Abstract
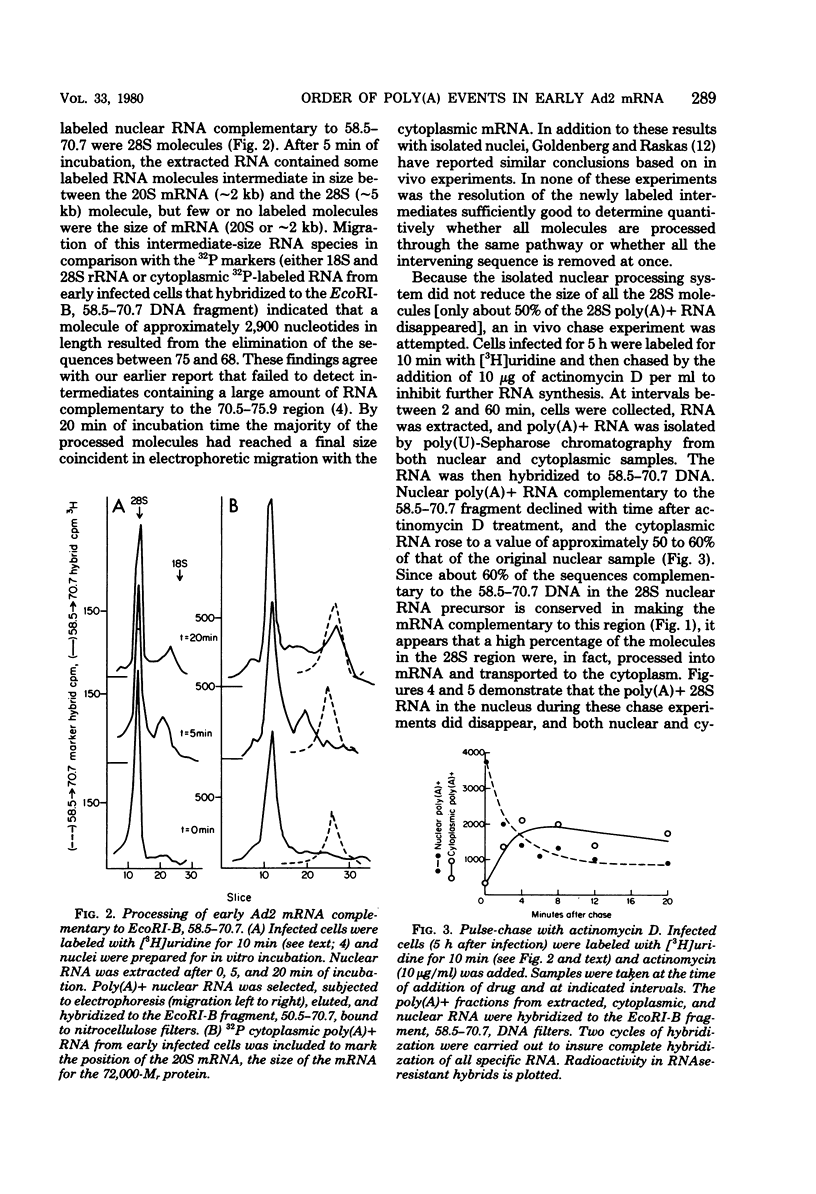
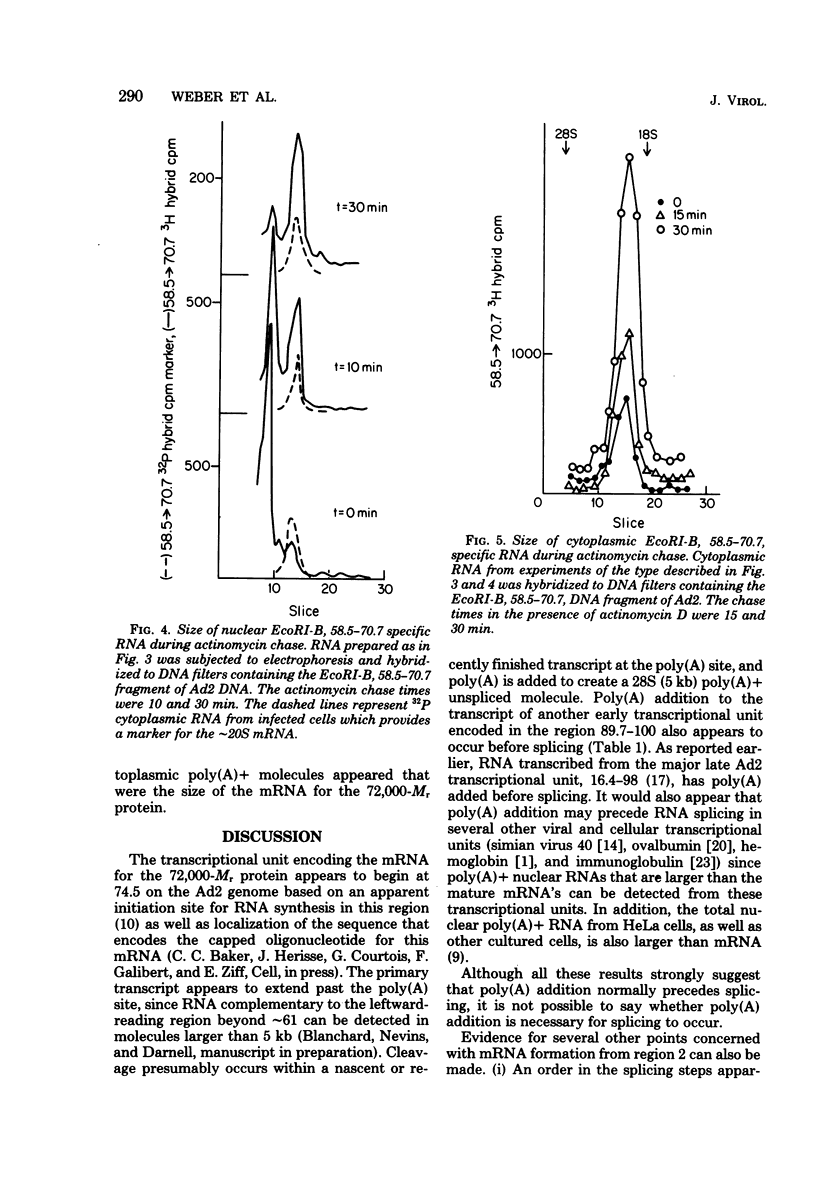
A study of the processing of mRNA from two early adenovirus type 2 transcription units (regions 2 and 4 of adenovirus type 2 genome; [J. Flint, Cell 10:153--166, 1977]) revealed that polyadenylic acid [poly(A)] was added in most if not all cases to an unspliced nuclear RNA molecule whose coordinates extended from the apparent initiation site for RNA synthesis to the single poly(A) site in each transcription unit. An intermediate RNA molecule in the processing of the mRNA for the 72,000-M, single-stranded DNA binding protein showed that the first of the two intervening sequences, the one closest to the 5' end of the molecule, was removed first at least in the majority of the processed molecules. Finally, in cells labeled for 10 min and then treated with actinomycin D to stop further RNA synthesis, the majority, if not all, of the poly(A)-terminated nuclear RNA specific for region 2 was successfully processed and transported to the cytoplasm.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr Hydrolysis of polyadenylic acid by pancreatic ribonuclease. J Biol Chem. 1960 Aug;235:2393–2398. [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Globin RNA precursor molecules: biosynthesis and process in erythroid cells. Cell. 1977 Jul;11(3):641–650. doi: 10.1016/0092-8674(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Blanchard J. M., Weber J., Jelinek W., Darnell J. E. In vitro RNA-RNA splicing in adenovirus 2 mRNA formation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5344–5348. doi: 10.1073/pnas.75.11.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Characteristics and significance of the polyadenylate sequence in mammalian messenger RNA. Prog Nucleic Acid Res Mol Biol. 1976;17:117–148. doi: 10.1016/s0079-6603(08)60068-9. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Nuclear transcripts larger than the cytoplasmic mRNAs are specified by segments of the adenovirus genome coding for early functions. Cell. 1976 Jun;8(2):205–213. doi: 10.1016/0092-8674(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Sayavedra M., Raskas H. J. Strand assignment of polyadenylated nuclear RNAs synthesized early in infection with adenovirus 2. Virology. 1977 Apr;77(2):545–555. doi: 10.1016/0042-6822(77)90480-9. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W., Puckett L., Derman E., Bachenheimer S. Biochemical events in mRNA formation in mammalian cells. Symp Soc Dev Biol. 1976;(34):53–74. [PubMed] [Google Scholar]
