Abstract
Objective
In mice and in humans, treatment with the second generation antipsychotic drug olanzapine (OLZ) produces excessive weight gain, adiposity and secondary metabolic complications, including loss of glucose and insulin homeostasis. In mice consuming a high fat (HF) diet, a similar phenotype develops, which is inhibited by the analgesic acetaminophen (APAP) and by the antioxidant tetrahydroindenoindole (THII). Therefore, we examined the ability of APAP and THII to prevent metabolic changes in mice receiving OLZ.
Design and Measurement
C57BL/6J mice received either a normal diet or a high fat diet, and were administered OLZ (3 mg/kg body weight/d), alone or with APAP (35 mg/kg body weight/d) or THII (4.5 mg/kg body weight), for 10 weeks. Parameters of body composition and metabolism, including glucose and insulin homeostasis and oxidative stress, were examined.
Results
OLZ treatment doubled the HF diet-induced increases in body weight and percent body fat. These increases were partially prevented by both APAP and THII, although food consumption was constant in all groups. The THII protection was associated with an increase in whole body and mitochondrial respiration. OLZ also exacerbated, and both APAP and THII prevented, HF diet-induced loss of glucose tolerance and insulin resistance. Since increased body fat promotes insulin resistance by a pathway involving oxidative stress, we evaluated production of reactive oxygen and lipid peroxidation in white adipose tissue (WAT). HF diet caused an increase in lipid peroxidation, NADPH-dependent O2 uptake and H2O2 production, which were further exacerbated by OLZ. APAP, THII, and the NADPH oxidase inhibitor, diphenyleneiodonium chloride (DPI) each abolished oxidative stress in WAT.
Conclusions
We conclude that both APAP and THII intervene in the development of obesity and metabolic complications associated with OLZ treatment.
Keywords: Acetaminophen, Diet, Mice, Obesity, Olanzapine, Oxidative stress
1. Introduction
Obesity poses a growing global health threat, due in large part to the consumption of diets high in fat content, coupled with sedentary lifestyles (1). Another risk factor for gain in body weight and adiposity are the use of drugs for treatment of psychotic disorders. These atypical (second-generation) antipsychotic drugs, such as olanzapine (OLZ; Zyprexa™; 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]-benzodiazepine) are used to treat millions of people suffering from psychotic episodes. While highly effective for their intended use, a high percentage of patients exhibit unfortunate secondary complications associated with the excess weight and obesity, including cardiovascular disease, dyslipidemia, and the development of insulin resistance and type 2 diabetes mellitus (T2DM), cardiovascular disease and stroke (2–4). It is therefore not surprising that a recent study found that persons with schizophrenia lose about 25 years of potential life due to premature cardiovascular mortality (5).
Standard anti-diabetic drugs such as metformin and thiazolidinediones (e.g., rosiglitazone, piaglitazone), or off-label drugs such as the anti-epileptic topiramate (Topamax™), are often prescribed with OLZ to prevent the development of metabolic complications, especially hyperglycemia (6). However, these prescription medications are expensive and have their own adverse side effects that tend to limit their use.
Mice are similar to humans by increasing body weight and adiposity when consuming a high fat diet (HF diet), and thus mouse models have proven useful for the study of metabolic disorders associated with a HF diet (7;8). In mice, acetaminophen (APAP; 20 mg/kg body weight/day) ameliorated the HF diet-induced gain in body weight and fat mass, and the associated metabolic complications (9;10). Histological examination of tissues that exhibit toxicity with high-dose APAP treatment (liver, kidney, olfactory epithelium) showed no evidence of toxicity under our dosing regimen (9). Similar to APAP, tetrahydroindenoindole (THII; 4b,5,9b,10-tetrahydroindeno[1,2-b]indole), prevented metabolic complications associated with excess weight and obesity that result from a HF diet (11). Therefore, we evaluated the potential for APAP and THII to protect against metabolic toxicity induced by OLZ in mice. APAP and/or THII may offer inexpensive alternatives or adjuvant therapy with drugs commonly used to treat metabolic disorders.
2. Materials and Methods
2.1. Chemicals
OLZ was obtained from Thermo Fisher Scientific (Pittsburgh, PA). THII was synthesized as described and was 98% pure as determined by NMR and GC/MS (12). All other chemicals and reagents were from Sigma-Aldrich Chemical Company (St. Louis, MO) as the highest available grades.
2.2. Animals and treatment
All experiments involving mice were conducted in accordance with the National Institutes of Health standards for care and use of experimental animals and the University of Cincinnati Institutional Animal Care and Use Committee. Female C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME), and mouse chow was from Research Diets, Inc., New Brunswick, NJ. Mouse groups were matched by initial body weight and maintained on a 12 h light/dark cycle. Mice were allowed ad libitum either a normal diet (AIN 93M) (8% energy derived from fat; 1.29 kJ from fat/g diet) or a HF diet (AIN93M supplemented with butter fat; Product D03082706; Tso’s high fat diet with butter fat) (13) (40% energy derived from fat; 7.74 kJ from fat/g diet) as previously described (9). Mice were given either tap water or tap water supplemented with 18.75 μg OLZ/ml, 0.25 mg APAP/ml, 100 μM THII, OLZ + APAP, or OLZ + THII. Water consumption for all groups was constant at about 3.5 ml/day for a 25 g mouse. Thus, the calculated daily dosages per kg body weight were about 3 mg OLZ, 35 mg APAP, and 4.5 mg THII. Drinking water was changed twice a week. The duration of treatment was 10 weeks, during which body weights, and food and water consumption, were measured twice weekly.
2.3. Glucose and insulin homeostasis
Glucose concentration was determined with a handheld glucometer (Ascensia Contour glucometer, Bayer) (13). Samples of blood (5 μl) were applied directly to the glucose strip from 8-h fasted mice to measure fasting levels of blood glucose (FBG). After initial FBG determinations, 1.5 mg D-glucose/g body weight was administered by i.p. injection, followed by glucose determinations at 20 min intervals for 120 min. Plasma insulin was measured using the Ultra Sensitive Mouse Insulin ELISA kit (catalog # 90080, Crystal Chem, Inc., Downers Grove, IL). Absorbance (450 nm – 630 nm) was determined on a Wallac Victor Multilabel Counter 1420 (Perkin Elmer, Waltham, MA). Insulin resistance was estimated by the Homeostasis Model Assessment for Insulin Resistance (HOMA-IR), calculated as the product of fasting plasma insulin levels (μU/ml) X fasting plasma glucose concentration (mg/dl) (14;15).
2.4. Metabolic parameters and body composition
In vivo oxygen consumption and CO2 release in non-fasted mice were determined using metabolic chambers. Non-fasting conditions were used to avoid any shift in metabolism toward fat utilization that may be attributable to food withdrawal, rather than APAP or THII. Oxygen consumption was determined as gas consumed in the presence of soda lime [Ca(OH)2:H2O:K/NaOH (75:21:4); Thermo Fisher Scientific, Pittsburgh, PA], to absorb CO2. CO2 release was calculated as [gas consumed in the presence of soda lime] minus [gas consumed in the absence of soda lime] (11). The entire process of measuring oxygen consumption and CO2 release required about 10 minutes for each mouse. Body composition was assessed in live, unanesthetized mice by nuclear magnetic resonance (EchoMRI; EchoMedical Systems, Houston TX). This method provides estimates of total fat tissue, lean tissue (muscle), and water (16;17).
