Abstract
This study tested the ability of MR Relaxography (MRR) to discriminate intra- (Nai+) and extracellular (Nae+) 23Na+ signals using their longitudinal relaxation time constant (T1) values. Na+-loaded yeast cell (Saccharomyces cerevisiae) suspensions were investigated. Two types of compartmental 23Na+ T1 differences were examined: a selective Nae+ T1 decrease induced by an extracellular relaxation reagent (RRe), GdDOTP5−; and, an intrinsic T1 difference. Parallel studies using the established method of 23Na MRS with an extracellular shift reagent (SRe), TmDOTP5−, were used to validate the MRR measurements. With 12.8 mM RRe, the 23Nae+ T1 was 2.4 ms and the 23Nai+ T1 was 9.5 ms (9.4T, 24°C). The Na+ amounts and spontaneous efflux rate constants were found to be identical within experimental error whether measured by MRR/RRe or by MRS/SRe. Without RRe, the Na+-loaded yeast cell suspension 23Na MR signal exhibited two T1 values, 9.1 (± 0.3) ms and 32.7 (± 2.3) ms, assigned to 23Nai+ and 23Nae+, respectively. The Nai+ content measured was lower, 0.88 (± 0.06); while Nae+ was higher, 1.43 (± 0.12) compared with MRS/SRe measures on the same samples. However, the measured efflux rate constant was identical. T1 MRR potentially may be used for Nai+ determination in vivo and Na+ flux measurements; with RRe for animal studies and without RRe for humans.
Keywords: 23Na MR, T1 relaxography, intracellular Na+, relaxation reagent
Introduction
23Na MR is the only technique that can potentially provide minimally invasive compartmental Na+ content measurement in intact organisms. Accurate in vivo intracellular Na+ concentration ([Nai+]) measurement could provide a biomarker of cellular viability [1]. In the heart, it is known that [Nai+] increases in ischemia [2; 3; 4] and, there is evidence that [Nai+] increases in both hypertrophy and in failure [5]. This is important because [Nai+] can have profound effects on cardiac function and arrhythmogenesis. In the brain, stroke increases total tissue [Na+] from values of less than 45 mM to approximately 70 mM [6]. Total tumor Na+ concentration was increased to 1.5 times that in contra-lateral normal brain [7]. A study by Sharma et al. of breast tumors employed an inversion recovery (IR) 23Na MRI sequence to null or minimize signals with the larger T1 values, which were presumed to be mostly 23Nae+[8]. The resulting image intensity therefore arose from magnetization with smaller T1 values. The increased tumor 23Na MRI intensity was thus attributed to increased 23Nai+.
The 23Nai+ and 23Nae+ resonances exhibit the same resonance frequency (ν) in biological samples. Some time ago, we and others introduced membrane impermeable extracellular shift reagents (SRes) to selectively change (“shift”) the Nae+ ν value [9; 10; 11; 12]. These SRes were originally applied to study [Nai+] in cell suspensions and perfused hearts [13; 14; 15]. There are two SRe, DyTTHA3− and TmDOTP5−, that have proven suitable for use in living animals[16; 17; 18; 19]. The use of 23Na MRS with SRe currently provides the best [Nai+] measurements in isolated organs or intact animals. The prospects for the use of these SRe in human studies are quite dim, however, because of the high doses required. This high [SRe] requirement will probably remain even if new SRes are developed.
The goal of this study was to develop and test MR methods for the measurement of Nai+ that do not require SRe. It is possible to discriminate cation resonances using their relaxation properties. This has been demonstrated for the longitudinal relaxation rate constant (R1 = T1−1) values of 39K+ resonances in perfused salivary gland [20] and in the isolated heart [21]. In those studies, the intrinsic T1 values of the Ki+ and Ke+ resonances (8 and 68 ms, respectively; B0 = 8.5T) were sufficiently different that inversion recovery (IR) pulse sequences could be used to selectively null the Ke+ resonances. Bi-exponential T2 relaxation was selectively detected using a double quantum filter pulse sequence and this edited for the dog red blood cell 23Nai+ resonance [22]. These methods require that compartmental 23Na Rx values (x = 1, 2) be sufficiently different. Alternatively, a (usually larger) Rx value difference can be induced with a relaxation reagent (RR). For example, Degani and Elgavish used GdEDTA− as an extravesicular RR to measure equilibrium Na+ and Li+ transport kinetics across phosphatidylcholine vesicle membranes catalyzed by the ionophore monensin [23]. An IR pulse sequence was used to null the 23Nae+ or 7Lie+ signal, which had a smaller T1 value and observe mostly 23Nai+ or 7Lii+, with the larger T1.
The present study used 23Na longitudinal (T1) MR Relaxography (MRR) to discriminate and measure Nai+ and Nae+. T1 relaxation decay data were subjected to an Inverse Laplace transform (ILT) (see Appendix 1 for a list of Abbreviation used) to produce a relaxogram, the apparent relaxation time constant (T1′) distribution. The relaxogram can have multiple peaks, depending on the number of spin populations. Relaxation decay data were also analyzed using appropriate exponential functions. The 23Na MRR measurements were validated with 23Na MRS in the presence of SRe. Na+-loaded yeast cell suspensions, which exhibit spontaneous Na+ efflux [13; 24], were used as model systems. Two methods for T1 relaxographic 23Nai+ and 23Nae+ discrimination were investigated. The first employed GdDOTP5− as an extracellular RR (RRe) to selectively increase 23Nae+ R1. The second used intrinsic 23Nai+ and 23Nae+ R1 differences.
Results
Measurement of Na+-loaded yeast suspension Na+ content and Nai+ efflux by 23Na MRS with SRe
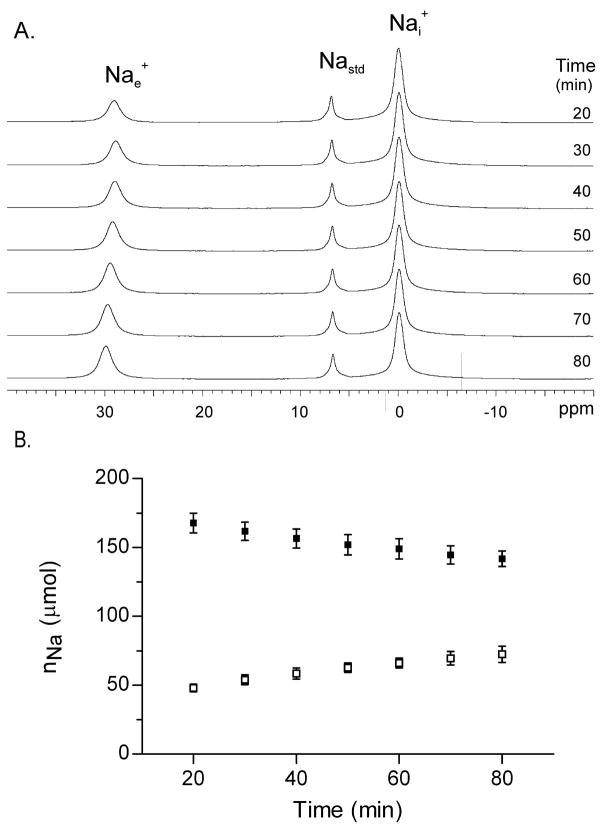
A stacked plot of 23Na MR spectra from a Na+-loaded yeast suspension with 12.8 mM TmDOTPe5− is shown in Figure 1A. Inspection of the shifted 23Nae+ peak reveals an increase in the area and frequency with time after re-suspension. The increase in 23Nae+ peak area results from the slow Nai+ efflux; the increase in 23Nae+ peak frequency may result from an uptake of divalent cations during the Nai+ efflux. The nNai and nNae amounts were derived from the spectral peak areas (Figure 1B). The nNai decreased and the nNae increased with time.
