Abstract
The native (pre-existing) collateral circulation minimizes tissue injury if obstructive vascular disease develops. Evidence suggests large differences in collateral extent exist among healthy individuals, presumably from as-yet unknown genetic and/or environmental factors. Little is known regarding when or how native collaterals form—information needed to identify these factors. We examined collateral development between the middle and anterior cerebral artery trees in BALB/c and C57BL/6 mouse embryos—strains with marked differences in adult collateral density and diameter (85% fewer, 50% smaller in BALB/c). The circulation was dilated, fixed and stained. By E15.5, a “primary collateral plexus” was beginning to form in both strains. By E18.5, plexus vessel number peaked, but was 60% less and diameter smaller in BALB/c (P<0.001). Earlier time-points were examined to determine if these differences correlated with differences in patterning of the general circulation. At ~E9.0, the primary capillary plexus was similar between strains, but by E12.5 branching was less and diameter larger in BALB/c (P<0.05). Between E12.5–E18.5—during pial artery tree development—small differences in tree size, branch number and distance between branches did not correlate with the large difference in collaterogenesis. Pruning of nascent collaterals between P1–P21 was comparable in both strains, yielding the adult density, but diameter and tortuosity increased less in BALB/c. Pericyte recruitment to nascent collaterals was comparable, despite lower VEGF-A and PDGF-B expression in BALB/c mice. These findings demonstrate that collaterals form late during vascular development and undergo postnatal maturation, and that differences in genetic background have dramatic effects on these processes.
Introduction
Native collaterals are pre-existing arteriole-to-arteriole anastomoses that bridge adjacent arterial trees in healthy tissues. Their density, diameter and capacity to enlarge following arterial obstruction (arteriogenesis), which are important determinants of their ability to protect against ischemic tissue injury, vary widely within and among species.1–4 Evidence suggests that conductance of the collateral circulation also differs greatly in healthy humans.5 This variance arises, presumably, from interactions among as-yet unidentified genetic and/or environmental factors that regulate formation (ie, collaterogenesis) and maintenance of the native collateral circulation (ie, collaterogenesis).
Surprisingly, no studies have examined when (or how) these unique vessels form. Does collaterogenesis occur in the embryo, neonate or during growth to adulthood? The cerebral arterial circulation and its collateral network reside in the pia/leptomeninges on the surface of the brain, making this tissue ideal for investigating these questions. Also, the extent of the pial collateral circulation greatly impacts the severity of stroke,4 adding relevance to studying collaterogenesis in brain. Early studies examining the development of the cerebral circulation noted the presence of rudimentary pial arteriole anastomoses.6–9 More recently, we reported that mouse pial collaterals are present at birth, and that targeted reduction in Vegfa or Clic4 expression reduces their density and increases postnatal pruning, resulting in a lower density in adults and increased severity of stroke.10,11 Little else is known about the time-course or mechanisms involved in formation and stabilization/remodeling (ie, maturation) of the native collateral circulation. Such information is not only fundamental, but is also required to identify mechanisms underlying individual variation in collateral extent and to develop therapies to induce formation of new collaterals in occlusive disease.
In the present study, we characterized collaterogenesis in the cerebral cortical circulation. To gain insight into genetic mechanisms underlying differences in this process, we compared two mouse strains—C57BL/6 and BALB/c—with large differences in collateral density and diameter in the brain and other tissues of adults.4 We hypothesized that natural, genetically determined differences in arterial tree patterning and/or postnatal maturation of nascent collaterals are major determinants of the wide variation in extent of the collateral circulation in healthy adults.
Materials and Methods
Offspring of C57BL/6 and BALB/c breeders from Jackson Laboratories were studied (embryonic, postnatal, and 10–12 weeks). Embryos were staged by crown-to-rump length. Experiments were conducted on at least two litters. The circulation was dilated, fixed, and stained for isolectin-B4, beta-galactosidase (ephrin-B2tLacZ)12 or NG2, or filled with casting material, followed by morphometry of both hemispheres.4,10,11 Quantitative RT-PCR was conducted on tissue samples taken from the “collateral zone” between the MCA and ACA trees and from the central MCA tree (non-collateral zone) Data are means ± SEM, with significance (P<0.05) determined by t-tests or ANOVA followed by Dunn-Bonferroni t-tests.
An expanded Materials and Methods is available in the Online Data Supplement at http://www.sciencedirect.com.
Results
Reduced branching in the early embryonic capillary plexus and middle cerebral artery tree of BALB/c mice
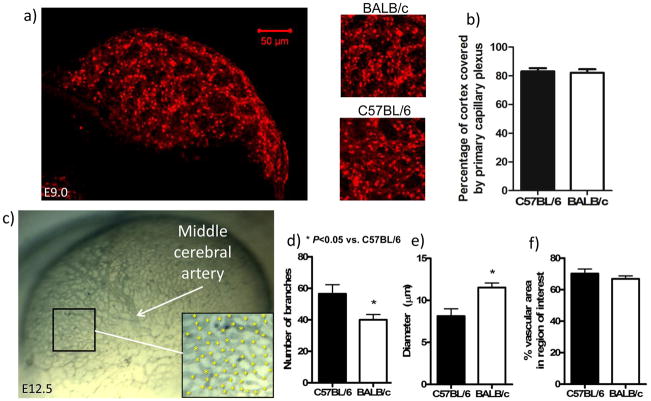
Preliminary studies showed that pial collaterals first appear in C57BL/6 and BALB/c strains at ~E15.5, with many fewer forming in BALB/c (detailed below). Therefore, we began by examining these strains at earlier time-points to determine if differences in vascular patterning of the cerebral circulation underlie the reduced formation in BALB/c. Differences in formation of the primary capillary plexus which begins at ~E8.5 in the pia,13,14 or its subsequent remodeling, could lead to variation in arterial tree patterning. This could result in variation in subsequent collateral formation. In agreement with others,14 at E8.5–9.5 the plexus did not have well-defined capillary tubes, preventing measurement of branch density and vessel diameter; however, percentage of cortex overlain by plexus material was comparable in both strains (Figure 1a,b). By E12.5, in BALB/c, plexus capillaries had remodeled into larger diameter vessels that branched less, but covered a similar area, compared to C57BL/6 (Figure 1c–f). These findings suggest that the primary plexus is composed of similar numbers of endothelial cells in both strains.
