Abstract
Paramyxovirus fusion (F) proteins promote both virus-cell fusion, required for viral entry, and cell-cell fusion, resulting in syncytia formation. We used the F-actin stabilizing drug, jasplakinolide, and the G-actin sequestrant, latrunculin A, to examine the role of actin dynamics in cell-cell fusion mediated by the parainfluenza virus 5 (PIV5) F protein. Jasplakinolide treatment caused a dose-dependent increase in cell-cell fusion as measured by both syncytia and reporter gene assays, and latrunculin A treatment also resulted in fusion stimulation. Treatment with jasplakinolide or latrunculin A partially rescued a fusion pore opening defect caused by deletion of the PIV5 F protein cytoplasmic tail, but these drugs had no effect on fusion inhibited at earlier stages by either temperature arrest or by a PIV5 heptad repeat peptide. These data suggest that the cortical actin cytoskeleton is an important regulator of fusion pore enlargement, an energetically costly stage of viral fusion protein-mediated membrane merger.
Introduction
The family Paramyxoviridae contains a number of important human pathogens. Measles remains an important cause of childhood mortality in the developing world, killing an estimated 345,000 children in 2005 alone (Wolfson et al., 2007). Respiratory syncytial virus (RSV) is the most common cause of hospitalization of infants and children in the United States (Black, 2003). Nipah virus first emerged in Malaysia and Singapore during 1998/9, resulting in an epidemic of encephalitis with 105 deaths from 265 reported cases (Chua et al., 1999; Paton et al., 1999). A series of more recent outbreaks of Nipah virus has been observed in southern and central Bangladesh, with higher mortality rates compared to the 1998 outbreak (up to 70%), and documented cases of human-to-human transmission (Eaton et al., 2006). The paramyxovirus family also contains animal pathogens, including parainfluenza virus 5 (PIV5), which causes respiratory infection in canines and also infects humans asympotomatically (Goswami et al., 1984; McCandlish et al., 1978).
Most paramyxoviruses have two major envelope glycoproteins that are essential for viral pathogenesis (Lamb and Parks, 2007). The attachment protein (HN, H, or G) binds to a viral receptor on the plasma membrane of the host cell and is hypothesized to trigger activation of the fusion protein (F). Conformational changes in the F protein then drive the merger of the viral envelope with the host cell membrane (Lamb and Jardetzky, 2007). Infection of host cells leads to expression of the viral fusion and attachment proteins at the plasma membrane. Cell surface expression of these viral proteins leads to fusion between adjacent cell membranes, as the cellular receptors on neighboring cells can be engaged by the fusion complex. As a result, multinucleated giant cells (termed syncytia), are observed in infections with many paramyxoviruses (Makino et al., 1994; Meyerholz et al., 2004; Paterson, Murray, and McCormack, 1998).
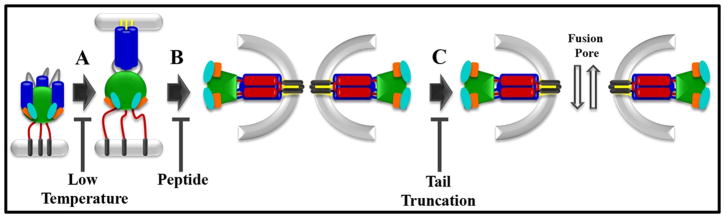
Paramyxovirus fusion proteins contain a series of conserved structural elements that are critical to the conformational rearrangements required to drive membrane fusion (Dutch, Jardetsky, and Lamb, 2000). These type I integral membrane proteins are synthesized as polypeptide precursors that trimerize in the endoplasmic reticulum, with subsequent proteolytic processing of the precursor protein required for the protein to become fusogenically active (Garten et al., 1994; Pager and Dutch, 2005; Scheid and Choppin, 1974). Proteolytic cleavage results in placement of a hydrophobic region termed the fusion peptide at the newly created N-terminus. Upon triggering, the fusion peptide is released and inserted intothe target membrane, resulting in a conformation that bridges the two membranes (Asano and Asano, 1985; Novick and Hoekstra, 1988) (Figure 1). Preventing activation of the fusion protein by inhibiting receptor binding or lowering the temperature blocks fusion upstream of this bridging-conformation (Russell, Jardetzky, and Lamb, 2001). These fusion proteins contain two highly conserved heptad repeat regions which do not interact in the prefusion form of the F protein (Yin et al., 2006). However, isolated peptides from these regions form an extremely stable six-helix bundle (Baker et al., 1999; Dutch, Leser, and Lamb, 1999). This bundle is observed in the post-fusogenic form of the F protein following conformation rearrangements associated with fusion (Yin et al., 2005) (Figure 1). Formation of this stable helical bundle brings the transmembrane domain and the fusion peptide into close proximity, and is hypothesized to provide at least a portion of the energy required for membrane fusion (Baker et al., 1999). Treatment with high concentrations of peptides corresponding to either of the heptad repeat regions disrupts the formation of the six-helix bundle and effectively blocks fusion (Russell, Jardetzky, and Lamb, 2001). Following six-helix bundle formation and initial pore formation, expansion of the fusion pore must occur. This expansion step is hypothesized to be the most energetically costlystage of the membrane fusion process (Chernomordik, Zimmerberg, and Kozlov, 2006). Interestingly, truncation of the cytoplasmic tail of the PIV5 protein significantly abrogated pore enlargement (Dutch and Lamb, 2001).
Figure 1.
Schematic representation of paramyxovirus F protein-promoted membrane fusion. A. Initial conformational changes lead to insertion of the fusion peptide into the target membrane, a step that is blocked by incubation at low temperature. B. Subsequent refolding leads to formation of a six-helix bundle, bringing the fusion peptide and the transmembrane domain regions into close proximity. This step can be blocked by addition of peptides corresponding to either of the heptad repeat regions. C. The fusion pore expands to allow passage of larger molecules. This step is affected by truncation of the cytoplasmic tail of the PIV5 F protein. Yellow = fusion peptide; dark blue = heptad repeat A; red = heptad repeat B; grey = transmembrane domain.
While extensive characterization of viral fusion proteins has led to a model of F protein mediated membrane fusion, much less is known about the contribution of target membranes and their associated proteins to fusion events. Lying just under the plasma membrane and intimately associated with it through a complex series of adaptor proteins is a dense network of actin filaments and associated proteins. Results from various non-viral fusion systems, including analysis of myoblast fusion and fusion events in exocytosis and endocytosis, suggest that the cortical actin cytoskeleton may be a modulator of membrane fusion events (Chernomordik, Zimmerberg, and Kozlov, 2006; Eitzen, 2003; Richardson, Nowak, and Baylies, 2008), however, the mechanism of action remains unclear. Studies of Drosphila myoblast fusion (Richardson et al., 2007), fusion during exocytosis (Muallem et al., 1995) and fusion between erythrocytes (Chernomordik and Sowers, 1991) have provided evidence that the actin cytoskeleton inhibits fusion by acting as a stiff scaffold preventing the deformation of the membrane required to allow lipid mixing or pore enlargement. However, other studies using similar model systems have supported a model by which actin polymerization stimulates fusion by generating force to drive the lipid membranes together and enlarge the nascent fusion pore (Massarwa et al., 2007; Zheng and Chang, 1991). Finally, recent research has implicated actin as important in a pre-fusion priming step during myoblast fusion (Kim et al., 2007).
