Abstract
Balancing intake of diverse nutrients is important for organismal growth, reproduction and survival. A shift in an organism’s optimal diet due to changes in nutritional requirements after developmental or environmental changes is referred to as dietary switch, and has been observed in several species [1]. We demonstrate that female D. melanogaster also undergo a dietary switch following mating that leads to an increased preference for yeast, the major source of protein in their diet. We demonstrate that S6 Kinase (S6K) and serotonin production are involved in the post-mating dietary switch. To further investigate the ability of D. melanogaster to balance nutrient intake, we examined the dietary preferences of adult flies following deprivation of yeast or sucrose in the diet. We observe that following conditioning on a diet deficient in either carbohydrates or yeast, D. melanogaster shows a strong preference for the deficient nutrient when permitted to choose its diet freely. Furthermore, flies with activated S6K or fed a serotonin precursor exhibit enhanced preference for yeast in this assay. Our results suggest that TOR signaling and serotonin may play an important role in maintaining nutrient balance in D. melanogaster. Hence, D. melanogaster can be used as a model organism to investigate the genetic basis for nutrient homeostasis which may contribute to our understanding of metabolic disorders such as obesity and diabetes [2].
RESULTS AND DISCUSSION
In order to maintain adequate nutritional status, organisms not only have to consume sufficient nutrients to meet energy needs, but also need to select a balanced diet from foods that vary widely in nutritional quality. Furthermore, in organisms ranging from insects to mammals, the required nutritional balance can be different depending on developmental stage or, changes in the environment [3, 4]. These distinct nutrient requirements can be satisfied by appropriately selecting from the various types of nutrients available to the organism [3, 4].
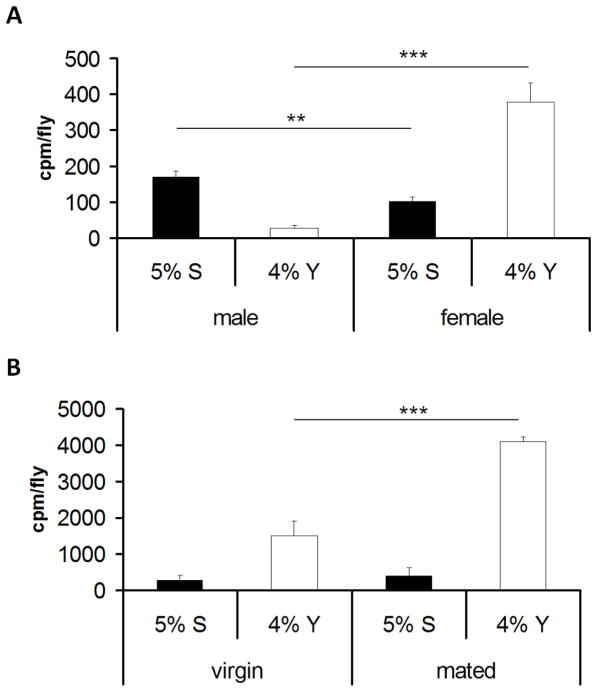
In order to test the ability of adult D. melanogaster to select different types of nutrients, a ‘nutrient preference’ assay was developed. In this assay, flies were given the freedom to choose two incomplete, yet complementary, diets in a sealed chamber. One food option consisted of a dietary mix lacking yeast extract (the major source of protein in the diet), while the other option consisted of a dietary mix lacking sucrose (the major source of carbohydrate in the diet). To measure ingestion of yeast, flies were given a choice between the two food options wherein the food containing yeast but no sucrose contained a radiolabeled tracer. To measure sucrose ingestion, a separate population of flies was given a choice between the two foods wherein the food containing sucrose but no yeast contained a radiolabeled tracer. This allowed the measurement of yeast and sucrose intake in the same strain of flies, albeit in separate experiments (for more details, see materials and methods and Figure S1). Using the radiolabeled food, we examined if both male and female flies ingested an optimal diet by consuming the supplied dietary mixes in a non-random fashion. The ‘nutrient preference’ assay demonstrated a significant sex-specific difference in food choice, with females eating a much larger amount of yeast than males and males consuming more sucrose relative to females (Figure 1A). This disparity in preferred nutrients between males and females is expected as females have a greater need for protein and other nutrients in yeast extract to effect egg production [5, 6]. Mating in female D. melanogaster leads to significant behavioral and physiological changes, including increased feeding [7], enhanced egg production and decreased longevity [8]. Therefore, we examined if the mating status of female flies was important for the increased preference for yeast observed in female flies over male flies. A ‘nutrient preference’ assay on mated and non-mated flies showed that mated females choose to eat significantly more yeast than their virgin counterparts (Figure 1B). These findings are consistent with the idea that mated female flies need to consume more protein to meet their increased investment in reproductive output [6, 7, 9].
