products in infected cells. J Virol. 1977 Oct;24(1):1–7. doi: 10.1128/jvi.24.1.1-7.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakower J. M., Barbacid M., Aaronson S. A. Radioimmunoassay for mammalian type C viral reverse transcriptase. J Virol. 1977 May;22(2):331–339. doi: 10.1128/jvi.22.2.331-339.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Mellon P., Pawson A., Bister K., Martin G. S., Duesberg P. H. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5874–5878. doi: 10.1073/pnas.75.12.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Anisowicz A., Scolnick E. M. Deletion mapping of moloney type C virus: polypeptide and nucleic acid expression in different transforming virus isolates. J Virol. 1976 May;18(2):491–503. doi: 10.1128/jvi.18.2.491-503.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzig K. J., Barbacid M., Aaronson S. A. Biological properties and translational products of three independent isolates of feline sarcoma virus. Virology. 1979 Jan 15;92(1):91–107. doi: 10.1016/0042-6822(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Sacks T. L., Deobagkar D. N., Stephenson J. R. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. doi: 10.1073/pnas.75.8.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K. C., Barbacid M., Porzig K. J., Aaronson S. A. Involvement of different exogenous feline leukemia virus subgroups in the generation of independent feline sarcoma virus isolates. Virology. 1979 Aug;97(1):1–11. doi: 10.1016/0042-6822(79)90367-2. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Okabe H., Tronick S. R., Gilden R. V., Aaronson S. A. Molecular mechanisms involved in the differential expression of gag gene products by clonal isolates of a primate sarcoma virus. J Virol. 1978 Feb;25(2):471–478. doi: 10.1128/jvi.25.2.471-478.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T. L., Reynolds F. H., Jr, Deobagkar D. N., Stephenson J. R. Murine leukemia virus (T-8)-transformed cells: identification of a precursor polyprotein containing gag gene-coded proteins (p15 and p12) and a nonstructural component. J Virol. 1978 Sep;27(3):809–814. doi: 10.1128/jvi.27.3.809-814.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Goldberg R. J., Williams D. Characterizatiion of rat genetic sequences of Kirsten sarcoma virus: distinct class of endogenous rat type C viral sequences. J Virol. 1976 May;18(2):559–566. doi: 10.1128/jvi.18.2.559-566.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Bishop J. M. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979 Aug;31(2):514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Sen A., Todaro G. J., Sliski A., Essex M. Pseudotypes of feline sarcoma virus contain an 85,000-dalton protein with feline oncornavirus-associated cell membrane antigen (FOCMA) activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1505–1509. doi: 10.1073/pnas.75.3.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Essex M., Hino S., Hardy W. D., Jr, Aaronson S. A. Feline oncornavirus-associated cell-membrane antigen (FOCMA): distinction between FOCMA and the major virion glycoprotein. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1219–1223. doi: 10.1073/pnas.74.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Boyars J. K., Parks W. P., Scolnick E. M. Friend strain of spleen focus-forming virus: a recombinant between mouse type C ecotropic viral sequences and sequences related to xenotropic virus. J Virol. 1977 May;22(2):361–372. doi: 10.1128/jvi.22.2.361-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Shih M. S., Gilden R. V., Hatanaka M. Sarcoma and helper-specific RNA tumor virus subunits in transformed nonproducer mouse cells activated to produce virus by treatment with bromodeoxyuridine. J Virol. 1974 Nov;14(5):1262–1267. doi: 10.1128/jvi.14.5.1262-1267.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]