Modulation of the actin cytoskeleton appears to also affect cell-cell fusion promoted by viral fusion proteins. RSV F protein-mediated syncytia formation was inhibited both by pharmacological treatments which alter the cytoskeleton (Kallewaard, Bowen, and Crowe, 2005) and by Clostridium C3toxin (Gower et al., 2005), an inhibitor of the RhoA protein which regulates the dynamic behavior of the actin cytoskeleton at the plasma membrane (Bishop and Hall, 2000). HIV env protein-promoted cell fusion was inhibited by both latrunculin A and jasplakinolide (Pontow et al., 2004). We have shown that transfection of various constitutively active Rho-family GTPases either stimulated or inhibited cell-cell fusion induced by paramyxovirus fusion proteins in a manner that is cell type specific, and dependent on whether the protein was expressed in target or effector cell populations (Schowalter et al., 2006). Two recent studies of cell-cell fusion mediated by the influenza hemagglutinin (HA) protein (Richard, Leikina, and Chernomordik, 2009) and the baculovirus gp64 protein (Chen et al., 2008) have addressed the potential role of actin. Data from the study of influenza HA-promoted fusion pore formation found no evidence for a role for actin in driving pore expansion, instead providing some evidence that the actin cytoskeleton restricts pore expansion (Richard, Leikina, and Chernomordik, 2009). Results from the gp64 study strongly indicated that the actin network is a barrier to fusion pore expansion (Chen et al., 2008).
To shed further light on the role of the actin cytoskeleton in membrane fusion, we have used various concentrations of latrunculin A and jasplakinolide to perturb the actin cytoskeleton during paramyxovirus glycoprotein-mediated membrane fusion. Latrunculin A depolymerizes cytoplasmic actin by binding to and sequestering actin monomers (Coue et al., 1987). Jasplakinolide stabilizes existing actin filaments and nucleates ectopic actin polymerization (Bubb et al., 1994). Both drugs stimulated fusion induced by the PIV5 F protein in a dose dependent manner at low concentrations. Experiments blocking fusion at defined points indicate that both drugs affect fusion after the stage of six helix bundle formation. Moreover, treatment with these drugs partially rescued a mutant which is deficient in pore enlargement, suggesting that disregulation of the cortical actin cytoskeleton may relieve a mechanical barrier to expansion of the nascent fusion pore.
Materials and Methods
Cell Lines
Baby hamster kidney (BHK) and Vero cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Invitrogen, Carlsbad, California) supplemented with fetal bovine serum (FBS [10%]), penicillin (1%), and streptomycin (1%) (P/S). BSR cells, derived from BHK cells and constitutively expressing the T7 polymerase (Buchholz, Finke, and Conzelmann, 1999) (kindly provided by Karl-Klaus Conzelmann, Max Pettenkofer Institute) were similarly passaged, by selection with 0.5 mg/mL G418 every third passage.
Drug Preparation
Latrunculin A and jasplakinolide were purchased from Calbiochem. Drugs were diluted to 50 μM working stocks in DMSO. C1 peptide was prepared as previously described (Joshi, Dutch, and Lamb, 1998).
Syncytium Assay
Subconfluent monolayers of BHK cells in six-well plates were transiently transfected with pCAGGS (2 μg) or pCAGGS PIV5 F (1 μg) and pCAGGS PIV5 HN (1 μg) using Lipofectamine Plus (Life Technologies) according to the manufacturer’s protocol. Cells were then returned to DMEM with FBS and P/S supplemented with drug or the drug carrier, DMSO. Syncytia were allowed to develop overnight and photomicrographs were taken the following day using a Nikon Diaphot inverted phase-contrast microscope.
Immunofluoresence
Vero cells were grown on polylysine coated coverslips. Cells were transfected and treated as in the syncytium assay. Syncytia were allowed to develop overnight. The following day cells were fixed in 4% paraformaldahyde. All cells were stained with rhodamine phalloidin (Invitrogen) and mounted in Vectashield with DAPI (Vector Labs). Cells were imaged on a Zeiss Axiovert 200M inverted fluorescence microscope (Carl Zeiss Microimaging, Thornwood, NY) using an Orca ER camera (Hamamatsu Corp. Bridgewater, NJ).
Reporter Gene Assay
Six well plates of subconfluent Vero cells were transfected with one μg pCAGGS PIV5 F, one μg pCAGGS PIV5 HN, and 0.8 μg T7-Control (containing the firefly luciferase gene under the control of a T7 promoter) on the day prior to performing the assay. Background signal was determined by replacing the fusion protein construct with empty pCAGGS vector. The day following transfection, BSR cells were trypsinized, washed once, and resuspended in DMEM + 10% FBS + 1% P/S supplemented with drug or DMSO. At the same time, the effector cell population was transferred to media supplemented with drug or DMSO. Both effector cell and target cell populations were maintained in drug for 3 hours prior to overlay. Target cells were maintained in suspension by periodic agitation. Target cells were then plated onto effector cells and three hours later the mixed cell population was lysed and assayed using the luciferase assay kit (Promega). Luminometry was performed on an Lmax luminometer (Molecular Devices, Sunnyvale, CA). For all reporter gene data, with the exception of the temperature inhibition study, background luciferase activity was subtracted from specific fusion induced activity. Each replicate was then normalized to signal measured with F and HN in the presence of DMSO, since significant changes in absolute time-integrated luminescence were seen between independent experiments while the trend remained consistent. Data was then averaged over all replicates and standard error of the mean and the statistical difference between populations were calculated using paired t-tests and ANOVA analysis using KaleidaGraph (Synergy Software, Reading, PA). Temperature blockade data was normalized as described, though without background subtraction, as signal was compared to background.
Temperature or peptide-mediated inhibition of membrane fusion
For studies on the effect of temperatures, reporter gene assays were performed as above with the exception that all media was maintained at room temperature and incubator temperature was lowered to 30°C for the drug incubation, and overlay. For studies on the effect of peptide addition, lyophilized C1 peptide, prepared as previously described (Joshi, Dutch, and Lamb, 1998) was dissolved in phosphate buffered saline and the peptide concentration was determined by 280 nm absorbance in 6 M guanidine. At the time of overlay the media was supplemented with PBS or PBS with C1 peptide to a final peptide concentration of 50 μM.
