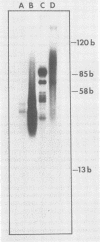
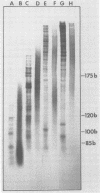
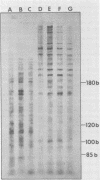
Abstract
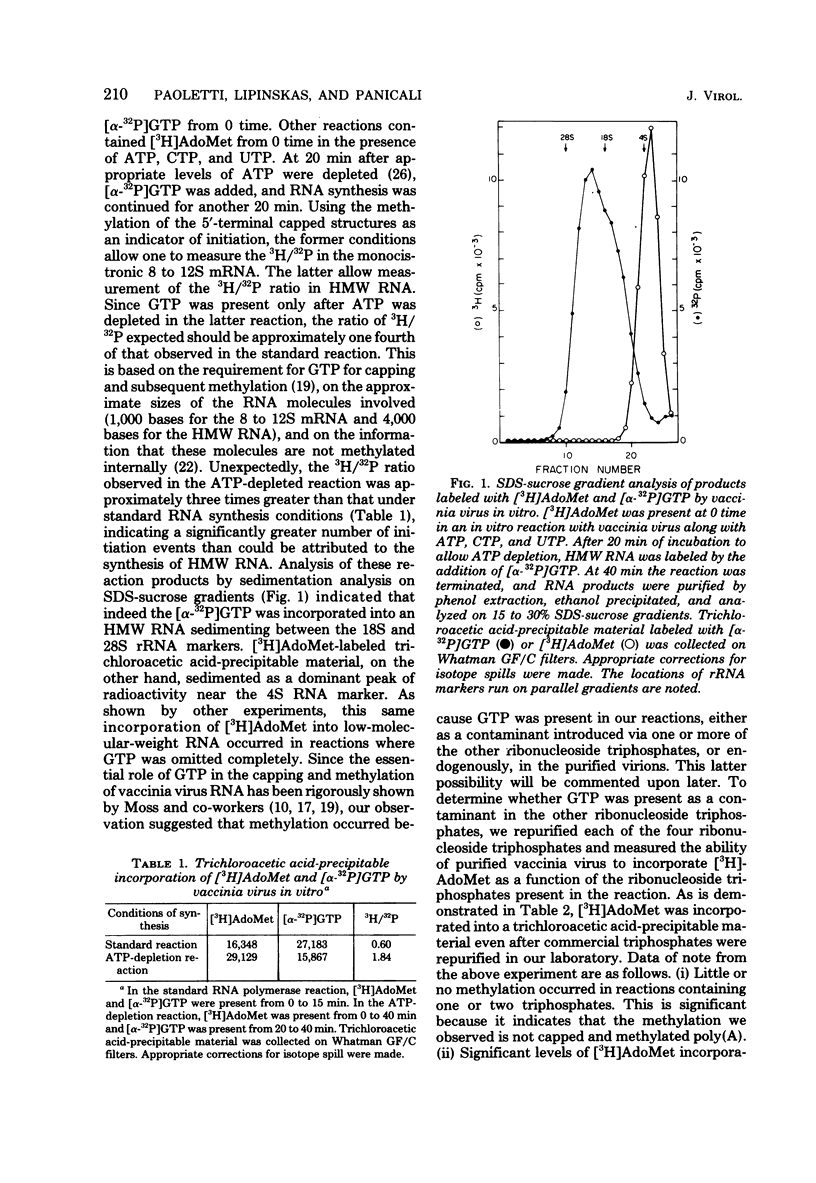
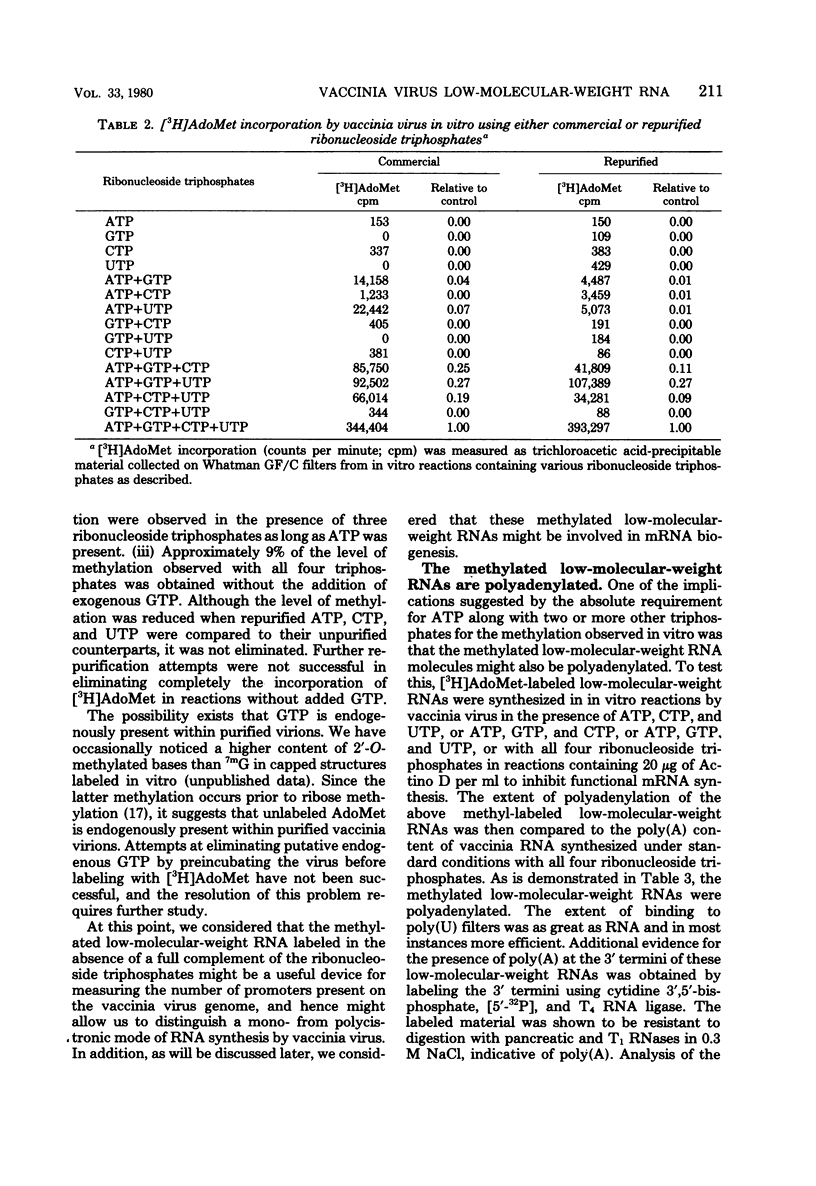
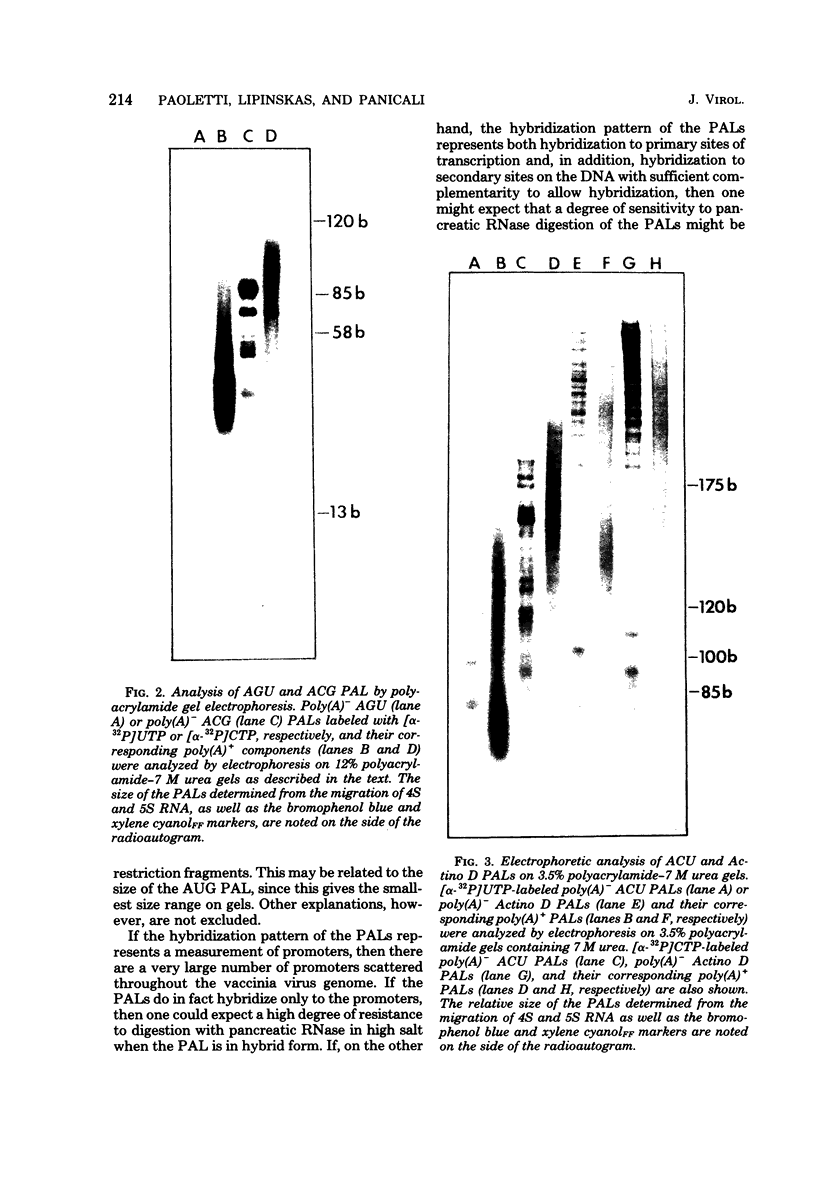
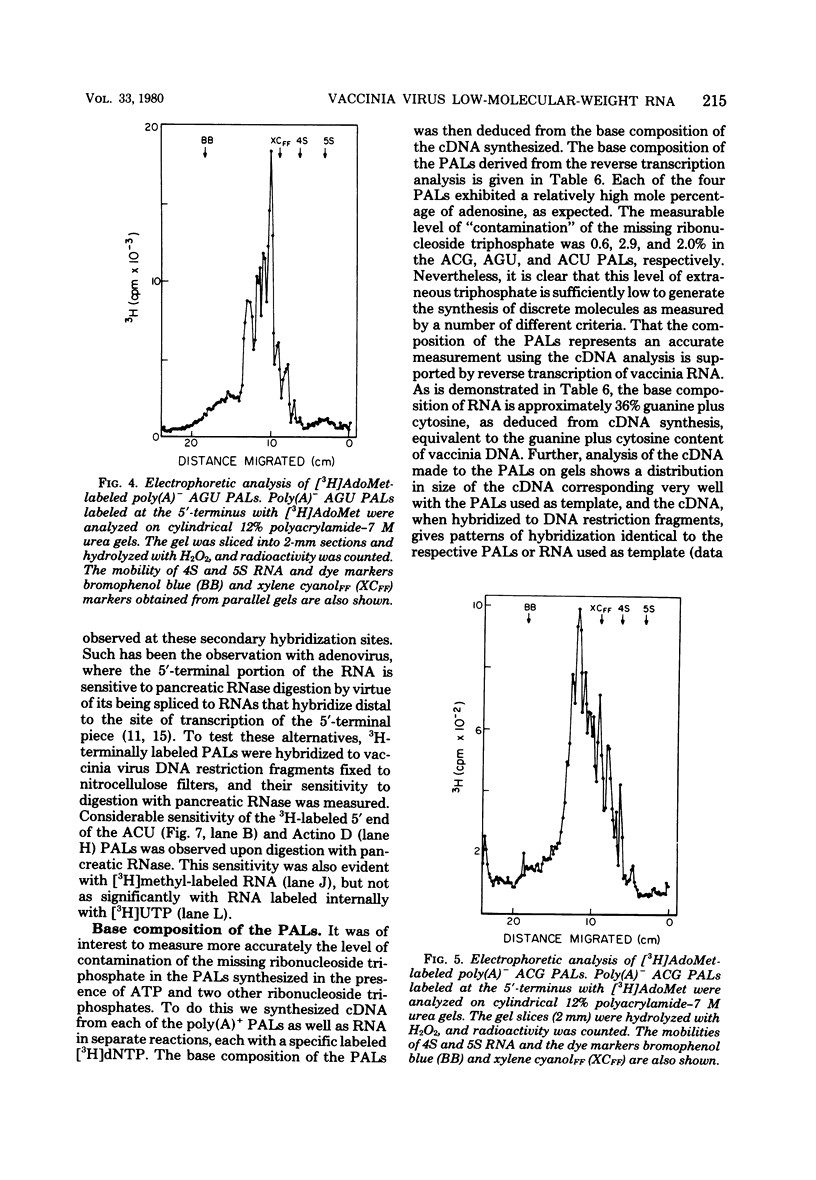
In the presence of ATP plus two other ribonucleoside triphosphates or in reactions containing all four ribonucleoside triphosphates and actinomycin D, vaccinia virus synthesizes in vitro discrete low-molecular-weight RNA molecules ranging in size from about 20 to several hundred bases. A novel feature of these small RNA molecules is that they are capped and methylated at the 5' terminus, containing both mGpppGm and mGpppAm type cap structures, and in addition these molecules are polyadenylated at the 3' terminus. Hybridization of these RNAs to restriction fragments derived from vaccinia virus DNA indicates a considerable degree of complexity, suggesting the presence of a large number of promoters throughout the genome. However, measurable sensitivity to pancreatic RNase of the 5' capped end of these RNAs while in hybrid form to the DNA suggests other possible roles for these small RNAs in vaccinia virus mRNA biogenesis.
