Abstract
Despite major advances in a variety of neuroscientific research fields, the majority of neurodegenerative and neurological diseases are poorly controlled by currently available drugs, which are largely based on a neurocentric drug design. Research from the past five years has established a central role of glia to determine how neurons function and – consequently – glial dysfunction is implicated in almost every neurodegenerative and neurological disease. Glial cells are key regulators of the brain’s endogenous neuroprotectant and anticonvulsant adenosine. This review will summarize how glial cells contribute to adenosine homeostasis and how glial adenosine receptors affect glial function. We will then move on to discuss how glial cells interact with neurons and the vasculature and outline new methods to study glial function. We will discuss how glial control of adenosine-function affects neuronal cell death and its implications for epilepsy, traumatic brain injury, ischemia, and Parkinson’s disease. Eventually, glial adenosine-modulating drug targets might be an attractive alternative for the treatment of neurodegenerative diseases. There are, however, several major open questions that remain to be tackled.
Keywords: astrocyte, adenosine receptor, adenosine kinase, epilepsy, excitotoxicity, neurodegeneration
1. Introduction
It is becoming increasingly clear that inflammatory processes play an important role in neurodegenerative disease, just as inflammation is becoming increasingly implicated in various systemic diseases elsewhere in the body1. Because the immune responses in brain show uncommon features it was long considered to be “immune privileged”. However, cells that are involved in adaptive immune reactions do enter the brain, and this can result in major CNS pathology. Multiple sclerosis is an important example. Furthermore, immune reactions, often attributable to the innate immune system, feature in many if not all neurodegenerative diseases. Microglial cells, astrocytes, endothelial cells, oligodendrocytes, and neurons all produce signals to orchestrate these reactions. Arachidonic acid metabolites, nitric oxide, cytokines, and chemokines all appear to play some role. Recently, the role of ATP and adenosine as critically important signaling molecules has become appreciated2,3. This awareness that a multiplicity of signals other than those principally involved in nerve-nerve communication play a role in neurodegenerative disease has also meant that we have to consider other cells than neurons as critically important players. The present brief review will focus on adenosine (and to a lesser extent ATP) signaling, and the role of glial cells in neurodegenerative disease. The review will feature results from the authors’ own work more prominently than would be motivated from an objective standpoint.
2. Glial control of adenosine homeostasis
In mammals, purine de novo synthesis proceeds via formation of IMP, which is then converted into AMP; however, there is no de novo synthesis pathway for adenosine. Physiologically, intracellular adenosine can be formed by either dephosphorylation of AMP by 5′-nucleotidase, or, alternatively, by hydrolysis of S-adenosylhomocysteine, whereas extracellular adenosine can be formed from released adenine nucleotides by a cascade of ectonucleotidases4. Two metabolic pathways are responsible for the removal of adenosine: deamination into inosine via adenosine deaminase (ADA; EC 3.5.4.4) and phosphorylation into AMP via adenosine kinase (ADK; EC 2.7.1.20). Based on its low KM for adenosine, ADK is considered to be the primary route of adenosine metabolism5. Recent findings indicate that extracellular levels of adenosine, and consequently the levels close to synapses, are largely regulated by astrocytes6–10, and an astrocyte-based adenosine-cycle has been proposed11,12.
2.1. Glial release of ATP as source for extracellular adenosine
ATP can be released from neurons and astrocytes, is identified as a neurotransmitter in both CNS and PNS, and exerts a multitude of largely excitatory effects by activation of specific ATP receptors (P2X and P2Y receptors)13. Vesicular release has also been clearly demonstrated from endocrine cells and here the release of ATP may differ in several ways from that of the hormone, because of so called kiss-and-run release14. Thus, ATP can be released even in situations when the vesicle fusion is too incomplete and transient to allow release of the stored hormone. Perhaps this can occur also in nerves. However, under physiological conditions the vesicular release of ATP, not from neurons, but from astrocytes, has been identified as a major source of synaptic adenosine15. Transgenic mice that express a dominant-negative SNARE domain selectively in astrocytes were characterized by the loss of the adenosine A1 receptor mediated tonic inhibition in synaptic slices, indicating that under physiological conditions astrocytic release of ATP (followed by degradation into adenosine via ectonucleotidases) is a major source of adenosine15 that affects synaptic transmission. A kiss and run like exocytotic release of ATP has been detected in astrocytes16. Another, possibly related, mechanism involves release of ATP from a lysosome pool in astrocytes17. However, astrocytes appear to use also other mechanisms to release ATP as a signal. One of those involve connexin hemichannels18, but recent work provides strong reason to assume that pannexin-1, rather than connexin-43, provides the most responsible channel19. Another proposal is that ATP is released via maxi-anion channels, and this may be particularly important in astrocytic swelling20. The P2X7 receptor may in some conformations allow ATP to be released. Finally, ATP may be released whenever there are increases in membrane volume via incorporation of intracellular vesicles into the membrane, or when there is a shedding of small vesicles21. In addition to the mechanisms described above, astrocytes can directly release adenosine, especially in response to hypoxic stimulation6,22, even though release of adenosine per se is more typical of neurons23. In that case the release depends on export of adenosine via the equilibrative nucleoside transporters.
Since astrocytes can contact thousands of synapses and coordinate synaptic networks8,24, it is conceivable that astrocytic release of ATP and its subsequent degradation into adenosine has a major regulatory function in setting a global adenosine-mediated inhibitory tone within a neuronal network. In addition, other glial cells can also contribute. For example, in retinal tissue stimulation of glial cells leads to an adenosine-mediated inhibition of neuronal activity, but in this case Muller cells rather than astrocytes appear to be most important25.
The distribution of adenosine formed from breakdown of released ATP will obviously also depend on the distribution of the enzymes that degrade the nucleotide. It was shown long ago that 5′ nucleotidase tended to accumulate in areas of a lesion26 and we now know that CD73, the ecto- 5′ nucleotidase, is highly expressed in microglial cells, as is the ecto-NTPDase, CD3927.
2.2. Elimination of adenosine via astrocytic adenosine kinase
Several lines of evidence indicate that astrocytic ADK is the key regulator for ambient levels of adenosine: (i) In adult brain, ADK is predominantly expressed in astrocytes10. (ii) Pharmacological inhibition of ADK is sufficient to prevent seizures in various models of epilepsy28. (iii) Genetic knockout or knockdown of ADK in cultured cells induces the secretion of adenosine into the medium29–32. (iv) Transgenic overexpression of ADK triggers seizures by reduction of ambient adenosine33. (v) Inhibition of ADK in hippocampal slices increases endogenous adenosine and depresses neuronal firing, whereas inhibition of ADA had no effect34. (vi) A substrate cycle between AMP and adenosine, which involves ADK and 5′-nucleotidase, enables minor changes in ADK activity to rapidly translate into major changes in adenosine35. (vii) ADK activity is regulated in response to brain injury and is subject to developmental regulation10,36,37. Based on these considerations, and based on the lack of a classical transporter-regulated-reuptake system for adenosine and the ubiquitous presence of bi-directional equilibrative nucleoside transporters38, ADK likely fulfills the role of a metabolic reuptake system for adenosine. Thus, tight regulation of ADK expression levels and of its specific activity becomes a necessity. Therefore, it is not surprising that ADK is highly conserved in evolution, that no naturally occurring mutations of the Adk-gene are known, and that a genetic disruption of the Adk-gene is lethal5,39.
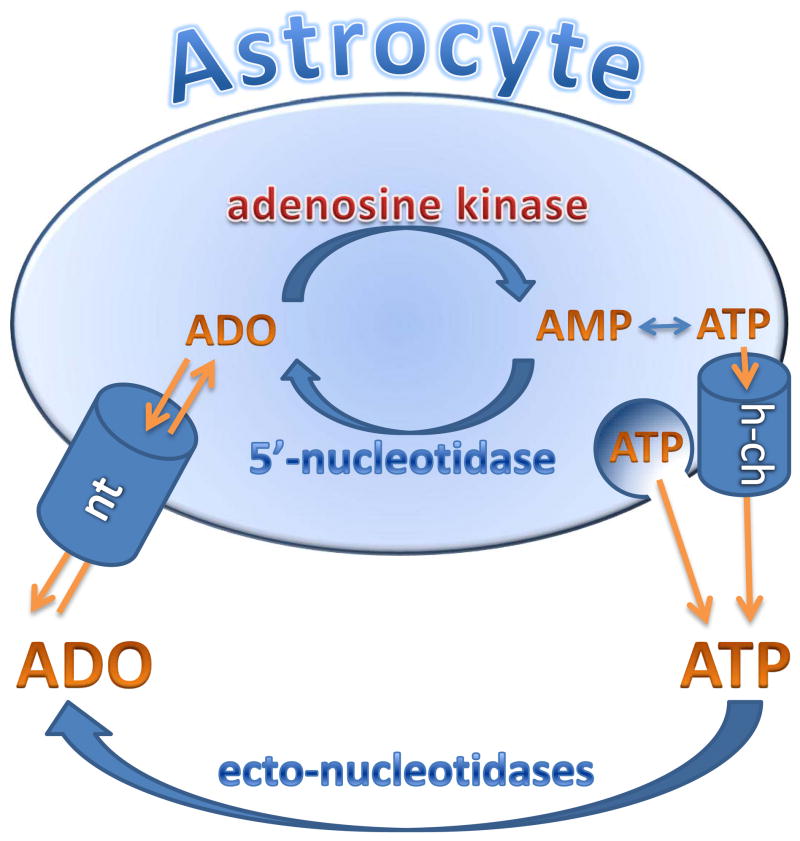
The fact that astrocytic ADK is of critical importance in regulating extracellular adenosine concentrations implies that the nucleoside transporters that facilitate adenosine uptake into astrocytes are important. Astrocytes express one concentrating (sodium-dependent) and two equilibrative nucleoside transporters40. Inhibition of this transport could potentially be used to elevate brain adenosine levels under conditions when extracellular adenosine is derived from extracellular ATP, but they would be less useful under conditions when adenosine is derived from intracellular production41. It is interesting to note that cannabinoids can block the equilibrative transporter and this effect can partly explain the immunosuppressive effects of these compounds42. The central role of astrocytes in regulating extracellular levels of adenosine is demonstrated in Figure 1.