2.5. Oxidative stress and NADPH oxidase activity in WAT
Mice were killed by CO2 asphyxiation, and a 10% whole homogenate emulsion of peri-uterine (visceral) white adipose fat was prepared in respiratory buffer (140 mM KCl, 0.1 mM EDTA, 2.5 mM KH2PO4, 2.5 mM MgCl2 and 0.05% bovine serum albumin, in 5 mM HEPES, pH 7.4.). Aliquots of this emulsion were removed for analysis of 4-hydroxyalkenals using the chromogenic probe, methylphenylindole (Bioxytech diagnostic kit LPO 586, OxisResearch, OXIS Health Products, Inc.). The remaining emulsion was broken by centrifuging at 1000g for 10 min. The upper fat layer was removed, and the post-nuclear homogenate evaluated for 6 mM succinate- or 0.4 mM NADPH-dependent O2 uptake (polarography) and H2O2 production (chemiluminescence using luminol), as previously described (18). In some experiments, 25 μM diphenyleneiodonium chloride (DPI) was used to inhibit NADPH oxidase.
2.6. APAP and THII as direct acting antioxidants
Four different assays were used to evaluate this parameter. First, catalase-inhibited luminol chemiluminescence measures the ability of horseradish peroxidase to oxidize luminol using H2O2. The reaction mixture consisted of 0–50 μM APAP, 5 μM luminol, 2.5 U horseradish peroxidase/ml, 20 mM glucose, 5 U glucose oxidase/ml, ± 500 U catalase/ml to scavenge H2O2, in 0.1 M potassium phosphate buffer, pH 7.25 (19). Second, the hydroxyl radical-mediated hydroxylation of salicylate to a chromogen was determined in a reaction mixture consisting of 0–10 μM APAP, 2.5 mM salicylate, 10 μM H2O2, 10 μM FeSO4, in potassium phosphate buffer, pH 7.4 (20). Third, the oxidation of 2-deoxyribose to thiobarbituric acid-reacting products was assayed in a reaction mixture consisting of 0–10 μM APAP, 16 mM 2-deoxyribose, 10 μM H2O2, 10 μM FeSO4, in 40 mM Tris-HCl buffer, pH 7.4 (21). Fourth, the peroxidation of asolectin phospholipid vesicles to thiobarbituric acid-reacting products, initiated by 10 μM Fe(NH4)2(SO4)2 in the presence of 100 μM ascorbate (22).
2.7. Statistics
Statistical significance of the differences between group sample mean values was determined by a three-way analysis of variance (ANOVA); the independent variables were diet type, APAP or THII treatment, and OLZ treatment. The ANOVA was followed by the Student-Newman-Keuls test for pairwise comparison of means. Where appropriate, a P-value < 0.05 was considered significant. Statistics were performed using SPSS software (SPSS Inc., Chicago, IL).
3. Results
3.1. OLZ effect on body composition and metabolism
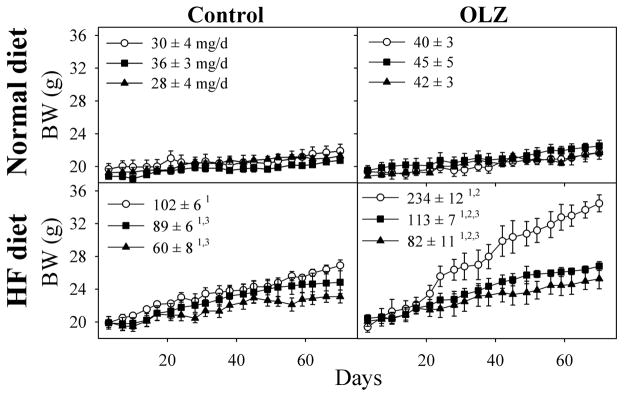
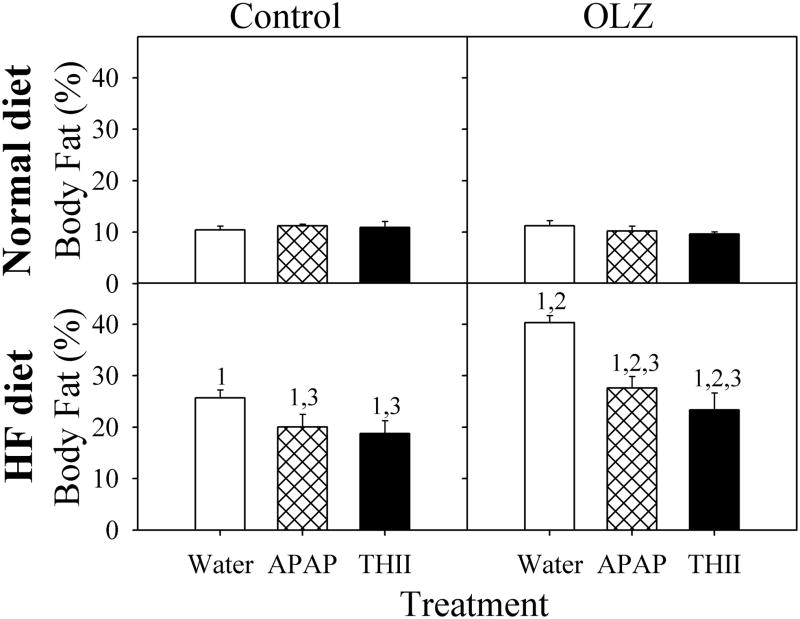
Mice fed a HF diet are a useful model for examining the changes that occur in body composition that contribute to future metabolic disorders, including insulin resistance and T2DM. Mice consuming the HF diet gained significantly greater weight that normal diet controls (Fig. 1). Although OLZ did not increase weight gain with normal diet mice, it doubled the increase in body weight in mice receiving the HF diet. APAP and THII prevented about 50% or 80%, respectively, of the weight gains associated with HF diet and with OLZ plus HF diet. Weight gain in HF diet and OLZ-treated mice was mostly a result of the significant increase in the percentage of body fat that developed during the course of treatment (Fig. 2). In mice fed the HF diet, APAP and THII treatment were both effective in reducing the accumulation of fat mass, with and without OLZ treatment.
Fig. 1. OLZ exacerbates, and APAP and THII decrease, the effects of a HF diet on body weight (BW) gain.
Mice were fed either a normal diet (top panels) or a HF diet (lower panels) for 10 weeks. Mice were provided drinking water (left panels), or water containing OLZ (right panels). Some mice were also administered APAP (filled squares) or THII (filled triangles) in the drinking water. The daily dosages were approximately 3 mg OLZ/kg body weight, 4.5 mg THII/kg body weight, and 30 mg APAP/kg body weight. Mean values ± SEM are indicated, with N=8 mice for groups depicted by open circles, and N=4 mice for all other groups. Linear regression analyses were performed for BW gain for each individual mouse, and the mean mg body weight gain/day ± SEM (shown in each panel) were used to evaluate statistical differences between groups.
1Significantly greater mean value than from corresponding normal diet mice (P<0.05).
2Significantly greater mean value than from corresponding mice not receiving OLZ (P<0.05).
3Significantly lower mean value than in corresponding mice not receiving APAP or THII (P<0.05).
Fig. 2. OLZ exacerbates, and APAP and THII decrease, the effects of a HF diet on body fat accumulation.