Flow Cytometry
Flow cytometry was performed as previously described (Schowalter et al., 2006). Briefly 6 cm plates of Vero cells were transfected with either 4μg of pCAGGS PIV5 F or pCAGGS empty vector. Cells were placed in media containing drug or DMSO three hours prior to staining. At the time of staining cells were moved to 4°C. Cells were stained with F1a monoclonal antibody (kindly provided by Dr. Richard Randall, University of St. Andrews) against PIV5 F and goat anti-mouse FITC conjugated secondary antibody. Mean fluorescence intensity was measured for 10,000 cells. Mean fluorescence intensity was normalized to DMSO treated cells. Independent replicates were averaged and standard error of the mean was calculated using Kaleidograph.
Expression and biotinylationof cell surface proteins
Verocells in six cm dishes were transiently transfected with 8 μg pCAGGS-PIV5 F or the empty pCAGGS expression vector using Lipofectamine 2000. At 18–24 h post-transfection, cells were starved in methionine- and cysteine-deficient DMEM for 45 min and then metabolically labeled with Trans[35S] label (100 μCi/ml; MP Biomedicals) for 1 hr. Cells were washed once with PBS, then incubated in 2 ml/dish DMEM plus FBS and P/S containing 0–200 nM latrunculin A or jazplakinolide for 3 hr. Cells were washed three times with cold pH 8 PBS and incubated with 1mg/ml EZ-Link Sulfo-NHS-Biotin (Pierce, Rockford, IL) diluted in pH 8 PBS for 10 min rocking at 4 C, then 20 min at room temperature. Cells were washed three times again with pH 8 PBS, then lysed in RIPA buffer containing 100 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100, 1% deoxycholic acid, protease inhibitors (1 KalliKrein inhibitory unit of aprotinin [Calbiochem, San Diego, Calif.], 1 mM phenylmethylsulfonyl fluoride [Sigma, St. Louis, Mo.]), and 25 mM iodoacetamide (Sigma). The lysates were centrifuged at 136,500 × g for 10 min at 4°C, and supernatants were collected. Antipeptide sera directed against the PIV5 F cytoplasmic tails and protein A-conjugated sepharose beads (Amersham, Piscataway, N.J.) were used to immunoprecipitate the F protein as previously described (Paterson, 1993). Sepharose beads were boiled twice in 10% SDS for 10 min to release protein. Fifteenpercent of total protein was removed for analysis, and the remaining 85% was incubated with immobilized streptavidin (Pierce) for 1 hour at 4 °C, then biotin-labeled protein bound to streptavidin was pulled-down and analyzed via SDS-15% polyacrylamide gel electrophoresis (SDS-PAGE) and visualized using the Typhoon imaging system.
Results
PIV5 F protein-mediated syncytia formation in the presence of jasplakinolide or latrunculin A
Analysis of paramyxovirus F protein-mediated membrane fusion is facilitated by the fact that expression of the viral attachment and fusion proteins is sufficient to induce cell-cell fusion events in tissue culture. Formation of multinucleated giant cells, termed syncytia, from repeated cell-cell fusion events provides a sensitive, but qualitative, measure of the relative efficiency of membrane fusion promotion under varying conditions.
To examine the effect of drugs which perturb the actin cytoskeleton on these membrane fusion events, BHK cells were transiently transfected toexpress the PIV5 F and attachment (HN) proteins using the pCAGGS expression system (Niwa, Yamamura, and Miyazaki, 1991). Following transfection, cells were moved to media containing either the drug carrier (DMSO) or varying concentrations of jasplakinolide, an F-actin stabilizing drug, or latrunculin A, a G-actin sequestrant. Syncytia formation was then analyzed twenty-four hours later.
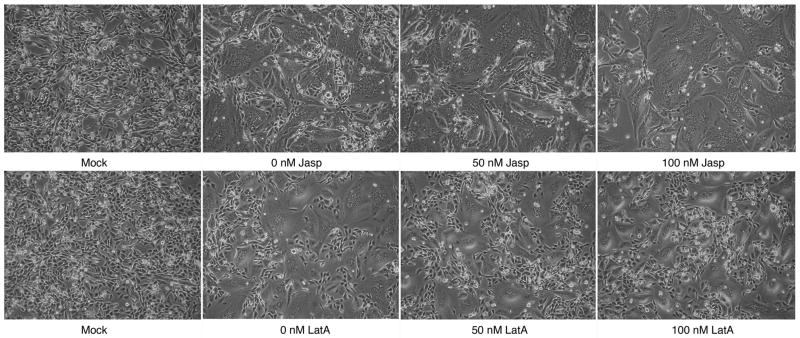
Interestingly, treatment of cells with doses of up to 100 nM jasplakinolide led to stimulation of syncytium formation by the PIV5 viral glycoproteins (Figure 2), suggesting that perturbation of the actin cytoskeleton can lead to increased cell-cell fusion. As concentrations were increased beyond 150 nM, a relative decrease in syncytium formation was observed, along with increasing cytotoxicity, marked cell rounding, and loss of cell-cell contact (data not shown). The observed stimulation at lower concentrations is in contrast to reports for HIV env-promoted syncytia formation (Pontow et al., 2004), though the inhibition observed at higher concentrations of jasplakinolide is consistent with results for both HIV env-promoted syncytia formation (Pontow et al., 2004) or RSV F-promoted viral entry (Kallewaard, Bowen, and Crowe, 2005).
Figure 2.
BHK -21 cells were transfected with pCAGGS PIV5 F and HN or mock transfected with empty pCAGGS vector. After transfection, cells were placed in media containing the described amount of jasplakinolide or latrunculin A. Syncytia were imaged the following day.
In contrast, treatment with latrunculin A produced no consistent changes in syncytia formation in cells expressing the PIV5 glycoproteins (Figure 2). Decreases in syncytia formation were again noted at higher concentrations (data not shown), consistent with reports from both the HIV and RSV systems (Kallewaard, Bowen, and Crowe, 2005; Pontow et al., 2004).
