Abstract
Reductions in alveolar oxygenation during lung hypoxia/reoxygenation (H/R) injury are common after gram-negative endotoxemia. However, the effects of H/R on endotoxin-stimulated cytokine production by alveolar macrophages are unclear and may depend upon thresholds for hypoxic oxyradical generation in situ. Here TNF-α and IL-β production were determined in rat alveolar macrophages stimulated with E. coli lipopolysaccharide (LPS, serotype O55:B5) while exposed to either normoxia for up to 24 h, to brief normocarbic hypoxia (1.5 h at an atmospheric PO2 = 10 ± 2 mm Hg), or to combined H/R. LPS-induced TNF-α and IL-β were reduced at the peak of hypoxia and by reoxygenation in LPS + H/R cells (P < 0.01) compared with normoxic controls despite no changes in reduced glutathione (GSH) or in PGE2 production. Both TNF-α mRNA and NF-κB activation were reduced by hypoxia that suppressed superoxide anion generation. Thus, dynamic reductions in the ambient PO2 of alveolar macrophages that do not deplete GSH suppress LPS-induced TNF-α expression, IL-β production, and NF-κB activation even as oxyradical production is decreased.
Keywords: Gram-negative bacterial sepsis, lung hypoxia/reoxygenation, hypoxic threshold, rat alveolar macrophages, systems biology
1. Introduction
Cellular hypoxia is a potent physiological signal with diverse biochemical, genomic, and proteomic effects on mammalian cells of the monocyte-macrophage lineage (Elbarghati et al., 2008; Hempel et al., 1994; Hockel & Vaupel, 2001; Leeper-Woodford & Detmer, 1999; Lewis et al., 1999; Ndengele et al. 2005; Nizet & Johnson, 2009; VanOtteren et al., 1995). Notable among these effects is the activation of hypoxia-inducible factors (HIFs) that subsequently accumulate in the nucleus, bind to hypoxia-responsive genetic elements, and thereafter regulate pathways influencing metabolism, vascular tone, apoptosis, angiogenesis, and inflammation (Elbarghati et al., 2008, Nizet & Johnson, 2009). Within the lungs, severe and/or prolonged hypoxia and subsequent reoxygenation (H/R) also elicit increased de novo oxyradical generation which can potently modulate signaling pathways of inflammation (Madjpour et al., 2003; Vuichard et al., 2005), culminating in the activation of the transcription factor NF-κ and the canonical cytokine genes TNF-α and IL-β (Leeper-Woodford & Detmer, 1999; Vuichard et al., 2005; Kunz et al., 2002). The interdependency of hypoxic and innate immune responses (Nizet & Johnson, 2009) is underscored by the fact that activation of NF-κB upregulates the hypoxiainducible factor 1A (HIF1A) gene (Frede et al., 2006). In vivo, pathophysiological reductions in O2 availability to lung cellular populations will vary along a continuum of temporal and spatial heterogeneity as well as severity in the distal airspaces, especially during acute lung injury (Gattinoni et al., 2006; Otto et al., 2008).
Alveolar macrophages orchestrate acute immune and inflammatory cytokine responses in the lungs to gram-negative microbial products, as typified by endotoxin from pneumonia or hematogenous pulmonary infection (Ndengele et al., 2005). Alveolar macrophages may encounter hypoxic stress as well as reoxygenation early after their exposure to endotoxin due to fluctuations in O2 availability caused by pulmonary edema, atelectasis, and other factors (Gattinoni et al., 2006; Otto et al., 2008). Despite the strategic location of alveolar macrophages at the air:blood interface, the effects on them of hypoxia/reoxygenation (H/R) are incompletely understood for several reasons. These include varying experimental levels of hypoxia, differing durations of reoxygenation (Koga et al., 1992; Leeper-Woodford & Detmer, 1999; Lewis et al., 1999; VanOtteren et al., 1995; Guida & Stewart, 1998) and even the pre-existing cellular oxidant tone (Hempel et al., 1994, 1996). Indeed, experimental hypoxia has rarely been coupled to antecedent inflammatory stimuli such as gram-negative bacterial endotoxin, or to differing doses and microbial origins of LPS (Leeper-Woodford & Detmer, 1999; Hempel et al., 1996). In certain reports, molecular cross-talk between LPS and secondary H/R sometimes increased TNF-α and IL-β production by alveolar macrophages (Leeper-Woodford & Detmer, 1999). Such results were attributed to enhanced oxyradical generation causing translocation and augmented nuclear binding of NF-κ and of activator-protein (AP)-1 to their respective cytokine promoter binding sites. Thus, human or rat alveolar macrophages stimulated by LPS before 2 – 24 h of hypoxia secreted more TNF-α and IL-β and showed increased NF-κ activation (LeeperWoodford & Detmer, 1999). Other studies found that secondary hypoxia decreased PGE2 production by LPS-stimulated alveolar macrophages, augmenting their cytokine production (Hempel et al., 1996). Brief, intermittent, or mild hypoxia may influence only some immunomodulatory responses of LPS-stimulated alveolar macrophages, without reducing O2-limited electron transport or stimulating robust inflammatory cytokine responses (Lewis et al., 1999).
In this context, we previously reported a novel suppressive effect of sequential co-stimulation by LPS + H/R on IL-β gene expression in murine macrophage RAW 264.7 cells, in which cytokine downregulation rather than upregulation occurred at the transcriptional level (Ndengele et al., 2000, 2006). These results raised the possibility that hypoxia modulates LPS-stimulated cytokine production by monocytes and macrophages bimodally, depending on the duration and severity of hypoxic exposure. It is conceivable that co-stimulation by LPS and hypoxia may affect cytokine gene expression differently among transformed cell lines vs. normal populations of monocytes and macrophages. Consequently we determined here the effects of a similar 1.5 h of secondary hypoxia, and of combined H/R, on TNF-α and IL-β production by rat alveolar macrophages stimulated by E. coli serotype O55:B5 LPS. We hypothesized that this brief hypoxic exposure would again suppress rather than enhance LPS-induced cytokine production, compared with levels in normoxic LPS controls. We simultaneously assessed intracellular levels of reduced glutathione (GSH) as an index of oxidative stress, and measured de novo production of PGE2 and superoxide anion, while determining in cell lysates the levels of NF-κ and AP-1 activation and DNA binding. We found that brief post-endotoxic hypoxia did not deplete GSH, nor did it kill alveolar macrophages or increase their PGE2 production. Even so, this hypoxia suppressed LPS-stimulated expression of canonical cytokines in these cells, likely via diminished transcription factor DNA binding and reduced oxyradical produc?gtion. These data underscore a hitherto unrecognized plasticity of alveolar macrophage responses to secondary moderate H/R in which the effects of their LPS co-stimulation are phenotypically attenuated rather than amplified.
2. Materials and Methods
2.1.Reagents
Purified LPS from E. coli serotype O55:B5, phenylmethylsulfonyl fluoride, leupeptin, aprotinin, proteinase K, and other tissue culture-grade chemicals were from Sigma (St. Louis, MO). Molecular biology-grade reagents (Sigma) were used for nuclear isolation, electrophoretic mobility shift assay (EMSA), and supershift assay procedures (Matuschak et al., 2004; Ndengele et al., 2005). The 32P labeled radiolabeled nucleotides were from Promega (Madison, WI). The PGE2 parameter assay kit was purchased from R&D Systems (Minneapolis, MN).