Mice were treated as described in the legend to Fig. 1. Data for control mice not receiving APAP or THII are shown as open bars, APAP-treated mice are shown with hatch-filled bars, and for THII-treated mice with solid-filled bars.
Mean values ± SEM (N=4 mice for APAP and THII-treatment groups; N=8 mice for other groups) are indicated
1 Significantly greater mean value than from corresponding normal diet mice (P<0.05)
2 Significantly greater mean value than from corresponding mice not receiving OLZ (P<0.05)
3 Significantly lower mean value than in corresponding mice not receiving APAP or THII (P<0.05).
While humans receiving OLZ tend to become hyperphagic, mice in our study did not consume more food by weight, although the HF diet afforded slightly more caloric intake (Fig. 3, center panels). Since there were no effects of OLZ, APAP or THII on calories consumed, and weight gain results from an imbalance in caloric intake and utilization, we examined energy homeostasis. While the increase in body weight from OLZ in mice eating the HF chow was associated with a decrease in oxygen utilization per kJ of food consumption, the prevention of OLZ-mediated body weight gain by APAP could not be explained by changes in metabolic rate (Fig. 3). In contrast, THII increased energy efficiency and decreased body weight gain per kJ of food consumed. These effects are related to the ability of THII to partially uncouple hepatic mitochondrial respiration and increase basal metabolic rate (11). THII-mediated partial uncoupling also occurs in white adipose tissue (WAT) mitochondria, where THII increased the rate of respiration using succinate, a mitochondrial-specific respiratory chain substrate (Table 1). While a HF diet decreased O2 consumption slightly, and CO2 production to a greater extent, THII increased oxygen consumption by about 35% and CO2 production by about 20% (Fig. 4). Both the HF diet and THII, but not APAP, produced a decrease in the respiratory quotient (CO2 produced/O2 consumed), which indicated a shift in catabolism toward lipid and away from carbohydrate. OLZ slightly decreased O2 consumption (Fig. 4), which could explain some of the weight gain associated with the drug.
Fig. 3. OLZ and APAP effects on parameters relating to energy utilization.
Mice were treated as described in the legend to Fig. 1. Data for control mice not receiving APAP or THII are shown as open bars, APAP-treated mice are shown with hatch-filled bars, and for THII-treated mice with solid-filled bars. Weekly BW gain per kJ weekly food consumption was averaged over the 10 week treatment for each animal. Those averages constituted a single value for each of 4 mice Mean values ± SEM (N=4 mice for APAP and THII-treatment groups; N=8 mice for other groups) are indicated
1 Significantly different mean value than from corresponding normal diet mice (P<0.05)
2 Significantly different mean value than from corresponding mice not receiving OLZ (P<0.05)
3 Significantly lower mean value than in corresponding mice not receiving APAP or THII (P<0.05).
Table 1.
APAP and THII reduce oxidative stress in white adipose tissue (WAT).
| Normal diet | |||||||
|---|---|---|---|---|---|---|---|
| − OLZ | + OLZ | ||||||
| Substrate | Parameters | Vehicle | APAP | THII | Vehicle | APAP | THII |
| - | 4-Hydroxyalkenals | 0.35 ± 0.08 | 0.12 ± 0.073 | 0.05 ± 0.033 | 0.46 ± 0.07 | 0.13 ± 0.053 | 0.04 ± 0.043 |
| Succinate | O2 uptake | 5.1 ± 1.0 | 4.3 ± 0.6 | 7.7 ± 1.13 | 5.1 ± 0.6 | 5.7 ± 0.9 | 6.7 ± 0.83 |
| Succinate | H2O2 | 0.08 ± 0.03 | 0.09 ± 0.04 | 0.04 ± 0.03 | 0.12 ± 0.05 | 0.10 ± 0.03 | 0.10 ± 0.03 |
| NADPH | O2 uptake | 14.3 ± 1.3 | 6.4 ± 0.83 | 7.0 ± 1.03 | 18.2 ± 1.6 | 7.8 ± 1.03 | 8.3 ± 1.23 |
| NADPH + DPI | O2 uptake | 7.1 ± 1.04 | 6.7 ± 0.9 | 6.7 ± 0.9 | 8.0 ± 1.34 | 8.1 ± 1.1 | 7.7 ± 1.1 |
| NOX activity | O2 uptake | 7.2 ± 0.6 | −0.3 ± 0.23 | 0.3 ± 0.23 | 10.2 ± 0.52 | −0.3 ± 0.13 | 0.6 ± 0.33 |
| NADPH | H2O2 | 1.2 ± 0.12 | 0.3 ± 0.063 | 0.1 ± 0.023 | 2.1 ± 0.292 | 0.4 ± 0.113 | 0.2 ± 0.143 |
| NADPH + DPI | H2O2 | 0.24 ± 0.054 | 0.11 ± 0.023,4 | 0.1 ± 0.023,4 | 0.24 ± 0.074 | 0.12 ± 0.084 | 0.1 ± 0.054 |
| High fat diet | |||||||
|---|---|---|---|---|---|---|---|
| − OLZ | + OLZ | ||||||
| Substrate | Parameters | Vehicle | APAP | THII | Vehicle | APAP | THII |
| - | 4-Hydroxyalkenals | 0.69 ± 0.111 | 0.14 ± 0.053 | 0.06 ± 0.043 | 1.43 ±0.191,2 | 0.12 ± 0.073 | 0.08 ± 0.063 |
| Succinate | O2 uptake | 4.9 ± 0.9 | 5.2 ± 0.6 | 7.2 ± 0.83 | 4.4 ± 0.7 | 4.7 ± 1.0 | 7.7 ± 1.23 |
| Succinate | H2O2 | 0.04 ± 0.03 | 0.04 ± 0.04 | 0.05 ± 0.04 | 0.10 ± 0.06 | 0.15 ± 0.05 | 0.05 ± 0.04 |
| NADPH | O2 uptake | 21.8 ± 4.01 | 9.7 ± 1.11,3 | 8.4 ± 0.91,3 | 33.1 ± 3.21,2 | 8.4 ± 1.23 | 9.2 ± 1.33 |
| NADPH + DPI | O2 uptake | 11.5 ± 1.94 | 9.0 ± 1.4 | 9.0 ± 1.4 | 12.6 ± 1.51,4 | 7.9 ± 1.63 | 10.4 ± 1.33 |
| NOX activity | O2 uptake | 10.3 ± 0.71 | 0.7 ± 0.33 | −0.6 ± 0.23 | 20.5 ± 0.91,2 | 0.5 ± 0.33 | −1.2 ± 0.53 |
| NADPH | H2O2 | 2.8 ± 0.231 | 0.4 ± 0.063 | 0.1 ± 0.043 | 6.2 ± 0.81,2 | 0.4 ± 0.18 | 0.1 ± 0.03 |
| NADPH + DPI | H2O2 | 0.39 ± 0.084 | 0.19±0.043,4 | 0.1±0.063,4 | 0.61 ± 0.191,4 | 0.21 ± 0.093 | 0.1 ± 0.063 |
Mice were fed either a normal or a HF diet for 10 weeks and provided drinking water, or water containing OLZ, APAP or OLZ + APAP. Parameters were determined using visceral WAT. Mean values ± SEM (N=4) are indicated. Succinate-dependent O2 uptake and H2O2 production (nmol/min/g fat) were determined under state 4 (ADP-limiting) conditions (55). Concentrations of substrates and inhibitor were 6 mM succinate, 0.4 mM NADPH and 25 μM DPI (diphenyleneiodonium chloride). Tissue concentrations of the lipid peroxidation product 4-hydroxyalkenals are shown as nmol/g WAT. NOX (NADPH oxidase) activity was calculated as the DPI-inhibited portion of the NADPH-supported O2 uptake, and expressed as nmol/min/g fat.
