Abstract
In the last four decades, advances in neurosurgical technique, delivery of radiation therapy (RT), supportive care, and use of chemotherapy have improved 5-year survival for children with central nervous system (CNS) malignancies. Currently, in the United States 74% of children will become 5-year survivors of their primary CNS malignancy. This improved outcome has resulted in a new and growing population of childhood cancer survivors. Surgery, RT and chemotherapy, while essential components of primary treatment for most childhood CNS malignancies, have also been associated with risk of long-term morbidity and late mortality. The Childhood Cancer Survivor Study, a retrospective cohort of over 14,000 survivors of childhood cancer diagnosed between 1970 and 1986, has been an important resource for quantification of associations between these therapeutic modalities and risk of long-term adverse health and quality of life outcomes. CNS malignancy survivors are at significant risk for late mortality, development of second neoplasms, as well as increased risk for multiple endocrinopathies and adverse neurologic health conditions. Importantly, the CCSS has identified a number of dose-response relationships between RT and development of subsequent malignant neoplasms of the central nervous system, abnormal timing of menarche and neurocognitive function. Ongoing study of childhood cancer survivors is needed to establish long-term risks and evaluate impact of newer techniques such as conformal RT or proton beam delivery that limit RT exposure and may reduce long-term effects.
Introduction
Children diagnosed with central nervous system (CNS) malignancies traditionally have had poor survival rates. Over the last four decades, improvements in neurosurgical technique, supportive care, radiation delivery and the use of combination chemotherapy have resulted in improvements in survival of these children. The most recent estimates from SEER suggest that in the modern era 74% will become 5-year survivors.1 The National Cancer Institute's Office of Cancer Survivorship estimates that as of January 1, 2005, there were over 328,000 survivors of childhood cancer in the United States, including large numbers of survivors of CNS tumors (51,650), acute lymphoblastic leukemia (ALL, 49,271), germ cell tumors (34,169) and Hodgkin lymphoma (31,598).2 The impact of this growing number of cancer survivors is made more apparent when one considers that 1 in 900 people in the United States is a survivor of childhood cancer and that within the 20-50 year age group that ratio decreases to 1 in 680 people.
This has led to a growing population who are now at risk for late mortality, second neoplasms, organ dysfunction, impaired growth and development, impaired cognitive function, difficulties obtaining employment and insurance and overall reduction in quality of life.3-13 While the causes of this increased risk for poor late outcomes are often multifactorial (including: underlying genetic predisposition, pre-morbid conditions, host demographic factors such as age, sex and race, and health behaviors), it is the location of the primary malignancy within the CNS and its subsequent treatment (surgery, radiation, chemotherapy) that are the most significant contributors to poor long-term health outcomes. Since 1994 the Childhood Cancer Survivor Study (CCSS), a retrospective cohort with longitudinal follow-up, has described many of these risks for poor outcome among survivors of CNS malignancies and identified specific associations with chemotherapy and CNS-directed RT.
Identification of Prevalence and Risk for Late-Effects: The Childhood Cancer Survivors Study
Established in 1994 as an NCI-funded resource, the CCSS is a retrospective cohort of 5-year survivors of childhood cancer from 26 institutions in the United States and Canada3. Eligibility criteria include: diagnosis between January 1, 1970 and December 31, 1986, survival at five years from date of diagnosis regardless of disease or treatment status, with restriction to specific diagnoses including: leukemia, central nervous system (CNS) cancer, Hodgkin and non-Hodgkin lymphoma, renal tumors, neuroblastoma, soft-tissue sarcomas and bone tumors. Of the 20,720 eligible survivors identified, 2,888 were survivors of CNS malignancies and were assessed for late mortality outcomes. Ultimately, 17% could not be located and 17.7% refused participation resulting in 1877 survivors of CNS malignancies who completed the baseline questionnaire.14 This time period (1970-1986) represents an era where surgery alone (431 patients, 26.0%) or surgery followed by radiation therapy (689 patients, 41.6%) were the predominant treatment modalities employed, and the use of chemotherapy (447 patients, 27%) as adjuvant therapy was in its infancy. To provide a comparison population, a randomly selected cohort of siblings of survivors was constructed. Information collected on the sibling cohort (n = 3899), with the exception of cancer-specific topics, was identical to that obtained from the survivor population.