Actin structures in syncytium treated with jasplakinolide or latrunculin A
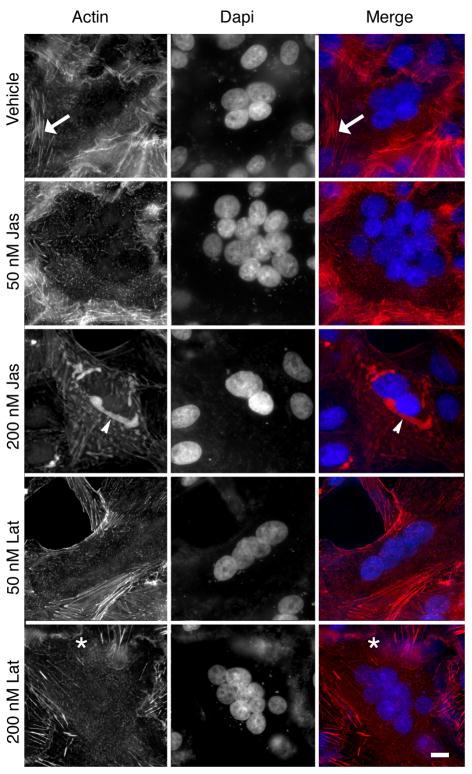
While jasplakinolide is known to promote polymerization of F-actin (Bubb et al., 1994) and latrunculin A inhibits actin polymerization (Spector et al., 1983) and sequesters monomeric actin (Coue et al., 1987), treatment with either jasplakinolide (Bubb et al., 2000) or latrunculin (Wakatsuki et al., 2001) can promote disordering of the cellular actin cytoskeleton, likely due to reduction in the pool of free monomeric actin needed for remodeling. To examine the specific effect of actin targeted drugs on the cellular cytoskeleton in cells expressing the viral glycoproteins, we induced syncytia formation using the PIV5 glycoproteins in the presence and absence of drug as above. Cells were fixed and stained with rhodamine-phalloidin and DAPI. Nuclei in the syncytium of the treated and untreated cells were almost exclusively found in an organized structure in the center of the cell. Syncytia displayed robust stress fiber labeling in the absence of drug treatment (note arrow in Figure 3). Syncytia treated with 50 nM jasplakinolide displayed decreased numbers of stress fibers as well as a relative decrease in cortical actin staining suggesting depletion of the actin network underneath the membrane. Treatment with 200nM jasplakinolide resulted in marked disturbance of the actin cytoskeleton and the presence of flocculent phalloidin-positive inclusions surrounding the nuclei (Figure 3, note arrowheads). These amorphous masses of F-actin have previously been reported upon treatment with jasplakinolide and result from ectopic actin polymerization (Bubb et al., 2000). Syncytia began to lose contact with substrate and retract, leading to a stellate appearance of the syncytium. Cells treated with this higher dose were fragile and easily fragmented or washed off the coverslips during staining (data not shown).
Figure 3.
Vero cells were transfected to express the PIV5 glycoproteins as described previously, and syncytia were allowed to develop in the presence of jasplakinolide or latrunculin. Cells were fixed in 4% paraformaldehyde, and stained with rhodamine-phalloidin and DAPI. The arrows in A–C identify a stress fiber in the control cells. The arrowhead in panels G–I indicate an actin aggregate formed due to ectopic polymerization and the asterisks in panels identifies a ruffled membrane in the latrunculin treated samples. Bar = 10 μm.
Cytoskeletal changes in syncytium treated with latrunculin A were more subtle than those seen with jasplakinolide. Treatment with 50 nM latrunculin A resulted in a modest loss of fine actin structures, leading to decreases in overall actin staining (Figure 3). Treatment with 200nM latrunculin A resulted in complete loss of fine F-actin structures, and a marked increase ruffling at the cell surface (Figure 3, note asterisk in panels G–H). An increased number of cells with two nuclei were noted (data not shown) after latrunculin A treatment, consistent with effects on cell division. A small number of multi-nucleated syncytia were also observed (Figure 3).
Reporter gene assays in the presence of jasplakinolide and latrunculin A
The observed increases in syncytium formation could be the result of an increase in the number of fusion events occurring. Alternatively, the rate of large scale cellular reorganization subsequent to the fusion event itself may have increased, allowing earlier visualization of the syncytia. To discriminate between these possibilities, we employed a reporter gene assay in which an effector cell population was cotransfected with the viral fusion and attachment proteins, as well asa firefly luciferase gene under the control of a T7 promoter. The effector cell population was then overlayed with a target cell population which stably expresses the T7 polymerase. Luciferase signal, therefore, requires only fusion pore enlargement sufficient to pass the T7 polymerase. In this assay both target and effector cell populations were pretreated with drug for three hours prior to overlay. Cells were then overlayed for three hours prior to cell lysis and assayedfor luciferase activity.
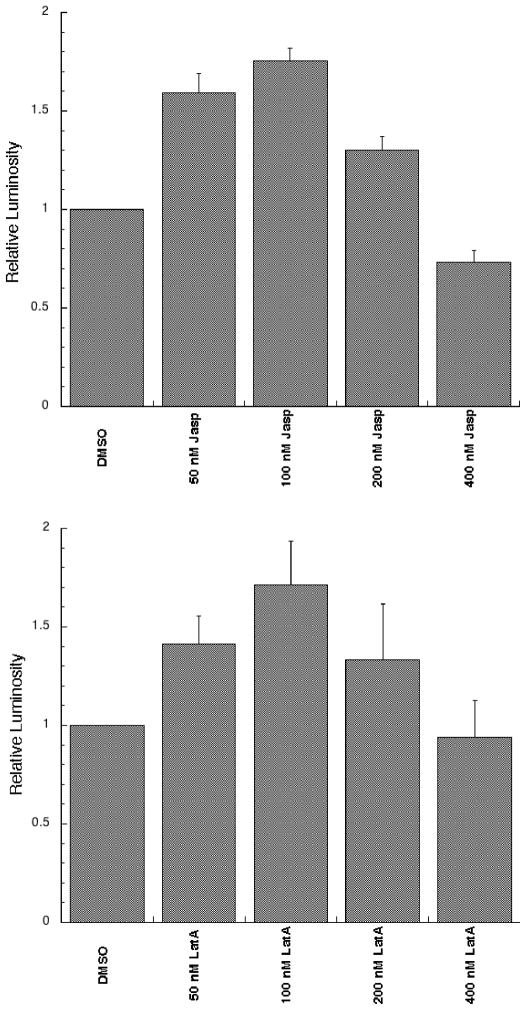
Treatment with relatively low doses of jasplakinolide resulted in a marked stimulation of luciferase activity with an approximate 1.8-fold stimulation in PIV5 F-promoted fusion (Figure 4A). This effect peaked at 100nM jasplakinolide, with the increases at this dose consistent with the stimulation observed in syncytia assays. Increasing concentrations beyond 100 nM resulted in a steady fall off in fusion activity, potentially due to the large changes in cell morphology noted earlier.
Figure 4.
Reporter gene assays were performed in the presence of jasplakinolide or latrunculin A at the indicated doses. Both target and effector cell populations were drug treated for 3 hours prior to and throughout the overlay. Six independent replicates of each experimentwere conducted. Background was subtracted, and the results were normalized to those of the DMSO treated control (RLU values for the DMSO treated control ranged from 3.46 – 15.48 for the jasplakinolide experiment and from 4.08 to 15.47 for the latrunculin experiments). Error bars represent the standard error of the mean.
Latrunculin A also stimulated fusion induced by the PIV5 glycoproteins at low doses (Figure 4B), as measured by the reporter gene assay, in contrast to the lack of obvious stimulation of syncytia formation (Figure 2B). Latrunculin A stimulation peaked at approximately 1.6 times the control signal at a concentration of 100 nM. Increasing doses above 100 nM also led to a gradual decrease in fusion at higher concentrations.