Effect of HF diet: Significantly different mean value than matched normal diet mice (P<0.05).
Effect of OLZ: Significantly different mean value than matched group not receiving OLZ (P<0.05).
Effect of APAP or THII: Significantly lower mean value than matched vehicle-treated mice (P<0.05).
Effect of DPI: Significantly different mean value than matched group not receiving DPI (P<0.05).
Fig. 4. Effect of treating mice with THII and OLZ on respiration.
Data for control mice not receiving APAP or THII are shown as open bars, APAP-treated mice are shown with hatch-filled bars, and for THII-treated mice with solid-filled bars. After 10 weeks, O2 consumption and CO2 production were evaluated in non-fasted mice. Shown are mean values for the parameters indicated ± SEM (N=4 mice for APAP and THII-treatment groups; N=8 mice for other groups).
1 Significantly different mean value for AUC than from corresponding normal diet mice (P<0.05).
2 Significantly different mean value for AUC than from corresponding mice not receiving OLZ (P<0.05).
3 Significantly different mean value for AUC than in corresponding mice not receiving APAP or THII (P<0.05).
3.2. OLZ, APAP and THII effects on glucose and insulin
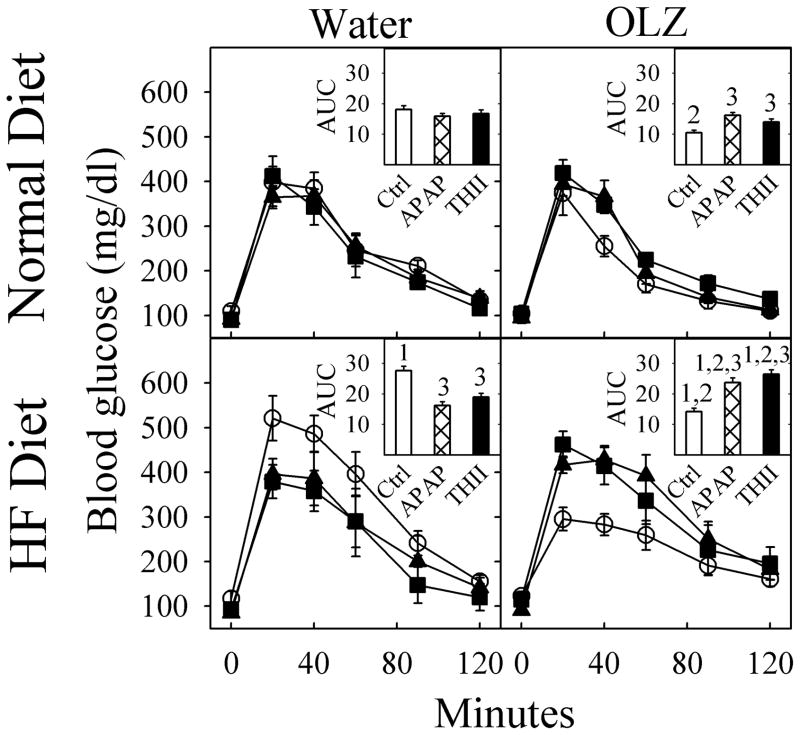
Since energy balance is closely related to glucose and insulin homeostasis, we examined the effect of OLZ on these parameters. Over the 10-week treatment schedule, fasting blood glucose levels did not change in any group (Fig. 5, zero time point). However, a glucose tolerance test revealed that HF diet reduced glucose tolerance, indicated by the greater area-under-the-curve (AUC) value, while OLZ actually increased glucose control, indicated by the lower AUC value (Fig. 5 insert histograms). Both APAP and THII completely prevented the increase in blood glucose due to the HF diet, as well as the decrease in blood glucose due to OLZ (Fig. 5).
Fig. 5. APAP prevents OLZ and HF diet induced effects on glucose tolerance.
Mice were treated as described in the legend to Fig. 1. Data for control mice not receiving APAP or THII are depicted with open circles, APAP-treated mice are shown with solid squares, and for THII-treated mice with solid-triangles. Fasted blood glucose (FBG) was determined after an 8-h fast. Then, glucose was injected i.p. and blood glucose concentration measured over the next 120 min. Mean values ± SEM (N=4 mice for APAP and THII-treatment groups; N=8 mice for other groups) are indicated.
The insert in each quadrant shows the calculated area-under-the-curve (AUC) for each line, in units of mg glucose/dl X min X 10−3. In the insert histograms, data for control mice not receiving APAP or THII are shown as open bars, APAP-treated mice are shown with hatch-filled bars, and for THII-treated mice with solid-filled bars.
1 Significantly different mean value for AUC than from corresponding normal diet mice (P<0.05).
2 Significantly different mean value for AUC than from corresponding mice not receiving OLZ (P<0.05).
3 Significantly different mean value for AUC than in corresponding mice not receiving APAP or THII (P<0.05).
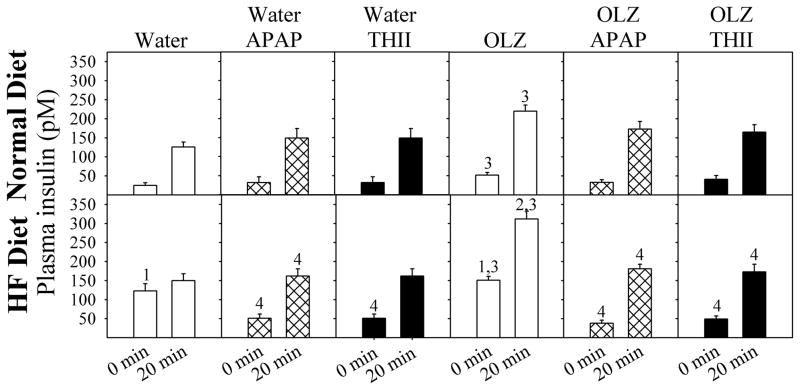
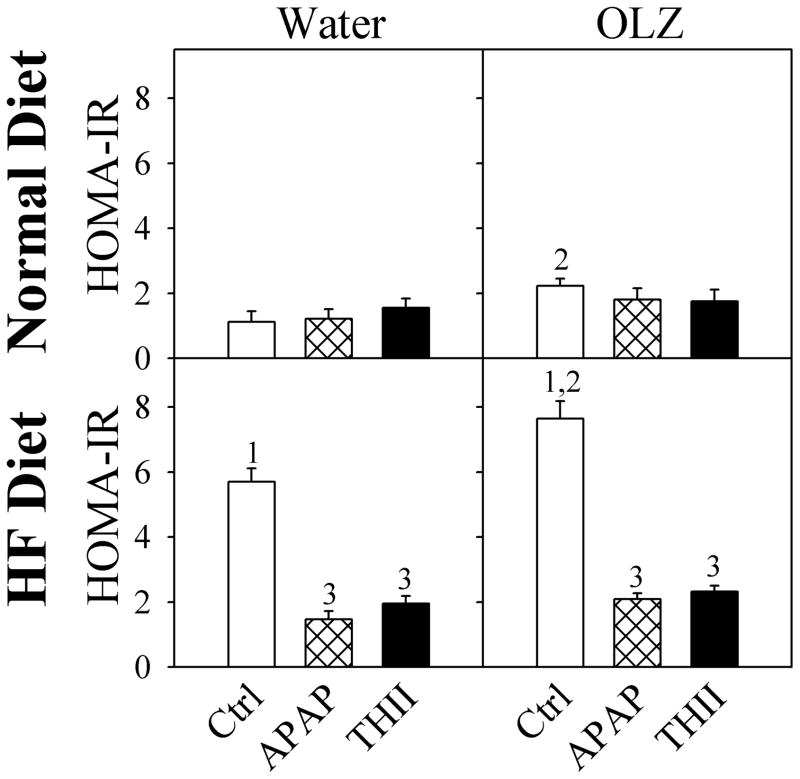
These changes in glucose tolerance can be explained by changes in insulin secretion. OLZ exacerbated the HF diet-induced increase in fasting plasma insulin levels, as well as increasing insulin following a glucose challenge (Fig. 6). Both APAP and THII restored fasting insulin levels to baseline, prevented the HF diet-induced increases in glucose-challenged insulin levels, and prevented most of the increase in glucose-challenged insulin levels resulting from OLZ treatment. These changes in glucose utilization and insulin secretion are useful for estimating insulin resistance, using the homeostasis model assessment of insulin resistance (HOMA-IR) values. OLZ exacerbated the HF diet-induced increase in HOMA-IR, and APAP and THII prevented these changes (Fig. 7).
