Abstract
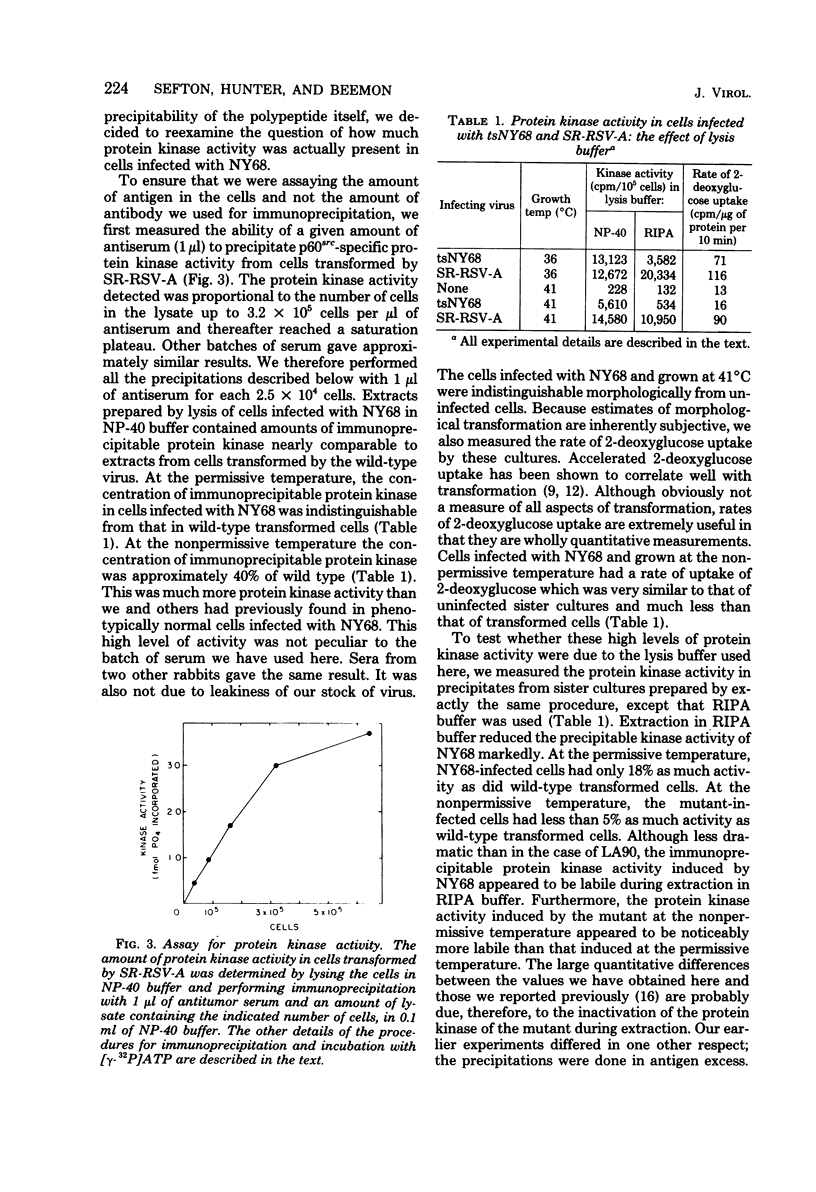
The transforming protein of Rous sarcoma virus, p60src, has associated with it a protein kinase activity. We examined whether a correlation exists between the cellular concentration of enzymatically active p60src and the degree to which chick cells are transformed by mutants of Rous sarcoma virus which are temperature-sensitive for transformation. Such a correlation does exist, but cells infected with some mutants could be shown to contain, at the nonpermissive temperature, an amount of protein kinase activity equal to 30 to 40% of that in a wild-type transformed cell. We quantified the amount of virus-induced protein kinase activity by precipitation of p60src with an excess of antitumor antiserum. Our initial measurements of activity were serious underestimates, due to the lability of the protein kinase activity associated with p60src of at least four temperature-sensitive mutants. In fact, no activity at all was associated with p60src of tsLA90 when immunoprecipitation was performed by standard means. However, when immunoprecipitation was performed with procedures which minimize inactivation, it became apparent both that cells transformed by tsLA90 contained protein kinase activity and that cells infected with either NY68 or BK5 contained at the nonpermissive temperature, one-third to one-half as much activity as wild-type transformed cells. This level of activity was much more than that arising from p60sarc in uninfected cells. In uninfected cells we found an amount of protein kinase activity which varied from 3 to 5% as much as that in a virally transformed cell. The lability of the protein kinase activity of each of these mutants is a further demonstration that this activity is essential for the transformation of cells by Rous sarcoma virus. So as to explain the high protein kinase levels in cells infected with NY68 and BK5 at the nonpermissive temperature, the idea that transformation may be a response to a small quantitative change in the total activity of p60src and the possibility that there may be more than one viral function which is essential for transformation are discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V. R., Chen L. B., Buchanan J. M. Surface ruffles as markers for studies of cell transformation by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3144–3148. doi: 10.1073/pnas.72.8.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., Kurth R., Critchley D., Friis R., Bauer H. Distinguishable transformation-defective phenotypes among temperature-sensitive mutants of Rous sarcoma virus. J Virol. 1977 Mar;21(3):1042–1055. doi: 10.1128/jvi.21.3.1042-1055.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Hayward W. S., Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. doi: 10.1073/pnas.76.7.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen H., Friis R. R., Bauer H. Src Gene product from different strains of avian sarcoma virus: Kinetics and possible mechanism of heat inactivation of protein kinase activity from cells infected by transformation-defective, temperature-sensitive mutant and wild-type virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):967–971. doi: 10.1073/pnas.76.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Halpern C. C., Nadel M., Hanafusa H. Recombination between viral and cellular sequences generates transforming sarcoma virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5812–5816. doi: 10.1073/pnas.75.12.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke J. A., Linial M. Temperature-sensitive avian sarcoma viruses: a physiological comparison of twenty mutants. Virology. 1973 May;53(1):152–161. doi: 10.1016/0042-6822(73)90474-1. [DOI] [PubMed] [Google Scholar]