2.2. Cell culture and experimental protocol
Mycoplasma-free rat alveolar macrophages (ATCC# CL-2192) NR8383 Sprague-Dawley strain (Hempel et al., 1994; McCourtie et al., 2008; Ofek et al., 2001) were obtained by whole lung lavage and cultured in the presence of gerbil lung cell-conditioned medium, and cultured at 37°C to 50-70% confluence (1 × 106 cells/well) in 24-well plates in Ham's F12K medium supplemented with 10% fetal bovine serum (FBS; 37°, 24 h). Culture medium was replaced with fresh Ham's F12K medium containing FBS at 300μL/well, and E. coli LPS serotype O55:B5 at 100 ng/mL or 1.0 μg/mL was added to stimulate adherent macrophages. As negative controls, isovolumetric Ham's F12K medium without LPS was added to 4 wells for baseline t = 0 values. Normoxic control cultures consisted of one of two 24-well plates of LPS stimulated cells continuously incubated in a 21% O2/5% CO2/74% N2 atmosphere for up to 24 h. Hypoxic stress was induced in concurrent cultures by switching to 0.3% O2/95% N2/5% CO2 incubating gas mixture within a controlled atmosphere chamber (Billups-Rothenberg, Delmar, CA) for 1.5 h starting immediately after LPS addition. Previous studies (Ndengele et al., 2000) showed that use of this gas mixture for 1.5 h in the apparatus chamber maintained a true steady-state hypoxic, rather than anoxic, atmospheric and liquid media conditions (see below). Non-LPS stimulated cells treated with vehicle (Ham's F12K medium) and subjected to hypoxia or to combined H/R, exhibited no significant cytokine production vs. normoxic vehicle-treated cells. Therefore, two major LPS-stimulated experimental groups were studied in quadruplicate cultures averaged over at least three experimental runs on different days: 1) LPS normoxic controls; and 2) LPS + hypoxia with a reoxygenation phase (LPS + H/R) that began at t = 1.5 h after completing hypoxic exposure and lasted for up to 24 h. As reported previously (Ndengele et al., 2000), the severity of hypoxic exposure was confirmed by measuring PO2 of the ambient gas phases during each experimental run (Instrumentation Labs-1600 Blood Gas Analyzer, Lexington, MA). These gas-phase PO2's averaged 10 ± 2 mm Hg (mean ± SD) at peak hypoxia, equivalent to a steady-state fractional oxygen concentration (FO2) = 0.0142 at 37°C and 100% relative humidity and the ambient barometric pressure of 753 mm Hg in St. Louis, MO. Corresponding PO2 values in the media during hypoxic intervals averaged 35± 6 mm Hg (FO2 = 0.05). Gas-phase and liquid-phase PO2's were all significantly less than normoxic control cells (P < 0.001). Hypoxic cultures were re-oxygenated by rapidly switching back to 21% O2/5% CO2/74% N2. Samples from these cultures for assays described below were obtained at: baseline before adding LPS (t = 0); at the peak of hypoxia (t = 1.5 h); 1 h after commencing reoxygenation (t = 2.5 h); after 4.5 h of reoxygenation (t = 6 h); and after 22.5 h of reoxygenation (t = 24 h).
2.3. Cytokine analyses
Immunoreactive TNF-α levels in cell culture supernatants and in alveolar macrophage cell lysates were determined in duplicate by ELISA (BioSource International, Camarillo, CA) as previously described (Ndengele et al., 2000, 2005). Reactions used a monoclonal anti-TNF-α capture antibody and a biotinylated polyclonal anti-murine TNF-α antibody, developed with streptavidin-horseradish peroxidase and absorbance measured at 450 nm. For cell-associated cytokine concentrations, lysates were chilled to 0°C for 20 min, centrifuged (14,000 g for 15 min), and diluted 1:50 in phosphate-buffered 0.9% NaCl (PBS). Total protein concentrations were measured in these lysates by the bicinchoninic method (Pierce, Rockford, IL) and expressed as ng TNF-α/mg cellular protein. Supernatant and lysate concentrations of immunoreactive IL-β were similarly determined in duplicate by solid-phase ELISA (Ndengele et al., 2000) sensitive to murine IL-β over a range of 50-3,200 pg/ml using a rabbit anti-murine anti-IL-β and a goat anti-rabbit IgG linked to horseradish peroxidase, followed by analysis at 450 nm (Bio-Tek EL-311, Winooski, VT). Recombinant murine TNF-α and the primary and secondary antibodies were obtained from Genzyme (Cambridge, MA). Inter-assay coefficients of variation for TNF-αand IL-α determinations were 1.4% and 8.0%, respectively. To confirm these results for immunoreactive TNF-α, bioactive [TNF] was measured in duplicate by the L929 cell cytotoxicity assay (Loftis et al., 2000) in selected samples of the same specimens for which immunoreactive cytokine concentrations were assayed.
2.4. Lactate dehydrogenase (LDH) assay
In addition to using trypan blue dye exclusion to assess the viability of normoxic LPS and LPS + H/R cell cultures, supernatant [LDH] were determined at each time point to confirm that cell membrane structural integrity was maintained throughout hypoxia with and without reoxygenation (Ndengele et al., 2000). Supernatants were evaluated for their LDH concentration with a standard kit assay (procedure 228-UV, Promega).
2.5. Intracellular [GSH]
Cellular [GSH], a sensitive indicator of induced oxidative stress, was measured in cell lysates over the 6 h from onset of hypoxia through 4.5h of reoxygenation (t = 6 h) using a modified Tietze assay as previously described (Ndengele et al., 2000; Matuschak et al., 2004; Loftis et al., 2000). Results are expressed as μmol GSH/mg cellular protein.
2.6. Isolation of RNA
Total RNA from 1 × 107 cells (Ndengele et al., 2000, 2006) was obtained by lysing them with Tri reagent (Sigma), followed by precipitation with isopropanol and washing with 75% ethanol. Precipitates were washed with 100% ethanol, resuspended in DEPC H2O, after which RNA was quantified by densitometry. Samples were analyzed for TNF-α and IL-β by the GEArray (Superarray, Inc.) using the manufacturer's instructions. Results were normalized to contemporaneous glyceraldehyde 3-phosphate dehydrogenase (GAPDH) activity.