Fig. 6. APAP prevents OLZ and HF diet induced changes in blood insulin.
Mice were treated as described in the legend to Fig. 1. Fasted plasma insulin was determined after an 8-h fast. Then, glucose was injected i.p. and plasma insulin concentration measured. Data for mice treated with APAP are shown with black filled bars. Mean values ± SEM (N=4 mice for APAP and THII-treatment groups; N=8 mice for other groups) are indicated.
1 Significantly greater mean value than from normal diet mice at 0 min (P<0.05).
2 Significantly greater mean value than from normal diet mice at 20 min (P<0.05).
3 Significantly greater mean value than from mice not receiving OLZ (P<0.05).
4 Significantly lower than corresponding mean value than in mice not receiving APAP or THII (P<0.05).
Fig. 7. APAP prevents OLZ and HF diet induced changes in insulin resistance.
Mice were treated as described in the legend to Fig. 1. Following an 8-h fast, plasma insulin (data from Fig. 5) and blood glucose (data from Fig. 4) were used to calculate insulin resistance as HOMA-IR (Homeostasis model assessment of insulin resistance). HOMA-IR units are (μU insulin/ml plasma) X (mg glucose/dl blood). Data for mice treated with APAP are shown with black filled bars. Mean values ± SEM (N=4 mice for APAP and THII-treatment groups; N=8 mice for other groups) are indicated.
1 Significantly greater mean value than from corresponding mice fed the normal diet (P<0.05).
2 Significantly greater mean value than from corresponding mice not treated with OLZ (P<0.05).
3. Significantly lower mean value than from corresponding mice not treated with APAP or THII (P<0.05).
3.3. Oxidative stress in WAT
Since oxidative stress, particularly in adipose tissue, is involve in initiating and aggravating insulin resistance, especially under obese conditions, we evaluated oxidative stress in WAT in our treatment groups. Levels of the endogenous product of lipid peroxidation, 4-hydroxyalkenals, were increased in WAT of mice receiving the HF diet, an effect exacerbated by OLZ (Table 1). The generation of 4-hydroxyalkenals was strongly inhibited by APAP and abolished by THII. We attempted to determine the source of oxidative stress in WAT. Succinate did not support mitochondrial-derived reactive oxygen production, even with the HF diet and/or OLZ, suggesting that the source for oxidative stress in WAT is non-mitochondrial.
Respiratory chain-independent production of reactive oxygen was estimated using NADPH as substrate. Since the plasma membrane-localized NADPH oxidase (NOX) is upregulated by a HF diet in WAT, and NOX is a potential source of superoxide, we examined the possibility that reactive oxygen in WAT is derived from NOX. The portion of NADPH-dependent oxygen metabolism mediated by NADPH oxidase (NOX) was evaluated using the NOX inhibitor, diphenylene iodonium chloride (DPI). The rates for NADPH-dependent O2 uptake and H2O2 production were increased in WAT by both the HF diet and by OLZ treatment (Table 1). DPI strongly inhibited both O2 consumption and H2O2 production, showing that NOX was increased in activity by OLZ and by the HF diet, and suggesting that NOX was the major source of reactive oxygen and oxidative stress produced by the HF diet and by OLZ (Table 1). APAP and THII strongly inhibited not only HF diet and OLZ-induced H2O2 production, but also DPI-inhibited NADPH-dependent O2 consumption, suggestive that both compounds not only scavenge reactive oxygen, but actually inhibit NOX activity.
3.4. APAP as a direct antioxidant
Even though APAP is phenolic in structure, there is little information regarding the ability of APAP to act directly as a reactive oxygen-quenching antioxidant. This would be important for these proposed studies, since antioxidants have been shown to diminish the development of metabolic diseases and diabetogenesis. Therefore, we tested APAP, relative to the known antioxidant THII, in four different cell-free systems. The results (Table 2) show that APAP is a good antioxidant in the three assays involving hydrophilic substrates (IC50 values of 2–10 μM), but not for the lipid peroxidation assay utilizing hydrophobic phospholipid substrate. APAP concentrations of 2–10 μM are in the range of blood concentrations maintained for several hours after an analgesic dosage of APAP (9).
Table 2.
APAP is a direct-acting antioxidant in cell-free systems.
| 50% Inhibition concentration (IC50) | ||
|---|---|---|
| Antioxidant assay | APAP (μM) | THII (nM) |
| Luminol | 10 | 395 |
| Salicylate | 3.5 | 810 |
| 2-Deoxyribose | 2 | 490 |
| Lipid peroxidation | N/A | 140 |
Four different assay systems were used to examine and compare the direct effect of APAP and the known antioxidant THII on reactive oxygen-mediated oxidation of substrates. The luminol assay consists of the glucose/glucose oxidase (H2O2)-dependent oxidation of luminol to chemiluminescent products (19). The salicylate assay shows the effect of APAP on hydroxyl-mediated hydroxylation of salicylate to a chromogen (20). The 2-deoxyribose assay shows the Fe/H2O2-dependent oxidation of 2-deoxyribose to thiobarbituric acid-reacting products (21), while the lipid peroxidation assay shows the iron/ascorbate-dependent production of thiobarbituric acid-reacting products derived from phospholipids (22). Values shown are the concentration of APAP or THII inhibiting a given assay by 50% (IC50). APAP did not inhibit lipid peroxidation by 50% at the highest concentration tested (100 μM). Each data point represents the average of 2 experiments.