Figure 1.
Sex and mating dependent differences in nutrient preference for sucrose or yeast in D. melanogaster. To measure ingestion of yeast, flies were given a choice between the two food options wherein only food deficient in sucrose but containing 4% yeast extract (4%Y) contained a radiolabeled tracer (white bars). To measure ingestion of sucrose, flies were given a choice between the two food options wherein only diet deficient in yeast but containing 5% sucrose (5% S) contained a radiolabeled tracer (black bars). (A) Results of a ‘nutrient preference’ assay demonstrating the differences in dietary preferences of males and females in the w1118 strain. (B) Differences in dietary consumption of yeast and sucrose in virgin and mated females in the w1118 strain. In each assay four sets of 15 to 20 whole body flies were analyzed. See also Figure S1. ***=p<0.001, **=p<0.01
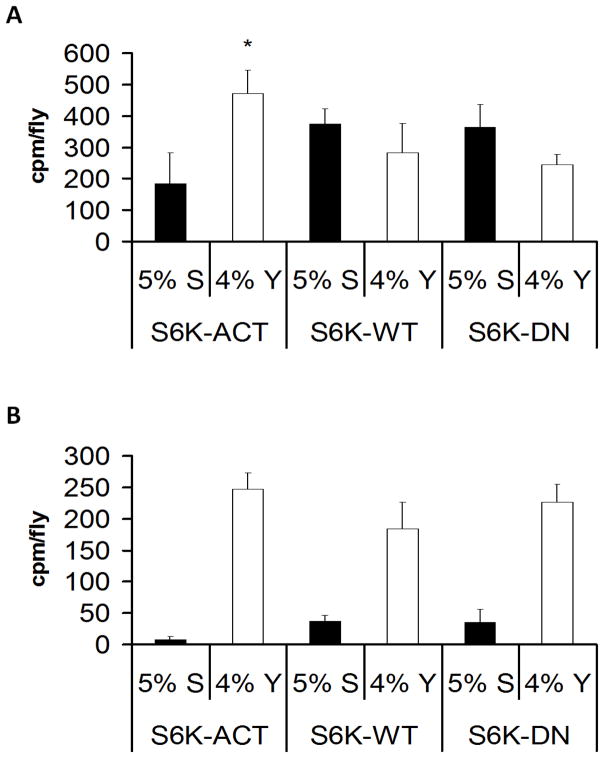
It has been shown that the target of rapamycin (TOR) pathway plays a conserved role in nutrient sensing and growth in multiple species [10]. Essential amino acids activate the TOR pathway after being transported into the cell [10]. S6K is a target of TOR that, in the presence of amino acids, is activated via phosphorylation and mediates downstream effects on enhancing mRNA translation and growth [10]. Given the role of the TOR pathway in nutrient sensing, we tested the possibility that S6K may modulate nutrient choice at the organismal level. We examined the role of S6K in post-mating dietary switch using different forms of dS6K: wild type, dominant negative, and constitutively active. The dominant negative form was previously generated by replacing a lysine with glutamine in the ATP binding site (UAS-S6KDN), and the constitutively active form by replacing multiple phosphorylation sites of S6K with acidic amino acids (UAS-S6KACT), respectively [11]. Overexpression of the dominant negative form has been previously shown to reduce growth, while the constitutively active form enhances growth [11]. We specifically examined the changes in nutrient preference of flies overexpressing these different forms of S6K in neuronal and fat tissues. Fat and neuronal tissues were chosen as they play a key role in sensing nutrient status and mediating physiological changes in the organism. Flies overexpressing the constitutively active dS6K using a pan-neuronal Gal4 enhancer trap, appl-GAL4, demonstrated an increase in yeast consumption for the virgin female population (Figure 2A). However, modulation of S6K in mated females did not show a significant change in nutrient preference (Figure 2B). The manipulation of S6K using appl-GAL4 is likely to alter gene expression both during development and adulthood such that changes in S6K activity during development cannot be ruled out as a contributing factor in the above experiments. Modulation of S6K in the fat body did not lead to any significant changes in yeast consumption (data not shown). To further assess the role of S6K in post-mating dietary switch, we tested the possibility that mated flies have increased S6K phosphorylation. However, the levels of S6K phosphorylation measured in the fly heads using an antibody that recognizes the phosphorylated form of S6K did not show any significant differences between virgin and mated flies (Figure S2). Though these experiments do not support a role for S6K activation leading to the increased preference for yeast in mated flies, it is possible that S6K activation in specific neurons may mediate this change but was not detectable in our experiments using whole fly heads. It has been previously shown that neuronal modulation of S6K plays an important role in mediating responses to hunger [12]. Our results show that in addition, S6K activation in neuronal cells enhances ingestion of food with a higher ratio of protein to sugar in unmated flies.
Figure 2.
The effect of S6K on nutrient preferences in virgin and mated D. melanogaster. Nutrient preference for sucrose (black bars) and yeast (white bars) was measured upon S6K manipulation. (A) Virgin female flies overexpressing either dominant negative (S6K-DN), wild-type (S6K-WT), or constitutively active (S6K-ACT) forms of S6 kinase (S6K) in the neurons driven by the pan neuronal appl-gal4 enhancer trap were tested for sucrose or yeast ingestion. The following genotypes were tested appl-GAL4/+; UAS-S6KSTDETE/+; +/+ (S6K-ACT), appl-GAL4/+; UAS-S6Kwt/+; +/+ (S6K-WT) and appl-GAL4/+; UAS-S6KKQ/+; +/+ (S6K-DN). S6K-ACT virgin flies were found to ingest significantly more yeast compared to the other two strains as calculated by one-way ANOVA (p<0.003) (B) Same as (A) but mated female flies were used for the experiment. 4 sets of 15 to 20 whole body flies were analyzed. See also Figure S2. **=p<0.01
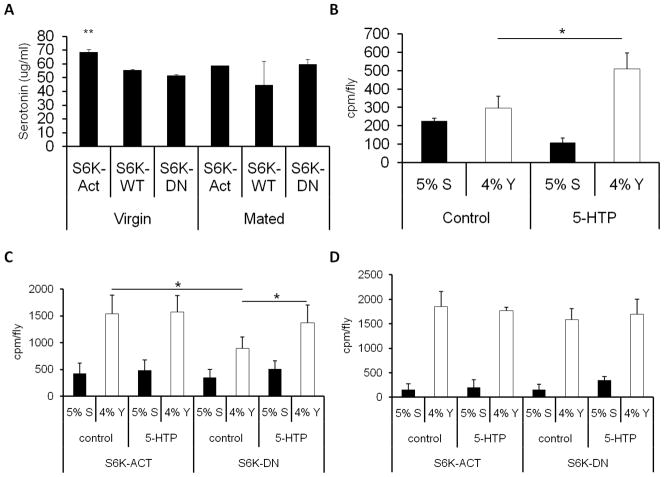
Serotonin has previously been implicated in changing the protein to carbohydrate ratio of ingested nutrients in animals such as cockroaches [13] and rats [14]. To determine the role of serotonin in post-mating dietary switch in D. melanogaster we examined whether serotonin levels are altered upon modulation of S6K. Increased neuronal S6K activation led to increased serotonin production (Figure 3A). However, no significant increase in serotonin levels were observed in fly heads upon mating (Figure S3A). To assess the relationship between S6K and serotonin in our paradigm we examined whether addition of a serotonin precursor, 5-hydroxy-L-tryptophan (5-HTP), to the food would alter S6K activity. 5-HTP has previously been used to study the effects of serotonin in D. melanogaster [15]. We observed that flies exposed to 5-HTP showed an increase in the level of serotonin in vivo (Figure S3A). Addition of 5-HTP to fly food or Drosophila embryonic S2 cells failed to change S6K phosphorylation as measured using an antibody that recognizes phosphorylated S6K (data not shown).
