Abstract
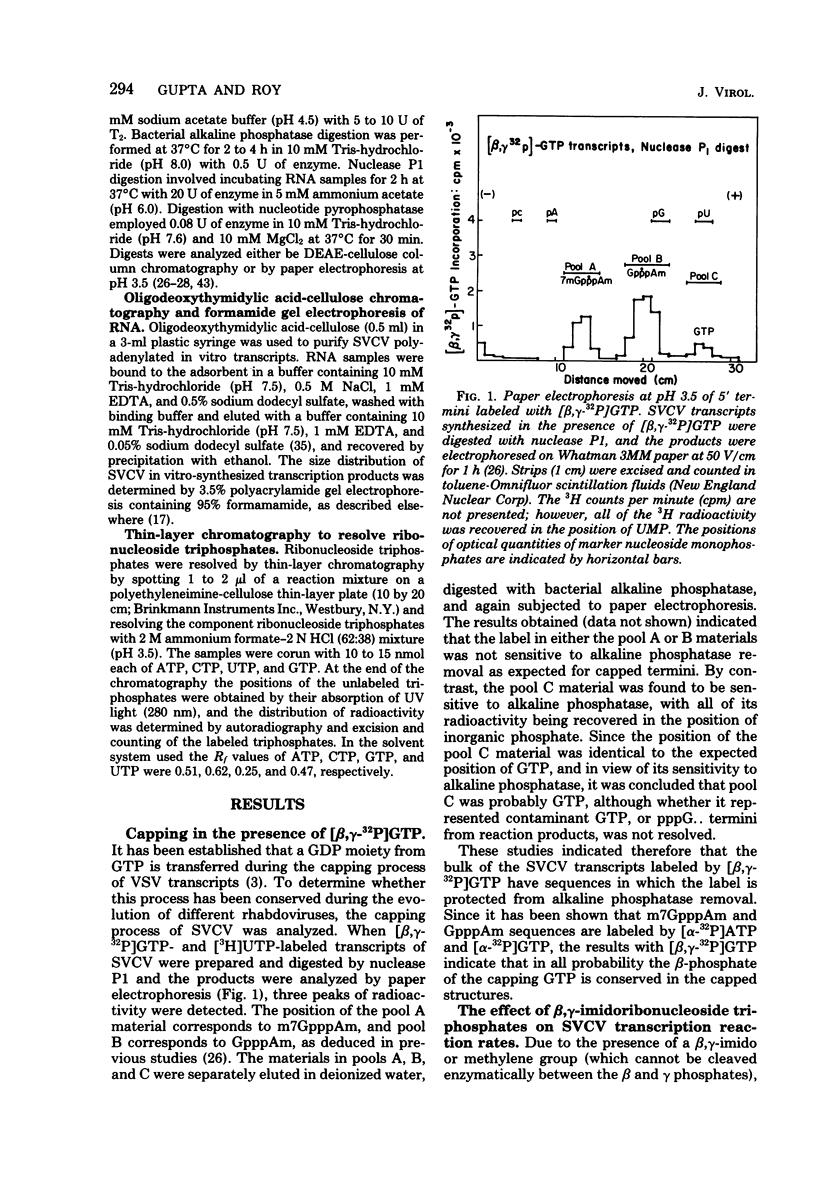
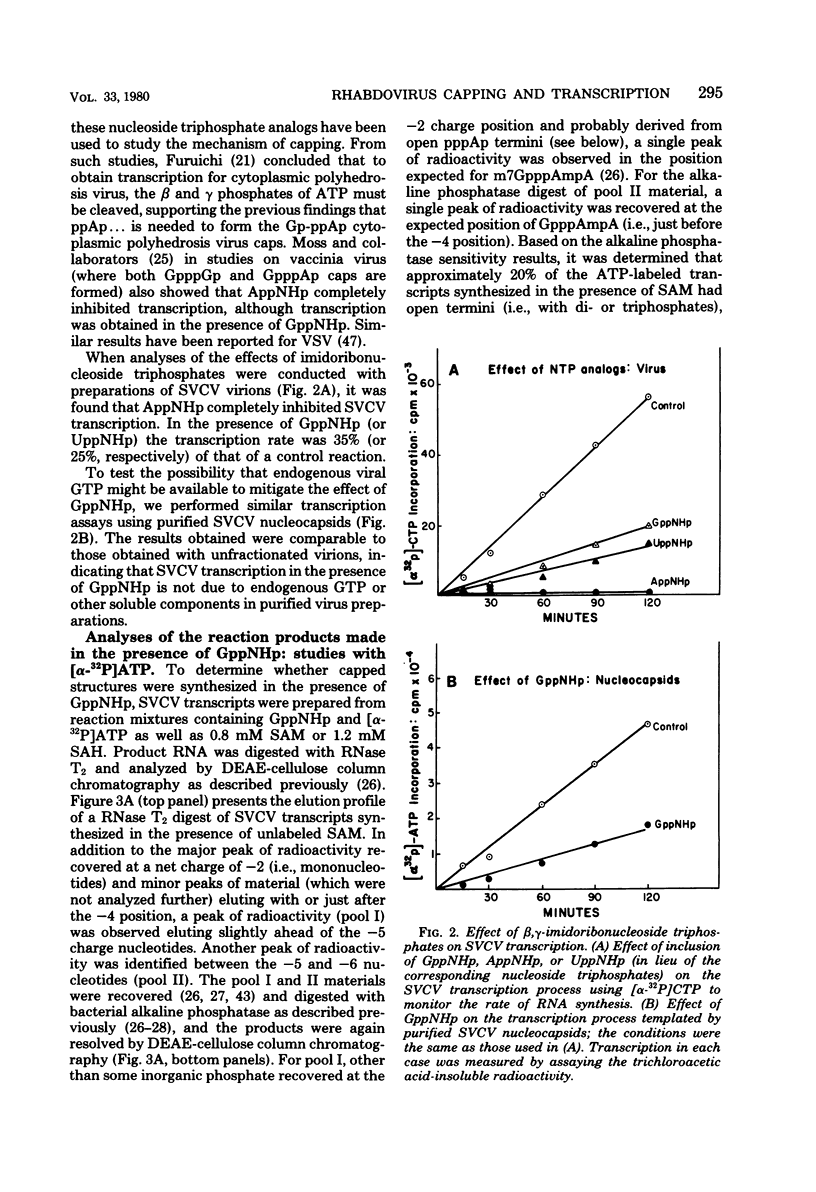
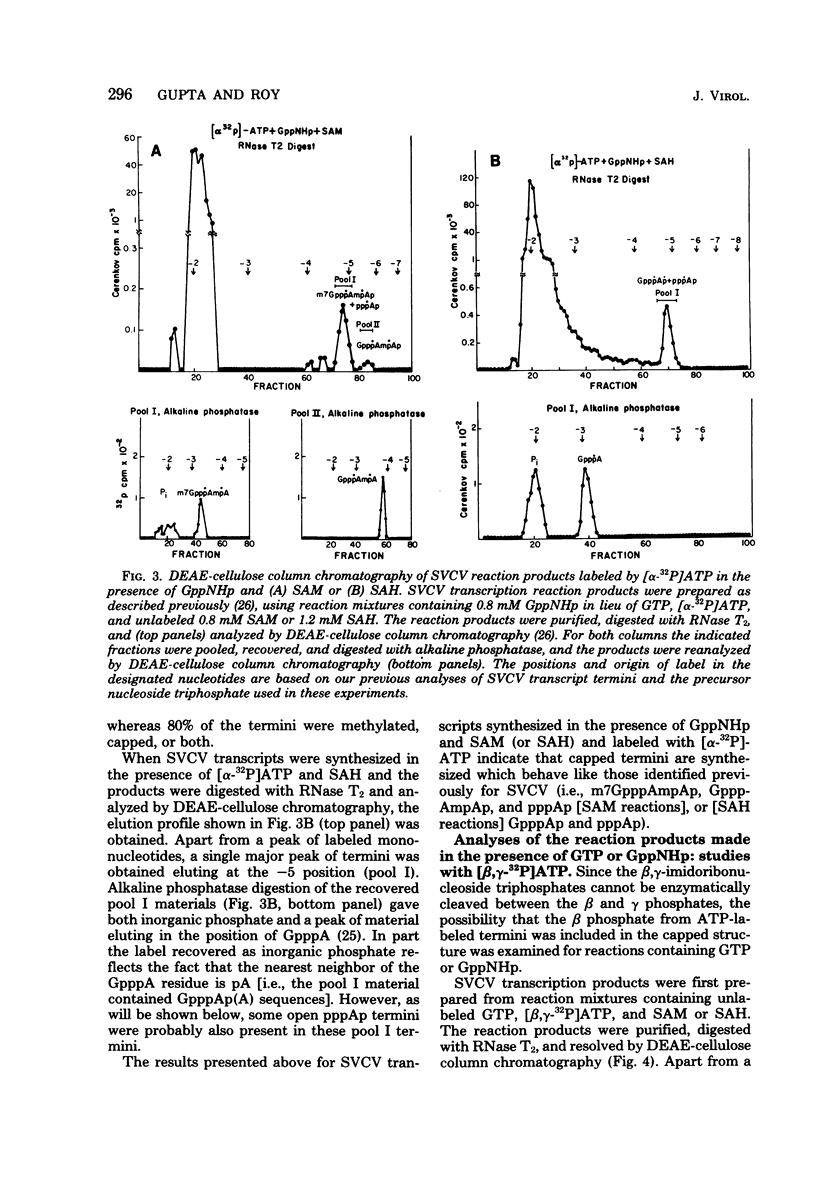
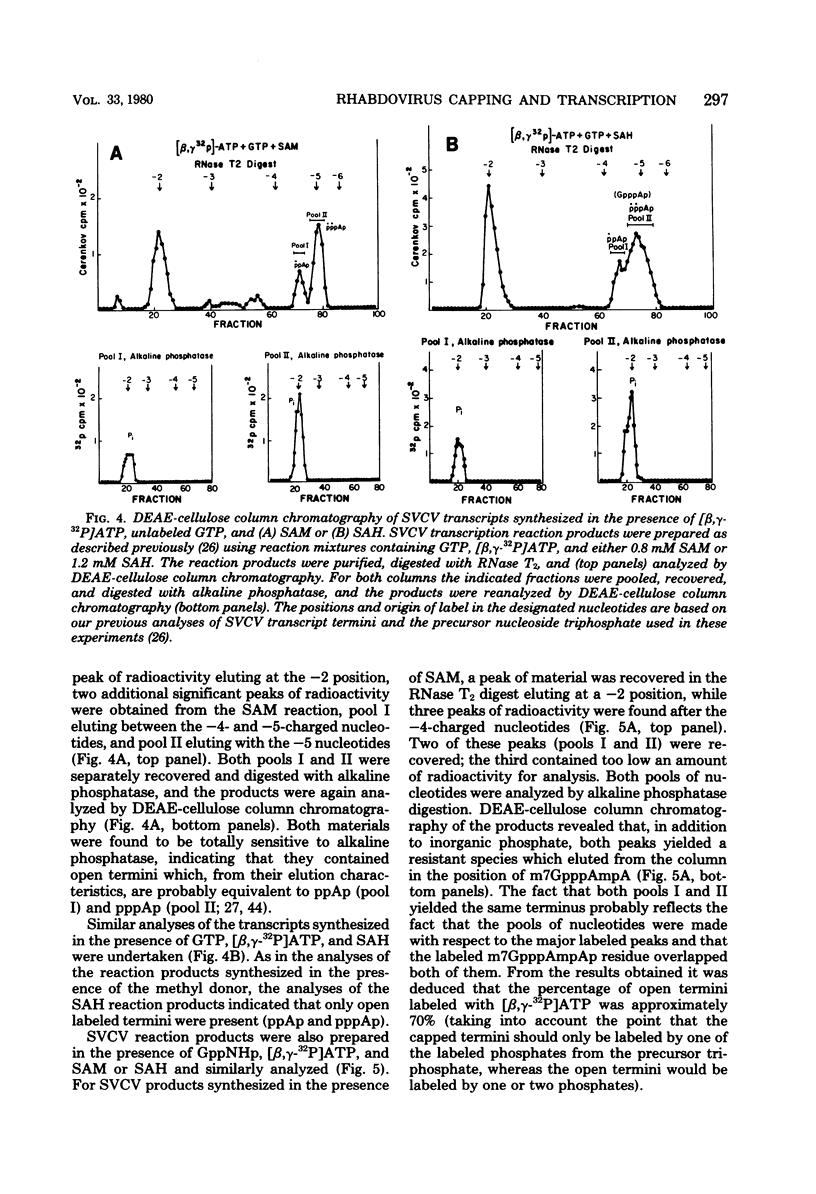
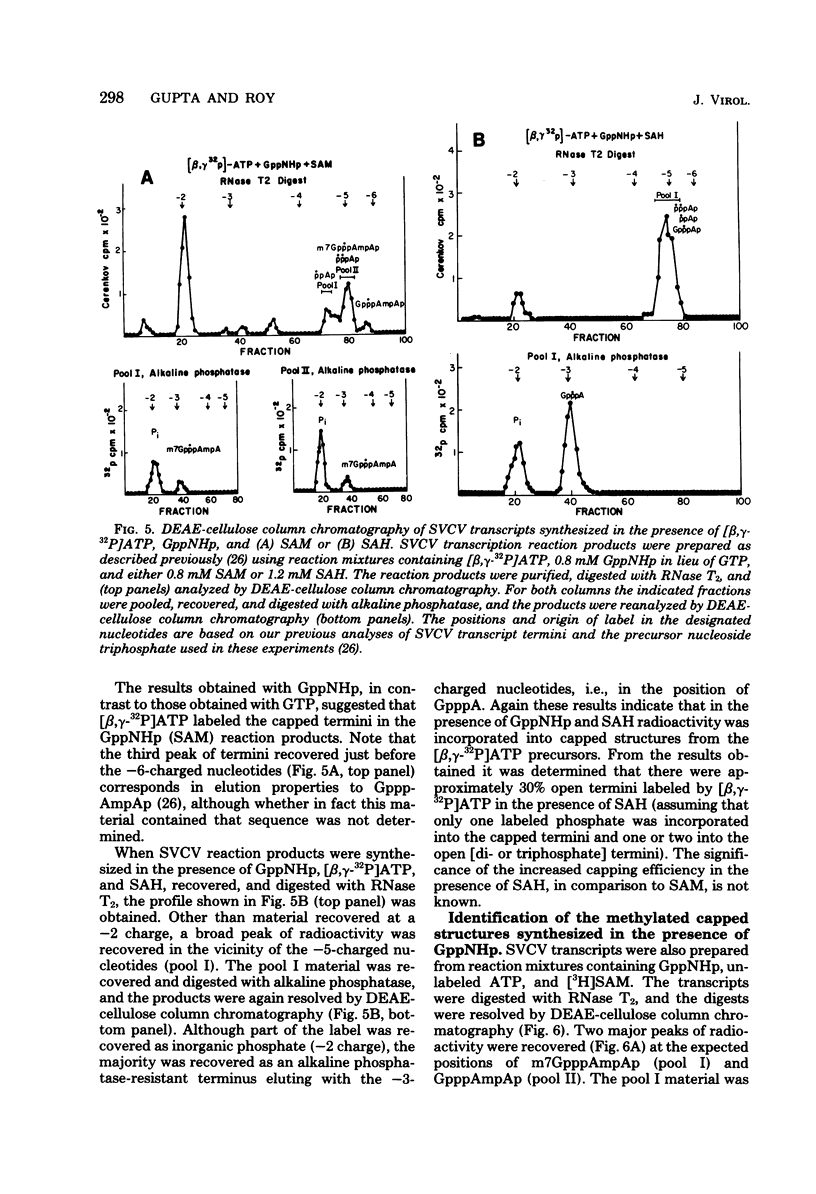
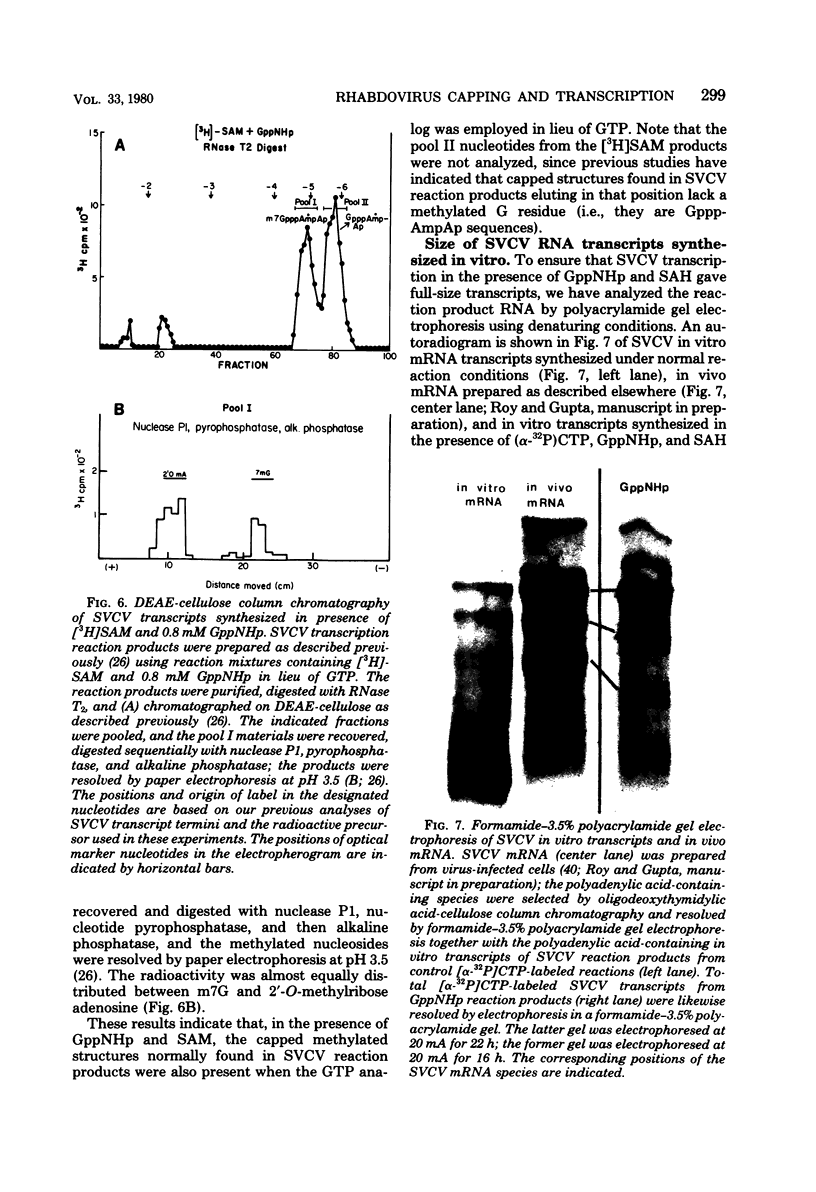
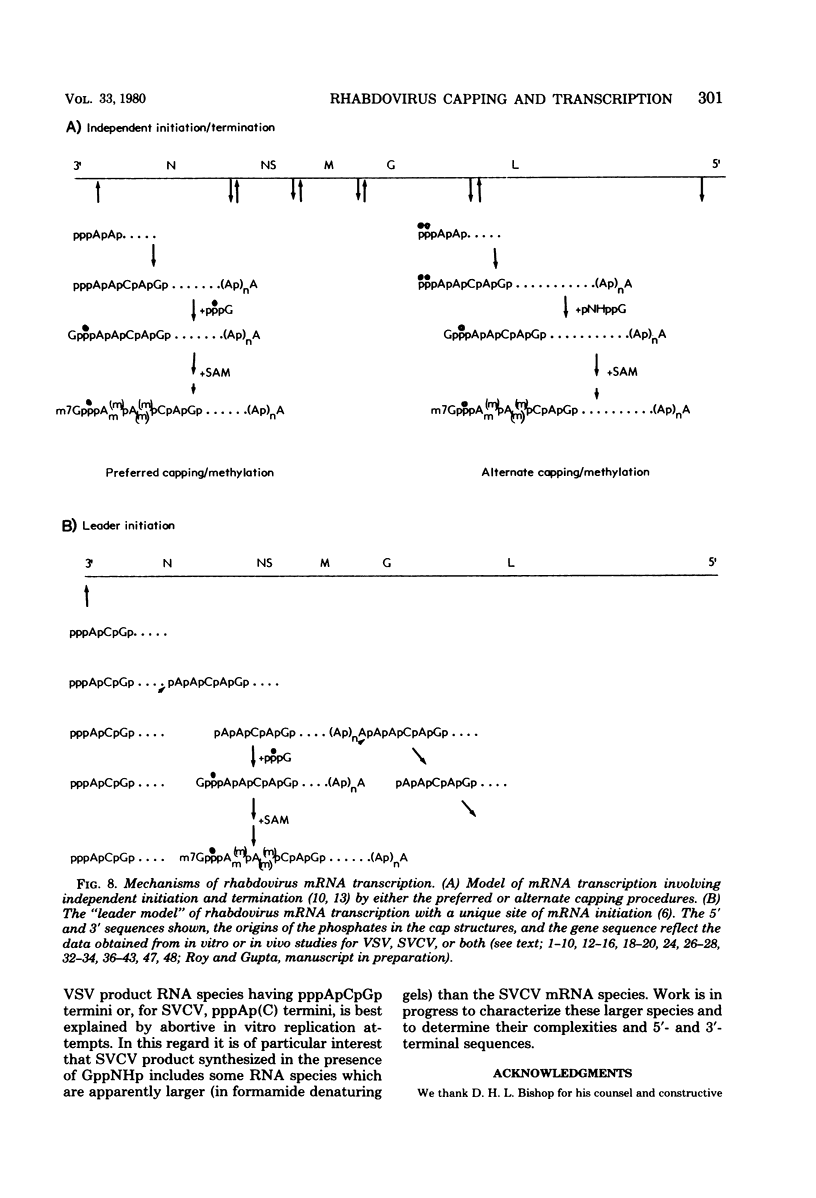
Two alternate mechanisms of mRNA capping for spring viremia of carp virus have been observed. Under normal reaction conditions, a ppG residue of the capping GTP is transferred to a pA moiety of the 5′ termini of mRNA transcripts. However, in reaction conditions where GppNHp is used instead of GTP, an alternate capping mechanism occurs whereby a pG residue of the capping GTP is transferred to a ppA moiety of the transcripts. The first mechanism is identical to that described previously for vesicular stomatitis virus (G. Abraham, D. P. Rhodes, and A. K. Banerjee, Nature [London] 255:37-40, 1975; A. K. Banerjee, S. A. Moyer, and D. P. Rhodes, Virology 61:547-558, 1974), and thus appears to be a conserved function during the evolution of rhabdoviruses. The alternate mechanism of capping indicates not only that capping can take place by two procedures, but also that the substrate termini have di- or triphosphate 5′ ends, indicating that they are probably independently initiated. An analog of ATP, AppNHp, has been found to completely inhibit the initiation of transcription by spring viremia of carp virus, suggesting that a cleavage