Treatment with jasplakinolide or latrunculin A doesnot affect PIV5 F surface density
Higher surface densities of the PIV5 F protein have been shown to increase the number of fusion events promoted by the PIV5 fusion system (Dutch, Joshi, and Lamb, 1998). Increasing surface density of the fusion proteins therefore represents a possible explanation for the stimulation of fusion we observed with actin drug treatment. We therefore employed both surface biotinylation and flow cytometry to investigate potential changes that drug treatment may have had on surface densities and cleavage state of the PIV5 F protein.
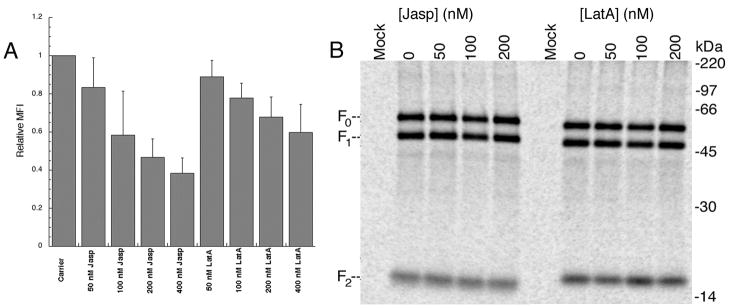
For flow cytometry, cells transiently expressing PIV5 F were treated with either latrunculin A or jasplakinolide, and analyzed using the F1a monoclonal antibody (Randall et al., 1987) (Figure 5A). Surface densities of PIV5 F showed only minor decreases at doses of both jasplakinolide and latrunculin A that produced stimulation of fusion. As the drug dose rose to levels which inhibit fusion, a progressive fall in surface density was seen, suggesting that the decrease in fusion may be the result of decreases in surface density of the fusion protein.
Figure 5.
A. Vero cells were transfected with pCAGGS PIV5 F. The following day, cells were treated with indicated doses of jasplakinolide or latrunculin A for 3 hours. Following drug treatment cells were stained with F1a primary, and goat anti-mouse FITC conjugated secondary, and analyzed by flow cytometry. Mean fluorescent intensity (MFI) was normalized to carrier treated control(n = 4, range of MFI valu es for DMSO-treated control from 99.82 – 348.6). Error bars representstandard error of the mean. B. Cells transiently expressing PIV5 F were treated with jasplakinolide or latrunculin A, and metabolically labeled with trans-35S. Surface biotinylation was performed and PIV5 F protein was immunoprecipitated, streptavidin pull-downs performed, and the resulting samples resolved with 15% SDS PAGE.
To confirm the flow cytometry results, and probe for alterations in proteolytic activation of the F protein that could result in fusion decreases, surface biotinylation was performed. Transfected cells were metabolically labeled with Trans-35S, and treated with drug. Surface populations were identified using a membrane impermeable biotinylation agent (Sulfo-NHS biotin). Cells were then lysed and total and surface protein amounts are compared. Treatment with the indicated doses of jasplakinolide and latrunculin A did not significantly alter surface expression levels of the PIV5 protein in the dose ranges examined (Figure 5B). There was no apparent change in either the uncleaved (F0) or the cleaved, active form of the protein (F1, F2) at the cell surface. These data demonstrate that the stimulation of fusion seen in the previous experiments are likely not due to alterations in cell surface concentrations or cleavage state of the PIV5 F protein. The decrease in PIV5 F cell surface expression seen with flow cytometry at high doses of jasplakinolide and latrunculin A, but not apparent with surface biotinylation may represent the increased sensitivity of the method to detect changes in the levels of cell surface proteins.
Jasplakinolide and lantrunculin A stimulate pore enlargment
Paramyxovirus fusion proteins go through a series of well-defined steps during the process of fusion promotion (Figure 1). After a proteolytic processing event, the fusion protein exists in a metastable state. Following a triggering event, conformational changes in the F protein lead to insertion of the fusion peptide into the target membrane, forming a membrane bridging conformation (Asano and Asano, 1985) (Figure 1). This initial triggering event can be blocked by incubation at lowered temperature (Russell, Jardetzky, and Lamb, 2001). The bridging conformation then undergoes a refolding process whereby the C-terminal heptad repeat regions fold back onto the N-terminal heptad repeat regions to form a very stable six-helix bundle (Figure 1). The refolding reaction can be inhibited by treatment with peptides derived from the heptad repeat regions which bind specifically to their complementary regions in the fusion protein, trapping the protein in a pre-hairpin conformation (Russell, Jardetzky, and Lamb, 2001). The final step in the fusion process is the enlargement of the nascent fusion pore (Figure 1). This final step is poorly understood, but truncation of the PIV5 cytoplasmic tail has been shown to specifically affect pore enlargement (Dutch and Lamb, 2001).
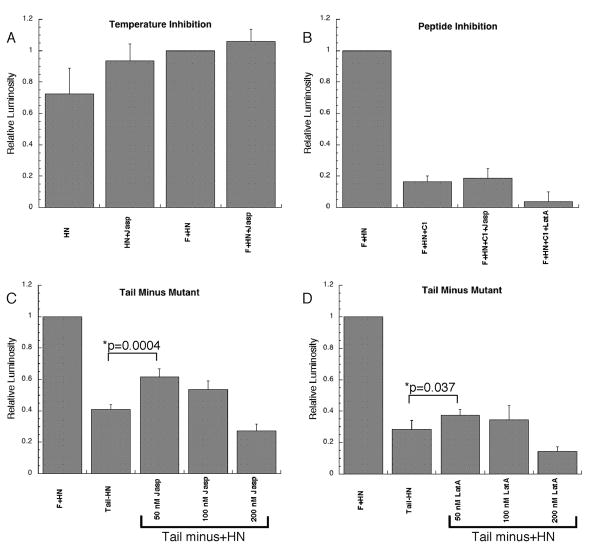
To characterize the mechanism by which actin destabilizing drugs stimulate fusion, a series of reporter gene experiments blocking specific steps of the PIV5 F protein fusion process were performed. Lowering the incubation temperature from 37°C to 30°C immediately following the mixing of the cell populations leads to a nearly complete inhibition of F protein specific cell-cell fusion events (West et al., 2005). The relationship between temperature and fusion is evident by the similarity of luciferase signal from F and HN expressing cells compared to negative control cell expressing HN alone (Figure 6a, Temperature Inhibition). Treatment with jasplakinolide did not significantly alter the low level of fusion, suggesting that our observed stimulation of fusion by this drug is not the result of lowering the energetic threshold for the activation of the fusion protein. In addition, these results indicate that jasplakinolide-induced stimulation of fusion is dependent on triggering of the fusion protein.
Figure 6.