Essential to the identification of associations between cancer treatment and long-term health outcomes is high-quality abstraction of cancer therapy. Each of the CCSS institutions was commissioned to abstract detailed records for surgical procedures and chemotherapy administration, including quantification of dose for many chemotherapeutics. For patients who had radiation therapy, the full radiation record was forwarded to the CCSS Radiation Physics Center at the MD Anderson Cancer Center for central review to quantify radiation exposure to the frontal, temporal, and occipital lobes of the brain as well as to the posterior fossa, with maximum radiation dose estimated for each region.15 The dose estimates were based on measurements in tissue-equivalent phantoms and calculations in three-dimensional mathematical phantom. Mathematical phantoms were designed to simulate children of any age and size. Details of the radiation dosimetry methods are described in Stovall et al.16 Acquisition of such detailed dose and location in such a large population has allowed CCSS investigators to identify key associations with late mortality, development of second neoplasms, as well as numerous other chronic health conditions.
Overall and Cause-Specific Mortality
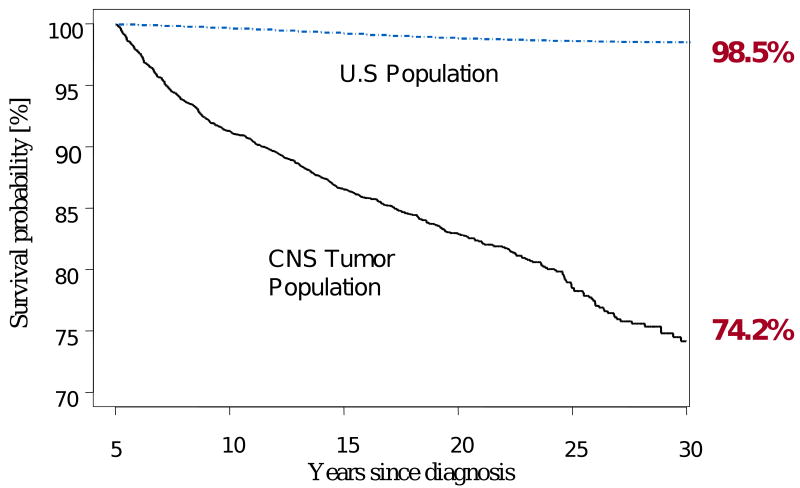
While survival to five years from the time of diagnosis is a common benchmark used for therapeutic trials, children with CNS tumors are at significant risk for late mortality well beyond the 5-year time point.17 Their mortality rate beyond five years from the time of survival is almost 13 times higher (standardized mortality ratio [SMR] 12.9, 95% confidence interval [95% CI] 11.8 - 14.0) than that of the age- and sex-matched U.S. population. The time of highest risk is the 5-9 years from diagnosis period (SMR 28.0, 95% CI 24.7 - 31.8) with decreasing risk thereafter, however, even at 30-34 years from diagnosis standardized deaths rates are elevated (SMR 8.4, 95% CI 2.7-19.6). In addition, children diagnosed in the first three years of life (SMR 17.2, 95% CI 14.7 to 20.1) are at highest risk, as well as females when compared to males (SMR 19.6, 95% CI 17.0-22.5 vs. 10.6, 9.5-11.8, Figure 1).
Figure 1.
All cause mortality among 5-year survivors of childhood CNS malignancies compared with age-adjusted US population
Unfortunately, the cumulative incidence of all-cause mortality continues to increase at a steady rate as this cohort ages (13.5%, 17.1%, 21.5%, and 25.8% at 15, 20, 25, and 30 years respectively, Figure 1) and this is largely a product of late recurrence or progression of the primary CNS malignancy.14 Recurrence or progression of the primary tumor was the underlying cause of death in 61% of the deaths in this cohort, resulting in a cumulative incidence of death of 14.0% (95% CI 12.5-15.6) at 30 years from diagnosis. While most recurrences occurred in the first 10 years from diagnosis, many deaths attributed to recurrence occurred beyond this point. In fact, the death rate due to recurrence or progression of primary disease was higher than all other causes of death until 20 years from diagnosis, when the rate of deaths attributable to subsequent neoplasms became predominant. Other studies have similarly noted a propensity for late occurring death from primary recurrence.18-20 Other medical causes of death included deaths attributable to subsequent neoplasms (cumulative incidence 2.8% at 30 years, 95% CI 1.8-3.8), and pulmonary (1.05, 95% CI 0.3-1.7) and cardiac (0.4%, 95% CI 0.2-0.7). When assessed by primary diagnosis, survivors with medulloblastoma (SMR 17.4, 95% CI 14.6-20.6) and ependymoma (SMR 15.9, 95%) have a significantly higher mortality rate than those with astrocytomas and glial tumors (SMR 11.2 10.1-12.5). As few patients with high grade glioma survive beyond the 5-year time point, most of these cases in this study were low grade lesions, and yet, the survival probability at 30 years (76.1%, 95%CI 73.3-79.0) documents that with time, even patients with low grade glioma with known excellent 5-year survival rates, have a significant risk of late death. At 30 years, survival probabilities for medulloblastoma (71.0%, 95% CI 64.5-76.7) and ependymoma (70.5%, 95% CI 62.5-78.5) were similar.