between the β and γ phosphates of ATP is essential for the initiation of transcription. However, in the presence of GppNHp, uncapped (ppAp and pppAp), capped (GpppAp), and capped methylated (m7GpppAmpAp and GpppAmpAp) transcripts are detected. Size analyses of oligodeoxythymidylic acid-cellulose-bound transcripts resolved by formamide gel electrophoresis demonstrated that full-size mRNA transcripts are synthesized as well as larger RNA species. The presence of GppNHp and S-adenosylhomocysteine in reaction mixtures did not have any effect on the type of unmethylated transcription products. Our results favor a transcription model postulated previously (D. H. L. Bishop, in H. Fraenkel-Conrat and R. R. Wagner, ed., Comprehensive Virology, vol. 10, Plenum Press, New York, 1977; D. H. L. Bishop and A. Flamand, in D. C. Burke and W. C. Russell, ed., Control Processes in Virus Multiplication, Cambridge University Press, Cambridge, 1975; D. H. L. Bishop and M. S. Smith, in D. Nayak, ed., The Molecular Biology of Animal Viruses, Marcel Dekker, New York, 1977; P. Roy and D. H. L. Bishop, J. Virol. 11:487-501, 1973) in which mRNA synthesis is initiated independently; they do not support a model for transcripts being synthesized by plus-strand cleavage (A. K. Banerjee, G. Abraham, and R. J. Colonno, J. Gen. Virol. 34:1-8, 1977; A. K. Banerjee, R. J. Colonno, D. Testa, and M. T. Franze-Fernandez, in B. M. J. Mahy and R. D. Barry, ed., Negative Strand Viruses and the Host Cells, Academic Press, London, 1978).