2.7.1 Electrophoretic Gel Mobility Shift Assay for NF-κB and AP-1
Nuclear protein concentrations were determined by the bicinchoninic acid assay (Pierce) with BSA as standard. Samples of nuclear protein (10 µg) were incubated as previously described (Loftis et al., 2000; Matuschak et al., 2004) in binding buffer (10 mM Tris, pH 7.5, 1 mM EDTA, 5 mM MgCl2, 25 mM NaCl, 5% glycerol, 5% sucrose, and 0.01% Nonidet P-40) for 10 min at 37°C. Poly(dIdC; 3 μg; Pharmacia, Piscataway, NJ) was added to samples before adding oligonucleotide probe. Double-stranded consensus oligonucleotides for NF-κ (5′-AGTTGAGGGACTTTCCCAGGC-3′) and for AP-1 (5′-CGCTTGATGAGTCAGCCGGAA-3′) (Promega) were end-labeled with [γ-32P]ATP using T4 polynucleotide kinase, and ~1 × 105 counts/min were added to reaction mixtures. For competition studies, a 100-fold excess was added of unlabeled NF-κB or AP-1 oligonucleotide or of a noncompetitive mutant oligonucleotide [5′-AGTTGAGGCGACTTTCCCAGGC-3′ (NF-κB mutant) and 5′-CGCTGATATTGGCGGAA-3′ (AP-1 mutant)] before adding radiolabeled probe. For supershift analyses of NF-κB, a 100-fold excess of antibodies cross-reactive to the rat p65 subunit of NF-κB was added to the reaction mixture. After addition of 1 μL of 10x gel loading buffer to the reaction mixture, samples were run through a 4% acrylamide-bis gel in 1x running buffer (0.025 M Tris and 0.2 M glycine) at 250 V for 3 – 4 h in a 4°C room. Gels were vacuum-dried and exposed to x-ray film (Hyperfilm, Amersham) for 24 – 72 h at −70°C before autoradiographs were developed. Individual bands were quantitated densitometrically over a linear range (Molecular Dynamics, Sunnyvale, CA), normalized to recombinant p50 (1 μL of a 0.135 gel shift units/μL solution, Promega) that was concomitantly loaded on each gel an as internal loading control, and averaged for subsequent analysis.
2.8. PGE2 Assay
PGE2 was determined in duplicate in culture supernatants and in cellular lysates by a specific immunoassay according to the manufacturer's instructions. PGE2 concentrations in supernatants are expressed as pg/mL, and in lysates as ng/mg cellular protein.
2.9. Superoxide anion generation
De novo superoxide anion production was assessed in quadruplicate at each designated time point by a luminol based assay (Calbiochem, San Diego, CA) modified to a 96-well microtiter plate format; the chemiluminescence of 5 × 106 cells was assessed according to the manufacturer's instructions. In additional experiments, the stimulatory effects of phorbol-12 myristate-13 acetate (PMA; 200 nmol) were determined on the time course of superoxide generation by LPS stimulated normoxic and hypoxic alveolar macrophages.
2.10. Statistical analyses
Data are means ± SD, with differences among results in normoxia and H/R evaluated by ANOVA or paired Student's t-test as appropriate. Significance was accepted for P values < 0.05.
3. Results
3.1. Hypoxia and combined H/R suppress LPS-induced TNF-α and IL-1β protein levels
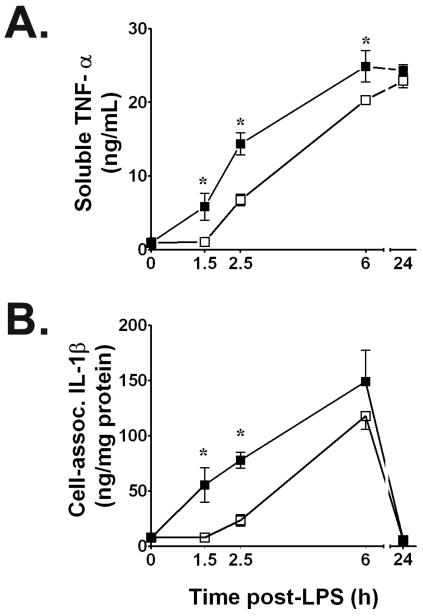
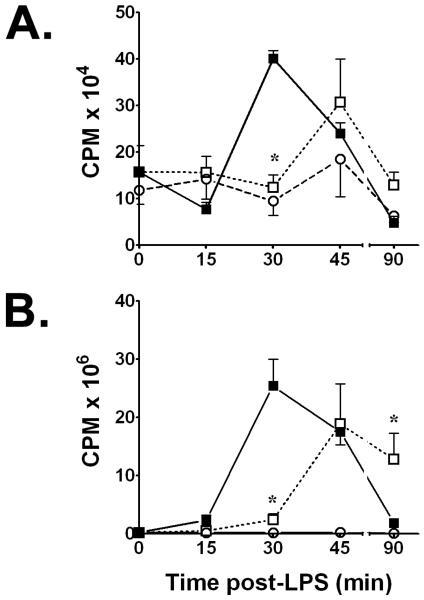
We initially assessed the time course of immunoreactive TNF-α and IL-1β release into culture supernatants from normoxic rat alveolar macrophages stimulated with 100 ng/mL of E. coli serotype O55:B5 LPS at t = 0. We compared those results with supernatant cytokine concentrations from time-matched macrophage cultures in which sequential treatments with LPS + hypoxia, or with LPS + combined H/R were performed. Compared to normoxic LPS control cells, exposing cultured macrophages to 90 min of acute hypoxia that began immediately after the start of LPS stimulation consistently reduced their secretion of TNF-α protein into culture supernatants, as typified by results at peak hypoxia (P < 0.01 vs. time-matched normoxic LPS controls at t = 1.5 h). This suppression of soluble [TNF-α] in supernatants by one 1.5 h episode of hypoxia persisted even after 60 min of reoxygenation (t = 2.5 h) and through at least t = 6 h (P < 0.05), but resolved by 24 h, when immunoreactive [TNF-α]'s between LPS normoxic controls and LPS + H/R cultures were indistinguishable (Figure 1A). Results for immunoreactive supernatant TNF-α were confirmed by the L929 cytotoxicity assay for bioactive [TNF] secreted by alveolar macrophages into culture supernatants in normoxic LPS controls vs. LPS + hypoxia or vs. LPS + combined H/R cultures. Thus, bioactive [TNF] in LPS normoxic controls at t = 1.5 h averaged 5,273 ± 1128 U/mL, vs. time-matched values of 579 ± 153 U/mL in LPS + hypoxia treated cells (P < 0.05). Likewise, LPS-induced supernatant bioactive [TNF] showed > 50% suppression by prior hypoxia during early reoxygenation at t = 2.5 h (8,686 ± 3,424 U/mL vs. 18,113 ± 6,057 U/mL in normoxic controls; P < 0.05). This disparity for bioactive [TNF] between normoxic LPS and time-matched LPS + H/R values was still evident at t = 6 h, being 65,815 ± 25,213 U/mL for normoxic LPS vs. 37,240 ± 6,480 U/mL in LPS + H/R treated cells.
Figure 1.
A. Brief hypoxia significantly suppresses soluble immunoreactive TNF-α secretion induced by E. coli serotype O55:B5 LPS (100 ng/mL) into supernatants of NR8383 rat alveolar macrophages. B. Time course of IL-1β production in E. coli LPS-stimulated cell lysates assayed by IL-1β-specific ELISA for total protein at time points shown after adding 100 ng LPS/mL. Values in both panels are means ± SD of quadruplicate determinations from at least three experiments per time point. Supernatants contained only trace amounts of IL-1β protein, while cell lysates contained only trace amounts of immunoreactive TNF-α protein. Open squares, LPS + hypoxia or LPS + combined hypoxia/reoxygenation (H/R) time-matched values. Filled squares, normoxic LPS control values; * P < 0.01 vs. time-matched normoxic LPS control value.