Subsequent Neoplasms
Development of subsequent neoplasms is an established late effect of childhood cancer and its treatment that, unfortunately, increases in risk with time from diagnosis.21 Within the CCSS cohort of survivors of CNS malignancies, there have been 76 subsequent malignant neoplasms (SMNs, self-reported, with histopathologic confirmation), among 1877 survivors of CNS malignancies, occurring at a median of 16 years from diagnosis. This represents a four-fold increase over the general population (standardized incidence ratio [SIR] 4.1, 95% CI 3.2-4.2).14 Most commonly seen are second malignancies in the CNS (20 observed, SIR 25.3, 95% CI 15.5-39.1), thyroid cancer (12 observed, SIR 11.2, 95% CI 5.8-19.6), and soft tissue sarcomas (8 observed, SIR 8.4, 95% CI 3.6-16.5).
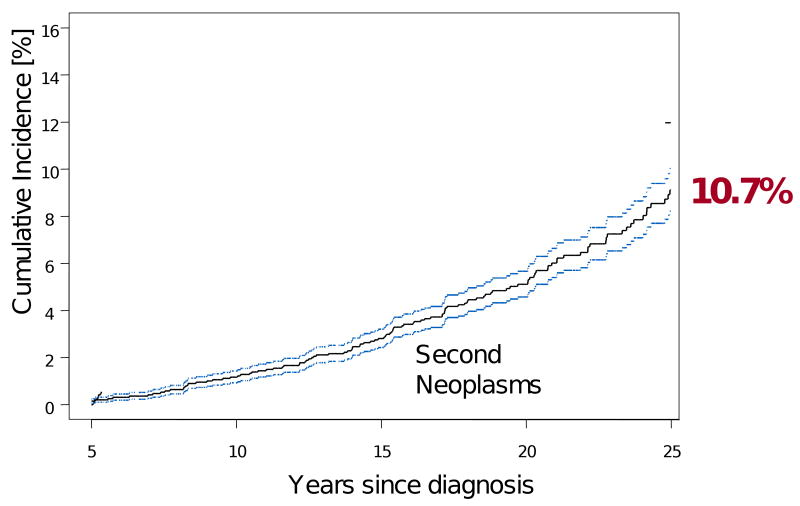
At 25 years from diagnosis, the cumulative incidence of all subsequent neoplasms is 10.7% (95% CI 8.8-12.6, Figure 2). However, benign second neoplasms make up a significant proportion of these second tumors. This cohort reported 112 non-melanoma skin cancers (cumulative incidence 2.9% at 25 years) and 59 benign meningiomas (cumulative incidence 3.3% at 25 years). Of concern, the incidence of these meningiomas increases sharply with time such that even among patients who are meningioma-free at 25 years, the 30 year cumulative incidence is 3.5% (95% CI 0.9-6.1). This increased rate over time clarifies the need for long-term follow-up of these patients and raises suspicion that screening neuroimaging modalities should be employed well beyond the immediate post-diagnosis period.
Figure 2.