Full text
PDF



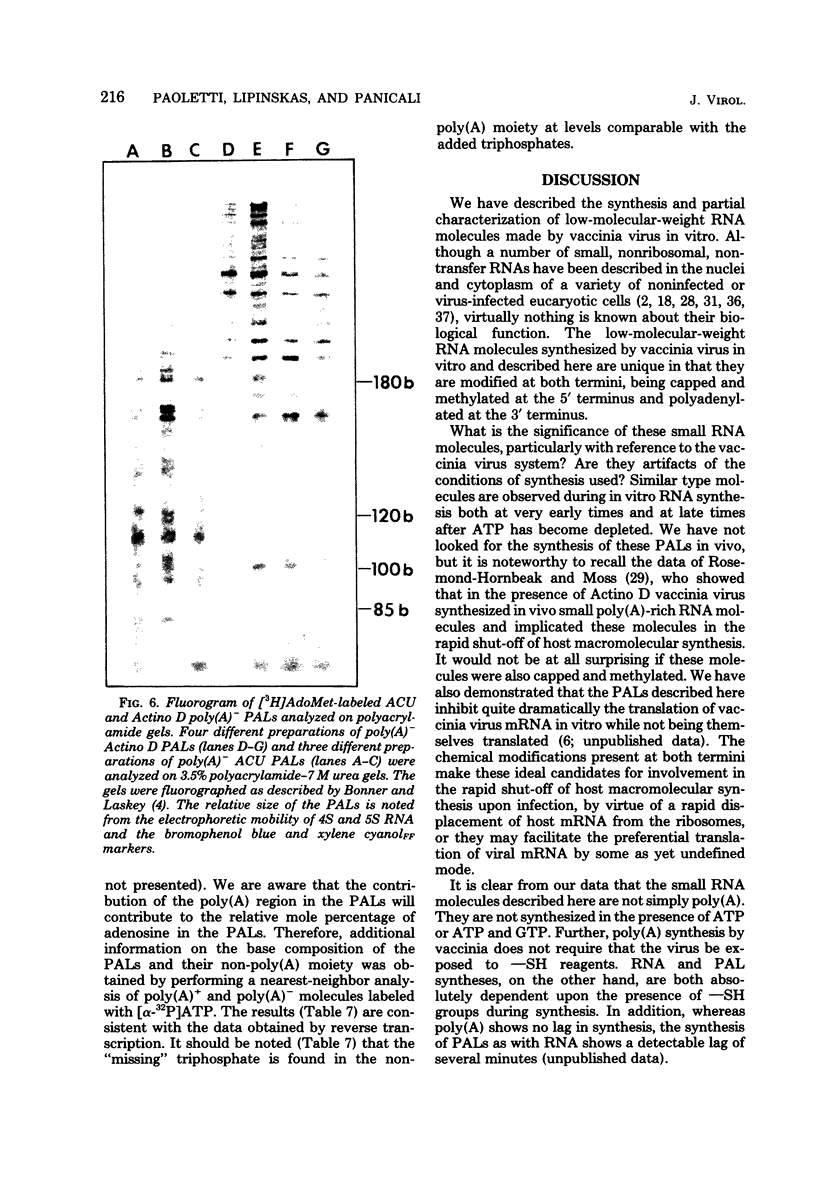
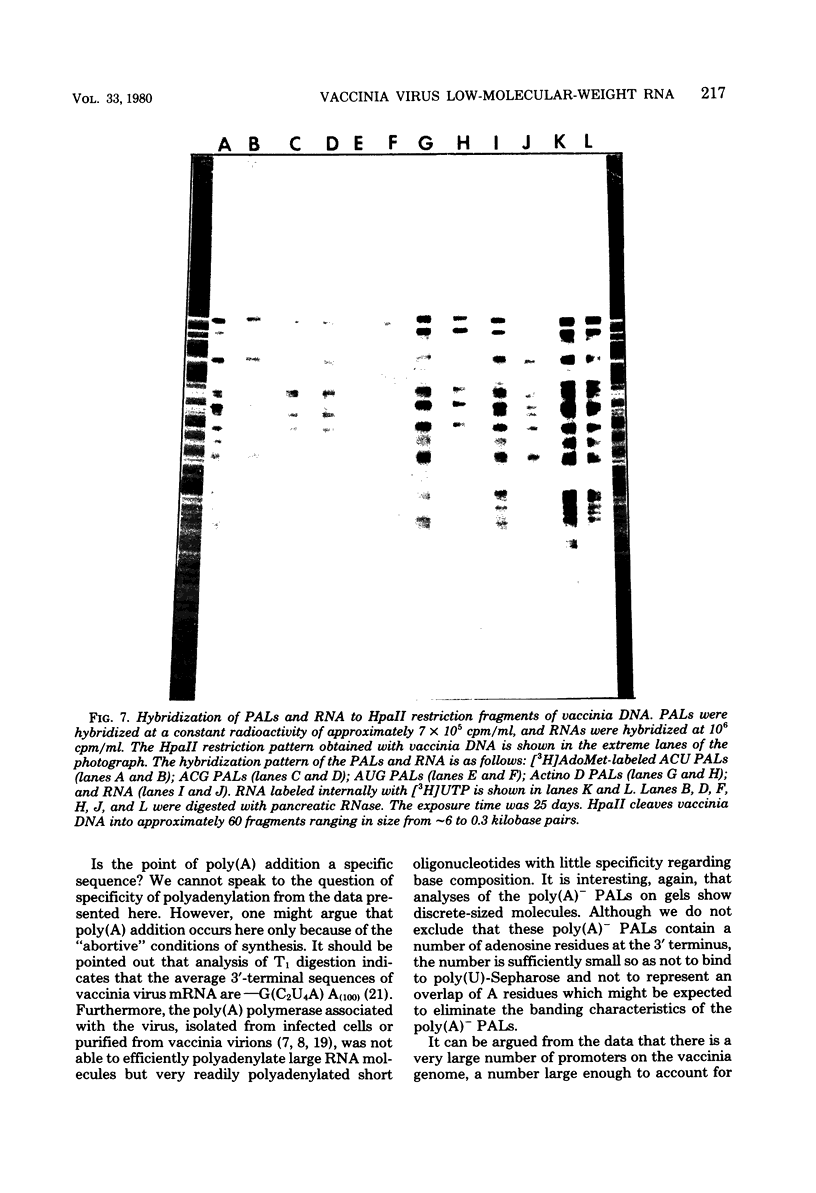


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Dhar R., Laub O., Horowitz M., Khoury G. Novel mechanism for RNA maturation: the leader sequences of simian virus 40 mRNA are not transcribed adjacent to the coding sequences. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3686–3690. doi: 10.1073/pnas.74.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benecke B. J., Penman S. A new class of small nuclear RNA molecules synthesized by a type I RNA polymerase in HeLa cells. Cell. 1977 Dec;12(4):939–946. doi: 10.1016/0092-8674(77)90158-1. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Bossart W., Paoletti E., Nuss D. L. Cell-free translation of purified virion-associated high-molecular-weight RNA synthesized in vitro by vaccinia virus. J Virol. 1978 Dec;28(3):905–916. doi: 10.1128/jvi.28.3.905-916.