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham G., Banerjee A. K. The nature of the RNA products synthesized in vitro by subviral components of visicular stomatitis virus. Virology. 1976 May;71(1):230–241. doi: 10.1016/0042-6822(76)90108-2. [DOI] [PubMed] [Google Scholar]
- Abraham G., Rhodes D. P., Banerjee A. K. Novel initiation of RNA synthesis in vitro by vesicular stomatitis virus. Nature. 1975 May 1;255(5503):37–40. doi: 10.1038/255037a0. [DOI] [PubMed] [Google Scholar]
- Abraham G., Rhodes D. P., Banerjee A. K. The 5' terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975 May;5(1):51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. D., Abraham G., Colonno R. J. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977 Jan;34(1):1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Moyer S. A., Rhodes D. P. Studies on the in vitro adenylation of RNA by vesicular stomatitis virus. Virology. 1974 Oct;61(2):547–558. doi: 10.1016/0042-6822(74)90289-x. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Rhodes D. P. In vitro synthesis of RNA that contains polyadenylate by virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3566–3570. doi: 10.1073/pnas.70.12.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Emerson S. U., Flamand A. Reconstitution of infectivity and transcriptase activity of homologous and heterologous viruses: vesicular stomatitis (Indiana serotype), Chandipura, vesicular stomatitis (New Jersey serotype), and Cocal viruses. J Virol. 