Cumulative incidence of subsequent neoplasms in 5-year survivors of childhood cancer
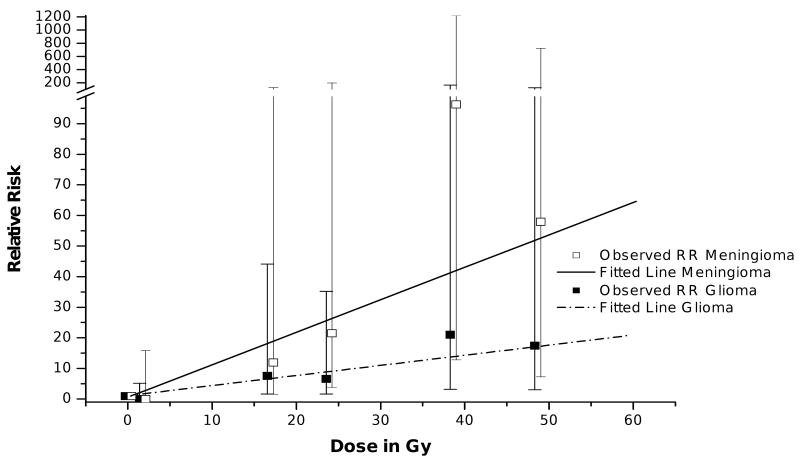
Radiation has been associated with an increased risk for subsequent neoplasms. The CCSS identified that survivors of CNS malignancies who received CNS directed radiation >50 Gy had a cumulative incidence of a subsequent CNS neoplasm of 7.1% (95% CI 4.5-9.6) at 25 years compared to 1.0% (95% CI 0.0-2.3) among those who received no CNS radiation.14 One of the major contributions from CCSS investigations has been demonstration of dose-response relationships, which improve the strength of evidence for a causal relationship between RT and the development of SMNs. To further investigate the dose effect of RT on the development of subsequent CNS tumors, investigators used a nested case-control method to match 116 individuals identified with a subsequent central nervous system neoplasms (from the entire CCSS cohort) with control subjects matched on age, sex, and time since original cancer diagnosis22. RT exposure was again associated with an increased risk for any subsequent CNS malignant neoplasm, and specifically for subsequent gliomas (odds ratio [OR] = 6.78, 95% CI = 1.54 -29.7) and meningiomas (OR = 9.94, 95% CI = 2.17-45.6). Importantly, linear dose-response relationships between RT dose and the development of both gliomas and meningiomas were identified and were statistically significant. The excess relative risk per Gy, equal to the dose of the linear response function, was 0.33 (95% CI=0.07-1.71) per Gy for gliomas, and 1.06 (95% CI=0.21-8.15) per Gy for meningiomas. After adjustment of radiation dose, there were no statistically significant associations between chemotherapy exposure and the development of a CNS subsequent neoplasm.
Chronic Medical Conditions
The location of a tumor within the CNS, the surgical procedure necessary to biopsy or resect these lesions, as well as subsequent radiation or chemotherapy, place patients at high risk for long-term neurological morbidity. Location of the tumor in the diencephalon, at or near the hypothalamus, may increase the risk for multiple endocrinopathies. In addition, use of craniospinal radiation, that may include some part of the heart border near the radiation field, may place certain patients at increased risk for subsequent cardiomyopathy or coronary vascular compromise. For these reasons, survivors of CNS tumors may have multiple chronic medical conditions developing any time from tumor presentation through treatment and long-term follow-up. The CCSS has used the Common Terminology Criteria for Adverse Events scoring system, developed by the National Cancer Institute, to determine the severity of these conditions.23 At the time of study eligibility (5 years from diagnosis), 82% of CNS tumor survivors reported at least one chronic medical condition of any grade and were more likely than siblings to have chronic medical conditions, including: endocrine complications (Prevalence Ratio [PR] 49.1, 95% CI 27.6-87.2), neurologic complications (PR 37.1, 95% CI 28.3-48.7), or any complication (PR 29.5, 95% CI 23.8-87.2).14 Importantly, however, 5-year survival does not mark the end of such medical problems. Beyond the 5-year time point, survivors are also at greater risk than their siblings of developing new onset of medical conditions including: endocrine complications (Hazard Ratio [HR] 19.8, 95% CI 14.5-27.1), neurologic complications (HR 5.6, 95% CI 4.8-6.7), and any condition (HR 6.4, 5.4-7.5).
Considering the multi-modality therapy CNS survivors receive, assessing the independent role of radiotherapy in development of late endocrine effects is difficult due to multiple confounding factors. Gurney et. al. showed that CNS survivors who had surgery alone had a lower risk for developing hypothyroidism (RR 0.3, 95% CI 0.2-0.7) and growth hormone deficiency (RR 0.2, 95% CI 0.1-0.5), compared to survivors who received surgery and radiation therapy.24 RT to the hypothalamic-pituitary axis was also demonstrated to be associated with an increased risk for short stature (ht <10th percentile of population norms).25 Notably, almost 40% of CNS tumor survivors had short stature.