4. Discussion
In this paper we show that, in C57BL/6J mice, as in humans, OLZ exacerbates the effects of a HF diet in eliciting obesity and a pre-diabetic phenotype. This mouse model is, therefore, suitable for mechanism and intervention studies regarding OLZ- and HF diet-induced obesity and associated metabolic complications. OLZ had no apparent adverse metabolic effects in mice receiving normal diet. However, when mice received a HF diet, OLZ increased body weight, body fat, hyperinsulemia and loss of glucose tolerance. All of these adverse effects were greatly ameliorated by APAP and by THII. The protection by THII from weight gain and increased adiposity was associated with an increase in basal metabolic rate. Normalization of glucose metabolism by APAP and THII were associated with inhibition of NOX and reactive oxygen scavenging, which abolished oxidative stress in WAT.
4.1. C57BL/6J mouse model for studies with OLZ, obesity and diabetes
A HF diet, even without excess caloric intake, leads to obesity and insulin resistance (23), major risk factors for the development of T2DM and hyperglycemia-derived tissue damage. Eventually, frank diabetes does develop in C57BL/6J mice fed a HF diet (24;25). A notable difference between mice and humans receiving OLZ is that humans become hyperphagic, with exaggerated cravings for carbohydrates. In our model, the mice show little change in voluntary food consumption, yet they exhibit an OLZ-dependent increase in weight and body fat. APAP and THII prevent these increases.
4.2. OLZ, HF diet, and the role of adipose tissue in diabetogenesis
White adipose is a complex, metabolically active tissue that is integrally involved in maintaining metabolic homeostasis (reviewed in (26)). In this study, feeding mice a HF diet caused an increase in body weight and adiposity, effects exacerbated by OLZ. Excessive amounts of visceral fat are associated with insulin resistance and glucose intolerance, dyslipidemia (elevated levels of plasma LDL cholesterol and triglycerides), and altered levels of hormones and cytokines involved in satiety, inflammation, oxidative stress, and intermediary metabolism. OLZ increased lipid accumulation in 3T3-L1 murine adipocytes by a mechanism involving an increase in sterol regulatory element binding protein-1 (SREBP-1) and peroxisome proliferator-activated receptorγ (PPARγ), resulting in higher activities of fatty acid synthase and triglyceride accumulation (27).
T2DM requires years to develop in humans after the onset of obesity, and results from an early progressive increase in insulin resistance, followed by a loss of β-cell mass and decreased insulin secretion. Hyperglycemia is a major causative factor for the development of diabetic microvascular diseases such as nephropathy, neuropathy, retinopathy and cardiovascular disease. In OLZ-treated dogs, pancreatic β-cell function is compromised with the development of insulin resistance, which may explain the diabetogenic effects of certain antipsychotics (28). Hyperglycemia in mice administered various antipsychotics was attributed to decreased glucose uptake from the blood (29). Although OLZ produces an increase in fasting blood esterified fatty acids in the form of cholesterol esters and triglycerides (30), an in vitro study did not support the direct antagonism of glucose uptake as the mechanism for development of OLZ-related hyperglycemia (31). A one-day clinical trial showed that the antioxidants N-acetylcysteine, vitamin E and vitamin C all improved glucose tolerance in patients with T2DM (32). This is an important consideration, since we show that APAP, and especially THII, are strong antioxidants.
4.3. APAP and THII and the role of oxidative stress and NADPH oxidase
Cellular reactive oxygen production and oxidative stress are important factors for the insulin resistance (25) and decline in β-cell mass (33) leading to the development of T2DM, and complications associated with hyperglycemic tissue damage (34). Our finding that APAP is similar to other phenolic antioxidants in its ability to scavenge reactive oxygen, as well as the report that APAP is particularly effective in scavenging peroxynitrite (35), suggests that one pathway for protection by APAP against obesity-related metabolic disease is via direct inhibition of oxidative stress by free radical scavenging. THII is an even more potent antioxidant that protects both animals and cells in culture against toxicity and carcinogenesis from a variety of chemicals (36–38). As an antioxidant, THII may act directly to reduce oxidative stress and inflammation associated with the development of insulin resistance.
Another potentially important pathway by which APAP and THII may reduce reactive oxygen production and inflammation associated with a HF diet is through NADPH oxidase. Normally, this enzyme (primarily NOX4 in WAT) is involved in important activities, such as oxygen signaling of pre-adipocyte differentiation to mature adipocytes (39). However, high NADPH oxidase activities can also generate an oxidative stress response in adipocytes, activate mitogen-activated protein (MAP) kinase pathways, decrease the availability of NO, and increase protein nitrosylation and lipid peroxidation (40). Obesity associated with a HF diet may also generate metabolic complications through oxidative stress pathways involving the increased expression of WAT NOX4 (41). Thus, our finding that APAP (10) and THII (11) abolished OLZ- and HF diet-mediated, NOX-dependent, production of toxic reactive oxygen species in WAT may be clinically relevant.
4.4. Prescription drugs currently used to treat metabolic complications resulting from OLZ
Humans who develop clinically significant metabolic disorders while receiving antipsychotics are typically prescribed secondary medication, often metformin, topiramate or rosiglitazone, to mitigate undesirable metabolic side effects. Metformin is widely used as an insulin sensitizing antihyperglycemic drug (42;43), acting by reducing hepatic gluconeogenesis (42), and by increasing peripheral glucose uptake and utilization (44). Side effects of metformin include gastrointestinal complications and lactic acidosis (45), and vitamin B12 deficiency and hyperhomocysteinemia (46). Topiramate is indicated for neurological pathologies (epilepsy, migraine) and psychiatric conditions (bipolar disorders, schizophrenia). Topiramate is also effective for weight reduction and improvement in glycemic control (47). A major side effect for topiramate is metabolic acidosis (48). Furthermore, intolerable cognitive side effects for topiramate were greater than for other antiepileptic drugs (49). Rosiglitazone and other thiazolidinediones are used clinically to improve glycemic control and dyslipidemia in patients with T2DM. These compounds are agonists of the peroxisome proliferator-activated receptor-gamma (PPARγ) (50;51). For T2DM patients, rosiglitazone reduces hyperglycemia by acting as an insulin sensitizer, and alleviates dyslipidemia by decreasing blood triglyceride levels. Adverse effects of rosiglitazone in rodents and in humans can include congestive heart failure, peripheral edema, weight gain, as well as stimulating bone marrow mesenchymal cells to differentiate into adipocytes (50;52). This latter effect diverts the differentiation of precursor cells to osteoblasts, causing loss of bone mass and skeletal abnormalities, as well as pancytopenia and anemia.
These considerations, in combination with the results from the current study, provide the rationale for future studies designed to examine the use of APAP and THII as adjunct therapeutic agents to improve the efficacy of metformin, topiramate or thiazolidinediones. This is because APAP and THII appear to act as antioxidants and through NOX-mediated oxidative stress, mechanisms distinct from other prescription medications used to treat obesity-related disorders,
4.5. OLZ does not alter metabolism in mice fed a normal diet
Although this paper focuses on the disturbances in metabolism produced by OLZ in mice fed a HF diet, it is important to note that mice fed a normal diet do not exhibit excessive gains in body weight or adiposity, and do not become glucose intolerant or insulin resistant. A possible explanation for this finding may be found in a clinical study, where weight gain in humans treated with OLZ was associated with single nucleotide polymorphisms (SNPs) in genes related to peripheral lipid homeostasis (53). In this way, the rate of adipose deposition may be proportional to the amount of dietary lipid consumed. Thus, the current study supports the recommendation that dietary counseling should be considered as a medical intervention for the unfortunate metabolic consequences of treatment with antipsychotic drugs (54).