Cell-associated immunoreative [TNF-α] in culture lysates increased from baseline due to LPS stimulation, peaking at 16,040 ± 4,850 ng/mL by t = 1.5 h in normoxic controls. In contrast to the hypoxia-induced effects on supernatant [TNF-α], the immunoreactive [TNF-α] in cell lysates did not differ at any time point between normoxic LPS-stimulated control cultures vs. macrophages exposed to LPS + secondary hypoxia, or to LPS + combined H/R.
The effects of acute secondary hypoxia on LPS-stimulated IL-1β secretion by rat NR8383 alveolar macrophages were opposite to those for TNF-α; supernatant [IL-1β] did not differ among normoxic LPS controls, LPS + hypoxia, or LPS + H/R treated cells. Also in contrast to TNF-α, the [IL-1β] in cell lysates from LPS + hypoxia and LPS + H/R treatments were significantly lower than for time-matched normoxic LPS controls, notably at both peak hypoxia and during early reoxygenation at t = 2.5 h (P< 0.01; Figure 1B). By t = 6 h and thereafter, no intergroup differences in cell lysate IL-1β levels were present.
Throughout these studies, we found no evidence of hypoxia- or H/R-mediated cell damage as assessed by trypan blue exclusion, with > 95 % viability among cells obtained from normoxic LPS controls as well as cells cultured in LPS + hypoxia or in LPS + combined H/R. Moreover, supernatant [LDH] at each time point for normoxic LPS controls did not differ from LPS + hypoxia or LPS + combined H/R (not shown).
3.2. Hypoxia suppresses LPS-induced cytokine gene expression
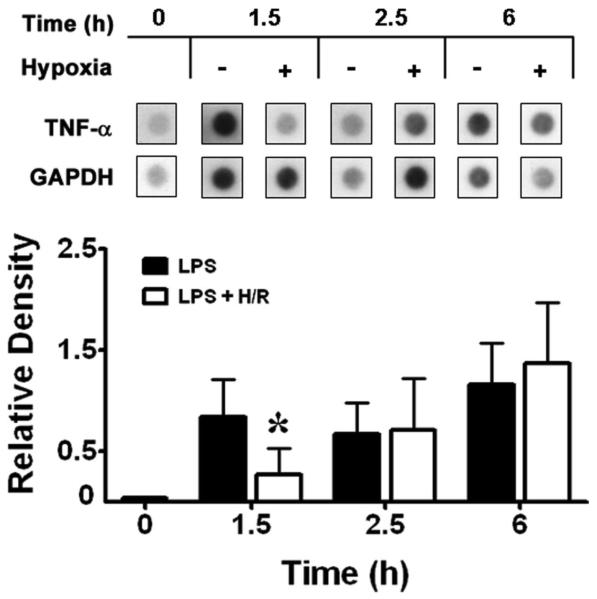
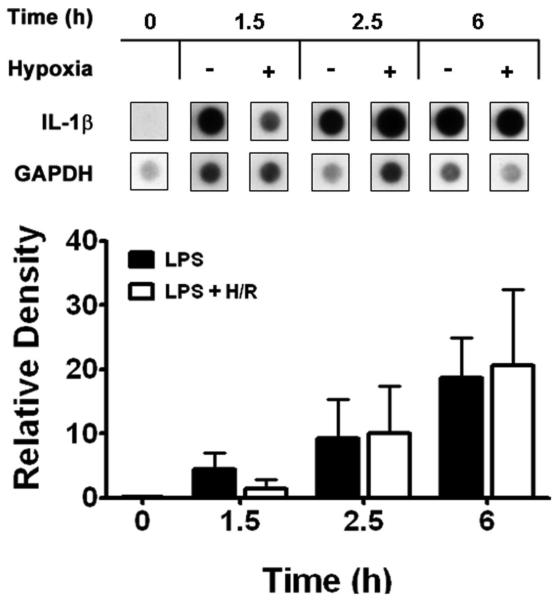
Having established that brief hypoxia suppresses LPS-induced TNF-α protein levels in the supernatants of alveolar macrophages, and [IL-1β] in cell lysates, we next determined the time course of comparative cytokine gene expression in normoxic LPS controls and in time-matched LPS + hypoxia or LPS + combined H/R. Using a highly specific assay system, we confirmed that secondary hypoxia maximally suppresses the number of LPS-induced TNF-α transcripts (Figure 2) at peak hypoxia (t = 1.5 h; P < 0.05). Subsequently, TNF-α [mRNA] was equivalent in LPS + H/R cells during the first hour of reoxygenation (at t = 2.5 h) and thereafter vs. time-matched normoxic LPS control macrophages. With respect to IL-1β [mRNA], the impressive LPS-induced increases in transcript levels in normoxic controls at t = 1.5 h appeared less in time-matched samples at peak hypoxia (Figure 3), but differences were not significant. Similarly to TNF-α, steady-state IL-1β [mRNA] did not increase during early reoxygenation at t = 2.5 h and thereafter, but remained unchanged vs. time-matched normoxic LPS control values.
Figure 2.
Top: Steady-state levels of TNF-α mRNA induced by E. coli serotype O55:B5 LPS were reduced by brief hypoxia. Total cellular RNA was loaded and run on agarose gels, blotted, and hybridized with murine 32P-labeled specific cDNA probes at the designated time points shown. +, presence; −, absence. Bottom: Densitometry of TNF-α signals was obtained from triplicate samples and normalized to those for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). * P < 0.05 vs. time-matched normoxic LPS control value
Figure 3.