Figure 1.
Area of the primary capillary plexus overlying the dorsal cerebrum is similar between C57BL/6 and BALB/c. Top: a,b, Confocal imaging of primary capillary plexus stained with isolectin-B4 and plexus area. b, n=5 C57BL/6, n=4 BALB/c; E8.5–E9.5 imaged. Bottom: BALB/c plexus has fewer branches of larger-diameter vessels at E12.5; collaterals not yet present. c, Lateral view of the cerebral vasculature at E12.5 stained with isolectin-B4. Rudimentary MCA tree is present. d–f, Number of plexus branches (* in inset in c), size of vessel diameters and percentage of cerebrum covered by vessels were determined in an anterior region of the cerebrum (box in c). n=4–5. Data here and in other figures are means±SE for n-number of embryos per strain (ie, per bar) from ≥ two litters per strain.
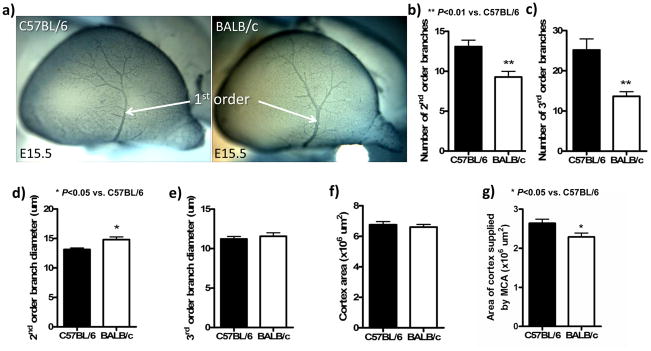
Between E13.5 and E14.5, second-order vessels began branching from the MCA trunk (which first became evident at E12.5—Fig 1c) in a similar pattern in both strains (data not shown). However, by E15.5, the BALB/c’s MCA had fewer second- and third-order branches, and second-order branches were larger in diameter (Figure 2a–e). These differences, which mimic those in the primary embryonic plexus (above), were confirmed with isolectin-B4 labeling of endothelium (data not shown). Cortex areas were comparable (Figure 2f).
Figure 2.
Branching in the MCA tree was less at E15.5 in BALB/c. a, Lateral view of the tree at E15.5. Staining with anti-neuron-glial 2 (NG2). b–e, Number of 2nd- and 3rd-order branches are reduced in BALB/c, while diameters of 2nd-order but not 3rd-order branches are larger in the BALB/c. f,g, Reduced branching in BALB/c is not due to a smaller cortex, but the MCA tree covers 14% less area. n=6–7/bar.
BALB/c embryos form fewer collaterals and intra-tree anastomoses
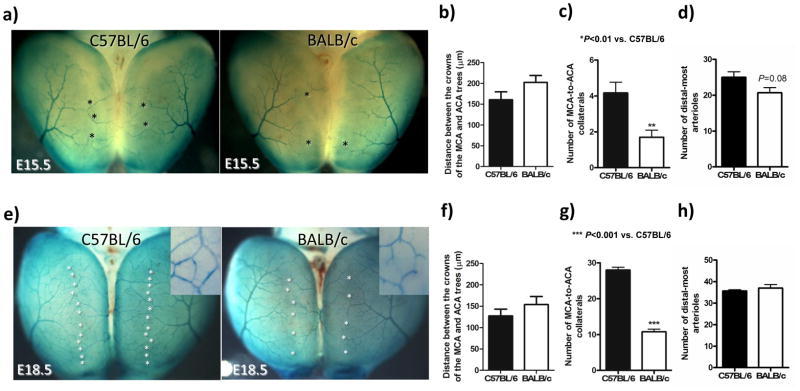
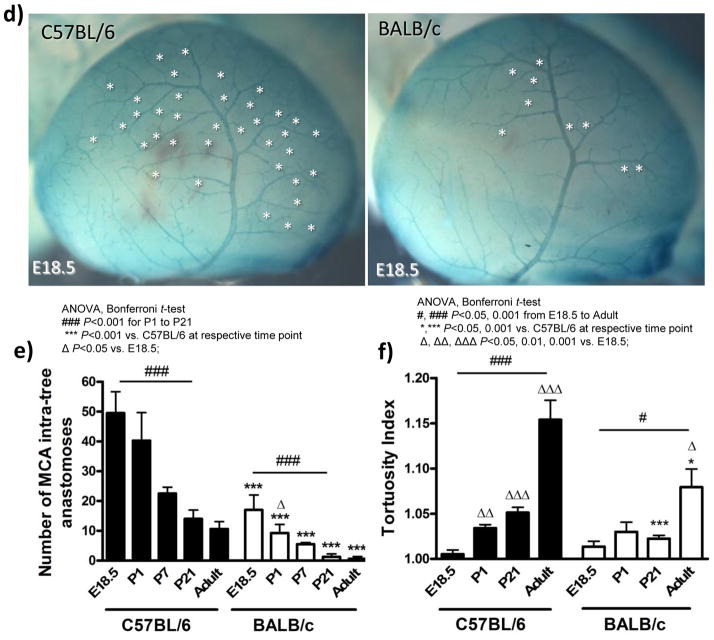
To aid differentiating developing arteries from veins, we back-crossed ephrin-B2tLacZ/+ mice12 (arterial endothelial cell marker) onto C57BL/6 and BALB/c strains for ≥10 generations (Figure 3a,e). Compared to C57BL/6 embryos at E15.5 and E18.5, respectively, BALB/c: had an MCA territory that was 14% smaller at both time-points (data not shown), tended to have 20% and 17% greater distance between the MCA and ACA crowns (Figure 3b,f), had 60% fewer collaterals (“primary embryonic collaterals”, ie, ring-like anastomoses–-see insets in Figure 3e and Supplemental figure 1) at both time-points between MCA and