Figure 1. Na+ efflux from Na+ loaded yeast 23Na MR spectroscopy.
Panel A: Stacked 23Na MR spectra obtained from a suspension of Na+ loaded yeast in which the SRe, [TmDOTPe5−] was 12.8 mM. The 3 23Na resonances are identified as follows (left to right) (1), the extracellular Na (Nae+); (2), the Na standard (Nastd), which contains TmDOTP5−; and, (3), the intracellular Na (Nai+).
Panel B: The Na+ contents, nNai (filled square) and nNae (open square), of suspensions of Na+ loaded yeast (n=4) measured by MRS with SRe. Mean ± (SD) values are plotted.
Measurement of Na+-loaded yeast suspension Na+ contents and Nai+ efflux by 23Na T1 MRR with RRe
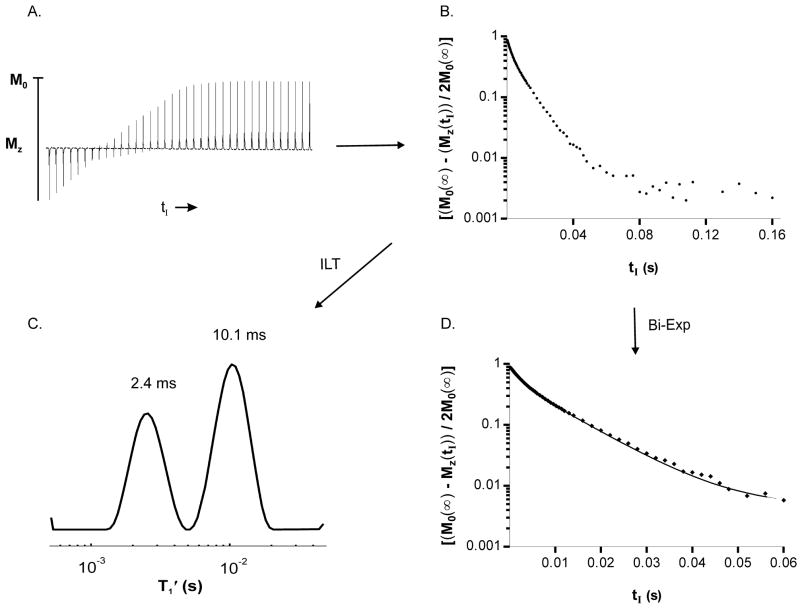
Figure 2 illustrates the T1 MRR data acquisition and processing used in this study (Methods). The IR spectral peak areas (panel A) obtained from a yeast sample 74 minutes after suspension of the yeast in medium with 12.8 mM GdDOTPe5− recover with tI. The IR peak area measurements plotted as the tI -dependence of [(MZ(∞) − MZ(tI))/(2MZ(∞))] are shown in panel B. The ILT of this decay is the 23Na T1′ relaxogram (panel C), which exhibits two distinct T1′ peaks, centered at 2.4 and 10.1 ms. With good quality relaxography the peak positions (T1′) and areas, (ai and ae), are reasonably reliable. Because the peak widths are subject to ILT regularization effects they are not very physically meaningful. The peak widths do, however, absorb some of the errors of the ILT process [25; 26; 27; 28]. Artifactual relaxographic peaks, which occasionally are present at very small or very large T1′ values, can distort ai and ae values. The tI decay may also be fitted with an empirical bi-exponential (Bi-exp) function, (panel D) to determine ai and ae. The T′1e and T′1i values obtained from the relaxogram were entered into the Bi-exp expression (Methods) and fixed; only ai, ae, and noise constant C values were varied. Two linear segments are evident at tI values less than 0.02 s (panel D). The small contribution at greater tI values is almost exclusively from the noise term C. The ai and ae values obtained from such bi-exponential fittings more closely matched MRS/SRe measured ai and ae values and are used in this report. The relaxogram aids in visualization.
Figure 2. 23Na MRR data acquisition and processing.
Panel A:f A plot of 37 23Na IR spectra (of a total of 74 tI values, 0.2 ms to 300 ms) acquired from a suspension of Na+ loaded yeast containing 12.8 mM [GdDOTPe5−]; Panel B: A semi log plot of [(MZ(∞) − MZ(tI))/2MZ(∞)] derived from integration of the 23Na yeast IR data; only the tI values to 160 ms are shown. Panel C: The ILT of the [(MZ(∞) − MZ(tI))/2MZ(∞)] decay yields the 23Na T1 relaxogram, i.e. the T1′ distribution with the Nae T1′ peak centered at 2.4 ms, ae/(ai + ae) = 0.337 and the Nai T1′ peak centered at 10.1 ms, ai/(ai + ae) = 0.663. Panel D: A semi log plot of [(MZ(∞) − MZ(tI))/2MZ(∞)] with a fit of a biexponential equation (Bi-Exp), aeexp(−tI/T′1e) + aiexp(−tI/T′1i) + C using the T1′ values obtained from the relaxogram: ae/(ai + ae) = 0.353 and ai/(ai + ae) = 0.647; all tI data were fit, only the tI values to 60 ms are shown.
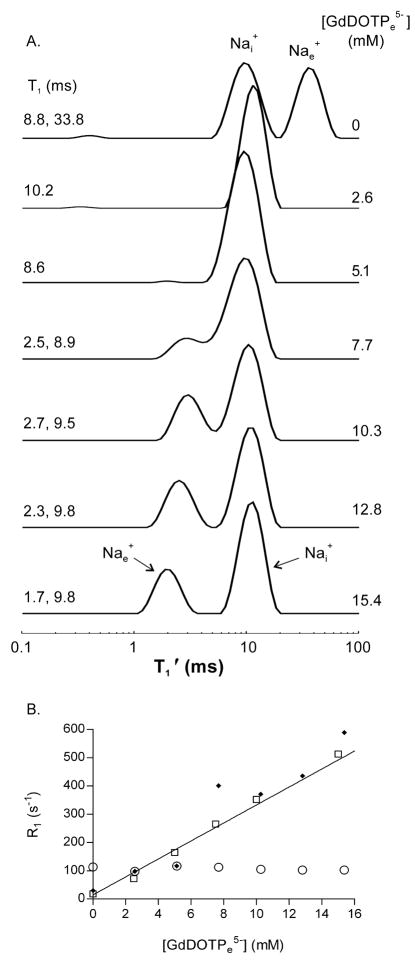
Figure 3A displays a stacked plot of relaxograms from a 50% wt/vol suspension of D273-10B Na+-loaded yeast with increasing [GdDOTPe5−]. The [RRe] values increase from top to bottom, and are given at the right sides of the relaxograms. One peak remains at ~10 ms in all relaxograms. Because the LnDOTP5− complexes do not enter cells [15; 29], this peak is assigned to 23Nai+. The other peak moves continually to smaller T1′ values with increasing [RRe] passing through the 10 ms peak when [RRe] is between 2 and 6 mM. By 7.7 mM [RRe] two relaxographic peaks begin to re-emerge. With greater [RRe] the resolution of these improves and the peak with the smaller T1′ (eventually less than 3 ms) value is assigned to Nae+. Whenever there are two distinct peaks in these relaxograms, the equilibrium transcytolemmal Na+ exchange system is in the slow-exchange-regime (SXR), the slow-exchange-limit (SXL), or the no-exchange-limit (NXL) condition. If one peak is present the system may be in the fast-exchange-regime (FXR) or the fast-exchange-limit (FXL) condition [30].