Top: Steady-state levels of IL-1β mRNA induced by E. coli serotype O55:B5 LPS were reduced by brief hypoxia. Total cellular RNA was loaded and run on agarose gels, blotted, and hybridized with murine 32P-labeled specific cDNA probes at the designated time points shown. +, presence; −, absence. Bottom: A mean densitometry of IL-1β signals was obtained from triplicate samples and then normalized to a similar mean densitometry value for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
3.3. Hypoxia suppresses LPS-induced NF-κB activation and DNA binding
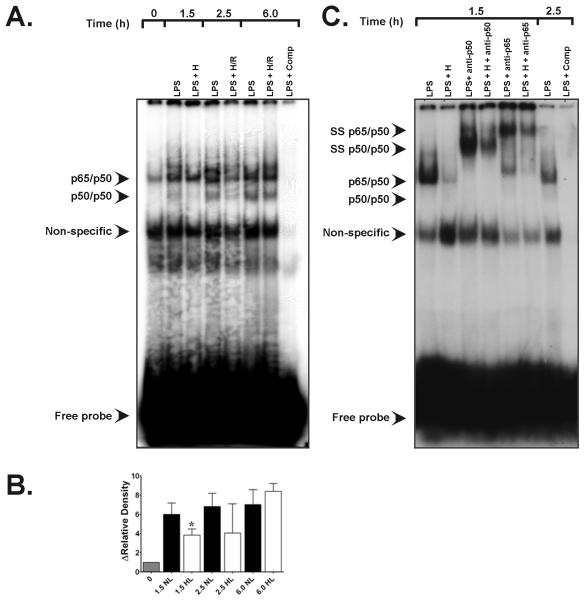
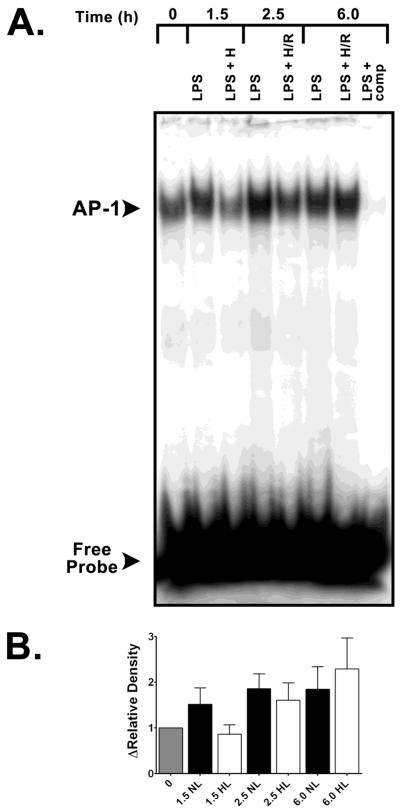
We next performed EMSA analyses for NF-κB to establish whether hypoxic suppressions of LPS-induced cytokine expression were associated with upstream reductions in the activation of early-acting redox-sensitive DNA binding proteins with multiple binding sites in the TNF-α and IL-1β promoters. As seen in Figure 4, the sequence of E. coli serotype O55:B5 LPS stimulation (1 μg/mL) followed by secondary brief hypoxia led to diminished rather than augmented activation and DNA binding of NF-κB. Supershift studies of an LPS + p65 antibody and an LPS + p50 antibody confirmed the heterodimeric nature of NF-κB in these experiments (Figure 4C.). Parallel AP-1 EMSA studies showed that neither LPS + hypoxia, nor LPS + H/R amplified the activation and binding of this redox-sensitive transcription factor vs. time-matched normoxic LPS controls. Signals for AP-1 were lower at peak hypoxia (t = 1.5 h) in LPS stimulated alveolar macrophages vs. normoxic LPS control signals but were not significant (Figure 5).
Figure 4.
A. Representative EMSA specific for NF-κB transactivation in rat alveolar macrophages showing the effects of hypoxic co-stimulation on E. coli serotype O55:B5 LPS-induced NF-κB DNA-binding activity. Bands of the NF-κB p65/p50 heterodimeric complex and of p50/p50 homodimers are depicted. Compared with normoxic LPS controls (90NL), 90 min of hypoxia (H) starting after LPS treatment at t = 0 (1.5 h) suppressed heterodimeric NF-κB activity as well as p50/p50 homodimeric signal. Lane 1, t = 0 baseline control without LPS or hypoxic co-stimulation; lane 2, signal from normoxic LPS control obtained 1.5 h after E. coli LPS stimulation (see Materials and Methods); lane 3, time-matched signal for LPS + H at peak hypoxia; lane 4, normoxic LPS control signal at 2.5 h; lane 5, time-matched signal for LPS + H/R cells at 2.5 h; lane 6, normoxic LPS control 6.0 h after initial LPS stimulation; lane 7, time-matched LPS + H/R at 6.0 h ; lane 8, (LPS + comp), competition of an LPS sample with excess unlabeled NF-κB oligonucleotide. B. Group-specific densitometric data (means ± SE) from the EMSA shown in panel A above, representing at least three separate experiments for each time point. * P < 0.05 vs. time-matched 1.5 h LPS normoxic LPS control. C. Representative EMSA depicting the results of supershift analyses for the NF-κB heterodimeric complex. Lane 1, signal from normoxic LPS control lysates obtained 1.5 h after E. coli LPS stimulation; lane 2, time-matched signal from LPS + hypoxia cell lysates at t = 1.5 h; lane 3, effects of anti-p65 antibody (Ab) treatment on normoxic LPS signal at t = 1.5 h; lane 4, effects of anti-p65 Ab treatment on LPS + 1.5 h hypoxia lysates; lane 5, effects of anti-p50 Ab treatment on normoxic LPS lysates at t = 1.5 h; lane 6, signal from t = 2.5 h normoxic LPS controls; lane 7, competition with excess unlabeled NF-κB oligonucleotide.
Figure 5.
A. Representative EMSA specific for activator protein (AP)-1 transactivation in rat alveolar cells showing the effects of hypoxic co-stimulation on E. coli serotype O55:B5 LPS-induced AP-1 DNA-binding activity. Lane 1, t = 0 baseline control without LPS or hypoxic co-stimulation; lane 2, signal from normoxic LPS control obtained 1.5 h after E. coli LPS stimulation (see Materials and Methods); lane 3, time-matched signal for LPS + H at peak hypoxia; lane 4, normoxic LPS control signal at 2.5 h; lane 5, time-matched signal for LPS + H/R cells at 2.5 h; lane 6, normoxic LPS control 6.0 h after initial LPS stimulation; lane 7, time-matched LPS + H/R at 6.0 h; lane 8, (LPS + comp), competition of an LPS sample with excess unlabeled AP-1 oligonucleotide. B. Group-specific densitometric data (means ± SE) from EMSA (A.) representing at least three separate experiments for each time point.
3.4. Hypoxia does not augment LPS-induced PGE2 production
Supernatant [PGE2] from macrophage cultures at t = 0 was < 39 pg/mL (the assay's lower limit of detection). Supernatant [PGE2] was similarly undetectable in normoxic LPS-stimulated cultures at t = 1.5 h or at 2.5 h, nor at peak hypoxia (t = 1.5 h) or early reoxygenation (t = 2.5 h) in hypoxic co-stimulated cultures. Cell lysate [PGE2] at t = 0 averaged 550 ± 472 ng/mg cell protein, and no significant changes occurred in lysates of LPS normoxic controls at t = 1.5 or 2.5 h, or in the co-stimulated cultures at peak hypoxia (706 ± 520 ng/mg cell protein) or at early reoxygenation (757 ± 621 ng/mg cell protein) (P = ns for all).
3.5. Hypoxia suppresses LPS-induced de novo oxyradical generation
We monitored the time course of de novo superoxide anion generation to assess whether hypoxic suppression of LPS-induced TNF-α and IL-1β production and NF-κB activation in rat alveolar macrophages results from altered oxyradical generation in this setting. We found that combining LPS stimulation with brief, non-lethal hypoxia significantly reduced early superoxide anion production at t = 30 min vs. time-matched normoxic LPS controls (Figure 6A).
Figure 6.