ACA trees (Figure 3c,g), and had no difference in the total number of distal-most MCA arterioles, ie, arterioles continuing on by either penetrating into the cortex or as a collateral connecting to the ACA tree (Figure 3d,h). The number of collaterals between the MCA and ACA trees increased 7-fold in both strains between E15.5 and E18.5 (compare ordinate values for Figures 3c and 3g). However, at E18.5 the number of distal-most arterioles in the MCA tree that continue on as collaterals was 80% in C57BL/6 and 30% in BALB/c (compare Figures 3d and 3h). Collateral diameters were 16% smaller at E18.5 in BALB/c (non-significant, n=5–6; data not shown).
Figure 3.
Collateral formation increases ~7-fold from E15.5-to-E18.5 in both strains but is less in BALB/c. c,g, BALB/c have 60% fewer collaterals at E15.5 and E18.5 (stars in a,e) than C57BL/6. a,e, LacZ staining (both strains are ephrin-B2tLacZ/+) of dorsal view of cortex; insets show ring-like morphology of early “primary” collateral plexus between MCA and ACA. b,f, Distance between the crowns of the MCA and ACA trees is 20% and 17% greater at E15.5 and E18.5, respectively, in BALB/c than C57BL/6 (non-significant). d,h, The number of distal-most arterioles at the crown of the MCA tree is not significantly different at E15.5 or E18.5. n=5–6/bar.
By E18.5, a plexus of ring-like arteriole-to-arteriole anastomoses had also formed within the MCA tree. This intra-tree plexus had the same morphology as the primary collateral plexus between the trees (Figure 4d and Supplemental figure 1). Like the 60% collaterals between the BALB/c trees at E18.5, BALB/c also had 65% fewer intra-tree anastomoses (Figure 4e). The density of these anastomoses within and between the cerebral artery trees did not change significantly from E18.5 to postnatal day 1 (P1) in either strain (Figures 3g, 4b,4e) except for a decline in BALB/c (Figure 4e).
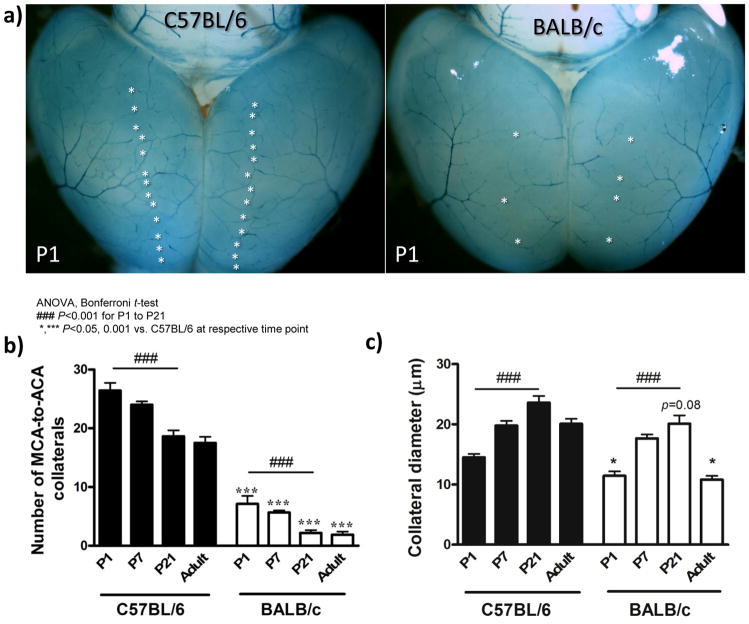
Figure 4.
Nascent collaterals and intra-tree anastomoses of C57BL/6 and BALB/c mice undergo comparable postnatal pruning. a, Dorsal view of postnatal day 1 (P1) vasculature of ephrin-B2tLacZ/+ mice. b, Fewer MCA-to-ACA collaterals (stars, panel a) in BALB/c versus C57BL/6 at all ages. c, Collateral diameters are smaller in BALB/c. d, Lateral view of cortex from ephrin-B2tLacZ/+ mice showing MCA intra-tree anastomoses (stars) at E18.5. e, Fewer intra-tree anastomoses in BALB/c versus C57BL/6 at all ages. f, Collateral tortuosity increases with age and is less in BALB/c. n=5–12/bar.