Figure 3. 23Na T1 MRR titration with [RRe].
Panel A: A stacked plot of relaxograms obtained from Na+ loaded yeast suspended in medium with increasing concentrations of the extracellular relaxation reagent (RRe) for Na+, GdDOTP5−. The [GdDOTPe5−] are shown on the right; The T1 values obtained for the peaks are shown on the left.
Panel B: The R1 (= T1−1) parameters obtained from relaxographic peaks (Fig 2A) as a function of the [GdDOTPe5−] are shown. The (open circle) reports the R1 of the peak at T1 ~ 9 – 10 ms, which is assigned to Nai+; the (filled circles) report the R1 of the peak assigned to Nae+. The R1 for a yeast cell free minimal medium solution containing 12.6 mM NaCl (open square) is also shown. The relaxivity of GdDOTP for 12.6 mM Na+ in cell free medium (Nar1CF ) obtained from the slope of the line fit to the R1 was 31.8 (± 1.2) s−1 mM−1, R2 = 0.99. The Nar1CF was 24.9 (± 0.52) s−1 mM−1 in 50mM Na+ medium and, 17.9 (± 0.2) s−1 mM−1 for 150mM Na+ medium (not shown).
Figure 3B shows the relaxivity plot, R1′ vs. [RRe], for the yeast suspension of Fig 3A. The Nai+ R1′ (filled circles) is constant as [RRe] increased, which is consistent with an SXL or NXL condition. The Nae+ R1′ (open circles) [RRe] dependence is reasonably linear. The data for a cell-free (CF) minimal medium solution with 12.6 mM Na+ (open squares) is also shown. The data show the linear behavior expected: the Na+ + RR5− ↔ NaRR4− system is in the FXL. The slope of the straight line, 31.8 (± 1.2) mM−1 s−1, is the CF GdDOTP5− relaxivity for 23Na (Nar1CF). The RR relaxivities for water (r1CF), although strictly proportional to [RR]/[H2O], are effectively [H2O] independent because of the very large H2O concentration (~50 M). This is not the case for Nar1CF values, which are quite dependent on both the [RR] numerator and the [Na+] denominator in [RR]/[Na+]. The Nar1CF values for 50 and 150 mM Na+ in minimal medium are decreased to 24.9 (± 0.52) and 17.9 (± 0.2) mM−1s−1, respectively (data not shown). Furthermore, since the interaction of the RR anion with the Na cation is that of an ion pair, the Nar1CF value is sensitive to the concentrations of competitive cations (e.g., Ca2+), anions, and the solution ionic strength. This is in addition to the B0 and temperature dependences that affect r1CF values. The general agreement of the R1′ for yeast suspension Nae+ and for Na+ in the CF minimal medium solution data is gratifying. The fact that the Fig. 3 Nai+ T1′ remains essentially [RRe]-independent confirms the robust nature of the ILT and is encouraging for the general quantification of the MRR/RRe approach.
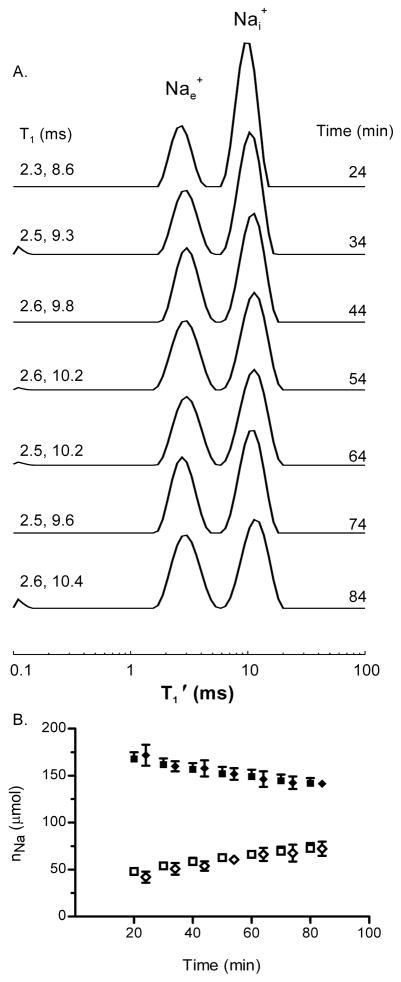
Figure 4 displays a stacked plot of sequential 23Na T1 relaxograms obtained from a yeast suspension containing 12.8 mM GdDOTPe5−. A decrease of the relaxographic Nai+ peak area and an increase of the Nae+ peak area are evident over time. The nNai and nNae values are plotted (diamonds) in Figure 4B, with the MRS/SRe nNai and nNae values (Fig. 1B) re-plotted (as squares) for comparison. The agreement is outstanding.
Figure 4. Na+ efflux from Na+ loaded yeast 23Na T1 MRR with RRe.
Panel A: A stacked plot of Na+ T1 relaxograms obtained from a suspension of Na+ loaded yeast with 12.8 mM [GdDOTPe−5] in the medium. The T1 values obtained for the relaxogram peaks are shown on the left, the elapsed time to the middle of the relaxogram acquisition is shown on the right.
Panel B: The time dependence of the Na+ contents, nNai (filled diamond) and nNae (open diamond), of suspensions of Na+-loaded yeast (n = 4) derived from the 23Na MRR/RRe measurements. Also shown for comparison are the MRS/SRe measured nNai (filled square) and nNae (open square) content of suspensions of Na+ loaded yeast (Fig. 1B). Mean ± (SD) values are plotted.