A. Time course of de novo superoxide anion generation from E. coli serotype O55:B5 LPS-stimulated rat alveolar macrophages with and without concurrent co-stimulation by hypoxia as assessed by chemiluminescence, indicating decreased de novo superoxide generation in LPS + hypoxia treated cells at t = 30 min that subsequently rebounds at t = 45 min. Open squares, LPS + hypoxia treated cells; filled squares, normoxic LPS controls; open circles, non-LPS treated hypoxic controls. * P < 0.05 vs. time-matched normoxic LPS control
B. Time course of phorbol myristate acetate (PMA) - induced chemiluminescence in LPS-stimulated rat alveolar macrophages with and without concurrent co-stimulation by hypoxia. Open squares, LPS + hypoxia treated cells; filled squares, normoxic LPS controls; open circles, non-LPS treated hypoxic controls. * P < 0.05 vs. time-matched normoxic LPS control
To determine whether LPS-treated alveolar macrophages could be stimulated by other agonists to generate superoxide anion despite prevailing hypoxia, PMA was added to cultures and samples were obtained for chemiluminescence studies. As depicted in Figure 6B, PMA did not alter the maximal hypoxic suppression of LPS-stimulated superoxide generation at t = 30 min (P < 0.05). Furthermore, cell lysate [GSH] as the principal intracellular antioxidant showed no inter-group differences through 6 h (Table 1), supporting the lack of significant de novo superoxide anion generation in LPS + hypoxia or LPS + combined H/R cells.
Table 1.
GSH Levels Are Unchanged over 6 h in E. coli LPS-Stimulated Alveolar Macrophages with and without Hypoxia and H/R
| Treatment and Time | GSH (nmol/mg cell protein) |
|---|---|
| Baseline pre-LPS, t = 0 | 3.10 ± 0.8 |
| Normoxic LPS, t = 1.5 h | 6.96 ± 3.9 |
| LPS + Hypoxia, t = 1.5 h | 6.32 ± 4.6 |
| Normoxic LPS, t = 2.5 h | 6.24 ± 2.9 |
| LPS + H/R, t = 2.5 h | 5.64 ± 3.3 |
| Normoxic LPS, t = 6 h | 8.36 ± 4.6 |
| LPS + H/R, t = 6 h | 6.14 ± 3.6 |
Abbreviations: GSH, reduced glutathione; H/R, hypoxia/reoxygenation.
Data are means ± SD of duplicate determinations from at least 3 experiments at each time point.
4. Discussion
In these studies, we have found that brief and otherwise well-tolerated hypoxia suppresses LPS-induced secretion of TNF-α and the cellular synthesis of IL-1β in rat alveolar macrophages during hypoxia as well as early after their reoxygenation (Figure 1). Using the E. coli LPS dose and the modest magnitude and duration of hypoxia employed in this model system, we also showed that TNF-α transcripts were reduced at peak hypoxia (Figure 2), and that activation and binding of NF-κB to DNA were diminished. Considering the redox-sensitivity of NF-κB (Lewis et al., 1999; Ndengele et al., 2005; Kunz et al., 2002) and the documented role of oxyradical signaling in its activation (Hockel & Vaupel, 2001), this latter result supports our finding of reduced de novo superoxide generation in LPS + hypoxia cultures (Figure 6). Reconciling our results with previous studies of enhanced cytokine production by alveolar macrophages under more stringent conditions of LPS and hypoxic co-stimulation (Koga et al., 1992; Leeper-Woodford and Detmer, 1999; Madjpour et al., 2003; Vuichard et al., 2005; Hempel et al., 1996), it appears that LPS-induced TNF-α and IL-1β production vary dynamically and bidirectionally according to the prevailing degree of oxygen limitation.
Hypoxic exposure of mammalian cells derived from monocyte-macrophage lineages elicits diverse immunological responses that are perhaps simplistically viewed as pro-inflammatory and thus predisposing to lung injury. However, significant heterogeneity exists among these reports as to compartmental origins of the mononuclear phagocytes, the severity and duration of hypoxia, variable reoxygenation paradigms, and LPS dosage as co-stimulatory signal. There has also been great variation in characterizing cellular redox status, O2-sensing mechanisms, and presumptive sources of oxyradicals such as mitochondrial NADH/NADPH. Thus, exposing human peripheral blood mononuclear phagocytes to a PO2 = 14 mm Hg for 9 h followed by normoxic reoxygenation for 6 – 9 h augmented their synthesis and release of bioactive IL-1 within ~ 3 h of reoxygenation, whereas neither TNF secretion nor IL-1 transcript numbers increased (Koga et al., 1992). Notably, the H/R-mediated enhancement of IL-1 secretion in that study was abrogated by allopurinol or oxyradical scavengers and amplified by H2O2 or the xanthine/xanthine oxidase oxyradical generating system. However, Hempel and colleagues reported that hypoxic exposure of human alveolar macrophages for 24 h (ambient PO2 < 1 mm Hg without reoxygenation) suppressed the PGH synthase-2 transcription induced by E. coli serotype 026:B6 LPS in normoxia (Hempel et al., 1994). Moreover, such co-stimulation with 1 μg/mL of LPS and hypoxia increased secretion of IL-1β by 30% above time-matched values in supernatants of normoxic LPS controls (Hempel et al., 1996). Even so, LPS + hypoxia co-stimulation did not alter intracellular IL-1β protein or IL-1β [mRNA], although both TNF-α protein and mRNA were increased in LPS + hypoxia cultures. Those authors further determined that hypoxia-mediated increases in LPS-induced TNF-α expression and IL-1β secretion were critically dependent on reduced synthesis of PGE2 as a tonic inhibitor of cytokine production, all being consequent to hypoxic downregulation of PGH-2 synthase (Hempel et al., 1996). In contrast to these findings, co-stimulation of rat alveolar macrophages with 1 μg/mL of LPS from Pseudomonas aeruginosa plus 2 h of acute hypoxia (1.8% O2 without reoxygenation) led to increased TNF bioactivity in culture supernatants as well as NF-κB activation and increased cytokine transcripts (TNF-α, IL-1α, IL-1β) in cell lysates (Leeper-Woodford & Detmer, 1999). That these LPS-induced cytokines and chemokines are differentially affected by H/R agrees with Bosco et al. (2004), who reported that 18 h of 1% O2 suppressed monocyte chemoattractant protein (MCP)-1 expression by murine ANA-1 macrophages, despite their co-stimulation by interferon-γ. Such hypoxic inhibition resulted from reduced MCP-1 gene transcription as well as reduced mRNA stability. Furthermore, MCP-1 release by hypoxic cell cultures co-stimulated with 10 μg/mL LPS (E. coli serotype O11:B4) was decreased by 68% vs. normoxic LPS controls and was subsequently reversed by 12 h of reoxygenation (Bosco et al., 2004).