The distance between the crowns of their MCA and ACA trees at E15.5 and E18.5 trended wider in BALB/c (~18%, Figure 3b,f). This could contribute to the 60% fewer collaterals formed. We thus wondered whether BALB/c also have a wider distance between branches of the MCA tree to account for their formation of 65% fewer intra-tree. Therefore, the length and number of all second-through-fourth order arterioles, and the territory that they circumscribe, were measured to permit determination of vessel length-density (n=6 for each strain and time-point). In BALB/c at E15.5 and E18.5, branch length averaged 19% and 7% less than C57BL/6 (P=0.01, P=0.45), and branch number averaged 2% and 15% fewer (non-significant). These small differences are expected since MCA territory of BALB/c was 14% smaller at both time-points, as noted above. Vessel length-density was 8% and 7% smaller in BALB/c at E15.5 and E18.5, respectively (both non-significant). These differences in length-density are considerably smaller than the differences in the distances separating the distal-most arterioles of the MCA and ACA trees at the same time-points (17% and 20%). Yet inter-tree and intra-tree anastomoses are reduced by similar amounts in BALB/c (60 and 65%, respectively). These results suggest that the somewhat greater distance between branches is not the main cause of the large decrease in inter- and intra-tree anastomoses forming in BALB/c mice. The above data also show that the MCA tree in BALB/c embryos grows out somewhat more slowly, resulting in slightly fewer distal-most arterioles that is rectified by E18.5. These two differences, which exist at a time when the primary collateral plexus is forming, though small in comparison to the large difference in collateral formation, could nevertheless contribute to the latter.
Nascent collaterals in BALB/c embryos undergo similar pruning but less growth in length and diameter after birth
C57BL/6 and BALB/c mice evidenced comparable decreases in the absolute number of collaterals between P1 and P21 (Figure 4b), whereas number of intra-tree anastomoses declined more in C57BL/6 (Figure 4e). In both strains, the number of collaterals and intra-tree anastomoses present at P21 are the same number observed in adults, in agreement with previous studies in CD-1 mice.10,11 Collateral diameter in both strains increased similarly, in absolute microns, between P1 and P21, but declined by ~50% in BALB/c during growth to adulthood (Figure 4c). Since acquisition of tortuosity—one of the signatures of native collaterals—has not been examined, we studied its development. Tortuosity increased from E18.5 through adulthood (compare Figure 3e insets with Supplemental figure 4), but by a smaller amount in BALB/c (Figure 4f). Thus, C57BL/6 and BALB/c mice undergo similar postnatal pruning but collaterals of BALB/c fail to increase their diameter and evidence smaller increases in length during growth to adulthood.
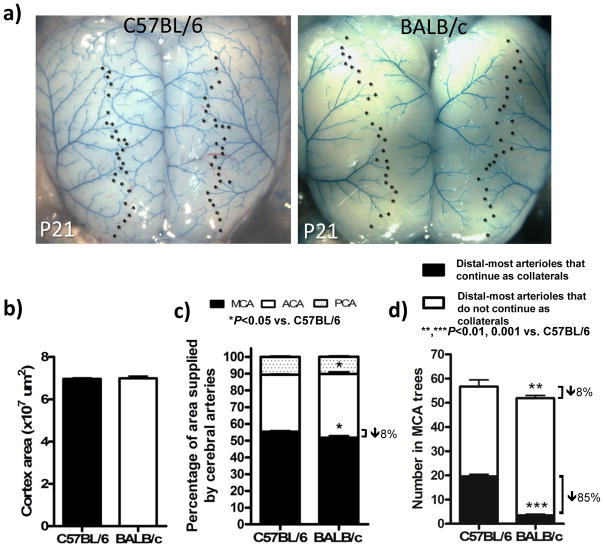
Adult collateral density was established by P21 in both strains (Figure 4b). Therefore, we examined the relationship between number of collaterals and distal-most arterioles of the MCA tree at this time-point. Compared to the 14% smaller size of the embryonic MCA tree of BALB/c (see above), the tree was now 8% smaller (Figure 5c); this remaining difference is abolished by adulthood.4 Consistent with this, the number of distal-most MCA arterioles was 8% lower in BALB/c (Figure 5d, sum of black and white bars), similar to the small differences in the embryo (Figure 3d,h). This contrasts with the much greater absolute and percentage decrease in collaterals in BALB/c (Figure 5d, black bars). Comparing E18.5 to P21, pruning reduced the number of distal-most arterioles in the MCA tree that continue on as collaterals from 80% to 35% in C57BL/6 and from 30% to 4% in BALB/c. Adult BALB/c had 9% fewer distal-most MCA arterioles, compared to adult C57BL/6 (data not shown).
Figure 5.
Smaller MCA territory and number of distal branches of MCA tree (both 8% smaller) are much less than the 85% lower collateral number in BALB/c. a, Dorsal view of cortex at P21. b,c, Dorsal cortical area and percentage of area supplied by cerebral artery trees. d, Total number of distal-most MCA arterioles adjacent to collateral zone (*, panel a) is 8% less in BALB/c, collateral number is ~85% less. n=8–11/bar.