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakel C., Kates J. R. Poly(A) polymerase from vaccinia virus-infected cells. II. Product and primer characterization. J Virol. 1974 Oct;14(4):724–732. doi: 10.1128/jvi.14.4.724-732.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Dorson J. W., Bollum F. J. Terminal riboadenylate transferase: a poly A polymerase in purified vaccinia virus. J Virol. 1973 Aug;12(2):203–208. doi: 10.1128/jvi.12.2.203-208.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C. V., Esteban M. Procedure for purification of intact DNA from vaccinia virus. J Virol. 1978 Jan;25(1):442–445. doi: 10.1128/jvi.25.1.442-445.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Martin S. A., Paoletti E., Moss B. Modification of the 5'-terminus of mRNA by soluble guanylyl and methyl transferases from vaccinia virus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2525–2529. doi: 10.1073/pnas.72.7.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas R. E., Roberts R. J. One predominant 5'-undecanucleotide in adenovirus 2 late messenger RNAs. Cell. 1977 Jul;11(3):533–544. doi: 10.1016/0092-8674(77)90071-x. [DOI] [PubMed] [Google Scholar]
- Gershowitz A., Boone R. F., Moss B. Multiple roles for ATP in the synthesis and processing of mRNA by vaccinia virus: specific inhibitory effects of adenosine (beta,gamma-imido) triphosphate. J Virol. 1978 Aug;27(2):399–408. doi: 10.1128/jvi.27.2.399-408.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. M., Parsonage M. T., Pelham H. R., Darby G. Heat inactivation of vaccinia virus particle-associated functions: properties of heated particles in vivo and in vitro. J Virol. 1978 Jun;26(3):646–659. doi: 10.1128/jvi.26.3.646-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Moss B. mRNA guanylyltransferase and mRNA (guanine-7-)-methyltransferase from vaccinia virions. Donor and acceptor substrate specificites. J Biol Chem. 1976 Dec 10;251(23):7313–7321. [PubMed] [Google Scholar]
- Mathews M. B., Pettersson U. The low molecular weight of RNAs of adenovirus 2-infected cells. J Mol Biol. 1978 Feb 25;119(2):293–328. doi: 10.1016/0022-2836(78)90439-4. [DOI] [PubMed] [Google Scholar]