A. Cells expressing either PIV5 HN protein alone or the PIV5 F and HN proteins together were utilized for reporter gene analysis, as previously described, except that an incubation temperature of 30o C was used. Jasplakinolide (50 nM) was added to the indicated samples. Six independent experiments were performed, and in each case luciferase activity was normalized to F + HN (RLU values for F + HN ranged from0.78 to 1.56). Error bars represent the standard error of the mean. B. Cells expressing the PIV5 F and HN proteins were treated with 50nM jasplakinolide or latrunculin A, and 50μM C1 peptide, and fusion measured by reporter gene analysis. Results from four independent experiments were each normalized to F+HN (RLU values for F + HN 4.47 to 5.11). C and D. Reporter gene assay using wild type PIV5 F or the mutant PIV5 F tail-. The assay was performed 11 times with the indicated doses of jasplakinolide (C) and six times for latrunculin A (D). Results were normalized to the F + HN samples (RLU values from 5.35 to 8.61 for the jazplakinolide experiments and from 7.22 to 17.56 for the latrunculin experiments). P values are indicated for statistically significant differences, and error bars represent standard error of the mean.
Insertion of the fusion peptide into membranes has a marked destabilizing influence on model membranes and is sufficient to promote fusion of small unilamellar vesicles (Rapaport and Shai, 1994). To determine if membrane destabilization caused by fusion peptide insertion is sufficient to allow for latrunculin A or jasplakinolide-induced stimulation, we examined the effect of these drugs in the presence of the heptad repeat 2 (HR2)-derived C1 peptide. Addition of this peptide does not block the initial conformational changes that result in fusion peptide insertion. Instead, the C1 peptide is hypothesized to arrest the fusion protein at a pre-hairpin conformation by interacting with the N-terminal heptad repeat coiled-coil formed after the initial conformational changes. Treatment with this peptide effectively blocks lipid mixing promoted by PIV5 F (Russell, Jardetzky, and Lamb, 2001). Addition of 50 μM C1 leads to an approximately 80% inhibition in fusion activity as measured by reporter gene activity (Figure 6, Peptide Inhibition). Treatment with 50 nM jasplakinolide or latrunculin A in the presence of the inhibitory peptide was unable to significantly increase fusion above these levels.
Deletion of the cytoplasmic tail of the PIV5 fusion protein (PIV5 Tail-) leads to a protein which promotes mixing of lipids and small aqueous dyes with kinetics similar to the wild-type protein (Dutch and Lamb, 2001). However, PIV5 F Tail- is significantly debilitated in reporter gene assays and syncytia formation, suggesting that the protein displays a defect in pore enlargement. To determine whether jasplakinolide or latrunculin A could rescue a defect in pore enlargement, we examined PIV5 F tail- in a reporter gene assay (Figure 6, Tail Minus Mutant). Interestingly, treatment with low dose jasplakinolide and latrunculin A resulted in a partial rescue of reporter gene activity (p=0.004 and p=0.037, respectively). Partial, rather than full restoration may indicatethat the cytoplasmic tail of the fusion protein plays an additional role in fusion unrelated to the actin cytoskeleton. Alternatively, these lower drug concentrations, while not toxic to the cell or affecting fusion protein surface expression, may not be sufficient to completely remove the actin barrier. This result does suggest that jasplakinolide and latrunculin A stimulate fusion by relieving a barrier to pore enlargement, which occurs naturally as a consequence of the dense cortical actin layer beneath the plasma membrane.
Discussion
We have shown that treatment with low doses of jasplakinolide can stimulate PIV5 glycoprotein-mediated fusion events. This stimulation requires the activation and subsequent conformational rearrangements of the PIV5 F protein (Figure 6), suggesting that the effect occurs late during the fusion process. We hypothesize that the stimulatory mechanism of these drugs may be to a decrease in a mechanical barrier at the membrane which is inhibitory to fusion pore enlargement by globally decreasing the bulk of cortical actin present at the membrane. Pore enlargement appears to be the most energetically costly step of viral mediated membrane fusion (Cohen and Melikyan, 2004), and a significant portion of the energy released upon formation of the six helix bundle is likely required for pore enlargement rather than membrane merger. Overcoming the physical barrier of the cortical cytoskeleton at the membrane may provide one possible explanation for this energetic barrier.
The observation that both latrunculin A and jasplakinolide stimulate PIV5 F protein promoted fusion, as measured by a reporter gene assay, is at first puzzling, as the drugs have very different mechanisms of action. Both drugs, however, do result in the depletion of the cellular free actin pool, and treatment with jaspkalinolide (Bubb et al., 2000) or latrunculin can result in disruption (Wakatsuki et al., 2001) of the actin cytoskeleton. Latrunculin A accomplishes this by directly sequestering G-actin, while jasplakinolide ties up cellular actin in F-actin inclusions within the cell. Even in contact-inhibited cells, the cortical actin meshwork undergoes rapid turnover (Ponti et al., 2003). By decreasing the pool of available actin monomers in the cell, the structure of the cortical actin cytoskeleton is rapidly altered. We hypothesize that these alterations to the cortical actin structures lead to the increased fusion that we see upon treatment with these drugs.
Interestingly, the effect of latrunculin treatment on PIV5 F protein-promoted fusion differed between the two assays utilized. No significant stimulation was observedin syncytia assays following latrunculin treatment (Figure 2), but stimulation was observed in the reporter gene assay at similar concentrations (Figure 4). While syncytia formation occurs between attached cells, reporter gene analysis measures fusionbetween attached cells expressing the viral glycoproteins and target cells which have been trypsinized and then added to the effector cells. Trypsin treatment has been reported to result in disorganization of the actin cytoskeleton (Pollack and Rifkin, 1975; Richard, Leikina, and Chernomordik, 2009), and thus the alterations to the actin cytoskeleton are likely greater in the target cells in the reporter gene assay than in the cells studied for syncytia formation.
Studies of myoblast fusion suggest that actin either serves as a barrier (Richardson et al., 2007), is involved in a priming step prior to fusion (Kim et al., 2007), or provides the needed force to expand the fusion pore (Massarwa et al., 2007). Conflicting conclusions have also been drawn concerning the role of actin in other fusion events (Eitzen, 2003). Recent studies of both the influenza HA protein (Richard, Leikina, and Chernomordik, 2009) and the baculovirus gp64 protein (Chen et al., 2008) suggest that the actin cytoskeleton serves as a barrier to fusion pore expansion, consistent with the findings presented here. Thus, the actin cytoskeleton restricts fusion promoted by three different type 1 fusion proteins from three viral families, suggesting a conserved mechanism of fusion.