Figure 1.
Extracellular adenosine levels are thought to be regulated by an astrocyte-based adenosine-cycle. Astrocytes can release ATP via vesicular release and/or by direct release through hemichannels (h-ch). Extracellular ATP is rapidly degraded into adenosine (ADO) by a series of ectonucleotidases. Adenosine can also be released directly via equilibrative nucleoside transporters (nt). Intracellularly adenosine levels are largely controlled by adenosine kinase, which is part of a substrate cycle between adenosine and AMP. Small changes in adenosine kinase activity rapidly translate into major changes in adenosine. Intracellular adenosine kinase is considered to be a metabolic reuptake system for adenosine. Only selected mechanisms and pathways are shown; for details please refer to main text.
3. Glial adenosine receptors
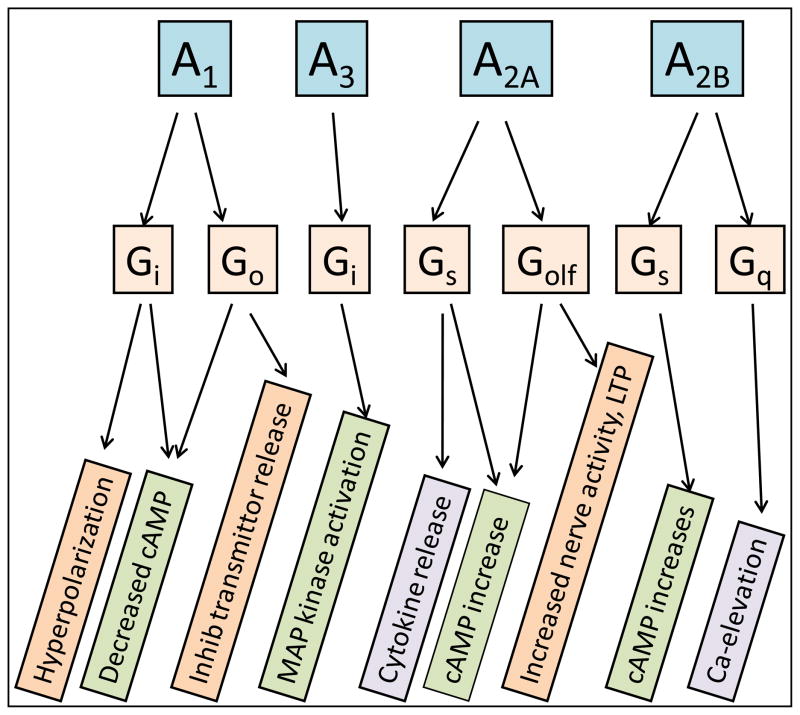
There are four types of evolutionarily conserved and pharmacologically well-characterized adenosine receptors called A1, A2A, A2B and A343 (Fig. 2). Adenosine is the endogenous agonist at all these receptors, but at A1 and A3 receptors inosine can act as a partial agonist44,45. The A1 and A3 receptors couple to the Gi family of G proteins and thus stimulate K+ channels, reduce transient voltage dependent Ca2+ channels and inhibit cAMP formation; A2A receptors couple to members of the Gs family (Golf in striatal neurons), whereas A2B receptors couple to many G proteins including Gs, Gq and G12. Adenosine is approximately equipotent on A1, A2A and A3 receptors, whereas A2B receptors require higher agonist concentrations, if cAMP changes is the readout45, but if MAP kinase activation is used to measure receptor activation adenosine is virtually equipotent on all four receptors46. All four adenosine receptors are detected in astrocytes22, and all have been reported to be expressed in microglial cells or microglial cell lines47–49.
Figure 2.
Adenosine receptors, their coupling to G-proteins and some of the down-stream consequences of receptor activation.
3.1. A1 receptors
A1 receptors on astrocytes reduce their proliferation rate in culture50. As in many other types of cells activation of A1 receptors can not only decrease cAMP accumulation but also stimulate phospholipase C, especially if this pathway is simultaneously activated by other stimuli51–53. A1 receptors help protect astrocytes from damage and cell death22,54,55, partly via activation of PI3K and Erk 1/2 phosphorylation. Nerve activity promotes myelination and it has been shown that this response is dependent on ATP56, but ATP acts on oligodendrocytes indirectly, because ATP acts on astrocytes to release leukemia inhibitory factor. This response may be modified by adenosine, as A1 receptors are present in oligodendroglia57 and stimulate their migration. It has been shown that A1 receptor activation leads to white matter loss, and that A1 receptors contribute to hypoxia-induced white matter loss58. A1 receptors on microglial cells are reported to reduce excessive activation of microglial cells upon immune activation59. Activation of these microglial receptors may secondarily affect oligodendroglial cells59 and also astrocyte proliferation60, which emphasizes the possibility of an extended glial network of signaling. A1 receptors on neurons (especially at nerve terminals) are critically important in mediating the dampening effect on neuronal activity mediated by adenosine generated from ATP released from astrocytes6,61. The highly abundant A1 receptors at nerve endings may preferentially signal via Go proteins to inhibit transient calcium channels, whereas the same receptors in nerve cell bodies and dendrites may preferentially regulate potassium channel conductance via G1 proteins.
3.2. A2A receptors
In brain, A2ARs are expressed at high levels in striatal neurons and at low levels in neurons outside of the striatum and in glial cells62,63. Many functional measurements (such as cAMP levels and cytokine release) coupled with pharmacological tools have clearly demonstrated the presence and function of A2ARs in glial cells64. A2AR binding densities are at the range ~30–60 fmole/mg protein in primary cultured microglial cells or in sorted microglial cells derived from striatum, as estimated by 3H-ligand binding studies65,66. Furthermore, the expression of the A2AR in glial elements in both the striatum and the solitary tract is confirmed by electronmicroscopic studies63,67. It should be noted that A2AR expression in microglia and astrocytes is usually low under physiological conditions and frequently below the detection limit of histological methods (i.e. immunohistochemistry, autoradiography, or in situ hybridization)62,64,68.
Importantly, the expression of A2ARs in glial cells is induced following brain insults. For example, LPS treatment induced A2AR mRNA and protein in primary cultures of (mixed) glial cells (mainly in microglial cells) at 16 hours and peaked at 48 hours after the treatment65. Recently, using double immunohistochemistry analysis, we demonstrated that A2AR expression is induced in microglial cells and astrocytes of mouse substantia nigra at 24 hours after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication. The induction of A2ARs in glial cells by brain insults and inflammatory signals, coupled with a local increase in adenosine and pro-inflammatory cytokines (such as IL-1β, which further induces A2AR expression), may serve as part of an important feed-forward mechanism to locally control neuroinflammatory responses in the brain. It has been shown that adenosine, acting on A2A receptors, can increase extracellular levels of glutamate, both by reducing glutamate uptake via GLT-1 and by direct release69,70. Thus, some of the reported effects of A2A receptors on glutamate release may be based on mechanisms mediated by astrocytes rather than by neurons.
A2ARs in glial cells may exert complex actions on neuronal cell death (both, potentially deleterious as well as neuroprotective) and possibly other functions such as modulation of synaptic transmission. In astrocytes, activation of A2ARs by extracellular adenosine increases astrocyte proliferation and activation71,72, but inhibits the expression of iNOS and the production of NO73, and regulates glutamate efflux by astrocytes69. Thus, modulation of astrogliosis by A2ARs is likely involved in brain repair processes, possibly via the formation of tissue scar. In microglial cells, activation of A2ARs has mixed effects on microglial proliferation, but has clear facilitating effects on the release of cytokines including up-regulation of cyclo-oxygenase 2 and the release of prostaglandin E2 (PGE2)74, and on increases in NOS activity and NO release65 and nerve growth factor expression75.
3.3. A2B receptors
It was shown early on that cyclic AMP accumulation in brain slices was due to a different adenosine receptor than that responsible for adenylate cyclase stimulation in striatum, and it is now clear that a major part of the brain slice cAMP response is due to A2B receptor activation on astrocytes76. A2B receptors can couple to two different classes of G proteins, Gq and Gs. Gq regulates intracellular calcium and vesicular release, whereas Gs affects a plethora of cAMP dependent signaling pathways. The A2B receptor may also activate phospholipase C77 and appears to be responsible for the adenosine-induced stimulation of Il-6 from astrocytes78. In airways, A2B receptors, via cAMP, regulate chloride channels79. Since this occurs also in the intestine, it is fair to predict that cells in the CNS likewise alter chloride flux via A2BR signaling. In particular, the possibility exists that adenosine is an important regulator of astrocytic swelling via modulation of volume-regulated anion channels. A2B receptors may also play an impiortant role in the development of the nervous system; it was intially proposed that the important regulator netrin required signaling via A2B receptors80, but later studies showed that netrin does not require simulataneous A2BR signaling81, but that netrin signaling requires prior A2B activation because the receptor is thereby regulated82.