- Moss B., Gershowitz A., Wei C. M., Boone R. Formation of the guanylylated and methylated 5'-terminus of vaccinia virus mRNA. Virology. 1976 Jul 15;72(2):341–351. doi: 10.1016/0042-6822(76)90163-x. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Gershowitz A. Characterization of a polyriboadenylate polymerase from vaccinia virions. J Biol Chem. 1975 Jun 25;250(12):4722–4729. [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Poly (A) sequences of vaccinia virus messenger RNA: nature, mode of addition and function during translation in vitra and in vivo. Virology. 1975 Jan;63(1):1–14. doi: 10.1016/0042-6822(75)90365-7. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Paoletti E. Methyl group analysis of virion-associated high-molecular-weight RNA synthesized in vitro by purified vaccinia virus. J Virol. 1977 Jul;23(1):110–116. doi: 10.1128/jvi.23.1.110-116.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Grady L. J. Transcriptional complexity of vaccinia virus in vivo and in vitro. J Virol. 1977 Sep;23(3):608–615. doi: 10.1128/jvi.23.3.608-615.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E. High molecular weight virion-associated RNA of vaccinia. A possible precursor to 8 to 12 S mRNA. J Biol Chem. 1977 Feb 10;252(3):872–877. [PubMed] [Google Scholar]