Figure 3.
The interaction between S6K and serotonin in modulating nutrient preferences in D. melanogaster. (A) Serotonin measurements were taken from the heads of 25 virgin and mated female flies overexpressing either dominant negative (S6K-DN), wild-type (S6K-WT), or constitutively active (S6K-ACT) forms of S6K in the neurons driven by the pan neuronal appl-gal4 enhancer trap. The following genotypes were tested appl-GAL4/+; UAS-S6KSTDETE/+; +/+ (S6K-ACT), appl-GAL4/+; UAS-S6Kwt/+; +/+ (S6K-WT) and appl-GAL4/+; UAS-S6KKQ/+; +/+ (S6K-DN). Serotonin levels were significantly increased in virgin flies overexpressing the constitutively active form of S6K in the neurons compared to the other virgin strains as calculated by one-way ANOVA (p<0.002). The results represent mean of three replicates. (B) The effect of feeding a serotonin precursor 5-HTP using a nutrient preference’ assay in virgin female D. melanogaster fed 5-HTP. Yeast (white bars) and sucrose (black bars) ingestion was measured as described in Figure 1. Four sets of 15 to 20 whole body flies were analyzed. The effect of 5-HTP on nutrient preference in males is shown in Figure S3B. (C) Nutrient preference upon treatment of 5-HTP in virgin female flies overexpressing the activated or dominant negative form of S6K in neurons. The strains S6K-ACT and S6K-DN were created as described above. Flies were fed 3 mg/mL 5-HTP for 48 hours prior to the ‘nutrient preference ‘assay and then examined for yeast and sucrose consumption as described in Figure 1. (D) Same as (C) except mated female flies were used for the experiment. Four sets of 15 to 20 whole body flies were analyzed in each experiment. See also Figure S3. **=p<0.01, *=p<0.05
To assess the role of serotonin in influencing nutrient choices, we analyzed the nutrient preference of flies conditioned on food containing 5-HTP. Virgin flies exposed to a diet containing 5-HTP showed a significant increase in yeast preference compared to control virgin females on a normal diet (Figure 3B). No significant differences of 5-HTP treatment in nutrient preference were observed in mated female (Figure 3D and data not shown) or male flies (Figure S3B). These experiments suggest that, as in other species, serotonin can also modulate dietary preferences in D. melanogaster under specific circumstances. In order to examine whether serotonin and S6K influence nutrient choice through the same pathway or in parallel, neuronally expressed, activated and dominant negative dS6K transgenic flies were fed 5-HTP and allowed to select their diet. Treatment with 5-HTP showed no effect on yeast preference in virgin female flies overexpressing the constitutively active dS6K, while there was an increase in preference for yeast in flies overexpressing the dominant negative form (Figure 3C). The effect on yeast preference with 5-HTP treatment in flies overexpressing the dominant negative form was not observed in mated flies (Figure 3D). Consistent with our finding that activated S6K leads to enhanced serotonin production, our results suggest that activation of S6K and serotonin enhance nutrient preference for yeast by overlapping mechanisms in unmated D. melanogaster. It is interesting to note that mated flies seem refractory to changes in nutrient preferences by S6K or serotonin manipulation suggesting a possible robust dietary switch upon mating that maybe hormonally induced as suggested before [9].