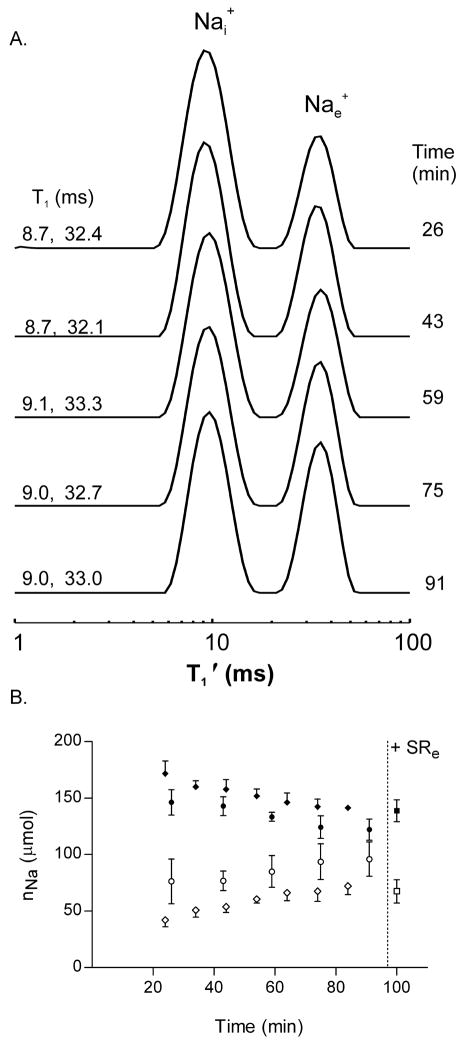
Measurement of Na+-loaded yeast suspension Na+ contents and Nai+ efflux by intrinsic 23Na T1 MRR
The potential for discrimination based on apparent intrinsic 23Nai+ and 23Nae+ T1′ differences is apparent in the 0 mM RRe relaxogram (Fig. 3A, top). 23Na T1′ relaxograms obtained from Na+-loaded yeast cells in RRe-free minimal medium are shown in Figure 5A. The relaxogram peak assignments are reversed from those obtained with RRe (Fig. 4A). These assignments for Fig. 5A are made on the basis of the RRe titration (Fig 3A); and, on the basis of T1 measurements made of the Nai+ resonances, T1′ = 9.5 ± (0.4 ms), made in the presence of SRe (not shown). A temporal decrease of the Nai+ peak area and an increase of the Nae+ peak are evident.
Figure 5. Na+ efflux from Na+ loaded yeast intrinsic T1 23Na MRR.
Panel A: A stacked plot of Na T1 relaxograms of a suspension of Na+ loaded yeast is shown. No RRe was added to the sample; the relaxogram peaks represent the distribution of intrinsic T1 values in the sample. The T1 values obtained from each relaxogram peak are shown on the left; the elapsed time to the middle of the relaxogram acquisition is shown on the right.
Panel B: The time dependence of the Na+ contents, nNai (filled circle) and nNae (open circle), of suspensions of Na+-loaded yeast (n=4) derived from 23Na intrinsic T1 MRR measures. At 97 min SR was added to the suspension and the MRS/SRe measured nNai (filled square) and nNae (open square) values were obtained. For comparison the MRR/RRe nNai (filled diamond) and nNae (open diamond) amounts (Fig 4B) are shown. All values are mean ± (SD).
The nNai and nNae values derived from intrinsic T1 MRR are shown in Figure 5B (circles). Inspection reveals that the nNai and nNae amounts are different from those found in the other studies (Figs. 1B and 3B). To validate these 23Na intrinsic T1 MRR measurements, at 97 min SR was added to each suspension for measurements by 23Na MRS (Fig 5B). The Nai+ and Nae+ MRS/SRe ai and ae values report that the intrinsic T1 MRR Nai+ is 0.88 (± 0.06) of the MRS Nai+ value; and, the MRR Nae+ is 1.43 (± 0.12) of the MRS Nae+ value. Thus, the Nai+ amount is 12% too low and, the Nae+ amount is 43% too high. A similar finding can be observed in the relaxograms obtained during the RRe titration (Fig. 3A). The ratio of Nai+ peak area in the 0 mM RRe relaxogram to that in the 12.8 mM RRe is 0.55/0.68 = 0.81 while the analogous ratio of the Nae+ peak areas is 0.45/0.32 = 1.4. Possible reasons for this are considered in the Discussion section. Whatever the cause, multiplying the intrinsic T1 MR nNai values by 1.14 and the intrinsic T1 MR nNae values by 0.70 (not shown) gives results that are in good agreement with the nNai MRR/RRe (filled diamonds) and the nNae MRR/RRe (open diamonds), Fig. 5B.
Na+ Efflux kinetics
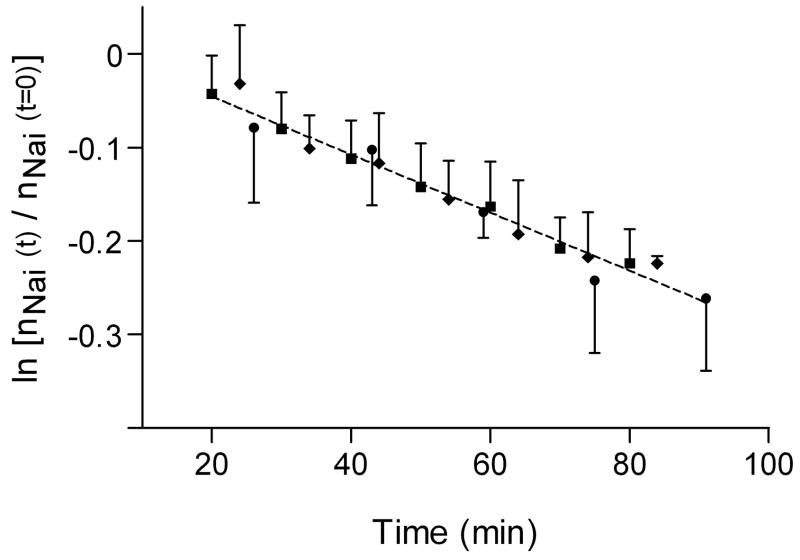
The transcytolemmal Na+ efflux was modeled as a first-order kinetic process (Methods). The ln[nNai(t)/nNai(0)] values for the MRS/SRe (squares), MRR/RRe (diamonds) and intrinsic T1MRR (circles) are shown in Figure 6. The data are clearly linear for the measurements of all three groups. The data sets were each individually fitted to lines with a slope of − 3.1 (± 0.3) ×10−3 min−1. No better fitting solution was found in fitting the three data sets to lines with different slopes. The pseudo-first order rate constant for Nai+ efflux was 3.1 (± 0.3) ×10−3 min−1. Thus, for changes in relative Nai+ (and presumably Nae+) amounts, the intrinsic T1 MRR approach returned a Na+ flux equal to that using MRS/SRe or MRR/RRe. This is an extremely encouraging result given the issue with the under-estimation of Nai+.
Figure 6. Na+ Efflux kinetics.
A plot of the time dependence of ln[nNai(t)/nNai(t=0)] from the MRS/SRe results (filled squares), MRR/RRe results (filled diamonds) and intrinsic T1 MRR results (filled circles). All three data sets were fit to straight lines. The hypothesis was tested that the slope was different for the three lines individually fit to each set of data (p = 0.98). Thus, the slopes are equal for all three data sets. The common slope was 3.1 (± 0.3) ×10−3 min−1; thus, the k = 3.1 (± 0.3) ×10−3 min−1. A single line with an intercept of 0.016 (± 0.015) and the above slope fit to the three sets of data (R2 = 0.67) is shown.