The number of distal-most arterioles was slightly larger in adult C57BL/6 and BALB/c (72±4 and 63±3, respectively) than at P21. This likely reflects the small additional brain growth that occurs between P21 and adulthood. The P21 and adult data show almost identical deficits in absolute number of distal-most MCA arterioles and MCA-ACA collaterals in BALB/c. This indicates that the cortex underlying the collateral zone of BALB/c mice is supplied mostly by penetrating arterioles descending from the ends of the distal-most arterioles of the MCA, ACA and PCA trees, plus those from the one or two collaterals present. This contrasts with abundant collaterals of C57BL/6 mice: on average, a collateral provides a “scaffold” for 2.8±0.3 and 2.7±0.7 penetrating arterioles in adult C57BL/6 and BALB/c (n=10 each), respectively, to help supply the collateral zone.15
Expression of PDGF-B and VEGF-A, but not angiopoietin-2 are reduced in BALB/c mice
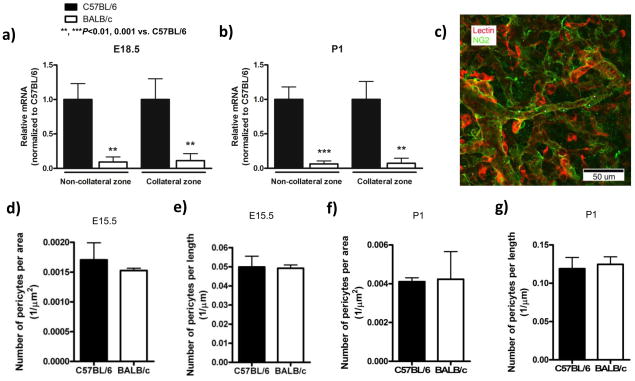
Differences in factors governing primary collateral plexus formation between C57BL/6 and BALB/c mice could underlie their subsequent differences in collateral density. VEGF-A, angiopoietin-2 and PDGF-B are implicated in formation, branching, stabilization, pericyte/smooth muscle cell recruitment (muralization) and regression (pruning) of nascent vessels.16–18 Therefore, we examined their expression in the cortex and overlying pia from two regions—the “collateral zone” between the MCA and ACA trees, and the regions overlain by the ACA and MCA trees (“non-collateral zone”). No significant differences were detected in individual VEGF-A isoforms; however overall VEGF-A expression was reduced at P1 in the collateral zone of BALB/c (Supplemental figure 2a,b). Angiopoietin-2 was elevated at E18.5 in the collateral zone of BALB/c (Supplemental figure 3). PDGF-B was reduced 90% in both sample sites at all time-points in BALB/c (Figure 6a,b). Expression of the endothelial cell marker, VE-cadherin, was comparable between strains (Supplemental figure 3c,d).
Figure 6.
BALB/c collaterals have reduced PDGF-B expression and similar pericyte coverage. a,b, Q-PCR in collateral and non-collateral zones of the cortex at indicated times. Data normalized to 18S rRNA and expressed as fold-change from C57BL/6; n=6/bar. c, Confocal image of isolectin-B4 (red), anti-NG2 (green); stars, pericytes. d-g, Number of pericytes per collateral length-x-width and per length at E15.5 and P1. n=3 mice/bar with 2–4 collaterals per brain.
BALB/c collaterals do not have a defect in pericytes
Reduced expression of VEGF-A and PDGF-B plus transient increase in angiopoietin-2 in BALB/c may contribute to impaired formation, stabilization, muralization or growth of nascent collaterals. Given the involvement of these molecules in pericyte function,18 we examined pericyte density on collaterals at E15.5 when collaterals are beginning to appear and at P1 when collateral density has passed its peak and pruning is beginning. Although VEGF-A and PDGF-B were lower in BALB/c, pericyte density on pial collateral arteries was comparable between strains (Figure 6d–g). Smooth muscle cells are not present on collaterals until after P7 (Zhang and Faber, unpublished, 2009). These findings do not support a defect in mural cell recruitment to explain formation of fewer collaterals of smaller diameter that exhibit less growth in length in BALB/c mice.
In vivo analysis of collateral flow
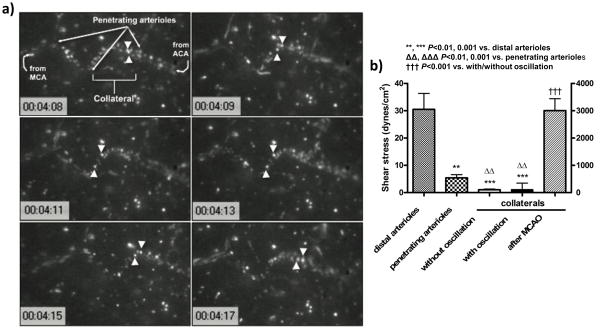
Collaterals are presumed to be subjected to unique hemodynamic forces during and after formation, given their position between two arterial trees, ie, low oscillatory flow and high circumferential wall stress. These forces could participate in formation and postnatal pruning and maturation in length and diameter of these vessels. However, no studies have measured velocity and shear stress in collaterals (albeit a study19 appeared just before submission of our findings). Therefore, we used intravital microscopy through an open cranial window over the collateral zone between the MCA and ACA to obtain collateral blood flow velocity, diameter and shear stress in anesthetized adult C57BL/6 mice. Velocity and shear stress averaged 150 μm/s and 2 dynes/cm2 (Figure 7), with some collaterals exhibiting very slow flow in one direction, while others having slow oscillatory flow (Supplemental videos 1,2). By comparison, velocity and shear stress were several-fold greater in adjacent penetrating arterioles and 15-fold greater in nearby distal-most arterioles with comparable diameters. After ligation of the MCA distal to the lenticulo-striate branches, velocity and shear stress abruptly increased to values measured in the distal-most arterioles (Figure 7). These findings document the unique hemodynamic environment in which native collaterals exist.