In light of such conflicting results, we previously noted hypoxic suppression of E. coli serotype O55:B5 LPS-induced IL-1β production in murine RAW 264.7 macrophages in which 1.5 h of hypoxic co-stimulation immediately followed the onset of LPS challenge (Ndengele et al., 2000). In that study we found that IL-1β but not TNF-α synthesis was downregulated at both protein and mRNA levels despite no change in cellular viability or redox status as measured by [GSH]. Our nuclear runoff analysis showed that downregulation of IL-1β was transcriptional, suggesting that the consequences of dynamic hypoxic cellular exposures may differ from a pro-inflammatory phenotype observed with more severe cellular O2 deprivation. Of special note, neither xanthine oxidase inhibition nor catalase treatment affected hypoxic modulation of IL-1β expression (Ndengele et al., 2000). In parallel studies, we found that activation and DNA binding profiles of NF-κB and AP-1 were similarly reduced by hypoxia in LPS-stimulated RAW cells vs. normoxic LPS controls. Our transfection studies with reporter vectors for NF-κB and AP-1 confirmed attenuated luciferase activity in LPS + hypoxia-treated RAW cell cultures (Ndengele et al., 2006). We also noted that LPS + hypoxia suppressed early activation of hypoxia-inducible factor (HIF)-1α vs. normoxic LPS or non-LPS hypoxia controls, respectively. In that study, de novo superoxide generation was reduced by LPS + hypoxia co-stimulation; hypoxic suppression of LPS-induced transcription factor:DNA binding was not reversed by allopurinol or catalase co-treatments, by inhibiting superoxide dismutase, or by pretreating with the antioxidant N-acetylcysteine (Ndengele et al., 2006). Extending these results, Lahat and colleagues recently confirmed in RAW 264.7 cells that 24 h of hypoxic exposure following stimulation by 1 μg/mL of E. coli LPS serotype O55:B5 reduced intracellular [TNF-α] 3-fold despite unchanged TNF-α mRNA levels and lack of cell apoptosis, due to enhanced TNF-α degradation in lysosomes and inhibited secretion via secretory lysosomes (Lahat et al., 2008). In addition to post-translational modulation by hypoxia of LPS-induced TNF-α secretion, increased binding of supernatant TNF-α by soluble receptor TNF-RII was also noted (Lahat et al., 2008).
Our results here on brief hypoxic co-stimulation of LPS-challenged rat alveolar macrophages agree with our previous work in murine RAW 264.7 cells. Even so, the differential responses of LPS-induced soluble vs. cell-associated TNF-α and IL-1β to modulation by hypoxia and H/R are worth noting. Considering that LPS-induced cell-associated [TNF-α] was not reduced by hypoxic stress even though TNF-α mRNA levels were decreased at peak hypoxia, we postulate that post-translational reductions in soluble [TNF-α] (Figure 1A) resulted from protein binding to co-secreted sTNF-RII receptors. This would have occurred even as inhibition of secretory lysosomal TNF-α trafficking and secretion prevented overall reductions in cell-associated cytokine concentrations (Lahat et al., 2008). Alternatively, the membrane activity of TNF-α cleaving enzyme (TACE) may have been diminished by the brief hypoxic stress used herein. These proposed mechanisms help to explain why the suppressive effects of hypoxia on LPS-induced TNF-α extended beyond the suppressive effect on TNF-α mRNA (Figure 2). We have extended that work here to confirm that the boundary conditions of superimposed oxygen limitation of LPS-stimulated alveolar cells here (atmospheric PO2 = 10 mm Hg and a liquid medium PO2 near that of mixed venous blood = 35 mm Hg) increased neither NF-κB or AP-1 activation (Figures 4 and 5), PGE2 production, nor de novo generation of superoxide anion (Figure 6A). These results also agree with our finding in a different model of brief post-endotoxic oxygen limitation at the whole organ level using perfused rat livers, in which post-bacteremic H/R after intraportal infection with E. coli serotype O55:B5 led to downregulated TNF-α, IL-1α, and IL-1β expression without altering intrahepatic GSH redox balance (Loftis et al., 2000; Matuschak et al., 2004). Our finding here of suppressed LPS-induced TNF-α production in alveolar macrophages at the protein and mRNA levels by brief, well-tolerated hypoxic exposure is especially supported by a correlative in vivo rat model of acute brief hypoxia following co-stimulation by hematogenous infection with 5 × 109 live E. coli serotype O55:B5 (Matuschak et al., 2005). Specifically, a 1.6-fold decreased expression was found for the TNF-α gene in the lungs of E. coli + hypoxia animals vs. normoxic E. coli controls by microarray analysis of 31,042 mRNA transcripts (Affymetrix Gene Chip rat genome 230 2.0 array) and subsequent Northern blot hybridization.
To what mechanism(s) can suppressed LPS-induced cytokine gene expression by brief, well-tolerated hypoxia in alveolar macrophages be ascribed that culminates in a modest hypoxia-dependent anti-inflammatory phenotype? Under the experimental conditions in this report, LPS stimulation had a “preconditioning” effect on thiol-rich, GSH-replete cells to subsequent brief hypoxic exposure. That effect led to reductions in spontaneous and PMA-induced oxyradical generation, NF-κB transactivation, TNF-α cytokine expression, and IL-1β production in cell lysates. Considerable evidence identifies oxyradicals as key signal transduction messengers in the redox regulation of LPS-induced cytokine expression and NF-κB transactivation in a variety of model systems (Hockel and Vaupel, 2001; Lewis et al. 1999, Hwang et al. 2003; Weir et al., 2005). However, the concomitant cellular redox status that presumably calibrates the threshold for such responses has not been consistently defined, while the PO2's in the liquid media phase remain poorly characterized.
At present, our data and those of others are most consistent with the concept of a bimodal relationship between the duration of hypoxia and severity in LPS-challenged cells on the one hand, and cellular redox status on the other. Thus, brief limitations of oxygen availability that do not perturb the threshold intracellular GSH:GSSG redox equilibrium appear to attenuate cytokine production by a non-PGE2-mediated mechanism. That attenuation occurs in conjunction with depressed superoxide anion generation that is then relatively insensitive to co-stimulation by the protein kinase C activator, PMA (Figure 6B). Presumably under a condition of more severe hypoxia, or when more oxyradicals are produced by higher doses of LPS, there may develop an altered intracellular thiol balance that augments redox-sensitive transactivation and cytokine expression. In support of this thesis, when we extended the duration of constant-flow hypoxia to 2 h in E. coli- infected, fasting perfused rat livers, indeed we found augmented rather than suppressed TNF-α production (Matuschak, personal observation). Moreover, novel mechanisms of tolerance to hypoxia have recently been described. These include selective inactivation of aminoacyl-tRNA synthetases (Anderson et al., 2009) and carbon monoxide-induced stabilization of HIF-1α, which in turn augment the expression of the anti-inflammatory cytokine transforming growth factor-β (Chin et al., 2007; Yu et al., 1998). Collectively these observations offer insight into the unexpected complexity of LPS-stimulated cytokine production at one end of the biological spectrum of reduced cellular oxygen availability. Our studies were not designed to evaluate these or other possible explanations, which will require further investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by NIH grant #GM-43153 (G.M.M.)