- Darnell J. E., Jr Transcription units for mRNA production in eukaryotic cells and their DNA viruses. Prog Nucleic Acid Res Mol Biol. 1979;22:327–353. doi: 10.1016/s0079-6603(08)60803-x. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Fraser N., Ziff E., Weber J., Wilson M., Darnell J. E. The initiation sites for RNA transcription in Ad2 DNA. Cell. 1977 Nov;12(3):733–739. doi: 10.1016/0092-8674(77)90273-2. [DOI] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Goldenberg C. J., Raskas H. J. Splicing patterns of nuclear precursors to the mRNA for adenovirus 2 DNA binding protein. Cell. 1979 Jan;16(1):131–138. doi: 10.1016/0092-8674(79)90194-6. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R., Lai S. P., Westphal H. Loop structures in hybrids of early RNA and the separated strands of adenovirus DNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4392–4395. doi: 10.1073/pnas.74.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Dhar R., Khoury G. Mapping the spliced and unspliced late lytic SV40 RNAs. Cell. 1978 Aug;14(4):971–982. doi: 10.1016/0092-8674(78)90351-3. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett L., Chambers S., Darnell J. E. Short-lived messenger RNA in HeLa cells and its impace on the kinetics of accumulation of cytoplasmic polyadenylate. Proc Natl Acad Sci U S A. 1975 Jan;72(1):389–393. doi: 10.1073/pnas.72.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop D. R., Nordstrom J. L., Tsai S. Y., Tsai M. J., O'Malley B. W. Transcription of structural and intervening sequences in the ovalbumin gene and identification of potential ovalbumin mRNA precursors. Cell. 1978 Oct;15(2):671–685. doi: 10.1016/0092-8674(78)90035-1. [DOI] [PubMed] [Google Scholar]
- Ross J., Knecht D. A. Precursors of alpha and beta globin messenger RNAs. J Mol Biol. 1978 Feb 15;119(1):1–20. doi: 10.1016/0022-2836(78)90266-8. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Jelinek W., Darnell J. E. 3'-Terminal addition to HeLa cell nuclear and cytoplasmic poly (A). J Mol Biol. 1977 Jun 15;113(1):219–235. doi: 10.1016/0022-2836(77)90051-1. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Van Der Vliet P. C., Levine A. J., Ensinger M. J., Ginsberg H. S. Thermolabile DNA binding proteins from cells infected with a temperature-sensitive mutant of adenovrius defective in viral DNA synthesis. J Virol. 1975 Feb;15(2):348–354. doi: 10.1128/jvi.15.2.348-354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Darnell J. E. Presence of cell and virus specific sequences in the same molecules of nuclear RNA from virus transformed cells. Nat New Biol. 1971 Jul 21;232(29):73–76. doi: 10.1038/newbio232073a0. [DOI] [PubMed] [Google Scholar]