While the larger picture of the role of actin in fusion appears similar, the specific drug effects noted differ between our studies and others. In particular, while we observed stimulation with jasplakinolide (Figures 2 and 3), inhibition with this drug was reported for gp64-mediated fusion (Chen et al., 2008) and HIV env-mediated fusion (Pontow et al., 2004). In the gp64 study (Chen et al., 2008) 500 nM jasplakinolide was employed, which is significantly higher than the concentrations used in our experiments. Indeed, we observed similar decreases in fusion with 400 nM jaspakinolide (Figure 4), though we also found alterations in cell morphology and reductions in cell surface expression of the F protein (Figure 5A) that complicate any conclusions on the direct effect of higher concentrations of jazplakinolide on PIV5 F protein-promoted fusion. Jasplakinolide inhibition of HIV env promoted cell-cell fusion also occurred at higher concentrations (500 nm – 1 μM), though no stimulation was observed with lower concentrations (Pontow et al., 2004), in contrast to our findings. Latrunculin A at two μM was found to greatly increase pore expansion in gp64-promoted membrane fusion, while similar concentrations completely inhibited HIV env-promoted fusion (Pontow et al., 2004). In our study, stimulation was observed at lower levels, but higher concentrations lead to inhibition and large changes in cell morphology (Figure 3). The inherent differences between the Sf9 host cells utilized in the gp64 experiments and the BHK and astroglioma cell lines utilized here and in the HIV env study may account for these differences. Alterations in the inhibitory/stimulatory effect of these drugs under different experimental conditions has been observed in studies of fusion in exocytosis (Eitzen, 2003).
Deletion of nineteen of the twenty amino acids of the cytoplasmic domain of PIV5 F leads to a specific defect in pore enlargement (Dutch and Lamb, 2001). It would be possible for this domain to interact with proteins in the cytosol of the target cell upon formation of the nascent fusion pore, thereby providing a platform for signaling to drive alterations in the cytoskeleton which could facilitate membrane fusion. Interestingly, recombinant viruses containing an F protein with an 18-amino acid C-tail deletion were only minorly debilitated for fusion, while those with a 20 amino acid deletion showed surface expression alterations for the F protein, and were therefore not examined for fusion (Waning et al., 2002). These results suggest that either other viral proteins, such as the matrix protein, can partially compensate for loss of the F protein cytoplasmic tail, or that thetwo cytoplasmic tail residues closest to the membrane are those critically important in fusion. Examination of this region for interactions with cellular proteins and study of the role of actin in the context of viral infection representsignificant areas worthy of further study.
Acknowledgments
We are grateful to members of the Dutch lab for critically reviewing this manuscript. Imaging studies were funded in part due to grants forthe imaging facility provided by NIH COBRE grant P20RR20171. This study was supported by NIAID grant R01A151517 to R.E.D., NEI grant R21 YE018112 to CLM, and NIH grant P20RR20171 to ROM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asano K, Asano A. Why is a specific amino acid sequence of F glycoprotein required for the membrane fusion reaction between envelope of HVJ (Sendai virus) and target cell membranes? Biochem International. 1985;10:115–122. [PubMed] [Google Scholar]
- Baker KA, Dutch RE, Lamb RA, Jardetzky TS. Structural basis for paramyxovirus-mediated membrane fusion. Mol Cell. 1999;3:309–319. doi: 10.1016/s1097-2765(00)80458-x. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–55. [PMC free article] [PubMed] [Google Scholar]
- Black CP. Systematic review of the biology and medical management of respiratory syncytial virus infection. Respir Care. 2003;48(3):209–31. discussion 231–3. [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269 (21):14869–71. [PubMed] [Google Scholar]
- Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem. 2000;275 (7):5163–70. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- Buchholz UJ, Finke S, Conzelmann KK. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J Virol. 1999;73:251–259. doi: 10.1128/jvi.73.1.251-259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Leikina E, Melikov K, Podbilewicz B, Kozlov MM, Chernomordik LV. Fusion-pore expansion during syncytium formation is restricted by an actin network. J Cell Sci. 2008;121 (Pt 21):3619–28. doi: 10.1242/jcs.032169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Sowers AE. Evidence that the spectrin network and a nonosmotic force control the fusion product morphology in electrofused erythrocyte ghosts. Biophys J. 1991;60(5):1026–37. doi: 10.1016/S0006-3495(91)82140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Zimmerberg J, Kozlov MM. Membranes of the world unite! J Cell Biol. 2006;175 (2):201–7. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, Zaki SR, Paul G, Lam SK, Tan CT. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354 (9186):1257–9. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- Cohen FS, Melikyan GB. The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement. J Membr Biol. 2004;199 (1):1–14. doi: 10.1007/s00232-004-0669-8. [DOI] [PubMed] [Google Scholar]
- Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213 (2):316–8. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Dutch RE, Jardetsky TS, Lamb RA. Virus membrane fusion proteins: biological machines that undergo a metamorphosis. Bioscience Reports. 2000;20 (6):597–612. doi: 10.1023/a:1010467106305. [DOI] [PubMed] [Google Scholar]
- Dutch RE, Joshi SB, Lamb RA. Membrane fusion promoted by increasing surface densities of the paramyxovirus F and HN proteins: comparison of fusion reactions mediated by simian virus 5 F, human parainfluenza virus type 3 F, and influenza virus HA. J Virol. 1998;72 (10):7745–53. doi: 10.1128/jvi.72.10.7745-7753.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch RE, Lamb RA. Deletion of the cytoplasmic tail of the fusion (F) protein of the paramyxovirus simian virus 5 (SV5) affects fusion pore enlargement. J Virol. 2001;75:5363–5369. doi: 10.1128/JVI.75.11.5363-5369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch RE, Leser GP, Lamb RA. Paramyxovirus fusion protein: characterization of the core trimer, a rod-shaped complex with helices in anti-parallel orientation. Virology. 1999;254:147–159. doi: 10.1006/viro.1998.9532. [DOI] [PubMed] [Google Scholar]
- Eaton BT, Broder CC, Middleton D, Wang LF. Hendra and Nipah viruses: different and dangerous. Nat Rev Microbiol. 2006;4 (1):23–35. doi: 10.1038/nrmicro1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641(2–3):175–81. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- Garten W, Hallenberger S, Ortmann D, Schafer W, Vey M, Angliker H, Shaw E, Klenk HD. Processing of viral glycoproteins by the subtilisin-like endoprotease furin and its inhibition by specific peptidylchloroalkylketones. Biochimie. 1994;76 (3–4):217–25. doi: 10.1016/0300-9084(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Goswami KKA, Lange LS, Mitchell DN, Cameron KR, Russell WC. Does simian virus 5 infect humans. J Gen Virol. 1984;65:1295–1303. doi: 10.1099/0022-1317-65-8-1295. [DOI] [PubMed] [Google Scholar]