1974 Jul;14(1):139–144. doi: 10.1128/jvi.14.1.139-144.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. H., Hefti E., Obijeski J. F., Bishop D. H. RNA transcription by the virion polymerases of five rhabdoviruses. J Virol. 1974 Mar;13(3):652–661. doi: 10.1128/jvi.13.3.652-661.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Mapping and initiation studies on the leader RNA of vesicular stomatitis virus. Virology. 1977 Mar;77(1):260–268. doi: 10.1016/0042-6822(77)90423-8. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E. Polyadenylation of vesicular stomatitis virus mRNA. J Virol. 1974 May;13(5):1055–1060. doi: 10.1128/jvi.13.5.1055-1060.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Summers D. F. Adenylate-rich sequences in vesicular stomatitis virus messenger ribonucleic acid. J Virol. 1972 Oct;10(4):683–688. doi: 10.1128/jvi.10.4.683-688.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze-Fernandez M. T., Banerjee A. K. In vitro RNA transcription by the New Jersey serotype of vesicular stomatitis virus. I. Characterization of the mRNA species. J Virol. 1978 Apr;26(1):179–187. doi: 10.1128/jvi.26.1.179-187.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y. "Pretranscriptional capping" in the biosynthesis of cytoplasmic polyhedrosis virus mRNA. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1086–1090. doi: 10.1073/pnas.75.3.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J. 5'-termini of reovirus mRNA: ability of viral cores to form caps post-transcriptionally. Virology. 1977 Apr;77(2):566–578. doi: 10.1016/0042-6822(77)90482-2. [DOI] [PubMed] [Google Scholar]
- Galet H., Prevec L. Polyadenylate synthesis by extracts from L cells infected with vesicular stomatitis virus. Nat New Biol. 1973 Jun 13;243(128):200–203. doi: 10.1038/newbio243200a0. [DOI] [PubMed] [Google Scholar]
- Gershowitz A., Boone R. F., Moss B. Multiple roles for ATP in the synthesis and processing of mRNA by vaccinia virus: specific inhibitory effects of adenosine (beta,gamma-imido) triphosphate. J Virol. 1978 Aug;27(2):399–408. doi: 10.1128/jvi.27.2.399-408.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. C., Bishop D. H., Roy P. 5'-terminal sequences of spring viremia of carp virus RNA synthesized in vitro. J Virol. 1979 Jun;30(3):735–745. doi: 10.1128/jvi.30.3.735-745.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti E., Bishop D. H. The 5' sequence of VSV viral RNA and its in vitro transcription product RNA. Biochem Biophys Res Commun. 1975 Sep 16;66(2):785–792. doi: 10.1016/0006-291x(75)90578-1. [DOI] [PubMed] [Google Scholar]
- Hefti E., Bishop D. H. The 5' sequences of VSV in vitro transcription product RNA (+/-SAM). Biochem Biophys Res Commun. 1976 Jan 26;68(2):393–400. doi: 10.1016/0006-291x(76)91158-x. [DOI] [PubMed] [Google Scholar]