23Na MR T1 Relaxography can detect multiple Nai+ populations in Na+-loaded yeast
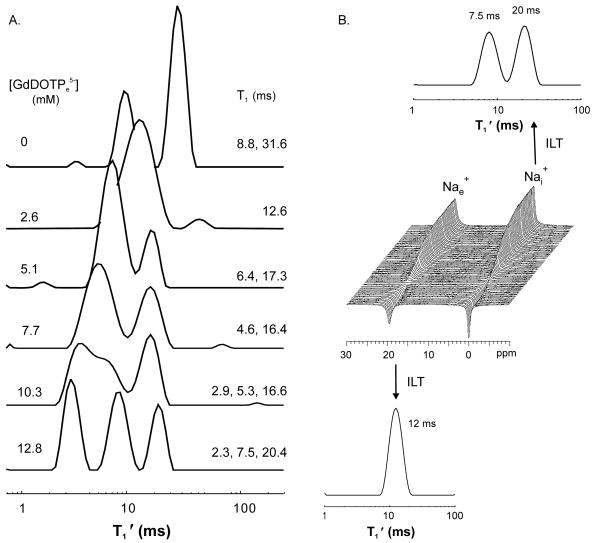
Multiple Nai+ relaxographic peaks were observed from suspensions of a Na+-loaded Baker’s yeast strain. This phenomenon was not seen with suspensions of D273 or Red Star Bakers Na+-loaded yeast. Figure 7A shows that during GdDOTPe5− titration of this Na+-loaded Baker’s yeast suspension, three relaxographic peaks are resolved with RRe ≥ 10.3 mM. In addition to the 2.3 ms 23Nae+ peak at 12.8 mM RRe, two other peaks are observed at 7.5 and 20.4 ms. Apparently, these two peaks report two different Nai+ (or RRe-inaccessible) populations. Although a single tissue Na+ population can exhibit two T1 values (i.e., relax bi-exponentially) when the 23Na system is out of the extreme narrowed condition, quantum mechanical constraints require that their relative contributions be 4:1 (component with smaller T1: component with larger T1) [31]. That is not the case here. To test the Nai+ assignment of the larger T1 peaks, 23Na IR measurements were made on another Na+-loaded Baker’s yeast suspension with SRe. The IR spectra are shown in the stacked plot (Fig. 7B). The IR data of the individual 23Nae+ and 23Nai+ resonances, discriminated by SRe, were subjected to ILT. The relaxogram of the SRe-inaccessible 23Nai+ resonance clearly exhibits two peaks (7.5 and 20 ms), while that for SRe-accessible 23Nae+ only one.
Figure 7. 23Na T1 MRR with [RRe] detects multiple Nai+ populations in Na+-loaded Bakers yeast.
Panel A: A stacked plot of relaxograms obtained from a suspension of Na+ loaded Bakers yeast during serial addition of increasing amounts of the RRe for Na+, GdDOTP5−. The [GdDOTPe5−] are shown on the left. The T1s determined from the center of the peaks are shown on the right. In the bottom relaxogram (12.8 mM RRe) the 2.3 ms peak reports Nae+. The peaks at 7.5 and 20.4 ms report Nai+.
Panel B: A stacked plot of T1 IR spectra obtained from a suspension of Na+ loaded bakers yeast with SRe in the medium (center) with relaxograms resulting from an ILT of the IR data from each resonance. The relaxogram (upper) from the Nai+ resonance exhibits two peaks at 7.5 ms and 20 ms, which matches the T1 values found from the Nai+ peaks in the bottom relaxogram (12.8 mM [GdDOTPe5−]) of panel A.
[Nai+] and [Nae+] calculations
The intra- and extracellular volumes inside the MR coil sensitive volume were estimated to be 0.53 and 3.06 mL, respectively for a 50% Na+-loaded yeast suspension (Methods). Using nNai and nNae amounts linearly extrapolated to t = 0 in Fig. 1B yields [Nai+]t=0 =175 (± 3) μmol/0.53 mL = 330 (± 6) mM and [Nae+]t=0 = 42 (± 2) μmol/3.06 mL = 13.5 (± 0.6) mM. The [Nae+] estimation is quite accurate: the minimal medium [Nae+] was 13 mM.
Discussion
The results of this study demonstrate that 23Na T1 MRR can discriminate and quantify Nai+ and Nae+ contents in Na+-loaded yeast suspensions. We are not aware that this has been previously reported. 23Na T1 MRR with RRe can report accurate absolute Nai+ and Nae+ contents, while intrinsic MRR reports accurate relative changes in Nai+ and Nae+ but not accurate absolute amounts.
In vivo 23Na T1 MRR measurements with RRe for Na+ would require an RRe that enabled differentiation of T1e and T1i at reasonable [RRe] values. For the RRe, GdDOTP5−, used in this study, RRe concentrations greater than 10 mM were required to resolve the T1e and T1i peaks. The ratio of [Nae+]/[GdDOTPe5−] was approximately 1, which would be problematic for in vivo use. The relaxivity of GdDOTP5− for 23Na+ is sensitive to the [Na+] value and to the presence of other cations, such as Mg2+, present in the medium that compete for the chelate Na+ binding site(s) and anions that compete for Na+. Unfortunately, the requirement for relatively high [RRe] may be hard to avoid. Gd(III) complexes that function as efficient RRs for 1H2O have a water molecule binding site within the Gd3+ inner coordination sphere. This close proximity to the Gd3+ atom results in efficient T1 relaxation of the 1H2O signal. Being also a cation, Na+ is unlikely to occupy a site so near Gd3+ inner coordination sphere. Na+ is more likely to bind to or be attracted to negatively charged sites on the ligand, the outer coordination sphere. Consequently, Na+ will be farther from the paramagnetic ion and, due to the dipolar nature of the interaction, not experience such large relaxation enhancement. Also, 23Na R1 values are inherently large because of the quadrupolar nature of this I = 3/2 nucleus. These characteristics will increase the [RRe] required for effective 23Na+ relaxation enhancement.
More promising for in vivo use, our results also demonstrate that intrinsic 23Na T1 MRR can differentiate Nai+ and Nae+ in Na+-loaded yeast suspensions. This approach accurately measures Na+ efflux kinetics and this suggests that intrinsic 23Na T1 MRR can accurately measure relative Nai+ and Nae+ amounts. However, there are issues in the accurate quantification of the absolute Nai+ and Nae+ amounts. The Nai+ content is underestimated while that of Nae+ is overestimated. We present three general hypotheses that could explain this discrepancy.