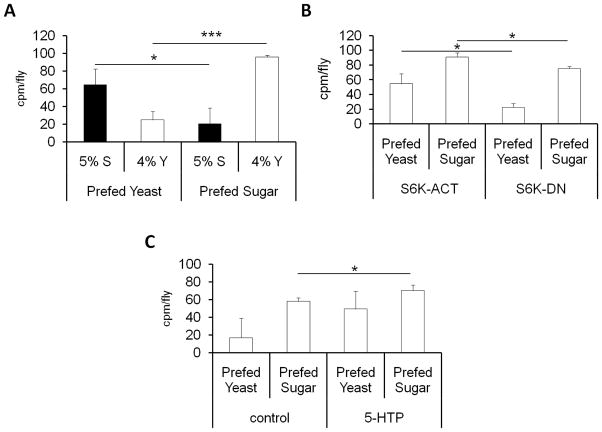
Many organisms show increased preference for diets that provide nutrients that they are lacking [3, 4, 16]. Using the ‘nutrient balance’ assay, we tested the ability of D. melanogaster to maintain macronutrient homeostasis (see Figure S4A and methods). Female flies were fed a diet lacking either sugar or yeast for 3 days prior to giving them a choice to select from complementary diets missing either sugar or yeast. Results showed that after conditioning on a sugar-rich diet, females consume a significantly increased amount of yeast, and conversely, when conditioned on a yeast-rich diet, females consume significantly increased levels of sugar (Figure 4A). A similar pattern was also seen in males (Figure S4B). These experiments demonstrate that D. melanogaster have the capability to select nutrients in a non-random manner to facilitate and retain proper nutrient balance and homeostasis.
Figure 4.
The effects of S6K and 5-HTP on nutrient balance in female D. melanogaster. A ‘nutrient balance’ assay was used (Figure S4) to assess the ability of D. melanogaster to compensate for deficiency of yeast or sucrose in their diet. The ingestion of yeast or sucrose was measured as described in Figure 1. (A) Results from a ‘nutrient balance’ assay in w1118 females following 3 days pre-feeding on incomplete diets. Sugar consumption (black bars) and yeast consumption (white bars) was measured following pre-feeding on a yeast deficient diet containing sugar (Prefed sugar) or a sucrose deficient diet containing yeast (Prefed yeast). Four sets of 15 to 20 whole body flies were analyzed. The data from male flies is shown in Figure S4B. (B) Results from a ‘nutrient balance’ assay showing yeast consumption (white bars) in female flies with altered neuronal levels of S6K activation following pre-feeding on incomplete diets. Nutrient intake of female flies overexpressing either dominant negative (S6K-DN) or constitutively active (S6K-ACT) forms of S6 kinase (S6K) in the neurons driven by the pan neuronal appl-gal4 enhancer trap was measured following pre-feeding in incomplete diets. The following genotypes were tested appl-GAL4/+; UAS-S6KSTDETE/+; +/+ (S6K-ACT) and appl-GAL4/+; UAS-S6KKQ/+; +/+ (S6K-DN). 4 sets of 15 to 20 flies were analyzed. Results from male flies are shown in Figure S4C. The corresponding data on sugar consumptions can be found in Figures S4D and S4E. (C) The effect of serotonin on nutrient balance. Yeast consumption (white bars) was measured in female flies fed 3 mg/mL 5-HTP for 48 hours. Flies were fed a diet lacking either sugar or yeast for 3 days prior to giving them a choice to select a fly diet containing either sucrose or yeast, with yeast containing diet containing the radiolabeled tracer (as described above). See also Figure S4. ***=p<0.001, *=p<0.05
Next, we examined whether the ability of D. melanogaster to balance nutrients is also regulated by S6K. Following deprivation of either yeast or sugar for 3 days, female flies with pan neuronally activated S6K choose to eat a significant amount more yeast than flies with dominant negative S6K (Figure 4B). Males also showed an increased preference for yeast when prefed a diet lacking yeast (Figure S4C). Sugar consumption in these flies was not altered significantly under these conditions (Figures S4B & S4E). These data support the notion that S6K modulation can alter nutrient balance. The effect of serotonin on balancing nutrients was also assessed. Female flies prefed a diet lacking yeast but containing sugar showed a greater preference for yeast upon on 5-HTP treatment (Figure 4C). No significant differences in sugar consumption were observed upon 5-HTP treatment (data not shown). These experiments suggest that both S6K and serotonin play a role in balancing the ingestion of macronutrients in D. melanogaster.