3.4. A3 receptors
Astrocytic A3 receptors appear to regulate chemokine release83. Activation of A3 receptors by endogenous adenosine protects astrocytes from cell death induced, e.g., by hypoxia22. Microglial cells have functional A3 receptors coupled to MSAP-kinase and p38 signaling47,84. Although adenosine itself has little effect on microglial migration, it was shown that the migration induced by ATP85, perhaps predominantly via P2Y12 receptors86 is lost when ATP hydrolysis via CD39 is eliminated and can be restored by adenosine27. Although it is not absolutely certain that the relevant adenosine receptor is the A3R, it is tempting to speculate that there is a similarity to the situation in neutrophil leucocytes where ATP acting on a P2Y receptor acts in concert with adenosine acting on A3 receptors to stimulate migration87. Microglial cells (and A3 receptors) may also be of particular importance in the regulation of chemokine release and chemokine actions88.
4. Glial cells and neurovascular coupling
Glial cells play important roles in coupling neuronal function to the cerebral microvasculature that controls cerebral blood flow (CBF)89,90 in the sense that increased neuronal activity requires corresponding increases in CBF. Apart from large processes that stain for intermediate filaments and give astrocytes their stellar appearance, astrocytes have a multitude of fine processes that have little overlap with processes from other astrocytes and that define individual astrocytic domains, which each contain 300–600 neuronal dendrites and 105 synapses in rodent hippocampus8,91–93. Thus, a single astrocyte can sense the activity, and integrate the function, of hundreds of neurons within its domain. In addition, each astrocyte extends at least one process with endfeet surrounding blood vessels of the microvasculature. Therefore, astrocytes are uniquely located to adjust regional CBF to regional energy metabolism.
The vasodilator adenosine has been identified as an important mediator that couples cerebral blood flow to neuronal activation94. Thus, adenosine was demonstrated to mediate glutamate-induced vasodilation in the cerebral cortex95,96. Topical application of glutamate dilated pial arterioles, an effect that could be reversed by an A2AR antagonist, but not by an A2AR antagonist95. Likewise topical superfusion AMPA on the cortical surface through a closed cranial window resulted in increases in pial arteriolar diameter, an effect that could be reversed by A2A and A2BR blockade, but not by inhibition of NO synthase, cyclooxygenase-2, or cytochrome P-450 epoxygenase96. Apart from the activation of vascular adenosine receptors, adenosine can exert important regulatory functions by activation of astrocytic adenosine receptors97. Thus, the adenosine-evoked calcium response in acutely isolated astrocytes was found to be coupled to the A2B receptor77; based on these findings adenosine could be implicated in promoting the propagation of calcium-increases throughout astrocytic processes. Increased Ca2+ in turn is also associated with the release of ATP through connexin hemichannels, a process that is potentiated by A2BR activation98,99. Through these mechanisms ATP-release and degradation into adenosine via ectonucleotidases appears to mediate arteriolar dilation in response to neuronal activation100. This process was dependent on astrocytes, since the application of the selective gliotoxin L-AAA, led to complete loss of arteriolar dilation in response to neuronal activation101.
5. Glial control of glutamate and excitotoxicity
Astrocytes play a fundamental role in the pathogenesis of ischemic neuronal death102. A large body of evidence indicates that astrocytes are involved in the control of glutamate homeostasis and the susceptibility of the brain to excitotoxic injury103. Glutamate transporters are expressed in many different types of brain cells, but astrocytes are primarily responsible for glutamate uptake. Studies using genetic deletion or antisense-oligonucleotide mediated knockdown of the astroglial glutamate transporter GLT-1 have demonstrated that this transporter is the predominant subtype responsible for the clearance of extracellular glutamate in the brain104,105. Affected animals were highly susceptible to glutamate-dependent excitotoxicity and developed epileptic seizures104,105. After uptake of glutamate into astrocytes the enzyme glutamine synthetase converts glutamate into glutamine, which is then transported into neurons, where it is converted back into glutamate. Interestingly, a loss of glutamine synthetase was found in the sclerotic hippocampus of human patients with temporal lobe epilepsy106 and the authors of that study concluded that reduced activity of the glutamate-glutamine cycle led to an accumulation of extracellular glutamate.
Apart from the mechanisms described above, astrocytes themselves can be a significant source of extracellular glutamate, which can be released by a variety of mechanisms107. It has been demonstrated that Ca2+ elevations in astrocytes induce the excitotoxic release of glutamate from these cells108. Most importantly, the Ca2+-dependent astrocytic release of glutamate was also dependent on the vesicular glutamate transporters (VGLUT1/2) and the vesicular SNARE protein, cellubrevin, and was consistent with a vesicular release mechanism of glutamate that was similar to synaptic release of glutamate108. Finally, it was shown that astrocyte-derived glutamate targets synaptic NMDA receptors109, providing a rational explanation for the astrocyte-based control of neurotoxicity. Given the emerging roles of astrocytes in the control of neuronal excitotoxicity neuroprotective efforts targeting the functional integrity of astrocytes may constitute a superior strategy for future neuroprotection.
6. Novel methods for studying the role of glia
Co-culture of glia and neurons
Many original insights into glio-transmission were discovered using co-culture systems, and then extended to brain slices or the intact brain. For example, the first evidence for gliotransmission came from studies in mixed cultures of astrocytes and neurons demonstrating that experimentally evoked Ca2+ elevation in astrocytes evoked the elevation of Ca2+ in adjacent neurons110,111. In this method, cultured cells from postnatal day 1–4 rodent brain are first enriched with one population of either glial or neuronal origin using specific culture conditions and then confirmed by immunohistochemistry. Glial or neuronal cells are then plated separately or together onto a coated substrate. The distinct morphology of glial cells and neurons allows the identification of these distinct cell types and permits the direct application of electric field potentials, micropipette tips, or neurochemical substrates to astrocytes in order to evoke a specific elevation of calcium in glial cells (e.g. in astrocytes)110,111. The spatiotemporal control over mechanical stimuli has permitted the selective stimulation of single astrocytes in mixed cultures of rat forebrain astrocytes and neurons. Using this strategy it was demonstrated that the elevation of calcium, triggered by focal electric field potentials in single astrocytes, induced a wave of calcium increase that was propagated from astrocyte to astrocyte, and importantly, this wave of calcium increase also evoked large increases in the concentration of cytosolic calcium in neurons depending on those astrocytes110,111. More recently, mixed co-cultures in multi-compartment dishes were equipped with the capability to provide electronic stimuli selectively to neurons in mixed cultures of oligodendrocytes and dorsal root ganglion neurons. This approach led to the finding that ATP and adenosine, released from neurons, acts as a potent neuron-glial transmitter that inhibited oligodendrocyte progenitor cell proliferation, stimulated their differentiation, and promoted the formation of myelin56,112.
Transgenic overexpression and targeted knockout of genes in defined glial populations
Glial cells have been shown to release gliotransmitters, including ATP9,17, glutamate111,113, and D-serine114 to coordinate synaptic networks. However, neurons and glia share these same chemical signaling molecules, making it difficult to define the role of gliotransmitters. To molecularly dissect the role of glial signaling molecules, genetic approaches have been developed to selectively manipulate the SNARE-dependent release of gliotransmitters using a glia-specific promoter. Pascual and Haydon developed a transgenic mouse line, which uses the tet-off system to allow conditional expression of the cytosolic portion of the SNARE domain of synptobrevin 2 [dominant-negative SNARE (dn-SNARE)] selectively in astrocytes15. The selective expression of dn-SNARE was achieved by the use of an astrocyte-specific glial fibrillary acid protein promoter. To confirm the cell type selectivity of astrocytic transgene expression, Pascual et al. used EGFP as a reporter system and showed that EGFP was visually detectable in 97% of the dnSNARE-transgene-expressing cultured astrocytes, and that EGFP-positive cells colocalized specifically with the astrocytic marker, but not with neuronal, NG2-glial, or oligodendroglial markers. This transgenic line has successfully been used to demonstrate the functional significance of gliotransmission on synaptic plasticity in hippocampus15 and more recently in the sleep-wake cycle61. Similarly, transgenic over-expression of a mutant disease-causing gene in a defined glial cell population has been used to study the role of SOD1 in astrocytes in the development of motor neuron death. Nagai et al demonstrated that the astrocyte selective expression of mutant human SOD1 (but not in spinal motor neurons, microglia or fibroblasts) killed spinal primary and embryonic mouse stem cell-derived motor neurons115.
As a complementary genetic approach, the selective deletion of signaling molecules in defined glial cell populations can be accomplished using the Cre-loxP strategy. For example, Boillee et al generated a transgenic line (LoxSOD1G37R) that carried a mutant human SOD1 gene flanked at both ends by a 34-base pair LoxP sequence116. These “floxed” mice were then cross-bred to two transgenic lines with expression of the Cre protein under control of (i) the promoter from the Islet-1 transcription factor, which directs the expression exclusively in progenitors of motor and dorsal root ganglion neurons, and (ii) the CD11b promoter, which directs the expression exclusively in the myeloid lineage (including macrophages and microglial cells). The establishment of these two novel transgenic lines allowed to demonstrate that the SOD1 mutation in motor neurons and microglial cells contributes distinctly to the onset and progression of amyotrophic lateral sclerosis: while expression of SOD1 in motor neurons is the primary signal for the initiation of motor neurodegeneration and an early sign of disease progression, the genetic inactivation of SOD in mciroglial cells had little effect on the early disease phase, but markedly attenuated disease progression116. Thus, these genetic approaches to selectively manipulate signaling molecules in defined glial (or neuronal) populations provide critical insights into the distinct role of glial cells in the development of neurodegeneration.
Flow cytometric analysis and fluorescence activated cell sorting (FACS)
The structural complexicity of brain tissues hampers the dissection of unique roles of glial cells under various physiological and pathological conditions. Glial cells are characterized by a unique morphology, which permits the distinction of neuronal versus glial cells in intact brain by immunohistochemistry. However, it is difficult to quantify immunohistochemical changes without performing labor-intensive stereological analysis. Furthermore, there is a critical need to isolate large numbers of pure glial cells from intact brain for detailed molecular analysis such as qPCR and microarray analysis. Flow cytometric and fluorescence activated cell sorting can be adapted to partially circumvent these limitations for the study of glial cell functions in brain.