The first hypothesis is related to the fact that the ILT is an “ill-conditioned” operation [25; 26; 28; 32]; it is not conducted analytically. It is accomplished numerically, usually with a smoothed (“regularized”) discretized (“grid”) method, which is effectively a multi-exponential analysis [25; 28; 32]. The numerical methods employed can cause errors when the apparent relaxation time constants of the two spin populations to be discriminated are too similar. The MRS/SRe validation experiments demonstrate that MRR/RRe is perfectly quantitative for the Na+ populations if the [RRe] value is great enough to cause a sufficiently large R′1e/R′1i ratio. The R′1e/R′1i is 4.3 when [RRe] = 12.8 mM and MRR/RRe is quantitative. Thus, one might suspect that the intrinsic R′1i/R′1e0 value (R′1i is here greater than R′1e0, the RRe-free value of R′1e) is insufficiently large to allow accurate determination of the apparent Nai+ and Nae+ populations. However, the intrinsic R′1i/R′1e0 value is reduced only to 3.6 (Fig. 5A). Simulations suggest that it is the smaller R′1 that is underestimated by ILT when the R′1 ratio is ≤ 5 [32]. Since we measure ai and ae using bi-exponential fitting with fixed ILT T1′ values, any ILT T1′ errors could propagate into our analysis.
The second hypothesis is as follows. In the absence of RRe the equilibrium transcytolemmal Na+ exchange system is not in the SXL condition. This would apply if the rate constant for exchange k is not sufficiently smaller that the applicable shutter speed: T −1 ≡ |R1e − R1i|, where the R1’s are the intrinsic values (i.e. those in the absence of exchange). There may be some evidence for this in the nature of the RRe titration relaxograms (Fig. 3A). Even though k [≡ kie + kei ≈ kie] would appear to be very small, the exchange system is forced to depart slower domains and pass through the FXL condition [T−1 ≪ k] during the RRe titration. This can be seen by expanding the T −1 expression as |Nar1e[RRe] + R1e0 − R1i|. Since R1i > R1e0, T−1 must pass through zero at some [RRe] value (≈ 3 mM in Fig. 3B). When 2SX systems are in the FXR or FXL conditions, it is the component with the greater R1 whose relative contribution is diminished by the exchange [31]. After the Nae+ peak has passed through the Nai+ peak and emerged well on the small T1 (large R1) side ([RRe] = 15.4 mM; Fig. 3A, bottom), the fractional relaxographic Nai+ area (ai) is essentially the same as the MRS value 20 minutes after re-suspension (Fig. 1B), so the system must be in the SXL. After the Nae+ peak passes through the Nai+ peak ([RRe] > 8 mM), however, its relative area (ae) is noticeably diminished. This is consistent with a shutter-speed (Nae+ ↔ Nai+ exchange) effect [31]. When two peaks emerge from relaxographic coalescence, the smaller T1 component fractional peak area (aS) is less than the mole fraction of the population that it represents (pe, in this case) unless the equilibrium intercompartmental exchange system is in the actual SXL condition [T−1 ≫ k], or the NXL. Since k seems so small in this case (Fig. 6; k = 3.1 × 10−3 min−1 or 5.2 × 10−5 s−1), the reversion to the FXR and FXL conditions would have to occur within a very tiny [RRe] range. Perhaps there are concomitant faster transcytolemmal cycling processes that result in equilibrium Na+ exchange, but do not contribute to the slower net Na+ efflux. A two-site-exchange analysis [33] suggests that the kie′ value resulting from faster equilibrium processes would have to be 5 s−1 (i.e., much greater than kie for the net efflux) to account for a 12% nNai underestimation.
Thus, the departure of the transcytolemmal Na+ exchange 23Na MR system from the SXL or NXL condition could explain why the ai and ae values from the RRe-free suspension relaxogram are not equal to the pi and pe values. If so, this could provide a way to determine pi and pe from ai and ae.
The third hypothesis is that 23Na MR intrinsic T1 relaxography inherently underestimates the smaller T1 component (Nai+) and overestimates the larger T1 component (Nae+). Using a phantom consisting of a sphere inside an NMR tube with compartmental Na+ T1s and a range of compartmental Na+ populations similar to those encountered in Na+ loaded yeast suspensions, we found the following: the sphere Na+ (modeling Nai+) population was underestimated 1–6%; and, the tube Na+ (modeling Nae+) population was overestimated 2 –4 %. Thus, under ideal conditions 23Na intrinsic T1 MRR areas do not equal the 23Na MRS determined populations. This population discrepancy was observed using all relaxographic analysis options: ILT, Bi-exp with T1s input from ILT (used in the analysis in this work), and a Bi-exp that determined both T1 and population. In general, the Bi-exp that determined T1 and population tended to be slightly more accurate. Although the Nai+ underestimation in the sphere/tube phantom (1–6%) was less than that observed in the yeast suspension (~12%) it probably accounts for 25 to 50% of that underestimation. The remaining discrepancy (~6 to 9%) could be due to more “noise” in the yeast suspension measurements and/or some of the equilibrium exchange effects outlined in the second hypothesis.
Despite the discrepancy between the RRe-free suspension relaxogram ai and ae values and the pi and pe values, the efflux kinetics are still accurately measured. This is evidenced by the excellent agreement shown in the Fig. 6 plot of the intrinsic MRR results with those from the MRS/SRe and MRR/Re studies. The intrinsic MRR approach measures relative nNai values (and presumably also relative nNae values) with high accuracy.
The capacity of intrinsic 23Na T1 MRR to discriminate Nai+ and Nae+ amounts, albeit with possibly reduced accuracy compared with the use of RRe, indicates the potential for application of this method in vivo, and even with human subjects. It may be possible to correct the Nai+ and Nae+ amounts, assuming that the content determination error is reproducible. Using the sphere/tube phantom described above we found that Nai+ of 5 to 19% of the total Na+, a reasonable range for Nai+ in vivo, was underestimated ~12% by MR relaxography. While this is greater than the underestimation observed at Nai+ of 50% in the same phantom it indicates that Nai+ can be discriminated and estimated in tissues in vivo. Since 23Na intrinsic T1 MRR accurately measures relative changes in Nai+ (Fig. 6), Nai+ contents of a region-of-interest may be compared with those of an appropriate control (reference) regions.
The ability of 23Na intrinsic T1 MRR to discriminate Nai+ and Nae+ will depend on the T1 values of the two populations, as well as the relative sizes of the two populations. Bansal and co-workers reported that in the in vivo rat liver (intact animal) Nai+ T1 = 21 ms in presence of SRe and Nae+ = 41 ms (estimated from measurements without SRe) [34]. When an IR 23Na MRI sequence was used with tI = 25–30 ms to null signals with T1 values of 36 – 43 ms, which were presumed to be 23Nae+, two fold increases in 23Na MRI intensity were observed in breast tumors in vivo [8]. This image intensity arose from magnetization with smaller T1 values (T1 ~ 20 ms) and was attributed to increased 23Nai+. Thus, there are indications from in vivo studies that Nai+ T1 ≈ 20 ms and Nae+ ≈ 40 ms. MRR simulations with pi ≈ 0.15, Nai+ T1 ≈ 20 ms, and Nae+ = 40 ms indicate that 23Na intrinsic T1 MRR will detect both populations.