The ability of serotonin to regulate nutrient choice has been previously implicated in various species including rats [17, 18] and cockroaches [13]. However, a causal role for serotonin in influencing macronutrient choice remains controversial [19, 20]. The mechanisms by which serotonin modulates nutrient choice have been debated. Previous studies in rats and Heliothis zea are consistent with our results that serotonin affects nutrient choice via changes in protein intake [21, 22], yet other studies suggest that serotonin influences carbohydrate intake in order to adjust the overall nutrient balance [13, 23]. Further investigation is merited to explore the role of serotonin in influencing nutrient choice. In addition, the mechanisms by which serotonin levels are increased or decreased in order to exert control over nutrient choice are also of great interest. Previous analyses in weanling rats and Heliothis zea have proposed an increase in plasma and brain serotonin levels, respectively, during times when the ingested food has low protein [21, 22]. Our observations corroborate the idea that increasing serotonin levels favor intake of protein-rich food. In D. melanogaster, serotonin has previously been shown to modulate several processes such as sleep, circadian rhythms, feeding, memory, and locomotion. The mechanisms by which serotonin influences nutrient choice may help us understand its impact on a variety of biological processes.
The TOR pathway’s involvement in nutrient sensing makes it an attractive candidate for influencing nutrient balance. Amino acids are sufficient to activate the highly conserved TOR pathway, thereby leading to phosphorylation of downstream effectors including S6K [10]. Varying nutrient compositions ingested by insects leads to changes in fat metabolism and lifespan [6, 24, 25]. It has been hypothesized that the ratio of protein to non-protein energy ingested is one of the key determinants of aging and fat metabolism which maybe under TOR regulation [26]. In support of this hypothesis, we demonstrate that S6K plays an important role in nutrient balance and in nutrient preference in response to mating in D. melanogaster. Hence, in addition to its role in sensing nutrients, TOR signaling also influences the composition of nutrients ingested. The TOR pathway has been shown to modulate fat metabolism and lifespan in worms, flies and even mice [27]. A better understanding of the mechanisms of nutrient homeostasis in simple model systems may contribute to our understanding of how TOR signaling influences human health, lifespan and other disease processes.
EXPERIMENTAL PROCEDURES
‘Nutrient preference’ assay
A nutrient preference assay to measure specific ingestion of yeast or sucrose was devised (Figure S1). 3–4 day-old virgin or mated flies (15–20 animals/vial) were conditioned for 2 days on the regular lab food (0.5% agar, 8% cornmeal, 1.8% yeast extract, and 10% sucrose). Flies were then transferred to a connected two-tube setup with sugar deficient yeast extract containing food - 4%Y (0.5% agar, 8% cornmeal and 4% yeast extract) in one tube and yeast deficient sucrose containing food - 5%S (0.5% agar, 8% cornmeal and 5% sucrose) in another tube. During the feeding assay period either the 4%Y or the 5%S food was labeled with dCTP[α-32P] (Perkin Elmer) to quantify yeast or sucrose ingestion, respectively. Two separate populations were assessed simultaneously for each experiment, one population for yeast consumption and one for sucrose consumption. All the labeled diets in the experiments contained 1 μL dCTP[α-32P] per 30 mL of food mix except for experiments in Figures 1B, 2A, and 3C. Figures 1B and 3C contain 1 μL dCTP[α-32P] per 20mL of food mix, and Figure 2A contains 1 μL dCTP[α-32P] per 30 mL in the 4%Y food mix and 2 μL dCTP[α-32P] per 30 mL in the 5%S food mix. These increases in dCTP[α-32P] concentration were performed to counteract the radioactive decay of the isotope. Flies were allowed to feed for 24 hours in dark (25°C), transferred to empty vials to groom for 10 minutes (to ensure removal of any cuticular radioactive deposits), anesthetized by cold, and assayed whole in 2 mL scintillation fluid (Research Products International Corp.) using a Beckman LS 5000 TA Liquid Scintillation System. The time of 24 hours was chosen for ‘nutrient preference’ assays as it has previously been shown that the Drosophila feeding behavior displays a 24 hour feeding clock [28] and the incorporation of the radiotracer is in the linear range within this time frame [29]. Radioactivity (cpm) of dCTP [α -32P]-labeled food ingested per fly in 24 hours is shown (mean ± s.d. of four replicate samples of 15–20 flies each). To examine the effects of serotonin, 3 mg/mL of the serotonin precursor, 5-HTP (SIGMA) was mixed with all the food throughout the feeding stages of the experiment.