Female CNS survivors who received RT are also at increased risk for abnormal timing of menarche.26 A population of 235 survivors diagnosed and treated prior to menarche had higher rates than siblings of both early onset of menarche (before 10 years of age, 11.9% vs. 1%, OR 14.1, 95% CI 7.0-30.9) and delayed menarche (10.6% vs. 1.9%, OR 6.6, 95% CI 3.4-11.4). Those who receive radiation directed at the hypothalamic-pituitary axis had a higher risk for early menarche (OR 3.8, 95% CI 1.2-16.5) than survivors with no radiation exposure. Those who had ≥50 Gy to this axis had an increased risk (OR 6.90, 95% CI 1.46-49.57) for delayed menarche, and this risk was almost doubled in those who additionally received spinal radiation (OR 12.40, 95% CI 2.66-89.64).
Late neurologic conditions are common in this population and continue to increase with time well beyond the 5-year time point. Survivors are at a continued increased risk for new onset of seizures (HR 15.1, 95% CI 10.7-21.2), weakness in arms or legs (HR 12.2, 95% CI 9.1-16.3), blindness (HR 7.5, 95% CI 4.1-13.5), and hearing loss (HR 21.0, 95% CI 12.9-34.2).14 In fact, among the population of survivors who had not had a previous seizure by 5 years from diagnosis, 33% would report a new onset of seizure beyond the 5-year time point. Similarly, 16% would report new onset of blindness. In additional analysis by Packer et. al. those who received more than 50 Gy radiation to the posterior fossa had a higher likelihood of developing hearing impairment (RR 3.7, 95% CI 1.8-7.8) compared to those who received <30 Gy.15 Additionally, radiation dose of ≥30 Gy or more to any cortical region was associated with a two-fold elevated risk of seizure.
Neurocognitive Outcomes
While CNS tumor survivors in the CCSS did not receive direct neuropsychological testing, assessment of self-reported neurocognitive function was achieved utilizing the CCSS Neurocognitive Questionnaire (NCQ).27 The CCSS NCQ was developed based on the Brief Rating Inventory of Executive Function, a multidimensional standardized rating inventory for children and adults.28 The CCSS NCQ has four domains that are reliable and valid: Task Efficiency (attention and processing speed), Emotional Regulation, Organization and Memory.27 The CCSS NCQ was also administered to siblings and the 10th percentile and below for sibling performance was defined as “impairment”. Survivors of astrocytomas who received no RT showed high rates of impairment in attention/processing speed (31.6%), with increases in impairment based on RT exposure: 1-50 Gy, 48.6%; >50 Gy, 53.7%. Similar increases in impairment in memory were seen with increasing RT dose: 15.8%, no RT; 30.0%, 1-50 Gy; 37.4%, >50Gy). Over 40% of medulloblastoma survivors had impairment in attention processing speed regardless of RT dose exposure. A more recent analysis of these data identified that in addition to RT exposure, presence of a ventriculoperitoneal shunt increased the risk of impairment in attention/processing speed and memory. In addition, survivors who experienced hearing impairment, paralysis or stroke reported a greater likelihood of impairment on all CCSS NCQ domains.29
Sociodemographic, Psychological, & Quality of Life Outcomes
The true impact of these neurocognitive deficits is not understood without properly assessing the current function of CNS tumor survivors in society. After controlling for age at diagnosis, sex, and race/ethnicity, siblings were more likely than survivors to report current employment (RR 1.4, 95% CI 1.3-1.5), an income greater than $20,000 (RR 1.2, 95% CI 1.1-1.3), marriage (RR 2.0,95% CI 1.8-2.2), and college graduation (RR 1.4, 95% CI 1.3-1.5).14 In addition, CNS tumor survivors frequently require use of special educations services during childhood, with increasing use based on younger age at diagnosis: age at diagnosis 0-5 years, 70% of survivors access special educational services; 6-10years, 58%; 11-15 years, 32%; 16-20 years, 24%.30
Fortunately, few survivors show signs of long-term psychological distress. Participants completed a standardized, self-report symptom inventory, designed to screen for depression, somatization, and anxiety, the Brief Symptom Inventory-18 (BSI-18). The prevalence of distress approximating clinically significant levels for both survivors (11%) and siblings (5%) reflected rates found in the general population.31 Survivors did report statistically significantly higher rates of global distress, depression and somatic distress than siblings. Notably, treatment intensity was not associated with poor psychological outcome. In general, survivors scored lower than population norms for most aspects of Health Related Quality of Life (HRQOL) as measured by the Medical Outcomes Short Form-36 (SF-36).32 Survivors of CNS tumors report more problems than siblings in specific domains, including physical function, mental function, and general health. While overall survivors of all childhood cancers report high levels of current and predicted life satisfaction, survivors of CNS tumors who predict lower levels of satisfaction 5 years into the future, compared to siblings.32
Conclusion
Improvements in supportive care, neurosurgical technique, RT delivery, as well as use of chemotherapy has improved the overall 5-year survival of children with CNS tumors. However, this new population of long-term survivors remains at high risk for late mortality, most commonly due to late recurrence or progression of the primary CNS tumor. In addition, the rate of development of subsequent neoplasms is increasing with time from diagnosis, and the development of new medial conditions beyond the five-year time point is common. Therefore, survivors of CNS tumors represent a high risk population in need of risk-based follow-up care by physicians aware of the problems they may face. There is a need for evidence-based guidelines to direct such care, though, currently, there is a paucity of data regarding the proper screening procedures for this exposed population. Future research should be directed at establishment of evidence-based guidelines for care of this population, as well as preventative strategies targeting therapeutic reduction where possible.