In the first application, defined glial cell populations (such astrocytes, microglia, or oligodendrocytes) are identified by labeling with a fluorescent antibody directed against specific cell surface markers. Quantitative changes of glial populations in normal and injured brains are determined by flow cytometry. For example, we recently utilized this analysis to evaluate the change of CD11b+ (a cell surface marker for microglial cells) and GFAP+ (a maker for astrocytes) cells in mouse striatum after treatment with MPTP and the A2AR antagonist KW6002117. This analysis not only provided an improved quantitative assessment of the effect of the A2AR antagonist on microglial activation at the very early phase of MPTP intoxication, but also identified a specific microglial cell population (i.e. CD11b+ cells with a large cell size representing fully activated microglial cells), which are most sensitive to KW6002 treatment in the brain117.
In the second application, fluorescence activated cell sorting permits isolation and purification of distinct populations of glial cells from neurons from brain tissues using fluorescent antibodies directed against cell surface markers. The sorted glial cell populations can then be used for detailed molecular analyses such as quantitative PCR and microarray analysis. For example, Lovatt et al. successfully performed microarray profiling of sorted astrocytes from mouse cortex using FACS and (surprisingly) demonstrated that most enzymes in the tricarboxylic acid cycle are expressed at higher relative levels in astrocytes than in neurons118.
7. Adenosine signaling in glial cells, excitotoxicity and cell death
As outlined above, synaptic levels of adenosine are largely controlled by an astrocyte-based adenosine cycle and the activity of the astrocyte-based enzyme ADK. Consequently, adenosine signaling in glial cells effects excitotoxicity and cell death in a variety of experimental paradigms. In addition, several forms of brain insult activate microglial cells. Here, ATP release is critically important85, but the ATP response in microglial cells is markedly enhanced by adenosine generated from ATP27.
7.1. Epilepsy
The adenosine kinase hypothesis of epileptogenesis implies that dysregulation of ADK is a major contributing factor to the epileptogenic cascade12 (Fig. 3). Consequently, ADK expression levels (that determine levels of ambient adenosine) determine the brain’s susceptibility to acute seizure-induced cell death. Mice with only moderate transgenic overexpression of ADK in brain (141% of normal) were highly susceptible to acute seizure-induced cell death and did not survive beyond 3 days following status epilepticus37, whereas engineered mice with reduced levels of ADK in forebrain (62% of normal) were completely resistant to seizure-induced cell death37. Resistance to seizure-induced excitotoxic cell death in ADK-deficient mice was dependent on adenosine and increased adenosine A1R activation, since blockade of A1Rs with its selective antagonist DPCPX restored wild-type like seizure-induced excitotoxic cell death37.
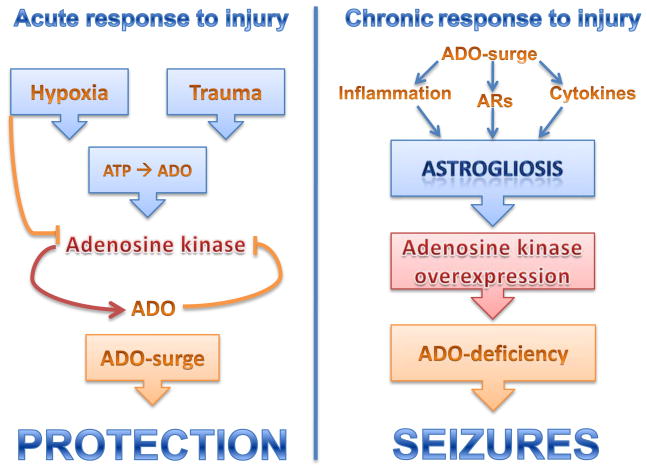
Figure 3.
Role of the adenosine (ADO) / adenosine kinase (ADK) system in regulating acute and chronic responses to injury. Left: Within hours after brain injury (e.g. stroke, trauma, prolonged seizures) a surge in micromolar levels of ADO results that protects the brain from further injury and from seizures. Hypoxia and trauma can directly lead to a rise in extracellular ATP that is rapidly degraded into adenosine. High levels of adenosine are known to inhibit ADK, further amplifying the adenosine surge. Right: The acute adenosine surge contributes to trigger astrogliosis via a variety of mechanisms that include modulation of astrocytic adenosine receptors (ARs), modulation of inflammatory processes and the release of cytokines. Astrogliosis leads to overexpression of ADK resulting in adenosine-deficiency, which contributes to seizure generation.
Astrogliosis is a pathological hallmark of the epileptic brain and contributes to seizure generation by a variety of mechanisms12,119,120. A recent study from our lab has identified the enzyme ADK in astrocytes as a molecular link between astrogliosis and neuronal dysfunction in epilepsy37. In a mouse model of CA3-selective epileptogenesis we found spatio-temporal co-localization of astrogliosis, upregulated ADK, and focal spontaneous electrographic seizures that were all restricted to the CA3-region, the site of the epileptogenesis precipitating acute injury; importantly, seizures could be suppressed pharmacologically by ADK inhibition37. In this model, the seizures remained highly localized and restricted to the astrogliotic scar, presumably due to normal adenosinergic control of the surrounding brain tissue. Transgenic overexpression of ADK, as well as genetic disruption of the A1R were sufficient to trigger spontaneous seizures, indicating that adenosine dysfunction rather than astrogliosis per se was responsible for seizure generation121. Conversely, mice with a genetically induced reduction of ADK in forebrain were completely resistant to the development of spontaneous seizures37. In vitro studies performed on hippocampal slices have subsequently demonstrated that reduction of the basal tone of adenosine by ADK is permissive to seizure generation, whereas ADK did not limit activity-dependant adenosine-release122. Together, these findings provide a neurochemical rationale for adenosine augmentation therapies (AATs). Consequently, several focal AAT-approaches – based on intracerebral adenosine-releasing implants – have demonstrated robust anticonvulsive and possibly antiepileptogenic efficacy in a variety of experimental paradigms that have been reviewed elsewhere123,124.
7.2. Traumatic brain injury
Traumatic brain injury triggers an acute surge in adenosine, presumably as a consequence of ATP release, and this may represent an endogenous neuroprotective mechanism. In one study, adenosine levels increased 61-fold following controlled cortical impact (CCI) in rats and peaked at 20 min following the impact125. The existence of an endogenous protective action of adenosine at A1 receptors early after experimental TBI was further corroborated by the finding of lethal status epilepticus in A1R knockout mice subjected to either controlled cortical impact126 or to kainic acid induced hippocampal injury127. In contrast to A1R knockout mice, A2AR knockout mice were largely protected from the adverse effects of CCI128. In line with these findings, increases in cerebrospinal fluid caffeine concentration were associated with favorable outcome after severe traumatic brain injury in humans, likely due to caffeine-mediated inhibition of A2ARs129. Likewise, chronic, but not acute caffeine attenuated the consequences of TBI in the mouse CCI model130. Although it probably does not contribute to the surge in adenosine following brain injury, acute downregulation of ADK in astrocytes has been described as a consequence of stroke36 or acute seizures131, and this may prolong the adenosine increase.
7.3. Parkinson’s Disease
The adenosine A2A receptor is a leading non-dopaminergic therapeutic target in Parkinson’s disease (PD) (Fig. 4). Interest in this receptor within the context of PD derives primarily from two lines of experimental and clinical investigations: First, decade-long preclinical studies demonstrated a unique co-localization of A2ARs and dopamine D2Rs in striatopallidal neurons. Antagonistic interactions between A2ARs and D2Rs at the molecular, neurochemical and behavioral level explain the motor stimulant effects of A2AR blockade 132–134. Thus, A2AR antagonists such as KW-6002 (istradefylline) and SCH420814 have now completed clinical phase IIB-III trials. Despite some limitations of these clinical trials and admittedly modest effects (“OFF” time reduced by one hour), these studies confirm that selective A2AR antagonists can stimulate motor activity by potentiating the L-dopa effect in advanced PD patients135,136. Second, in addition to symptomatic relief, A2AR antagonists appear to more directly attenuate dopaminergic neurodegeneration, as suggested by convergent epidemiological and experimental evidence. Following an initial report from the Honolulu Heart Program137 by Ross and colleagues, several large-cohort prospective studies have confirmed a similar inverse relationship between the consumption of caffeinated coffee and the risk of developing PD. Including the Health Professionals' Follow-Up Study and the Nurses’ Health Study these studies involved a total of 47,351 men and 88,565 women138, whereas a more recent study conducted by the Finnish Mobile Clinic Health Examination Survey included 19518 men and women139. These studies firmly established a relationship between increased caffeine consumption and decreased risk of developing PD in males. In addition, studies with animal models of PD provide a compelling clue about the potentially protective effects of caffeine, by demonstrating that pharmacological blockade (by caffeine or selective A2AR antagonists) or genetic depletion of the A2AR attenuates dopaminergic neurotoxicity and neurodegeneration140–142. These studies provide a neurobiological basis for the inverse relationship between increased caffeine consumption and reduced risk of developing PD.
Figure 4.
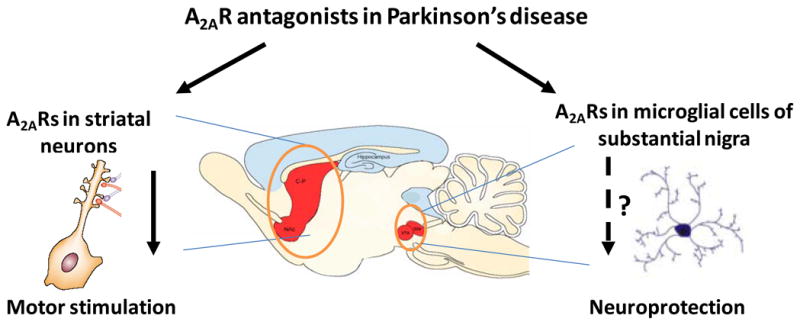
The dual functions of A2A receptor antagonists in Parkinson’s disease models: A2AR antagonists act at the A2AR in striatal neurons to stimulate motor activity. Furthermore, it is postulated that A2AR antagonists may modulate microglial activation in substantia nigra to exert a possible neuroprotective effect in an animal model of Parkinson’s disease.