Figure 7.
Slow, oscillatory velocity and shear stress in native collaterals under baseline, quantified using intravital microscopy of 1μm diameter fluorescent microspheres injected intravenously. a, Tracking of two microspheres (arrowheads); time-base in 0.01 second. Mice were anesthetized with ketamine and xylazine. b, Shear stress and velocity; calculations in Supplement. n=3/bar.
Several additional observations in this study are noteworthy. (1) We did examine the time-course of formation of the pial cerebral vein trees. However formation, remodeling and localization of these trees occur later and differ significantly from artery tree development (Supplemental figure 5). (2) Unlike the developing artery trees overlying the cerebral cortex which have a dendritic pattern similar to the newborn and adult (eg, Figures 2–5, Supplemental figure 5, reference 4), the pattern of the early artery trees overlying the cerebellar cortex dramatically changes with development (Supplemental figure 6 versus reference 4). (3) Ephrin-B2-LacZ background staining differed significantly for the cerebral cortex versus the midbrain and cerebellum (Figures 3,4, Supplemental Figures 1c, 6), suggesting that within the cortex, but not other brain regions, endothelial cells or neurons/glia express ephrin-B2.
Discussion
This is the first study to examine development of the native collateral circulation. To facilitate this, we compared C57BL/6 and BALB/c mice—strains with large differences in collateral extent (ie, density and diameter) in adult brain, hindlimb and intestine.4 These strains have the greatest extremes in collateral extent among 15 inbred strains,15 and BALB/c suffer more severe tissue injury than C57BL/6 in models of stroke and hindlimb ischemia.1,4,15 Significant differences in extent of the native collateral circulation also appear to exist in healthy humans.5 Deficient collateral conductance in adult BALB/c could be from fewer collaterals formed, reduced stabilization (ie, increased pruning) or impaired lumen/wall growth of nascent collaterals, or due to a decline in collateral density, diameter or increase in length (tortuosity) during tissue growth to adulthood. We examined these questions in the cerebral circulation because of its imaging advantages and because genetically-determined differences in collateral extent in this circulation are shared in other tissues.4,10,11 Our findings show that pial collaterals begin to form in both strains at ~E15.5, after the MCA has extended across the cerebral cortex. At the time of peak collateral formation (E18.5), BALB/c form 60% fewer. In both strains, these nascent collaterals undergo comparable pruning that is complete by P21, yielding the adult density. Our findings suggest that the deficiency in collateral formation in BALB/c results from slower outgrowth of the cerebral artery trees and altered expression of genes important in forming and stabilizing newly formed anastomoses.
Before this study, information about the early collateral circulation was confined to comments in several early studies examining the postnatal cerebral and cerebellar circulations6–9. In newborn rats, the presence of “ring-like anastomoses” between and within pial artery trees was noted, followed by pruning over the first ~7 weeks after birth.7,8 In Swiss-Webster mice, Wang et al. noted that anastomoses between the MCA and ACA were present at birth but, like the BALB/c strain studied herein, were almost absent within two weeks.8
Our findings show that BALB/c mice form fewer collaterals than C57BL/6. Identifying this difference and the responsible mechanisms required determining when these vessels develop. Pial collaterals begin to form in both strains at ~E15.5 as an abruptly appearing plexus of ring-like “primary” collaterals composed of endothelial cells expressing the ephrin-B2 arterial phenotype. Outgrowth of this plexus peaks at ~E18.5. A similar plexus arises between branches within the cerebral artery trees (intra-tree anastomoses) over this same relatively short time-window. Compared to formation of the general arterial circulation, this period of primary collateral plexus formation occurs relatively late, ie, after the cerebral artery trees have extended across the cortex. BALB/c have 60–65% fewer inter- and intra-tree plexus vessels at both time-points. These nascent anastomoses subsequently undergo a similar absolute amount of pruning in both strains, such that by P21 when the adult density is achieved, BALB/c have 85% fewer collaterals and 95% fewer intra-tree anastomoses (increases in percentage, compared to E18.5, are a consequence of changes in total numbers of anastomoses).
We investigated possible mechanisms for these large differences in collateral formation. No difference existed between the strains in the total area of the primary embryonic capillary plexus at E9.0 and 12.5. This suggests no difference in size of the initial endothelial cell population, which is determined by vasculogenesis and sprouting angiogenesis. This is not surprising, since both processes are tightly regulated by genes (eg, Vegfa, angiopoietins) whose expression, if altered beyond certain amounts, cannot be tolerated without major or often lethal vascular abnormalities.16–18
We also hypothesized that differences in branching morphogenesis, leading to differences in branch density at the crowns of the cerebral artery trees that collaterals interconnect, could also be important. Both the embryonic capillary plexus at E12.5 and early MCA tree at E15.5 of BALB/c mice displayed less branching and vessels with larger diameters. However, no difference in the number of distal-most arterioles was evident between the strains during collateral plexus formation (E15.5–18.5), although there was a trend toward slightly (14%) fewer at E15.5 in BALB/c.