- Paoletti E. In vitro synthesis of a high molecular weight virion-associated RNA by vaccinia. J Biol Chem. 1977 Feb 10;252(3):866–871. [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R. Soluble endoribonuclease activity from vaccinia virus: specific cleavage of virion-associated high-molecular-weight RNA. J Virol. 1978 Jun;26(3):822–824. doi: 10.1128/jvi.26.3.822-824.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R. The role of ATP in the biogenesis of vaccinia virus mRNA in vitro. Virology. 1978 Jun 15;87(2):317–325. doi: 10.1016/0042-6822(78)90137-x. [DOI] [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Moss B. Inhibition of host protein synthesis by vaccinia virus: fate of cell mRNA and synthesis of small poly (A)-rich polyribonucleotides in the presence of actinomycin D. J Virol. 1975 Jul;16(1):34–42. doi: 10.1128/jvi.16.1.34-42.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J., Losick R. Cloned Bacillus subtilis DNA containing a gene that is activated early during sporulation. Cell. 1977 Aug;11(4):751–761. doi: 10.1016/0092-8674(77)90289-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stafford D. W., Bieber D. Concentration of DNA solutions by extraction with 2-butanol. Biochim Biophys Acta. 1975 Jan 6;378(1):18–21. doi: 10.1016/0005-2787(75)90132-x. [DOI] [PubMed] [Google Scholar]
- Söderlund H., Pettersson U., Vennström B., Philipson L., Mathews M. B. A new species of virus-coded low molecular weight RNA from cells infected with adenovirus type 2. Cell. 1976 Apr;7(4):585–593. doi: 10.1016/0092-8674(76)90209-9. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. A micromethod for base analysis of 32P-labeled oligoribonulcleotides. Anal Biochem. 1977 Nov;83(1):222–227. doi: 10.1016/0003-2697(77)90530-9. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylated nucleotides block 5'-terminus of vaccinia virus messenger RNA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):318–322. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Zieve G., Penman S. Small RNA species of the HeLa cell: metabolism and subcellular localization. Cell. 1976 May;8(1):19–31. doi: 10.1016/0092-8674(76)90181-1. [DOI] [PubMed] [Google Scholar]