Despite consistent demonstration that A2AR antagonists afford neuroprotection against MPTP or 6-hydroxydopamine -induced dopaminergic neurotoxicity, the mechanism by which A2AR inactivation protects against the loss of dopaminergic neurons remains unknown. The particular challenge lies in explaining the apparent dichotomy between restricted expression of the A2AR in striatopallidal neurons and neuroprotection against degeneration of dopaminergic neurons in the substantia nigra where only a scattered expression of A2ARs is detected. An additional challenge is to identify the cellular mechanism, which allows A2AR inactivation to protect neurons against a broad spectrum of brain insults, from ischemia to excitotoxicity to mitochondrial toxicity143. In this context, the involvement of the glial A2AR becomes an attractive possibility, since glial function and neuroinflammation is commonly associated with diverse pathological conditions as mentioned above. Indeed, we recently demonstrated that MPTP treatment markedly upregulates A2AR expression in microglial cells at 24–48 hours after treatment65,66, which may result in further amplification of the A2AR-mediated modulation of neuroinflammation in PD models. Consistent with this notion, an immunohistochemical study showed that KW-6002 reduced the loss of striatal dopamine contents and nigral cell bodies, and this coincided with inhibition of microglial activation144. Furthermore, we demonstrated by flow cytometery that KW-6002 attenuated MPTP-induced microglial activation at 48 hours after MPTP treatment117. In conclusion, A2AR antagonists may confer neuroprotection by acting at A2ARs in glial cells, at least in the MPTP model of PD.
7.4. Ischemia
During ischemia, an imbalance between ATP degradation and resynthesis brings about a rapid and marked increase in extracellular level of adenosine in the brain during ischemia 145–154. In addition hypoxia will increase ATP release, resulting in further adenosine production. While no clinical reports with purinergic compounds in human stroke exist, it is widely believed that adenosine and its receptors function as an endogenous neuroprotectant under these conditions155–161. Indeed, adenosine162 or adenosine-potentiating agents (such as inhibitors of ADA or ADK163–167 or of adenosine transport151,166,168–173) offer protection against ischemic neuronal damage in different in vivo ischemia models. Furthermore, transgenic overexpression of ADK aggravates cell death, while reduction of ADK in the hippocampus increases protection after transient focal ischaemia36,174.
Such a protective effect is attributed to stimulation of adenosine A1 receptors that exert a protective role in ischemia by presynaptic reduction of Ca2+ influx, by inhibition of the release of excitatory neurotransmitters175,176, and by postsynaptic hyper-polarization and reduction of neuronal activity through increases in K+ and Cl− ion conductances177. The efficacy of A1 receptors stimulation on neuroprotection depends on the model used and no protective effect was observed in a global ischemia model178. Since adenosine does influence glutamate release it is suggested that this is not critically important in some ischemic models. However, adenosine (probably acting at the A2A receptor) may in fact contribute to neurotoxicity, neuronal damage, and cell death. The potential neuroprotection by A2AR antagonists was first reported in a global ischemia model with the less selective antagonist CGS 15943179,180. Further studies substantiated this finding in different models of ischemia with the selective A2A receptor antagonist 8-(3-chlorostyryl)caffeine (CSC) and SCH 58261181,147,182 in various animal models of stroke183,184. Studies in genetically manipulated mice confirmed the neuroprotective role of A2A receptor antagonists on ischemic brain damage185. Major protective effects of A2A receptor antagonists in stroke have been attributed to reduced glutamate outflow183,186,187. It should be considered however, that in several studies, A2A receptor agonists have been found protective in the global ischaemia model in the gerbil180 and that A2A knockout mice show aggravated hypoxic ischaemic injury in neonatal mice188. Possible mechanisms are not clear yet, but include A2AR-mediated protection via inhibition of platelet aggregation, vasodilation163,189, or anti-inflammatory actions. Lastly, activation of A3Rs produced mixed results and the exact contribution of A3Rs to ischemic brain injury is not clear190.
So far, support for a role for glia in the neuroprotective effect of A2AR antagonism in ischemia comes from the observation that the A2A receptor antagonist SCH 58261 reduces p38 MAPK activation in microglial cells184 and phospho-JNK in neurons and oligodendrocytes191 in the ischaemic hemisphere 24 hours after permanent MCAO. Since p38 MAPK and JNK are activated up to 24 hours after ischemia192,193 and are involved in neuronal death194,195, this correlation indicates that A2AR antagonists may confer neuroprotection against ischemic brain injury through modulation of glial function and neuroinflammation. However, reduced MAPK activation might be secondary to a reduction in the excitotoxic cascade that primes p38 and JNK activation, since reduction glutamate outflow in the ischemic brain by A2AR blockade is believed to be one of the main underlying mechanisms196. Further studies with selective manipulation of glial adenosine receptors or glial function in vivo are critical to our understanding to what extend adenosine regulation of glial signalling and function is responsible for ischemic brain injury.
8. Conclusions and major open questions
The discussion above has shown that much is now known about a role of glial cells in mediating the effects of adenosine (and other purines) in different neurodegenerative states. It is clear that many of the actions of endogenous or exogenous adenosine (and ATP) are in fact due to actions on glial cells. This makes it much more complicated to understand precisely how adenosine acts, and it has become apparent that the often diverse actions reported are due to the fact that several different receptors, located on many cell types are involved. In order to get a better understanding, there are several major questions that require an answer. Among the questions that need to be addressed in future research we find:
Is the acute adenosine surge that follows brain injury a trigger for subsequent astrocyte activation?
Do all the proposed mechanisms of ATP release from glial cells in fact occur, and are the triggers for astrocytic ATP release via these mechanisms different?
To what extent does regional variation in the expression of ecto-nucleotidases control the distribution of adenosine in brain?
Can adenosine-signals propagate within the brain via astrocyte-astrocyte communication?
Given the major increase in glial cells in humans, how far can we extrapolate from rodents to man?
When several adenosine receptors, with at least partly opposing signaling, appear to regulate a single biological response, are the receptors located on different cells or on very different parts of the same cell?
Given that two GPCR molecules can form dimers, but apparently only one of them can actually signal, is there any functional significance of heterodimers between two types of purine receptors?
Can activation of endothelial cells at the vascular interphase signal to synapses?
Are microglial cells directly involved in synaptic transmission or do they mainly help prune synaptic contacts?
Why are effects of A2ARs on inflammation different in CNS and peripheral organs?
Acknowledgments
The work of the authors was supported by NIH-grants NS058780, MH083973, NS061844, NS057538, NS057475, and by the CURE Foundation in collaboration with the Department of Defense (to D.B.), by NIH-grants NS48995, NS41083, DA19362, and a grant from the Department of Defense W81XWH-071-1-0012 (to J.F.C), and by grants from the Swedish Science Research Council and Hjärnfonden (to B.B.F.).