- Gower TL, Pastey MK, Peeples ME, Collins PL, McCurdy LH, Hart TK, Guth A, Johnson TR, Graham BS. RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J Virol. 2005;79(9):5326–36. doi: 10.1128/JVI.79.9.5326-5336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SB, Dutch RE, Lamb RA. A core trimer of the paramyxovirus fusion protein: parallels to influenza virus hemagglutinin and HIV-1 gp 41. Virology. 1998;248:20–34. doi: 10.1006/viro.1998.9242. [DOI] [PubMed] [Google Scholar]
- Kallewaard NL, Bowen AL, Crowe JE., Jr Cooperativity of actin and microtubule elements during replication of respiratory syncytial virus. Virology. 2005;331 (1):73–81. doi: 10.1016/j.virol.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, Gonzalez GA, Chen EH. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12 (4):571–86. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Lamb RA, Jardetzky TS. Structural basis of viral invasion: lessons from paramyxovirus F. Curr Opin Struct Biol. 2007;17 (4):427–36. doi: 10.1016/j.sbi.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA, Parks GD. Paramyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 1. Vol. 2. Lippincott: Williams and Wilkins; 2007. pp. 1449–1496. [Google Scholar]
- Makino S, Yamaguchi F, Sata T, Urushibata O, Kurata T, Nishiwaki M. The rash of measles is caused by a viral infection in the cells of the skin: a case report. J Dermatol. 1994;21 (10):741–5. doi: 10.1111/j.1346-8138.1994.tb03280.x. [DOI] [PubMed] [Google Scholar]
- Massarwa R, Carmon S, Shilo BZ, Schejter ED. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell. 2007;12 (4):557–69. doi: 10.1016/j.devcel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- McCandlish IA, Thompson H, Cornwell HJ, Wright NG. A study of dogs with kennel cough. Vet Rec. 1978;102(14):293–301. doi: 10.1136/vr.102.14.293. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Grubor B, Fach SJ, Sacco RE, Lehmkuhl HD, Gallup JM, Ackermann MR. Reduced clearance of respiratory syncytial virus infection in a preterm lamb model. Microbes Infect. 2004;6 (14):1312–9. doi: 10.1016/j.micinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128 (4):589–98. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants by a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Novick SL, Hoekstra D. Membrane penetration of Sendai virus glycoproteins during the early stage of fusion with liposomes as determined by hydrophobic affinity labeling. Proc Natl Acad Sci USA. 1988;85:7433–7437. doi: 10.1073/pnas.85.20.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pager CT, Dutch RE. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J Virol. 2005;79 (20):12714–20. doi: 10.1128/JVI.79.20.12714-12720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DL, Murray PK, McCormack JG. Zoonotic disease in Australia caused by a novel member of the paramyxoviridae. Clin Infect Dis. 1998;27 (1):112–8. doi: 10.1086/514614. [DOI] [PubMed] [Google Scholar]
- Paterson RG, Lamb RA. The molecular biology of influenza viruses and paramyxoviruses. In: Davidson A, Elliott RM, editors. Molecular virology: a practical approach. IRL Oxford University Press; Oxford, England: 1993. pp. 35–73. [Google Scholar]
- Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE, Ling AE, Chew SK, Ang B, Rollin PE, Umapathi T, Sng I, Lee CC, Lim E, Ksiazek TG. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. 1999;354(9186):1253–6. doi: 10.1016/S0140-6736(99)04379-2. [DOI] [PubMed] [Google Scholar]
- Pollack R, Rifkin D. Actin-containing cables within anchorage-dependent rat embryo cells are dissociated by plasmin and trypsin. Cell. 1975;6:495–506. [Google Scholar]
- Ponti A, Vallotton P, Salmon WC, Waterman-Storer CM, Danuser G. Computational analysis of F-actin turnover in cortical actin meshworks using fluorescent speckle microscopy. Biophys J. 2003;84(5):3336–52. doi: 10.1016/S0006-3495(03)70058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontow SE, Heyden NV, Wei S, Ratner L. Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion. J Virol. 2004;78 (13):7138–47. doi: 10.1128/JVI.78.13.7138-7147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RE, Young DF, Goswami KKA, Russell WC. Isolation and characterization of monoclonal antibodies to simian virus 5 and their use in revealing antigenic differences between human, canine and simian isolates. J Gen Virol. 1987;68:2769–2780. doi: 10.1099/0022-1317-68-11-2769. [DOI] [PubMed] [Google Scholar]
- Rapaport D, Shai Y. Interaction of fluorescently labeled analogues of the amino-terminal fusion peptide of Sendai virus with phospholipid membranes. J Biol Chem. 1994;269(21):15124–31. [PubMed] [Google Scholar]
- Richard JP, Leikina E, Chernomordik LV. Cytoskeleton reorganization in influenza hemagglutinin-initiated syncytium formation. Biochim Biophys Acta. 2009;1788(2):450–7. doi: 10.1016/j.bbamem.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BE, Beckett K, Nowak SJ, Baylies MK. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 2007;134 (24):4357–67. doi: 10.1242/dev.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BE, Nowak SJ, Baylies MK. Myoblast fusion in fly and vertebrates: new genes, new processes and new perspectives. Traffic. 2008;9 (7):1050–9. doi: 10.1111/j.1600-0854.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CJ, Jardetzky TS, Lamb RA. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 2001;20(15):4024–34. doi: 10.1093/emboj/20.15.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A, Choppin PW. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974;57:475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schowalter RM, Wurth MA, Aguilar HC, Lee B, Moncman CL, McCann RO, Dutch RE. Rho GTPase activity modulates paramyxovirus fusion protein-mediated cell-cell fusion. Virology. 2006;350:323–334. doi: 10.1016/j.virol.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219(4584):493–5. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Wakatsuki T, Schwab B, Thompson NC, Elson EL. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J Cell Sci. 2001;114 (Pt 5):1025–36. doi: 10.1242/jcs.114.5.1025. [DOI] [PubMed] [Google Scholar]
- Waning DL, Schmitt AP, Leser GP, Lamb RA. Roles for the cytoplasmic tails of the fusion and hemagglutinin-neuraminidase proteins in budding of the paramyxovirus simian virus 5. J Virol. 2002;76 (18):9284–97. doi: 10.1128/JVI.76.18.9284-9297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DS, Sheehan MS, Segeleon PK, Dutch RE. Role of the simian virus 5 fusion protein N-terminal coiled-coil domain in folding and promotion of membrane fusion. J Virol. 2005;79 (3):1543–51. doi: 10.1128/JVI.79.3.1543-1551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson LJ, Strebel PM, Gacic-Dobo M, Hoekstra EJ, McFarland JW, Hersh BS. Has the 2005 measles mortality reduction goal been achieved? A natural history modelling study. Lancet. 2007;369 (9557):191–200. doi: 10.1016/S0140-6736(07)60107-X. [DOI] [PubMed] [Google Scholar]
- Yin HS, Paterson RG, Wen X, Lamb RA, Jardetzky TS. Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc Natl Acad Sci U S A. 2005;102(26):9288–93. doi: 10.1073/pnas.0503989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HS, Wen X, Paterson RG, Lamb RA, Jardetzky TS. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature. 2006;439(7072):38–44. doi: 10.1038/nature04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QA, Chang DC. Reorganization of cytoplasmic structures during cell fusion. J Cell Sci. 1991;100 ( Pt 3):431–42. doi: 10.1242/jcs.100.3.431. [DOI] [PubMed] [Google Scholar]