‘Nutrient balance’ assay
A ‘nutrient balance’ assay was devised to measure the effect of consumption of yeast or sucrose following deficiency of either of these nutrients (Figure S4A). 3–4 day-old flies (15–20 animals/vial) were pre-conditioned for 3 days on food deficient in sucrose or yeast. The sugar deficient yeast extract containing food −4%Y (0.5% agar, 8% cornmeal and 4% yeast extract) or the yeast deficient sucrose containing food labeled −5%S (0.5% agar, 8% cornmeal and 5% sucrose) was used. Flies were then transferred to the same double-tube complementary food setup as described above for the ‘nutrient preference’ assay. Either 4%Y or 5%S food was labeled by dCTP[α-32P] (Perkin Elmer). All the labeled diets in the experiments contained 1 μL dCTP[α-32P] per 30 mL of food mix. Flies were allowed to feed for 4 hours in the dark (25°C). The short feeding assay time was critical to measure the acute changes in response to the deprivation of specific nutrients (data not shown). The test flies were then transferred to empty vials to groom for 10 minutes, anesthetized by cold and assayed whole in 2mL scintillation fluid (Research Products International Corp.) using a Beckman LS 5000 TA Liquid Scintillation System. Radioactivity (cpm) of dCTP[α -32P]-labeled food ingested per fly in 4h is shown (mean ± s.d. of four replicate samples of 15–20 flies each).
Statistics
Student t test (two tails) was used for comparative analysis of two groups of values, and is displayed in the figures by a connecting line between groups. One-way analysis of variance (ANOVA) was used for comparative analysis containing more than two groups of values, and is displayed by asterisks above the furthest outlying value.
Highlights
Mated femaleD. melanogastershow an increased preference for yeast in the diet.
Neuronal expression of activated S6K enhances preference for yeast.
Neuronal expression of activated S6K enhances serotonin production.
Activation of S6K and serotonin enhance preference for yeast by similar mechanisms.