- Moss B., Gershowitz A., Wei C. M., Boone R. Formation of the guanylylated and methylated 5'-terminus of vaccinia virus mRNA. Virology. 1976 Jul 15;72(2):341–351. doi: 10.1016/0042-6822(76)90163-x. [DOI] [PubMed] [Google Scholar]
- Moss B., Martin S. A., Ensinger M. J., Boone R. F., Wei C. M. Modification of the 5'-terminals of mRNAs by viral and cellular enzymes. Prog Nucleic Acid Res Mol Biol. 1976;19:63–81. doi: 10.1016/s0079-6603(08)60908-3. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Banerjee A. K. In vivo methylation of vesicular stomatitis virus and its host-cell messenger RNA species. Virology. 1976 Apr;70(2):339–351. doi: 10.1016/0042-6822(76)90276-2. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Banerjee A. K. Messenger RNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell. 1975 Jan;4(1):37–43. doi: 10.1016/0092-8674(75)90131-2. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Grubman M. J., Ehrenfeld E., Banerjee A. K. Studies on the in vivo and in vitro messenger RNA species of vesicular stomatitis virus. Virology. 1975 Oct;67(2):463–473. doi: 10.1016/0042-6822(75)90447-x. [DOI] [PubMed] [Google Scholar]
- Rhodes D. P., Abraham G., Colonno R. J., Jelinek W., Banerjee A. K. Characterization of vesicular stomatitis virus mRNA species synthesized in vitro. J Virol. 1977 Mar;21(3):1105–1112. doi: 10.1128/jvi.21.3.1105-1112.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. P., Banerjee A. K. 5'-terminal sequence of vesicular stomatitis virus mRNA's synthesized in vitro. J Virol. 1975 Jan;17(1):33–42. doi: 10.1128/jvi.17.1.33-42.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. P., Moyer S. A., Banerjee A. K. In vitro synthesis of methylated messenger RNA by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell. 1974 Dec;3(4):327–333. doi: 10.1016/0092-8674(74)90046-4. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Heterogneeous 5'-terminal structures occur on vesicular stomatitis virus mRNAs. J Biol Chem. 1975 Oct 25;250(20):8098–8104. [PubMed] [Google Scholar]
- Rose J. K., Knipe D. Nucleotide sequence complexities, molecular weights, and poly(A) content of the vesicular stomatitis virus mRNA species. J Virol. 1975 Apr;15(4):994–1003. doi: 10.1128/jvi.15.4.994-1003.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Lodish H. F., Brock M. L. Giant heterogeneous polyadenylic acid on vesicular stomatitis virus mRNA synthesized in vitro in the presence of S-adenosylhomocysteine. J Virol. 1977 Feb;21(2):683–693. doi: 10.1128/jvi.21.2.683-693.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Initiation and direction of RNA transcription by vesicular stomatitis virus virion transcriptase. J Virol. 1973 Apr;11(4):487–501. doi: 10.1128/jvi.11.4.487-501.