Figure 3.
Relative Risk of subsequent glioma and meningioma within the CCSS by radiation dose (open boxes, mean observed relative risk for meningioma; closed boxes, mean observed relative risk for glioma; solid line, fitted line for meningioma risk; hatched line, fitted line for glioma risk)
Acknowledgments
This work was supported by the National Cancer Institute (grant number U24-CA55727, to L.L.Robison, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ries L, Eisner MP, Kosary CL. SEER cancer statistics review, 1975-2002. 2005. [Google Scholar]
- 2.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-Term Survivors of Childhood Cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 3.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-Supported Resource for Outcome and Intervention Research. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–27. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Liu Q, Yasui Y, et al. Late Mortality Among 5-Year Survivors of Childhood Cancer: A Summary From The Childhood Cancer Survivor Study. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27:2339–55. doi: 10.1200/JCO.2008.21.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–62. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan PC, Ford JS, Henderson TO, et al. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2363–73. doi: 10.1200/JCO.2008.21.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green DM, Sklar CA, Boice JD, Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374–81. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ness KK, Hudson MM, Ginsberg JP, et al. Physical performance limitations in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2382–9. doi: 10.1200/JCO.2008.21.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2390–5. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2396–404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson MM, Mulrooney DA, Bowers DC, et al. High-risk populations identified in Childhood Cancer Survivor Study investigations: implications for risk-based surveillance. J Clin Oncol. 2009;27:2405–14. doi: 10.1200/JCO.2008.21.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:946–58. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21:3255–61. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 16.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–57. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 17.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–79. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins MM, Kingston JE, Kinnier Wilson LM. Late deaths after treatment for childhood cancer. Arch Dis Child. 1990;65:1356–63. doi: 10.1136/adc.65.12.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor DD, Potish RA. Late deaths following radiotherapy for pediatric tumors. Am J Clin Oncol. 1985;8:472–6. doi: 10.1097/00000421-198512000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson HS, Fears TR, Byrne J. Death during adulthood in survivors of childhood and adolescent cancer. Cancer. 1994;73:3094–102. doi: 10.1002/1097-0142(19940615)73:12<3094::aid-cncr2820731231>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–29. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 22.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–37. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 23.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 24.Gurney JG, Kadan-Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97:663–73. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 25.Gurney JG, Ness KK, Stovall M, et al. Final height and body mass index among adult survivors of childhood brain cancer: childhood cancer survivor study. J Clin Endocrinol Metab. 2003;88:4731–9. doi: 10.1210/jc.2003-030784. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong GT, Whitton JA, Gajjar A, et al. Abnormal timing of menarche in survivors of central nervous system tumors: a Report From the Childhood Cancer Survivor Study. Cancer. 2009;115:2562–2570. doi: 10.1002/cncr.24294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krull KR, Gioia G, Ness KK, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113:2188–97. doi: 10.1002/cncr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gioia G, I PK, G SC, et al. Behavior Rating Inventory of Executive Function. Lutz, Florida: Psychological Assessment Resources, Inc.; 2000. [Google Scholar]
- 29.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009 doi: 10.1037/a0016674. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitby PA, Robison LL, Whitton JA, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2003;97:1115–26. doi: 10.1002/cncr.11117. [DOI] [PubMed] [Google Scholar]
- 31.Zebrack BJ, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2004;22:999–1006. doi: 10.1200/JCO.2004.06.148. [DOI] [PubMed] [Google Scholar]
- 32.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2008;17:435–46. doi: 10.1158/1055-9965.EPI-07-2541. [DOI] [PubMed] [Google Scholar]