The attractiveness of intrinsic T1 MRR is that it does not require use (or development) of an RRe for 23Na+. This could be especially valuable in neurological 23Na MR studies, where RR access to the extracellular interstitial space is limited by low blood-brain barrier permeability.
The observation of two different relaxographic T1′ peaks for SRe-inaccessible Na+ populations in suspensions of Na+-loaded Baker’s yeast cells also results from intrinsic T1 differences. These two T1 peaks represent Na+ populations for which the exchange system is in the SXR or SXL condition. This means a k ≪ 30 s−1 (from the difference in the R1′ values). It is possible that these are two Nai+ populations separated by membranes, i.e., Na+ in two different compartments, such as, the cytoplasm and vacuoles. Gupta, et al, reported an intracellular Na+ resonance with two T1 components in the 23Na MR spectrum of a Rana oocyte suspension [35]. As Na+ efflux from this strain of Baker’s yeast proceeded, the area of the T1 ~ 8 ms peak generally remained relatively constant, while the area of the T1 ~ 18 ms peak decreased, at least up until the two peaks merged into one.
23Na MR spectroscopy and relaxography measure Na+ amounts. To determine [Nai+] and [Nae+] values, the intracellular and extracellular volumes must also be measured or estimated. Labadie et. al. demonstrated that 1H2O T1 MR relaxography with an RRe (or contrast reagent), GdDTPA2−, allows discrimination of the intra- (1H2Oi) and extracellular (1H2Oe) signals [25]. The two peak relaxogram measures the volume fractions, if the shutter-speed effects of equilibrium transcytolemmal water exchange kinetics are accounted for and quantified. Using this method to determine the volumes enabled us to estimate [Nai+], and [Nae+] values and, thus, the Na+ concentration gradient. Immediately upon re-suspension, [Nai+]/[Nae+] was 24 for Na+-loaded yeast cells. After one hour of spontaneous efflux, the [Nai+]/[Nae+] ratio had decreased to 13.
The potential diagnostic utility of Nai+ measurements has led to the development of other Na+ compartmental discrimination methods. As described above, 23Na MRS/SRe currently provides the best [Nai+] measurements in isolated organs or intact animals. The most extensively studied alternative method has been the 23Na multiple-quantum MR coherence filters. The Nai+ discrimination is, however, usually not as complete as with SRe. This, combined with the more than 90% signal intensity reduction from that of single quantum coherences used here, has limited the usefulness of the multi-quantum-filter [31; 36; 37]. Studies of perfused heart concluded that Nai+ content may be reliably determined from SR-free triple-quantum-filtered spectra when the Nae+ contribution does not vary appreciably, such as during constant pressure perfusion [38]. Studies of the liver in vivo, which used SRe to aid interpretation, found that Nae+ contributed significantly to the total triple-quantum-filtered signal in live animals, and that the intensity of this signal did not change postmortem. However, the triple-quantum-filtered Nai+ signal increased by approximately 380% over a one hour postmortem period, whereas the single quantum Nai+ increased by only 90% [29]. Thus, it is difficult to quantify Nai+ in 23Na multi-quantum-filtered spectra.
This study explored the potential for compartmental T1 differences to allow Na+ discrimination. The findings demonstrate that 23Na MRR can discriminate and measure Nai+ and Nae+ on this basis. Two types of ΔT1 situations were investigated. The first imposed a T1e reduction with an RRe. This method was accurate but required relatively large [RRe] values. With a more effective Nae+ RRe, this method might be used in vivo. The second method exploited intrinsic Nai+ and Nae+ T1 differences in the yeast cell suspension. This method was less accurate in Nai+ content determination but did accurately measure the spontaneous Nai+ efflux rate constant. It offers the potential advantage of not requiring the use of exogenous agents to enable relaxographic discrimination in 23Na MRI, and could thus be used for human studies.
Experimental
Na+-loaded yeast cells were prepared as follows. Typically, 16 g of yeast cells, either D273-10B (American Type Culture Collection, Manassas, VA) or baker’s yeast (obtained from a local bakery or Red Star yeast from a supermarket) were added to 800 mL of a 0.2 M Na3citrate, 5% weight/volume (wt/vol) glucose solution and bubbled with 95% O2/5% CO2. This mixture was stirred at room temperature for 2 h (± 15 min) [24]. After Na+ loading, the yeast cells were centrifuged (T = 4°C) and washed twice with 50 mL of cold (T = 0°C) minimal medium containing: 4 mM MgSO4, 13 mM KCl, 13 mM Na+ (added as NaOH to adjust pH to 6.6), and 50 mM 3-N-morpholino-propanesulfonic acid. After the second washing the centrifuged yeast pellet was re-suspended in minimal medium to make up the experimental samples to be 50% wet wt/vol. Where noted stock solutions containing 100 mM Tris4HTmDOTP (a 23Na+ SR[15]) or Tris4HGdDOTP (a 23Na+ RR) were added to the minimal medium before making up the 50% wt/vol yeast suspension. The final total suspension volume required to bring cell density to 50% wt/vol varied slightly but was 10 mL or greater. The final extracellular SR or RR concentrations were ~12.8 mM unless otherwise noted.
In studies where [RRe] was varied, the following steps occurred after each set of MR measurements: (1) the yeast suspension was centrifuged; (2) the supernatant was discarded; (3) the cell pellet was re-suspended in 50 mL of cold minimal medium and re-centrifuged; and (4) the packed cells were re-suspended in ~10 mL minimal medium containing necessary RR stock volume to achieve the next [RRe] value.
Yeast suspension MR measurements were conducted in 20 mm o.d. NMR tubes (Wilmad-Labglass, Buena, NJ). Before positioning in the magnet, the tube was fitted with a home-made apparatus consisting of two lengths of Clay Adams PE 90 tubing (Becton-Dickinson, Franklin Lakes, NJ) that extended into the tube to near its bottom. The 95% N2/5% CO2 gas flowed constantly through the tubing. The gas flow was adjusted to keep the yeast cells suspended during the MR measurements.
23Na MR measurements
23Na MR free-induction-decays (FIDs) were acquired at 105.5 MHz (9.4T) using a 20 mm Broad Band probe (Nalorac Inc., Martinez, CA) in a Varian Inova spectrometer (Varian Inc., Palo Alto, CA). For 23Na MRS one-pulse measurements, 208 FIDs were acquired and averaged over a 1 min period using 90° RF pulses and a recycle time of 0.266 s: the time between the end of the pulse and the start of FID digitization (the dead time) was 115 μs. 23Na MR T1 relaxation time constants of yeast suspension in presence of RR were measured using an IR pulse sequence with the following parameters: 16 FIDs were averaged for each incremented time from inversion (tI) between the 180° and 90° pulses; there were 74 tI values (minimum, 0.2 ms; maximum, 300 ms); the recycle time minimum was 0.306 s; the dead time was 115 μs; and the total time was 7 min. In studies that measured intrinsic 23Na T1 relaxation time constants, 8 FIDs were averaged for each of 74 tI values (minimum, 0.5 ms and maximum, 600 ms); the recycle time was 0.6 s; the dead time was 115μs; and the total time was 7min. The mid-point of the spectral acquisition was taken as the measurement time. Time zero was defined as that of the re-suspension of the yeast in cold minimal medium to a 50% wt/vol cell density. The MR probe air temperature was 24 (± 1) °C.