Abbreviations
- AAT
adenosine augmentation therapy
- ADA
adenosine deaminase
- ADK
adenosine kinase
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- AR
adenosine receptor
- CBF
cerebral blood flow
- CCI
controlled cortical impact
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- EGFP
enhanced green fluorescent protein
- DR
dopamine receptor
- FACS
fluorescence activated cell sorting
- MAP
microtubule associated protein
- NMDA
N-methyl-D-aspartic acid
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NOS
nitric oxide synthase
- NTPDase
nucleoside triphosphate diphosphohydrolase
- PD
Parkinson’s disease
- SNARE
soluble NSF attachment protein
- SOD
superoxide dismutase
References
- 1.Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29:518–27. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Geiger JD, Buscemi L, Fotheringham JA. Role of adenosine in the control of inflammatory events associated with acute and chronic neurodegenerative disorders. In: Hasko G, Cronstein BN, Szabo C, editors. Adenosine Receptors. Therapeutic Aspects for Inflammatory and Immune Diseases. CRC. Taylor and Francis; 2007. pp. 213–236. [Google Scholar]
- 3.Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 5.Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, et al. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- 7.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27:6473–7. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological Reviews. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 10.Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, et al. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boison D. The adenosine kinase hypothesis of epileptogenesis. Progress in Neurobiology. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald PE, Braun M, Galvanovskis J, Rorsman P. Release of small transmitters through kiss-and-run fusion pores in rat pancreatic beta cells. Cell Metab. 2006;4:283–90. doi: 10.1016/j.cmet.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–6. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Wang L, Zhou Y, Zheng LH, Zhou Z. “Kiss-and-run” glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J Neurosci. 2005;25:9236–43. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, et al. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–53. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 18.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, et al. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–11. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu HT, Sabirov RZ, Okada Y. Oxygen-glucose deprivation induces ATP release via maxi-anion channels in astrocytes. Purinergic Signal. 2008;4:147–54. doi: 10.1007/s11302-007-9077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Bjorklund O, Shang MM, Tonazzini I, Dare E, Fredholm BB. Adenosine A(1) and A(3) receptors protect astrocytes from hypoxic damage. European Journal of Pharmacology. 2008;596:6–13. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Parkinson FE, Sinclair CJ, Othman T, Haughey NJ, Geiger JD. Differences between rat primary cortical neurons and astrocytes in purine release evoked by ischemic conditions. Neuropharmacology. 2002;43:836–46. doi: 10.1016/s0028-3908(02)00083-7. [DOI] [PubMed] [Google Scholar]
- 24.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–66. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreutzberg GW, Barron KD. 5′-Nucleotidase of microglial cells in the facial nucleus during axonal reaction. J Neurocytol. 1978;7:601–10. doi: 10.1007/BF01260892. [DOI] [PubMed] [Google Scholar]
- 27.Farber K, Markworth S, Pannasch U, Nolte C, Prinz V, Kronenberg G, et al. The ectonucleotidase cd39/ENTPDase1 modulates purinergic-mediated microglial migration. Glia. 2008;56:331–41. doi: 10.1002/glia.20606. [DOI] [PubMed] [Google Scholar]
- 28.Kowaluk EA, Jarvis MF. Therapeutic potential of adenosine kinase inhibitors. Expert Opin Investig Drugs. 2000;9:551–64. doi: 10.1517/13543784.9.3.551. [DOI] [PubMed] [Google Scholar]
- 29.Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp Neurol. 2007;208:26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Güttinger M, Padrun V, Pralong W, Boison D. Seizure suppression and lack of adenosine A1 receptor desensitization after focal long-term delivery of adenosine by encapsulated myoblasts. Exp Neurol. 2005;193:53–64. doi: 10.1016/j.expneurol.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Fedele DE, Koch P, Brüstle O, Scheurer L, Simpson EM, Mohler H, et al. Engineering embryonic stem cell derived glia for adenosine delivery. Neurosci Lett. 2004;370:160–165. doi: 10.1016/j.neulet.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Huber A, Padrun V, Deglon N, Aebischer P, Mohler H, Boison D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc Natl Acad Sci USA. 2001;98:7611–6. doi: 10.1073/pnas.131102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rulicke T, et al. Astrogliosis in epilepsy leads to overexpression of adenosine kinase resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- 34.Pak MA, Haas HL, Decking UKM, Schrader J. Inhibition of adenosine kinase increases endogenous adenosine and depresses neuronal activity in hippocampal slices. Neuropharmacol. 1994;33:1049–1053. doi: 10.1016/0028-3908(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 35.Arch JR, Newsholme EA. Activities and some properties of 5′-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978;174:965–77. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2008;28:17–23. doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- 37.Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–43. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 39.Boison D, Scheurer L, Zumsteg V, Rülicke T, Litynski P, Fowler B, et al. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA. 2002;99:6985–6990. doi: 10.1073/pnas.092642899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng L, Huang R, Yu AC, Fung KY, Rathbone MP, Hertz L. Nucleoside transporter expression and function in cultured mouse astrocytes. Glia. 2005;52:25–35. doi: 10.1002/glia.20216. [DOI] [PubMed] [Google Scholar]
- 41.King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol Sci. 2006;27:416–25. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- 44.Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–57. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–8. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 46.Schulte G, Fredholm BB. Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol. 2000;58:477–82. [PubMed] [Google Scholar]
- 47.Hammarberg C, Schulte G, Fredholm BB. Evidence for functional adenosine A3 receptors in microglia cells. J Neurochem. 2003;86:1051–4. doi: 10.1046/j.1471-4159.2003.01919.x. [DOI] [PubMed] [Google Scholar]
- 48.van Calker D, Biber K. The role of glial adenosine receptors in neural resilience and the neurobiology of mood disorders. Neurochem Res. 2005;30:1205–17. doi: 10.1007/s11064-005-8792-1. [DOI] [PubMed] [Google Scholar]
- 49.Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiology & Behavior. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 50.Ciccarelli R, Di Iorio P, Ballerini P, Ambrosini G, Giuliani P, Tiboni GM, et al. Effects of exogenous ATP and related analogues on the proliferation rate of dissociated primary cultures of rat astrocytes. J Neurosci Res. 1994;39:556–66. doi: 10.1002/jnr.490390507. [DOI] [PubMed] [Google Scholar]
- 51.Peakman MC, Hill SJ. Adenosine A1 receptor-mediated changes in basal and histamine-stimulated levels of intracellular calcium in primary rat astrocytes. Br J Pharmacol. 1995;115:801–10. doi: 10.1111/j.1476-5381.1995.tb15004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biber K, Klotz KN, Berger M, Gebicke-Harter PJ, van Calker D. Adenosine A1 receptor-mediated activation of phospholipase C in cultured astrocytes depends on the level of receptor expression. J Neurosci. 1997;17:4956–64. doi: 10.1523/JNEUROSCI.17-13-04956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cormier RJ, Mennerick S, Melbostad H, Zorumski CF. Basal levels of adenosine modulate mGluR5 on rat hippocampal astrocytes. Glia. 2001;33:24–35. doi: 10.1002/1098-1136(20010101)33:1<24::aid-glia1003>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 54.Ciccarelli R, D’Alimonte I, Ballerini P, D’Auro M, Nargi E, Buccella S, et al. Molecular signalling mediating the protective effect of A1 adenosine and mGlu3 metabotropic glutamate receptor activation against apoptosis by oxygen/glucose deprivation in cultured astrocytes. Mol Pharmacol. 2007;71:1369–80. doi: 10.1124/mol.106.031617. [DOI] [PubMed] [Google Scholar]
- 55.D’Alimonte I, Ballerini P, Nargi E, Buccella S, Giuliani P, Di Iorio P, et al. Staurosporine-induced apoptosis in astrocytes is prevented by A1 adenosine receptor activation. Neurosci Lett. 2007;418:66–71. doi: 10.1016/j.neulet.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 56.Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–32. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Othman T, Yan H, Rivkees SA. Oligodendrocytes express functional A1 adenosine receptors that stimulate cellular migration. Glia. 2003;44:166–72. doi: 10.1002/glia.10281. [DOI] [PubMed] [Google Scholar]
- 58.Kim M, Yu ZX, Fredholm BB, Rivkees SA. Susceptibility of the developing brain to acute hypoglycemia involving A1 adenosine receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E562–9. doi: 10.1152/ajpendo.00112.2005. [DOI] [PubMed] [Google Scholar]
- 59.Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, et al. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. Journal of Neuroscience. 2004;24:1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Synowitz M, Glass R, Farber K, Markovic D, Kronenberg G, Herrmann K, et al. A1 adenosine receptors in microglia control glioblastoma-host interaction. Cancer Res. 2006;66:8550–7. doi: 10.1158/0008-5472.CAN-06-0365. [DOI] [PubMed] [Google Scholar]
- 61.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–96. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 63.Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–46. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 64.Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005;26:511–6. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saura J, Angulo E, Ejarque A, Casado V, Tusell JM, Moratalla R, et al. Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J Neurochem. 2005;95:919–29. doi: 10.1111/j.1471-4159.2005.03395.x. [DOI] [PubMed] [Google Scholar]
- 66.Yu L-Q, Shen H-Y, Coelho J, Araújo I, Huang Q-Y, Day Y-J, et al. A2A receptor antagonists exert motor and protective effects by distinct cellular mechanism in MPTP model of PD. Annals of Neurology. 2008 doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]
- 67.Pickel VM, Chan J, Linden J, Rosin DL. Subcellular distributions of adenosine A1 and A2A receptors in the rat dorsomedial nucleus of the solitary tract at the level of the area postrema. Synapse. 2006;60:496–509. doi: 10.1002/syn.20326. [DOI] [PubMed] [Google Scholar]
- 68.Cunha RA. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Puringergic Signalling. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li XX, Nomura T, Aihara H, Nishizaki T. Adenosine enhances glial glutamate efflux via A2a adenosine receptors. Life Sci. 2001;68:1343–50. doi: 10.1016/s0024-3205(00)01036-5. [DOI] [PubMed] [Google Scholar]