We found that the cerebral artery trees form somewhat more slowly in BALB/c mice (MCA tree territory was 14% smaller at E15.5 and E18.5, 8% smaller at P21, and not different in adult). This caused BALB/c to have a wider distance between the MCA and ACA trees (17–20%) and between branches within the MCA tree (ie, a 7–8% lower branch length-density) during the E15.5–E18.5 interval of plexus formation. This difference could contribute to the fewer anastomoses that form at both sites in BALB/c. However, the much larger deficits (60 and 65%) in collaterals and intra-tree anastomoses that formed suggests an additional mechanism may play a larger role in collateral development. In addition, a very weak correlation was found between differences in cerebral artery tree territories and collateral number (r2 = 0.05) in an analysis of 15 inbred mouse strains exhibiting large genetic differences both traits.15 We therefore postulated that the large difference in collateral formation in BALB/c and C57BL/6 mice may involve genetically determined differences in expression/activity of factors within the signaling pathway(s) driving formation and stabilization of the primary collateral plexus.
To begin to examine this hypothesis, we measured expression of three potential factors—VEGF-A, angiopoietin-2 and PDGF-B. These were chosen because of their known roles in vessel formation, growth, stabilization and remodeling.16–18,20 plus our previous eQTL analysis suggesting the presence of a polymorphism in or near the VEGF-A gene linking lower VEGF-A4 and lower PDGF-B expression in BALB/c compared to C57BL/6. Tissue samples were taken from the “collateral zone” and from the center of the MCA and ACA trees (“non-collateral zone”). Thus, a limitation is that these samples unavoidably included RNA from both the pial vasculature and the underlying neurons and glia. Overall VEGF-A isoform expression was lower in both zones at P1 (significant for the collateral zone). BALB/c are known to express less VEGF-A during ischemia/hypoxia.4,21,22 Moreover, VEGF-A expression positively regulates collateral density in the newborn and hence adult in a gene-dose-dependent manner.10 Thus, the present data support the hypothesis that VEGF-A signaling is involved in driving formation and outgrowth of the primary collateral and intra-tree anastomotic plexuses. Previous studies have also shown that expression of Clic4, which may reside in the VEGF-A signaling pathway, positively regulates collateral density in the newborn and adult in a gene-dose-dependent manner.11
Our inability to detect differences in VEGF-A expression until P1 may have resulted from its known high expression in the neurons and glia before birth.23,24 This could mask detection of levels in and near the pial vasculature before birth, compared to after birth. Our angiopoietin-2 and PDGF-B data support this notion, since these predominantly vascular/mesenchymal factors are expressed at much lower levels or not at all by neurons and glia.25,26 Moreover, they support the above hypothesis concerning their involvement in collateral plexus formation: Angiopoietin-2 was elevated at E18.5 in the collateral zone samples, at a time when PDGF-B (discussed below) was reduced. Unlike VEGF-A, angiopoietin-2 is highly expressed in endothelial cells, compared to neurons and glia.17,25 Also, VEGF-A augments angiopoietin-2 expression, and angiopoietin-2 is a key controller of angiopoietin-1—Tie-2-mediated stabilization of nascent vessel formation.17 Elevated angiopoietin-2 levels promote instability of nascent vessels.17 PDGF-B, which is downstream of VEGF-A—angiopoietin signaling16,18 and is much more strongly expressed in vascular than brain tissue,26 was reduced by more than 90% at all time-points in BALB/c mice. Because of this, we hypothesized that lower PDGF-B in BALB/c mice could result in less mural cell recruitment to nascent collaterals, resulting in less stabilization and thus many fewer forming in BALB/c. However, pericyte numbers were comparable in both strains. PDGF-B is highly expressed by sprouting tip-cells.27 Thus, another possiblility is that lower expression in BALB/c could result in less branching density and fewer collaterals being formed.
Based on these and our previous studies,4,10,11 we propose that levels of VEGF-A, angiopoietin-2 and PDGF-B are important in determining formation of the primary collateral plexus in the embryo and maturation in the neonate, and thus the extent of the collateral circulation in the adult. Therefore, variants in genetic elements impacting the expression or activity of these factors could contribute to differences in the native collateral circulation among species and individuals. Future studies localizing expression and activity are needed to clarify the role of these and undoubtedly other factors involved in collateral formation.
Compared to previous work in adult C57BL/6 and BALB/c mice4 that were confirmed herein, collateral density in P1 pups was greater in both strains, and C57BL/6 collaterals had smaller diameters. To identify mechanisms for this difference, we examined additional postnatal time-points. In both strains, comparable postnatal pruning of collaterals (and intra-tree anastomoses) occurred through P21, as did comparable increases in collateral diameter. In support of the similar pruning observed, no difference was seen at P1 in pericyte density on collaterals between the strains. This is consistent with the similar angiopoietin-2 expression in both strains at P1, which may, along with potential compensations in BALB/c, prevent enhanced pruning of nascent vessels that is favored by their lower VEGF and PDGF-B expression.
BALB/c collaterals developed less tortuosity after birth and during growth to adulthood (3 months). This may result from an effect of lower VEGF and PDGF-B expression on proliferation of collateral endothelial and mural cells, and could also explain why diameter declines between P21 through adulthood in BALB/c. It is unclear why collaterals acquire their characteristic tortuous morphology and why tortuosity continues to increase beyond P21 when density (and diameter in C57BL/6) have reached adult values. A similar increase in pial collateral tortuosity from P10 to P56 was reported in Wistar rats.28 We speculate that this characteristic, which increases resistance and is thus counterproductive for collateral function, is caused by the low and oscillatory shear stress environment in which native collaterals normally reside (Figure 7). Such conditions may stimulate a higher level of tonic endothelial cell proliferation than in non-collateral arterioles, especially after birth when arterial pressure and pulsatility from heart rate rapidly rise, resulting in progressively increasing tortuosity. Understanding the adaptive mechanisms that permit endothelial and mural cells of the native collateral to tolerate such a disturbed hemodynamic environment is an intriguing area for future investigation.