Supplementary Material
Acknowledgments
We thank Seymour Benzer, Phi Nguyen, Ursula Edman, the anonymous reviewers and other members of the Kapahi laboratory for helpful discussions. We thank Dr. Stewart for the kind gift of fly strains expressing various forms of S6K. This work was funded by grants from the Ellison Medical Foundation, American Foundation for aging research, Hillblom foundation, a Nathan Shock Startup award, and the NIH (RL1AAG032113, 1R21AG028241-01) (P.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waldbauer GP, Friedman S. Self-selection of optimal diets by insects. Annual Review of Entomology. 1991;36:43–63. [Google Scholar]
- 2.Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. 2005;6:133–142. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 3.Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res Rev. 1997;10:151–179. doi: 10.1079/NRR19970009. [DOI] [PubMed] [Google Scholar]
- 4.Simpson SJ, Batley R, Raubenheimer D. Geometric analysis of macronutrient intake in humans: the power of protein? Appetite. 2003;41:123–140. doi: 10.1016/s0195-6663(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 5.Chippindale AK, Leroi AM, Saing H, Borash DJ, Rose MR. Phenotypic plasticity and selection in Drosophila life history evolution. 2. Diet, mates and the cost of reproduction. Journal of Evolutionary Biology. 1997;10:269. [Google Scholar]
- 6.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the Sex Peptide of Drosophila. Curr Biol. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 9.Barton Browne L, editor. Regulatory Mechanisms of Insect Feeding. Chapman & Hall; 1995. [Google Scholar]
- 10.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Barcelo H, Stewart MJ. Altering Drosophila S6 kinase activity is consistent with a role for S6 kinase in growth. Genesis. 2002;34:83–85. doi: 10.1002/gene.10132. [DOI] [PubMed] [Google Scholar]
- 12.Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci U S A. 2005;102:13289–13294. doi: 10.1073/pnas.0501914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen RW. Diet Balancing in the Cockroach Rhyparobia madera: Does Serotonin Regulate this Behavior? Journal of Insect Behavior. 2001;14:99. [Google Scholar]
- 14.Wurtman JJ, Wurtman RJ. Fenfluramine and fluoxetine spare protein consumption while suppressing caloric intake by rats. Science. 1977;198:1178–1180. doi: 10.1126/science.929195. [DOI] [PubMed] [Google Scholar]
- 15.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the rat. Appetite. 1997;28:201–213. doi: 10.1006/appe.1996.0077. [DOI] [PubMed] [Google Scholar]
- 17.Mullen BJ, Martin RJ. The effect of dietary fat on diet selection may involve central serotonin. Am J Physiol. 1992;263:R559–563. doi: 10.1152/ajpregu.1992.263.3.R559. [DOI] [PubMed] [Google Scholar]
- 18.Li ET, Anderson GH. 5-Hydroxytryptamine : a modulator of food composition but not quantity? Life Sci. 1984;34:2453–2460. doi: 10.1016/0024-3205(84)90281-9. [DOI] [PubMed] [Google Scholar]
- 19.Fernstrom JD. Food-induced changes in brain serotonin synthesis: is there a relationship to appetite for specific macronutrients? Appetite. 1987;8:163–182. doi: 10.1016/0195-6663(87)90014-6. [DOI] [PubMed] [Google Scholar]
- 20.Booth DA. Central dietary “feedback onto nutrient selection”: not even a scientific hypothesis. Appetite. 1987;8:195–201. doi: 10.1016/0195-6663(87)90016-x. [DOI] [PubMed] [Google Scholar]
- 21.Ashley DVM, Anderson GH. Correlation between the Plasma Tryptophan to Neutral Amino Acid Ratio and Protein Intake in the Self-selecting Weanling Rat. J Nutr. 1975;105:1412–1421. doi: 10.1093/jn/105.11.1412. [DOI] [PubMed] [Google Scholar]
- 22.Cohen RW, Friedman S, Waldbauer GP. Physiological control of nutrient self-selection in Heliothis zea larvae: The role of serotonin. Journal of Insect Physiology. 1988;34:935. [Google Scholar]
- 23.Wurtman JJ, Wurtman RJ. Drugs that enhance central serotoninergic transmission diminish elective carbohydrate consumption by rats. Life Sciences. 1979;24:895. doi: 10.1016/0024-3205(79)90339-4. [DOI] [PubMed] [Google Scholar]
- 24.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Raubenheimer D, Bonduriansky R, Brooks RC. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr Biol. 2008;18:1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 26.Simpson SJ, Raubenheimer D. Macronutrient balance and lifespan. Aging (Albany NY) 2009;1:875–880. doi: 10.18632/aging.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapahi P, Vijg J. Aging--lost in translation? N Engl J Med. 2009;361:2669–2670. doi: 10.1056/NEJMcibr0909815. [DOI] [PubMed] [Google Scholar]
- 28.Xu K, Zheng X, Sehgal A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.