References
- Anderson LL, Mao X, Scott BA, Crowder CM. Survival from hypoxia in C. elegans by inactivation of aminoacyl-tRNA synthetases. Science. 2009;323:630–633. doi: 10.1126/science.1166175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco MC, Puppo M, Pastorino S, Mi Z, Mellillo G, Massazza S, Rapisarda A, Varesio L. Hypoxia selectively inhibits monocyte chemoattractant protein-1 production by macrophages. J. Immunol. 2004;172:1681–1690. doi: 10.4049/jimmunol.172.3.1681. [DOI] [PubMed] [Google Scholar]
- Chin BY, Jiang G, Wegiel B, Wang HJ, MacDonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Otterbein LE. Hypoxia inducible factor 1-α stabilization by carbon monoxide results in cytoprotective preconditioning. Proc. Natl. Acad. Sci. USA. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbarghati L, Murdoch C, Lewis CE. Effects of hypoxia on transcription factor expression in human monocytes and macrophages. Immunobiology. 2008;213:899–908. doi: 10.1016/j.imbio.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Frede S, Stockman C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-κB. Biochem. J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with acute respiratory distress syndrome. New Engl. J. Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- Guida E, Stewart A. Influence of hypoxia and glucose deprivation on TNF-α and GM-CSF in human cultured monocytes. Cell. Physiol. Biochem. 1998;8:75–87. doi: 10.1159/000016272. [DOI] [PubMed] [Google Scholar]
- Hempel SL, Monick MM, He B, Yano T, Hunninghake GW. Synthesis of PGH synthyase-2 by human alveolar macrophages in response to lipopolysaccharide is inhibited by decreased cell oxidant tone. J. Biol. Chem. 1994;52:32979–32984. [PubMed] [Google Scholar]
- Hempel SL, Monnick MM, Hunninghake GW. Effect of hypoxia on release of IL-1 and TNF by human alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1996;14:170–176. doi: 10.1165/ajrcmb.14.2.8630267. [DOI] [PubMed] [Google Scholar]
- Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- Hwang TL, Wu CC, Guh JH, Teng CM. Potentiation of TNF-α expression by YC-1 in alveolar macrophages through a cyclic GMP-independent pathway. Biochem. Pharmacol. 2003;66:149–156. doi: 10.1016/s0006-2952(03)00202-8. [DOI] [PubMed] [Google Scholar]
- Koga S, Ogawa S, Kuwabara K, Brett J, Leavy JA, Ryan J, Koga Y, Plocinski J, Benjamin W, Burns DK, Stern D. Synthesis and release of interleukin 1 by reoxygenated human mononuclear phagocytes. J. Clin. Invest. 1992;90:1007–1015. doi: 10.1172/JCI115913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M, Bloss G, Gillitzer R, Gross G, Goebeler M, Rapp UR, Ludwig S. Hypoxia/reoxygenation induction of MCP-1 in melanoma cells: involvement of NF-κB, stimulatory protein-1 transcription factors and mitogen-activated protein kinase pathways. Biochem. J. 2002;366:299–306. doi: 10.1042/BJ20011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat N, Rahat MA, Kinarty A, Weiss-Cerem L, Pinchevski S, Bitterman H. Hypoxia enhances lysosomal TNF-α degradation in mouse peritoneal macrophages. Am. J. Physiol. 2008;295:C2–C12. doi: 10.1152/ajpcell.00572.2007. [DOI] [PubMed] [Google Scholar]
- Leeper-Woodford SK, Detmer K. Acute hypoxia increases alveolar macrophage TNF activity and alters NF-κB expression. Am. J. Physiol. 1999;276:L909–L916. doi: 10.1152/ajplung.1999.276.6.L909. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Lee JA, Underwood CE, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J. Leukocyte Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- Loftis L, Johanns CA, Lechner AJ, Matuschak GM. Brief hypoxic stress suppresses postbacteremic NF-κB activation and TNF-α bioactivity in perfused liver. Am. J. Physiol. 2000;279:R99–R108. doi: 10.1152/ajpregu.2000.279.1.R99. [DOI] [PubMed] [Google Scholar]
- Madjpour C, Jewell UR, Kneller S, Ziegler U, Schwendener R, Booy C, Klausli L, Pasch T, Schimmer RC, Beck-Schimmer B. Decreased alveolar oxygen induces lung inflammation. Am. J. Physiol. 2003;284:L360–L367. doi: 10.1152/ajplung.00158.2002. [DOI] [PubMed] [Google Scholar]
- Matuschak GM, Lechner AJ, Chen Z, Todi S, Doyle TM, Loftis LL. Hypoxic suppression of E. coli-induced NF-κB and AP-1 transactivation by oxyradical signaling. Am. J. Physiol. 2004;287:R437–R445. doi: 10.1152/ajpregu.00404.2003. [DOI] [PubMed] [Google Scholar]
- Matuschak GM, Doyle TM, Chen Z, Lechner AJ. Microarray analysis of early postbacteremic liver and lung gene expression following hypoxic stress. Crit. Care Med. 2005;32:A489. [Google Scholar]
- McCourtie AS, Farivar AS, Woolley SM, Merry HE, Wolf PS, Mackinnon-Patterson B, Keech JC, Fitzsullivan E, Mulligan MS. Alveolar macrophage secretory products effect type 2 pneumocytes undergoing hypoxia/reoxygenation. Ann. Thorac. Surg. 2008;86:1774–1779. doi: 10.1016/j.athoracsur.2008.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndengele MM, Bellone CJ, Lechner AJ, Matuschak GM. Brief hypoxia differentially regulates LPS-induced IL-1β and TNF-α gene transcription in RAW 264.7 cells. Am. J. Physiol. 2000;278:L1289–L1296. doi: 10.1152/ajplung.2000.278.6.L1289. [DOI] [PubMed] [Google Scholar]
- Ndengele MM, Muscoli C, Wang ZQ, Doyle TM, Matuschak GM, Salvemini D. Superoxide potentiates NF-κB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock. 2005;23:186–193. doi: 10.1097/01.shk.0000144130.36771.d6. [DOI] [PubMed] [Google Scholar]
- Ndengele MM, Doyle TM, Salvemini D, Lechner AJ, Matuschak GM. Superimposed brief hypoxia modulates endotoxin-induced activation of p38 mitogen-activated protein kinase and AP-1 DNA binding in RAW 264.7 macrophages. Am. J. Respir. Crit. Care Med. 2006;174:A136. [Google Scholar]
- Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nature Rev. Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I, Mesika A, Kalina M, Keisari Y, Podschun R, Sahly H, Chang D, McGregor D, Crouch E. Surfactant protein D enhances phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect. Immun. 2001;69:24–33. doi: 10.1128/IAI.69.1.24-33.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto CM, Markstaller K, Kajikawa O, Karmrodt J, Syring RS, Pfeiffer B, Good VP, Frevert CW, Baumgardner JE. Spatial and temporal heterogeneity of ventilator-associated lung injury after surfactant depletion. J. Appl. Physiol. 2008;104:1485–1494. doi: 10.1152/japplphysiol.01089.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanOtteren GM, Standiford TJ, Kunkel SL, Danforth JM, Strieter RM. Alterations of ambient oxygen tension modulate the expression of tumor necrosis factor and MIP-1α from murine alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1995;13:399–409. doi: 10.1165/ajrcmb.13.4.7546769. [DOI] [PubMed] [Google Scholar]
- Vuichard D, Ganter MT, Schimmer RC, Suter D, Booy C, Reyes L, Pasch T, Beck-Schimmer B. Hypoxia aggravates lipopolysaccharide-induced lung injury. Clin. Exp. Immunol. 2005;141:248–260. doi: 10.1111/j.1365-2249.2005.02835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. New Engl. J. Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am. J. Physiol. 1998;275:L818–L826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]