- 70.Nishizaki T, Nagai K, Nomura T, Tada H, Kanno T, Tozaki H, et al. A new neuromodulatory pathway with a glial contribution mediated via A(2a) adenosine receptors. Glia. 2002;39:133–47. doi: 10.1002/glia.10100. [DOI] [PubMed] [Google Scholar]
- 71.Hindley S, Herman MA, Rathbone MP. Stimulation of reactive astrogliosis in vivo by extracellular adenosine diphosphate or an adenosine A2 receptor agonist. J Neurosci Res. 1994;38:399–406. doi: 10.1002/jnr.490380405. [DOI] [PubMed] [Google Scholar]
- 72.Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia. 2003;43:190–4. doi: 10.1002/glia.10243. [DOI] [PubMed] [Google Scholar]
- 73.Brodie C, Blumberg PM, Jacobson KA. Activation of the A2A adenosine receptor inhibits nitric oxide production in glial cells. FEBS Lett. 1998;429:139–42. doi: 10.1016/s0014-5793(98)00556-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fiebich BL, Biber K, Lieb K, van Calker D, Berger M, Bauer J, et al. Cyclooxygenase-2 expression in rat microglia is induced by adenosine A2a-receptors. Glia. 1996;18:152–60. doi: 10.1002/(SICI)1098-1136(199610)18:2<152::AID-GLIA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 75.Heese K, Fiebich BL, Bauer J, Otten U. Nerve growth factor (NGF) expression in rat microglia is induced by adenosine A2a-receptors. Neurosci Lett. 1997;231:83–6. doi: 10.1016/s0304-3940(97)00545-4. [DOI] [PubMed] [Google Scholar]
- 76.Peakman MC, Hill SJ. Adenosine A2B-receptor-mediated cyclic AMP accumulation in primary rat astrocytes. Br J Pharmacol. 1994;111:191–8. doi: 10.1111/j.1476-5381.1994.tb14043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pilitsis JG, Kimelberg HK. Adenosine receptor mediated stimulation of intracellular calcium in acutely isolated astrocytes. Brain Res. 1998;798:294–303. doi: 10.1016/s0006-8993(98)00430-2. [DOI] [PubMed] [Google Scholar]
- 78.Schwaninger M, Neher M, Viegas E, Schneider A, Spranger M. Stimulation of interleukin-6 secretion and gene transcription in primary astrocytes by adenosine. J Neurochem. 1997;69:1145–50. doi: 10.1046/j.1471-4159.1997.69031145.x. [DOI] [PubMed] [Google Scholar]
- 79.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol. 2009;9:262–267. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corset V, Nguyen-Ba-Charvet KT, Forcet C, Moyse E, Chedotal A, Mehlen P. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–50. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- 81.Stein E, Zou Y, Poo M, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science. 2001;291:1976–82. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- 82.McKenna WL, Wong-Staal C, Kim GC, Macias H, Hinck L, Bartoe JL. Netrin-1-independent adenosine A2b receptor activation regulates the response of axons to netrin-1 by controlling cell surface levels of UNC5A receptors. J Neurochem. 2008;104:1081–90. doi: 10.1111/j.1471-4159.2007.05040.x. [DOI] [PubMed] [Google Scholar]
- 83.Wittendorp MC, Boddeke HW, Biber K. Adenosine A3 receptor-induced CCL2 synthesis in cultured mouse astrocytes. Glia. 2004;46:410–8. doi: 10.1002/glia.20016. [DOI] [PubMed] [Google Scholar]
- 84.Hammarberg C, Fredholm BB, Schulte G. Adenosine A3 receptor-mediated regulation of p38 and extracellular-regulated kinase ERK1/2 via phosphatidylinositol-3′-kinase. Biochem Pharmacol. 2004;67:129–34. doi: 10.1016/j.bcp.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 85.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 86.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–9. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 87.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–5. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 88.Miller RJ, Rostene W, Apartis E, Banisadr G, Biber K, Milligan ED, et al. Chemokine action in the nervous system. J Neurosci. 2008;28:11792–5. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–76. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 90.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–9. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 91.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103:17513–8. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, et al. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–76. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–53. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 94.Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol. 1994;267:H296–301. doi: 10.1152/ajpheart.1994.267.1.H296. [DOI] [PubMed] [Google Scholar]
- 95.Iliff JJ, D’Ambrosio R, Ngai AC, Winn HR. Adenosine receptors mediate glutamate-evoked arteriolar dilation in the rat cerebral cortex. Am J Physiol Heart Circ Physiol. 2003;284:H1631–7. doi: 10.1152/ajpheart.00909.2002. [DOI] [PubMed] [Google Scholar]
- 96.Ohata H, Cao S, Koehler RC. Contribution of adenosine A2A and A2B receptors and heme oxygenase to AMPA-induced dilation of pial arterioles in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R728–35. doi: 10.1152/ajpregu.00757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jimenez AI, Castro E, Mirabet M, Franco R, Delicado EG, Miras-Portugal MT. Potentiation of ATP calcium responses by A2B receptor stimulation and other signals coupled to Gs proteins in type-1 cerebellar astrocytes. Glia. 1999;26:119–28. [PubMed] [Google Scholar]
- 99.Alloisio S, Cugnoli C, Ferroni S, Nobile M. Differential modulation of ATP-induced calcium signalling by A1 and A2 adenosine receptors in cultured cortical astrocytes. Br J Pharmacol. 2004;141:935–42. doi: 10.1038/sj.bjp.0705707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol. 2007;92:647–51. doi: 10.1113/expphysiol.2006.036863. [DOI] [PubMed] [Google Scholar]
- 101.Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol. 2008;294:H622–32. doi: 10.1152/ajpheart.00530.2007. [DOI] [PubMed] [Google Scholar]
- 102.Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40:S8–12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eid T, Williamson A, Lee TS, Petroff OA, de Lanerolle NC. Glutamate and astrocytes--key players in human mesial temporal lobe epilepsy? Epilepsia. 2008;49(Suppl 2):42–52. doi: 10.1111/j.1528-1167.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 105.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 106.Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, et al. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- 107.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–40. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 108.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–20. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 109.Lee SY, Haydon PG. Astrocytic glutamate targets NMDA receptors. J Physiol. 2007;581:887–8. doi: 10.1113/jphysiol.2007.134676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–71. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 111.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–7. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 112.Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–68. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–9. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 114.Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, et al. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A. 2003;100:15194–9. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 117.Yu L, Shen HY, Coelho JE, Araujo IM, Huang QY, Day YJ, et al. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol. 2008;63:338–46. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]
- 118.Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, et al. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–66. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tian GF, Azmi H, Takano T, Xu QW, Peng WG, Lin J, et al. An astrocytic basis of epilepsy. Nature Medicine. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–68. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- 121.Li T, Lan JQ, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology. 2007;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, et al. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boison D. Adenosine augmentation therapies (AATs) for epilepsy: prospect of cell and gene therapies. Epilepsy Res. 2009 doi: 10.1016/j.eplepsyres.2009.03.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boison D. Engineered adenosine-releasing cells for epilepsy therapy: human mesenchymal stem cells and human embryonic stem cells. Neurotherapeutics. 2009;6:278–283. doi: 10.1016/j.nurt.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bell MJ, Kochanek PM, Carcillo JA, Mi Z, Schiding JK, Wisniewski SR, et al. Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J Neurotrauma. 1998;15:163–70. doi: 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- 126.Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, et al. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:565–575. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- 127.Fedele DE, Li T, Lan JQ, Fredholm BB, Boison D. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol. 2006;200:184–190. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- 128.Li W, Dai S, An J, Xiong R, Li P, Chen X, et al. Genetic inactivation of adenosine A2A receptors attenuates acute traumatic brain injury in the mouse cortical impact model. Exp Neurol. 2009;215:69–76. doi: 10.1016/j.expneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 129.Sachse KT, Jackson EK, Wisniewski SR, Gillespie DG, Puccio AM, Clark RS, et al. Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li W, Dai S, An J, Li P, Chen X, Xiong R, et al. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience. 2008;151:1198–207. doi: 10.1016/j.neuroscience.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 131.Gouder N, Scheurer L, Fritschy J-M, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A. 1991;88:7238–41. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Richardson PJ, Gubitz AK, Freeman TC, Dixon AK. Adenosine receptor antagonists and Parkinson’s disease: actions of the A2A receptor in the striatum. Adv Neurol. 1999;80:111–9. [PubMed] [Google Scholar]
- 134.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–54. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 135.Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, Chen JF. Adenosine, adenosine A(2A) antagonists, and Parkinson’s disease. Parkinsonism Relat Disord. 2009 doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 136.Schwarzschild MA. Adenosine A2A antagonists as neurotherapeutics: crossing the bridge. Prog Neurobiol. 2007;83:261–2. doi: 10.1016/j.pneurobio.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. Jama. 2000;283:2674–9. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 138.Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 139.Saaksjarvi K, Knekt P, Rissanen H, Laaksonen MA, Reunanen A, Mannisto S. Prospective study of coffee consumption and risk of Parkinson’s disease. Eur J Clin Nutr. 2008;62:908–15. doi: 10.1038/sj.ejcn.1602788. [DOI] [PubMed] [Google Scholar]
- 140.Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, et al. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21:RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ikeda K, Kurokawa M, Aoyama S, Kuwana Y. Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. J Neurochem. 2002;80:262–70. doi: 10.1046/j.0022-3042.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- 142.Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, et al. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol. 2002;52:160–7. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