4.6. Conclusions
Obesity and obesity-related diseases result from excessive food and fat consumption, an increasing sedentary lifestyle, and prescription medications, such as certain antipsychotic drugs, including OLZ. Major prescription drugs that are used to treat metabolic disorders associated with OLZ have their own potentially serious side effects. Mice consuming a HF diet, like humans, exhibit increased body weight and fat gain, and loss of glucose homeostasis, effects exacerbated by OLZ. In mice consuming a normal diet, OLZ had little effect on any parameters examined in this study. However, when mice were fed a HF diet, both APAP and THII mitigated the development of OLZ-mediated metabolic disorders, in part by inhibiting oxidative stress by acting as direct-acting antioxidants, as well as by inhibiting WAT NOX to ameliorate production of reactive oxygen. Preventing oxidative stress in WAT would intervene in the development of WAT inflammation, important in the etiology of T2DM. THII, but not APAP, acts as a partial mitochondrial uncoupling agent to increase the rate of basal metabolism, thus reducing body weight gain and fat deposition. Since it is recognized that the chronic usage of APAP has hepatotoxic potential, its application to treat metabolic disorders in humans would require periodic evaluation of liver function. Nevertheless, APAP and THII may have clinical application as inexpensive alternatives or supplements to drugs commonly used to treat metabolic disease associated with OLZ.
Acknowledgments
Role of funding source
This study was supported by NIEHS Center for Environmental Genetics Grant P30 ES06096 (H.G.S., M.B.G.), and NIEHS training grants T32 ES117051 and T32 ES016646 (E.L.K.). Funded also in part by NARSAD (H.G.S., M.B.G.), the world’s leading charity dedicated to mental health research, and through the University of Cincinnati Medical College Dean’s Bridge Funds Program (M.B.G.). Besides funding, there was no additional input from any funding source.
We thank Jennifer Schurdak for her technical assistance.
Abbreviations
- APAP
acetaminophen
- DPI
diphenyleneiodonium chloride
- FBG
fasting blood glucose
- HF diet
high fat diet
- NOX
NADPH oxidase
- OLZ
olanzapine, 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]-benzodiazepine
- T2DM
type 2 diabetes mellitus, non insulin dependent diabetes mellitus
- THII
4b,5,9b,10-tetrahydroindeno[1,2-b]indole
- WAT
white adipose tissue
Footnotes
Contributors: All authors have made significant scientific contributions to the planning, experimentation and/or writing of this manuscript. All authors approved the final manuscript.
Howard G. Shertzer: Conceptualized the project, secured funding, and wrote the initial manuscript;
Eric L. Kendig: Performed much of the experimentation and edited the manuscript;
Henry A. Nasrallah: contributed to the conceptualization of the study, and edited the manuscript;
Elisabet Johansson: Animal care and treatment, and implementation of experiments;
Mary Beth Genter: Helped conceive the project, secured funding, implemented experiments, edited and revised the manuscript.
References
- 1.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999 Oct 27;282(16):1530–8. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 2.Nasrallah H. A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology. 2003 Jan;28( Suppl 1):83–96. doi: 10.1016/s0306-4530(02)00114-2. [DOI] [PubMed] [Google Scholar]
- 3.Goudie AJ, Cooper GD, Halford JC. Antipsychotic-induced weight gain. Diabetes Obes Metab. 2005 Sep;7(5):478–87. doi: 10.1111/j.1463-1326.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005 Sep 22;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 5.Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008 Oct;105(1–3):175–87. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006 Dec;163(12):2072–9. doi: 10.1176/ajp.2006.163.12.2072. [DOI] [PubMed] [Google Scholar]
- 7.Coccurello R, Caprioli A, Ghirardi O, Conti R, Ciani B, Daniele S, et al. Chronic administration of olanzapine induces metabolic and food intake alterations: a mouse model of the atypical antipsychotic-associated adverse effects. Psychopharmacology (Berl) 2006 Jul;186(4):561–71. doi: 10.1007/s00213-006-0368-5. [DOI] [PubMed] [Google Scholar]
- 8.Cope MB, Nagy TR, Fernandez JR, Geary N, Casey DE, Allison DB. Antipsychotic drug-induced weight gain: development of an animal model. Int J Obes (Lond) 2005 Jun;29(6):607–14. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- 9.Shertzer HG, Schneider SN, Kendig EL, Clegg DJ, D’Alessio DA, Genter MB. Acetaminophen normalizes glucose homeostasis in mouse models for diabetes. Biochem Pharmacol. 2008 Mar 15;75(6):1402–10. doi: 10.1016/j.bcp.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Kendig EL, Schneider SN, Clegg DJ, Genter MB, Shertzer HG. Over-the-counter analgesics normalize blood glucose and body composition in mice fed a high fat diet. Biochem Pharmacol. 2008 Jul 15;76(2):216–24. doi: 10.1016/j.bcp.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Shertzer HG, Schneider SN, Kendig EL, Clegg DJ, D’Alessio DA, Johansson E, et al. Tetrahydroindenoindole inhibits the progression of diabetes in mice. Chem Biol Interact. 2009 Jan 15;177(1):71–80. doi: 10.1016/j.cbi.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shertzer HG, Sainsbury M, Graupner PR, Berger ML. Mechanisms of chemical mediated cytotoxicity and chemoprotection in isolated rat hepatocytes. Chem Biol Interact. 1991;78(2):123–41. doi: 10.1016/0009-2797(91)90009-v. [DOI] [PubMed] [Google Scholar]
- 13.Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003 Apr;133(4):1081–7. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004 Jun;27(6):1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 16.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res. 2004 Jan;12(1):150–60. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- 17.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006 Apr;55(4):978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 18.Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, et al. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic Biol Med. 2002 Nov 1;33(9):1268–78. doi: 10.1016/s0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- 19.Shertzer HG, Clay CD, Genter MB, Chames MC, Schneider SN, Oakley GG, et al. Uncoupling-mediated generation of reactive oxygen by halogenated aromatic hydrocarbons in mouse liver microsomes. Free Radic Biol Med. 2004 Mar 1;36(5):618–31. doi: 10.1016/j.freeradbiomed.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Zhu H, Bannenberg GL, Moldeus P, Shertzer HG. Oxidation pathways for the intracellular probe 2′,7′-dichlorofluorescein. Arch Toxicol. 1994;68(9):582–7. doi: 10.1007/s002040050118. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, He M, Bannenberg GL, Moldeus P, Shertzer HG. Effects of glutathione and pH on the oxidation of biomarkers of cellular oxidative stress. Arch Toxicol. 1996;70(10):628–34. doi: 10.1007/s002040050321. [DOI] [PubMed] [Google Scholar]