In summary, this study shows that the collateral circulation forms in the mouse initially as a plexus late in embryogenesis, after the general arterial-venous circulation has formed. This plexus then undergoes pruning and maturation during the first three postnatal weeks, at which time collaterals acquire their characteristic tortuosity and adult density and diameter. Genetic background is capable of having dramatic effects on these processes. Lastly, we present evidence supporting the hypothesis that individual differences in the extent of this circulation may arise from natural genetic polymorphisms that alter expression of genes driving formation of the collateral plexus. These findings are not only fundamental, but they may also help identify genetic mechanisms underlying differences in collateral abundance in humans and aid development of therapies to promote formation of new collaterals in individuals that have or are prone to occlusive disease.
Supplementary Material
Acknowledgments
We thank Dr. Jennifer Lucitti for advice on histology and critical review of the manuscript, Pranay Prabhakar for determining collateral tortuosity, and Katy Liu for assistance with confocal microscopy.
Sources of Funding
NIH grants HL62584, HL090655 (JEF), F32-HL080847, K99-HL093609 (DC).
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–6. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 2.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–51. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 3.Sherman JA, Hall A, Malenka DJ, De Muinck ED, Simons M. Humoral and cellular factors responsible for coronary collateral formation. Am J Cardiol. 2006;98:1194–7. doi: 10.1016/j.amjcard.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–91. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 5.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–83. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida Y, Ikuta F, Watabe K, Nagata T. Developmental microvascular architecture of the rat cerebellar cortex. Anat Embryol (Berl) 1985;171:129–38. doi: 10.1007/BF00341407. [DOI] [PubMed] [Google Scholar]
- 7.Bär T, Miodoński A, Budi Santoso AW. Postnatal development of the vascular pattern in the rat telencephalic pia-arachnoid. A SEM study. Anat Embryol (Berl) 1986;174:215–23. doi: 10.1007/BF00824337. [DOI] [PubMed] [Google Scholar]
- 8.Wang DB, Blocher NC, Spence ME, Rovainen CM, Woolsey TA. Development and remodeling of cerebral blood vessels and their flow in postnatal mice observed with in vivo videomicroscopy. J Cereb Blood Flow Metab. 1992;12:935–46. doi: 10.1038/jcbfm.1992.130. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zhao Y, Zhou JW. The three-dimensional structure and the relationship between external and internal vascularizations in the brain of rat embryos. Chin Med J (Engl) 2004;117:280–5. [PubMed] [Google Scholar]
- 10.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–36. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalothorn D, Zhang H, Smith JE, Edwards JC, Faber JE. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res. 2009;105:89–98. doi: 10.1161/CIRCRESAHA.109.197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–9. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 14.Walls JR, Coultas L, Rossant J, Henkelman RM. Three-dimensional analysis of vascular development in the mouse embryo. PLoS One. 2008;3:e2853. doi: 10.1371/journal.pone.0002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010 Feb 3; doi: 10.1038/jcbfm.2010.10. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–45. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 17.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–77. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 18.Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–8. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 19.Toriumi H, Tatarishvili J, Tomita M, Tomita Y, Unekawa M, Suzuki N. Dually Supplied T-Junctions in Arteriolo-Arteriolar Anastomosis in Mice. Key to Local Hemodynamic Homeostasis in Normal and Ischemic States? Stroke. 2009;40:3378–83. doi: 10.1161/STROKEAHA.109.558577. [DOI] [PubMed] [Google Scholar]
- 20.Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17:377–86. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward NL, Moore E, Noon K, Spassil N, Keenan E, Ivanco TL, LaManna JC. Cerebral angiogenic factors, angiogenesis, and physiological response to chronic hypoxia differ among four commonly used mouse strains. J Appl Physiol. 2007;102:1927–35. doi: 10.1152/japplphysiol.00909.2006. [DOI] [PubMed] [Google Scholar]
- 22.Vasquez-Pinto LM, Landgraf RG, Bozza PT, Jancar S. High vascular endothelial growth factor levels in NZW mice do not correlate with collagen deposition in allergic asthma. Int Arch Allergy Immunol. 2007;142:19–27. doi: 10.1159/000095995. [DOI] [PubMed] [Google Scholar]
- 23.Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–32. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 24.Ogunshola OO, Stewart WB, Mihalcik V, Solli T, Madri JA, Ment LR. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res Dev Brain Res. 2000;119:139–53. doi: 10.1016/s0165-3806(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 25.Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157:1473–83. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasahara M, Amano S, Sato H, Yang JG, Hayase Y, Kaneko M, Sato I, Suzaki M, Hazama F. Normal developing rat brain expresses a platelet-derived growth factor B chain (c-sis) mRNA truncated at the 5′ end. Oncogene. 1998;16:1571–8. doi: 10.1038/sj.onc.1201679. [DOI] [PubMed] [Google Scholar]
- 27.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coyle P. Interruption of the middle cerebral artery in 10-day-old rat alters normal development of distal collaterals. Anat Rec. 1985;212:179–82. doi: 10.1002/ar.1092120212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.