- 143.Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, et al. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol. 2007;83:310–31. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 144.Pierri M, Vaudano E, Sager T, Englund U. KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse. Neuropharmacology. 2005;48:517–24. doi: 10.1016/j.neuropharm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 145.Hagberg H, Andersson P, Lacarewicz J, Jacobson I, Butcher S, Sandberg M. Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J Neurochem. 1987;49:227–231. doi: 10.1111/j.1471-4159.1987.tb03419.x. [DOI] [PubMed] [Google Scholar]
- 146.Kopf SR, Melani A, Pedata F, Pepeu G. Adenosine and memory storage: effect of A(1) and A(2) receptor antagonists. Psychopharmacology (Berl) 1999;146:214–9. doi: 10.1007/s002130051109. [DOI] [PubMed] [Google Scholar]
- 147.Gianfriddo M, Corsi C, Melani A, Pezzola A, Reggio R, Popoli P, et al. Adenosine A(2A) antagonism increases striatal glutamate outflow in the quinolinic acid rat model of Huntington’s disease. Brain Res. 2003;979:225–9. doi: 10.1016/s0006-8993(03)02942-1. [DOI] [PubMed] [Google Scholar]
- 148.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–84. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 149.Latini S, Bordoni F, Corradetti R, Pepeu G, Pedata F. Temporal correlation between adenosine outflow and synaptic potential inhibition in rat hippocampal slices during ischemia-like conditions. Brain Res. 1998;794:325–8. doi: 10.1016/s0006-8993(98)00304-7. [DOI] [PubMed] [Google Scholar]
- 150.Matsumoto K, Graf R, Rosner G, Shimada N, Heiss WD. Flow thresholds for extracellular purine catabolite elevation in cat focal ischemia. Brain Res. 1992;579:309–14. doi: 10.1016/0006-8993(92)90066-i. [DOI] [PubMed] [Google Scholar]
- 151.Dux E, Fastbom J, Ungerstedt U, Rudolphi K, Fredholm BB. Protective effect of adenosine and a novel xanthine derivative propentofylline on the cell damage after bilateral carotid occlusion in the gerbil hippocampus. Brain Res. 1990;516:248–56. doi: 10.1016/0006-8993(90)90925-2. [DOI] [PubMed] [Google Scholar]
- 152.Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem. 2007;101:1400–13. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Phillis JW, Perkins LM, Smith-Barbour M, O’Regan MH. Transmitter amino acid release from rat neocortex: complete versus incomplete ischemia models. Neurochem Res. 1994;19:1387–92. doi: 10.1007/BF00972467. [DOI] [PubMed] [Google Scholar]
- 154.Phillis JW, Smith-Barbour M, O’Regan MH. Changes in extracellular amino acid neurotransmitters and purines during and following ischemias of different durations in the rat cerebral cortex. Neurochem Int. 1996;29:115–20. doi: 10.1016/0197-0186(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 155.Schwarzschild MA, Chen JF, Ascherio A. Caffeinated clues and the promise of adenosine A(2A) antagonists in PD. Neurology. 2002;58:1154–60. doi: 10.1212/wnl.58.8.1154. [DOI] [PubMed] [Google Scholar]
- 156.Ribeiro JA, Sebastiao AM, de Mendonca A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2002;68:377–92. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- 157.Ongini E, Adami M, Ferri C, Bertorelli R. Adenosine A2A receptors and neuroprotection. Ann N Y Acad Sci. 1997;825:30–48. doi: 10.1111/j.1749-6632.1997.tb48412.x. [DOI] [PubMed] [Google Scholar]
- 158.Fredholm BB, Cunha RA, Svenningsson P. Pharmacology of Adenosine A(2A) Receptors and Therapeutic Applications. Curr Top Med Chem. 2003;3:413–26. doi: 10.2174/1568026033392200. [DOI] [PubMed] [Google Scholar]
- 159.Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–25. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 160.Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Neuroprotective role of adenosine in cerebral ischemia. Trends Pharmacol Sci. 1992;13:439–445. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- 161.Fredholm BB. Adenosine and neuroprotection. In: Green AR, Cross AJ, editors. Neuroprotective agents and cerebral ischemia. Academic Press; London: 1996. pp. 259–80. [Google Scholar]
- 162.Kitagawa H, Mori A, Shimada J, Mitsumoto Y, Kikuchi T. Intracerebral adenosine infusion improves neurological outcome after transient focal ischemia in rats. Neurol Res. 2002;24:317–23. doi: 10.1179/016164102101199819. [DOI] [PubMed] [Google Scholar]
- 163.Phillis JW, O’Regan MH. Deoxycoformycin antagonizes ischemia-induced neuronal degeneration. Brain Res Bull. 1989;22:537–40. doi: 10.1016/0361-9230(89)90107-x. [DOI] [PubMed] [Google Scholar]
- 164.Phillis JW, Walter GA, Simpson RE. Brain adenosine and transmitter amino acid release from the ischemic rat cerebral cortex: effects of the adenosine deaminase inhibitor deoxycoformycin. Journal of neurochemistry. 1991;56:644–650. doi: 10.1111/j.1471-4159.1991.tb08198.x. [DOI] [PubMed] [Google Scholar]
- 165.Lin Y, Phillis JW. Deoxycoformycin and oxypurinol: protection against focal ischemic brain injury in the rat. Brain Res. 1992;571:272–80. doi: 10.1016/0006-8993(92)90665-v. [DOI] [PubMed] [Google Scholar]
- 166.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 167.Miller LP, Jelovich LA, Yao L, DaRe J, Ugarkar B, Foster AC. Pre- and peristroke treatment with the adenosine kinase inhibitor, 5′-deoxyiodotubercidin, significantly reduces infarct volume after temporary occlusion of the middle cerebral artery in rats. Neurosci Lett. 1996;220:73–6. doi: 10.1016/s0304-3940(96)13234-1. [DOI] [PubMed] [Google Scholar]
- 168.DeLeo J, Schubert P, Kreutzberg GW. Protection against ischemic brain damage using propentofylline in gerbils. Stroke. 1988;19:1535–9. doi: 10.1161/01.str.19.12.1535. [DOI] [PubMed] [Google Scholar]
- 169.Andine P, Rudolphi KA, Fredholm BB, Hagberg H. Effect of propentofylline (HWA 285) on extracellular purines and excitatory amino acids in CA1 of rat hippocampus during transient ischaemia. Br J Pharmacol. 1990;100:814–8. doi: 10.1111/j.1476-5381.1990.tb14097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kano T, Katayama Y, Kawamata T, Hirota H, Tsubokawa T. Propentofylline administered by microdialysis attenuates ischemia-induced hippocampal damage but not excitatory amino acid release in gerbils. Brain Res. 1994;641:149–54. doi: 10.1016/0006-8993(94)91829-5. [DOI] [PubMed] [Google Scholar]
- 171.Matsumoto K, Sakaki T, Kohmura E, Hayakawa T, Yamada K. Amelioration of ischemic brain damage by the preischemic administration of propentofylline (HWA285) in a rat focal ischemia. Brain Res. 1996;723:228–30. doi: 10.1016/0006-8993(96)00258-2. [DOI] [PubMed] [Google Scholar]
- 172.Fritschy J-M, Johnson DJ, Mohler H, Rudolph U. Independent assembly and subcellular targeting of GABAA-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci Lett. 1998;249:99–102. doi: 10.1016/s0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- 173.Parkinson FE, Xiong W, Zamzow CR. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol Res. 2005;27:153–60. doi: 10.1179/016164105X21878. [DOI] [PubMed] [Google Scholar]
- 174.Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- 175.Zetterstrom T, Fillenz M. Adenosine agonists can both inhibit and enhance in vivo striatal dopamine release. Eur J Pharmacol. 1990;180:137–43. doi: 10.1016/0014-2999(90)90601-2. [DOI] [PubMed] [Google Scholar]
- 176.Corradetti R, Lo Conte G, Moroni F, Passani MB, Pepeu G. Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur J Pharmacol. 1984;104:19–26. doi: 10.1016/0014-2999(84)90364-9. [DOI] [PubMed] [Google Scholar]
- 177.Greene RW, Haas HL. The electrophysiology of adenosine in the mammalian central nervous system. Prog Neurobiol. 1991;36:329–41. doi: 10.1016/0301-0082(91)90005-l. [DOI] [PubMed] [Google Scholar]
- 178.Olsson T, Cronberg T, Rytter A, Asztely F, Fredholm BB, Smith ML, et al. Deletion of the adenosine A1 receptor gene does not alter neuronal damage following ischaemia in vivo or in vitro. Eur J Neurosci. 2004;20:1197–204. doi: 10.1111/j.1460-9568.2004.03564.x. [DOI] [PubMed] [Google Scholar]
- 179.Phillis JW. The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res. 1995;705:79–84. doi: 10.1016/0006-8993(95)01153-6. [DOI] [PubMed] [Google Scholar]
- 180.Von Lubitz DK, Lin RC, Jacobson KA. Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist. Eur J Pharmacol. 1995;287:295–302. doi: 10.1016/0014-2999(95)00498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bona E, Aden U, Gilland E, Fredholm BB, Hagberg H. Neonatal cerebral hypoxia-ischemia: the effect of adenosine receptor antagonists. Neuropharmacology. 1997;36:1327–38. doi: 10.1016/s0028-3908(97)00139-1. [DOI] [PubMed] [Google Scholar]
- 182.Monopoli A, Lozza G, Forlani A, Mattavelli A, Ongini E. Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. Neuroreport. 1998;9:3955–9. doi: 10.1097/00001756-199812010-00034. [DOI] [PubMed] [Google Scholar]
- 183.Pedata F, Gianfriddo M, Turchi D, Melani A. The protective effect of adenosine A2A receptor antagonism in cerebral ischemia. Neurol Res. 2005;27:169–74. doi: 10.1179/016164105X21913. [DOI] [PubMed] [Google Scholar]
- 184.Melani A, Gianfriddo M, Vannucchi MG, Cipriani S, Baraldi PG, Giovannini MG, et al. The selective A(2A) receptor antagonist SCH 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia. Brain Res. 2006;1073–1074:470–80. doi: 10.1016/j.brainres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 185.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann N Y Acad Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- 187.Marcoli M, Raiteri L, Bonfanti A, Monopoli A, Ongini E, Raiteri M, et al. Sensitivity to selective adenosine A1 and A2A receptor antagonists of the release of glutamate induced by ischemia in rat cerebrocortical slices. Neuropharmacology. 2003;45:201–10. doi: 10.1016/s0028-3908(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 188.Aden U, Halldner L, Lagercrantz H, Dalmau I, Ledent C, Fredholm BB. Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke. 2003;34:739–44. doi: 10.1161/01.STR.0000060204.67672.8B. [DOI] [PubMed] [Google Scholar]
- 189.Phillis JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol. 2004;16:237–70. doi: 10.1615/critrevneurobiol.v16.i4.20. [DOI] [PubMed] [Google Scholar]
- 190.von Lubitz DKJE. Adenosine and cerebral ischemia: therapeutic future or death of a brave concept? Eur J Pharmacol. 1999;365:9–25. doi: 10.1016/s0014-2999(98)00788-2. [DOI] [PubMed] [Google Scholar]
- 191.Chen JF, Pedata F. Modulation of ischemic brain injury and neuroinflammation by adenosine A(2A) receptors. Current Pharmaceutical Design. 2008;14:1490–1499. doi: 10.2174/138161208784480126. [DOI] [PubMed] [Google Scholar]
- 192.Irving EA, Barone FC, Reith AD, Hadingham SJ, Parsons AA. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 2000;77:65–75. doi: 10.1016/s0169-328x(00)00043-7. [DOI] [PubMed] [Google Scholar]
- 193.Wu DC, Ye W, Che XM, Yang GY. Activation of mitogen-activated protein kinases after permanent cerebral artery occlusion in mouse brain. J Cereb Blood Flow Metab. 2000;20:1320–30. doi: 10.1097/00004647-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 194.Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, et al. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med Res Rev. 2001;21:129–45. doi: 10.1002/1098-1128(200103)21:2<129::aid-med1003>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 195.Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, et al. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- 196.Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, et al. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J Biol Chem. 1997;272:18518–21. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]