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Nucleoside triphosphate phosphotransferase. A new enzyme activity of oncogenic and non-oncogenic "budding" viruses. Biochim Biophys Acta. 1971 Apr 14;235(1):191–206. doi: 10.1016/0005-2744(71)90047-7. [DOI] [PubMed] [Google Scholar]
- Schibler U., Perry R. P. Characterization of the 5' termini of hn RNA in mouse L cells: implications for processing and cap formation. Cell. 1976 Sep;9(1):121–130. doi: 10.1016/0092-8674(76)90058-1. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Miura K. i. The process of formation of the 5' -terminal modified structure in messenger RNA of cytoplasmic polyhedrosis virus. FEBS Lett. 1976 Apr 15;64(1):204–208. doi: 10.1016/0014-5793(76)80284-0. [DOI] [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. Initiation of RNA synthesis in vitro by vesicular stomatitis virus. Role of ATP. J Biol Chem. 1979 Mar 25;254(6):2053–2058. [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Characterization and translation of methylated and unmethylated vesicular stomatitis virus mRNA synthesized in vitro by ribonucleoprotein particles from vesicular stomatitis virus-infected L cells. J Virol. 1976 Feb;17(2):477–491. doi: 10.1128/jvi.17.2.477-491.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Moss B. 5'-Terminal capping of RNA by guanylyltransferase from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3758–3761. doi: 10.1073/pnas.74.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]