- 22.Shertzer HG, Berger ML, Tabor MW. Intervention in free radical mediated hepatotoxicity and lipid peroxidation by indole-3-carbinol. Biochem Pharmacol. 1988 Jan 15;37(2):333–8. doi: 10.1016/0006-2952(88)90737-x. [DOI] [PubMed] [Google Scholar]
- 23.Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004 Apr;53(4):454–7. doi: 10.1016/j.metabol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988 Sep;37(9):1163–7. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008 Aug;57(8):1071–7. doi: 10.1016/j.metabol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008 Sep;34(4 Pt 1):317–27. doi: 10.1016/j.diabet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Yang LH, Chen TM, Yu ST, Chen YH. Olanzapine induces SREBP-1-related adipogenesis in 3T3-L1 cells. Pharmacol Res. 2007 Sep;56(3):202–8. doi: 10.1016/j.phrs.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Ader M, Kim SP, Catalano KJ, Ionut V, Hucking K, Richey JM, et al. Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease: a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes. 2005 Mar;54(3):862–71. doi: 10.2337/diabetes.54.3.862. [DOI] [PubMed] [Google Scholar]
- 29.Dwyer DS, Donohoe D. Induction of hyperglycemia in mice with atypical antipsychotic drugs that inhibit glucose uptake. Pharmacol Biochem Behav. 2003 May;75(2):255–60. doi: 10.1016/s0091-3057(03)00079-0. [DOI] [PubMed] [Google Scholar]
- 30.Coccurello R, Caprioli A, Conti R, Ghirardi O, Borsini F, Carminati P, et al. Olanzapine ( LY170053, 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5] benzodiazepine), but not the novel atypical antipsychotic ST2472 (9-piperazin-1-ylpyrrolo[2,1-b][1,3]benzothiazepine), chronic administration induces weight gain, hyperphagia, and metabolic dysregulation in mice. J Pharmacol Exp Ther. 2008 Sep;326(3):905–11. doi: 10.1124/jpet.108.137240. [DOI] [PubMed] [Google Scholar]
- 31.Robinson KA, Yacoub Wasef SZ, Buse MG. At therapeutic concentrations, olanzapine does not affect basal or insulin-stimulated glucose transport in 3T3-L1 adipocytes. Prog Neuropsychopharmacol Biol Psychiatry. 2006 Jan;30(1):93–8. doi: 10.1016/j.pnpbp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Neri S, Signorelli SS, Torrisi B, Pulvirenti D, Mauceri B, Abate G, et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: a single-blind, 15-day clinical trial in patients with untreated type 2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clin Ther. 2005 Nov;27(11):1764–73. doi: 10.1016/j.clinthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004 Feb;53( Suppl 1):S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000 Apr 13;404(6779):787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 35.Schildknecht S, Daiber A, Ghisla S, Cohen RA, Bachschmid MM. Acetaminophen inhibits prostanoid synthesis by scavenging the PGHS-activator peroxynitrite. FASEB J. 2008 Jan;22(1):215–24. doi: 10.1096/fj.06-8015com. [DOI] [PubMed] [Google Scholar]
- 36.Brown DW, Graupner PR, Sainsbury M, Shertzer HG. New antioxidants incorporating indole and indoline chromophores. Tetrahedron. 1991;47:4383–408. [Google Scholar]
- 37.Shertzer HG, Sainsbury M, Reilman R, Warshawsky D. Retardation of benzo[a]pyrene-induced epidermal tumor formation by the potent antioxidant 4b,5,9b,10-tetrahydroindeno[1,2-b]indole. Cancer Lett. 1994 Nov 11;86(2):209–14. doi: 10.1016/0304-3835(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 38.Westerlund C, Ostlund-Lindqvist AM, Sainsbury M, Shertzer HG, Sjoquist PO. Characterization of novel indenoindoles. Part I. Structure-activity relationships in different model systems of lipid peroxidation. Biochem Pharmacol. 1996 May 17;51(10):1397–402. doi: 10.1016/0006-2952(96)00080-9. [DOI] [PubMed] [Google Scholar]
- 39.Mouche S, Mkaddem SB, Wang W, Katic M, Tseng YH, Carnesecchi S, et al. Reduced expression of the NADPH oxidase NOX4 is a hallmark of adipocyte differentiation. Biochim Biophys Acta. 2007 Jul;1773(7):1015–27. doi: 10.1016/j.bbamcr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007 Aug;293(2):C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 41.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004 Dec;114(12):1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995 Aug 31;333(9):550–4. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 43.Cheng JT, Huang CC, Liu IM, Tzeng TF, Chang CJ. Novel mechanism for plasma glucose-lowering action of metformin in streptozotocin-induced diabetic rats. Diabetes. 2006 Mar;55(3):819–25. doi: 10.2337/diabetes.55.03.06.db05-0934. [DOI] [PubMed] [Google Scholar]
- 44.Patane G, Piro S, Rabuazzo AM, Anello M, Vigneri R, Purrello F. Metformin restores insulin secretion altered by chronic exposure to free fatty acids or high glucose: a direct metformin effect on pancreatic beta-cells. Diabetes. 2000 May;49(5):735–40. doi: 10.2337/diabetes.49.5.735. [DOI] [PubMed] [Google Scholar]
- 45.Strack T. Metformin: a review. Drugs Today (Barc ) 2008 Apr;44(4):303–14. doi: 10.1358/dot.2008.44.4.1138124. [DOI] [PubMed] [Google Scholar]
- 46.Sahin M, Tutuncu NB, Ertugrul D, Tanaci N, Guvener ND. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J Diabetes Complications. 2007 Mar;21(2):118–23. doi: 10.1016/j.jdiacomp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Khanna V, Arumugam S, Roy S, Mittra S, Bansal VS. Topiramate and type 2 diabetes: an old wine in a new bottle. Expert Opin Ther Targets. 2008 Jan;12(1):81–90. doi: 10.1517/14728222.12.1.81. [DOI] [PubMed] [Google Scholar]
- 48.Wallace KB, Eells JT, Madeira VMC, Cortopassi G, Jones DP. Mitochondria-Mediated Cell Injury. Fundam Appl Toxicol. 1997;38:23–37. doi: 10.1006/faat.1997.2320. [DOI] [PubMed] [Google Scholar]
- 49.Arif H, Buchsbaum R, Weintraub D, Pierro J, Resor SR, Jr, Hirsch LJ. Patient-reported cognitive side effects of antiepileptic drugs: predictors and comparison of all commonly used antiepileptic drugs. Epilepsy Behav. 2009 Jan;14(1):202–9. doi: 10.1016/j.yebeh.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Werner AL, Travaglini MT. A review of rosiglitazone in type 2 diabetes mellitus. Pharmacotherapy. 2001 Sep;21(9):1082–99. doi: 10.1592/phco.21.13.1082.34615. [DOI] [PubMed] [Google Scholar]
- 51.Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochim Biophys Acta. 2007 Aug;1771(8):999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004 Jan;145(1):401–6. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruano G, Goethe JW, Caley C, Woolley S, Holford TR, Kocherla M, et al. Physiogenomic comparison of weight profiles of olanzapine- and risperidone-treated patients. Mol Psychiatry. 2007 May;12(5):474–82. doi: 10.1038/sj.mp.4001944. [DOI] [PubMed] [Google Scholar]
- 54.Aquila R, Emanuel M. Interventions for Weight Gain in Adults Treated With Novel Antipsychotics. Prim Care Companion J Clin Psychiatry. 2000 Feb;2(1):20–3. doi: 10.4088/pcc.v02n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen D, Dalton TP, Nebert DW, Shertzer HG. Glutathione redox state regulates mitochondrial reactive oxygen production. J Biol Chem. 2005 Jul 8;280(27):25305–12. doi: 10.1074/jbc.M500095200. [DOI] [PubMed] [Google Scholar]