Na+ amounts from 23Na MR
The areas of MRS 23Na+ resonances were measured using both the Varian software frequency spectral integration routine (VNMR 6.1c) and Bayesian FID Analysis (Bayesian Analysis Software, G.L. Bretthorst Washington University, St. Louis, MO). The Bayesian Analysis yields the FID amplitude for each resonance [39], which we term the Bayes number. With the same spectrometer settings, the Bayes numbers report the relative signal sizes (Na+ amounts) in different samples. The absolute Na+ content was calculated as follows. A capillary containing 8.425 μmol of Na5Dy(PPP)2, a SR[11], was placed inside a 20 mm NMR tube containing Na+-free minimal medium (KOH was used to alter pH). The capillary was contained completely within the RF coil sensitive volume. Bayesian analysis of the one pulse MRS FID of this standard sample provided a conversion factor, μmol Na+/Bayes number. Thus, for each MRS/SRe measurement, the Bayes numbers for the Nai+ and Nae+ resonances were converted to amounts (μmol Na+). For MRR studies, the Bayes number of a one-pulse MRS FID was obtained to yield the total Na+ amount (nNa). One-pulse and IR measurements were interleaved throughout the study.
23Na Relaxographic data analysis
In the IR data sets, integrated using Varian software, the longitudinal magnetization (Mz) values at the three longest tI values were averaged to estimate the Boltzmann equilibrium magnetization [<MZ(tI)> = MZ(∞)]. The tI-dependent quantity [(MZ(∞) − MZ(tI))/(2MZ(∞))] was then calculated from the IR data and processed using a 1D ILT algorithm [28; 40] written in Matlab (TwoDLaplaceInverse, Magritek Limited, Wellington, New Zealand). The output of the ILT is the apparent relaxation time constant distribution, or T1′ relaxogram. A two peak relaxogram yielded presumed Nai+ T1′ (T′1i) and Nae+ T1′ (T′1e) values. Relaxograms were plotted and analyzed to obtain the T1′ (center of a peak) and relative area values using Matlab routines (MathWorks Inc, Natick, MA).
The T1′ values obtained from the relaxogram were used in a least squares fitting of an empirical (phenomenological) bi-exponential expression, aeexp(−tI/T′1e) + aiexp(−tI/T′1i) + C, to the [(MZ(∞) − MZ(tI))/(2MZ(∞))] IR decay (GraphPad Prism version 5.0 for Windows, GraphPad Software, San Diego, CA). Only the apparent mole fraction, ai and ae and noise constant, C, values were varied. The Nai+ (nNai) and Nae+ (nNae) amounts were then calculated as follows:
| [1] |
Yeast suspension intra- and extracellular volumes
Total sample intracellular and extracellular volumes (Vi and Ve, respectively) were calculated as follows. 1H2O MRR (398.8 MHz; 9.4T) with GdDTPAe2− and two-site-exchange (2SX) analysis [25] for equilibrium transcytolemmal water interchange in a 50% wt/vol Na+-loaded yeast suspension found a 1H2Oi mole fraction (population) [pi(w)] of 0.148 and a 1H2Oe mole fraction [pe(w)] of 0.852.
The yeast cell pellet dry to wet weight ratio was 0.17 (± 0.02). Thus, 5 g (wet wt) yeast contains 0.85 g dry wt. The final yeast suspension sample volume was 10 mL. Correcting for yeast cell mass gives water mass (10 − 0.85 = 9.15 g) - assuming unit density, this is 9.15 mL; 9.15 × 0.852 = 7.796 mL ≈ 7.8 mL = Ve. This Ve was used to calculate [SRe] and [RRe].
The [Nai+] and [Nae+] values were calculated using the intra- and extracellular volume fractions detected, dVi and dVe. The RF coil sensitive volume was 3.92 mL; thus, 0.392 of the total sample volume. Therefore, 0.333 g (dry wt) yeast was inside the RF coil and, 3.92 mL RF coil volume − 0.333 mL yeast = 3.59 mL H2O total inside the RF coil volume. This results in the following detected volumes, 3.59 mL H2O × pi (0.148) = 0.53 mL = dVi; and, dVe = 3.06 mL.
Kinetics
After re-suspension, Na+ spontaneously exits the Na+-loaded yeast cells. We analyze this by assuming that transcytolemmal Na+ efflux is an effectively irreversible first-order kinetic process: that is kei ≪ kie, where kie is first-order rate constant for Na+ efflux and kei is that for Na+ influx. If so a plot of ln[nNai(t)/nNai(0)] vs. t will yield a straight line with slope equal to − kie. The nNai(0) values were estimated by fitting nNai (t) with an effective straight line.
Statistical analysis
Results are presented as the mean (± one standard deviation (SD)) values. GraphPad Prism version 5.0 for Windows, GraphPad Software, San Diego, CA was used for graphs and data fittings and comparison of data fitting parameters. Differences were declared statistically significant if p < 0.05.
Acknowledgments
NIH Grants RO1 HL78634 (to J.A.B), and RO1 EB00422 and RO1 NS40801 (to C.S.S.) supported this work. We enjoyed stimulating discussions with Dr. Xin Li.
Appendix 1
Abbreviations
- ai
fractional peak area of intracellular Na+ obtained by relaxography
- ae
fractional peak area of extracellular Na+ obtained by relaxography
- aL
fractional area of peak with larger T1 obtained from relaxography
- aS
fractional area of peak with smaller T1 obtained from relaxography
- Bi-exp
bi-exponential function (aeexp(−tI/T′1e) + aiexp(−tI/T′1i) + C)
- FXR
Fast eXchange Regime
- FXL
Fast eXchange Limit ([T−1 ≪ k])
- ILT
Inverse Laplace Transform, the apparent relaxation time constant (T1′) distribution
- MRR
Magnetic Resonance Relaxography
- nNae
amount of extracellular Na+
- nNai
amount of intracellular Na+
- NXL
No eXchange Limit
- pe
fractional population of extracellular Na+
- pi
fractional population of intracellular Na+
- Nar1CF
Relaxivity of Na+ in cell free solution
- R1e0
longitudinal relaxation rate extracellular Na+ in absence of RRe
- R1e
longitudinal relaxation rate of extracellular Na+ (in the absence of exchange)
- R1i
longitudinal relaxation rate of intracellular Na+ (in the absence of exchange)
- Relaxogram
the apparent relaxation time constant (T1′) distribution produced by ILT
- RRe
extracellular relaxation reagent
- SRe
extracellular shift reagent
- SXL
Slow eXchange Limit ([T−1 ≫; k])
- SXR
Slow eXchange Regime
- T−1
shutter speed ≡ |R1e − R1i|
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parrish TB, Fieno DS, Fitzgerald SW, Judd RM. Theoretical basis for sodium and potassium MRI of the human heart at 1.5 T. Magnetic Resonance in Medicine. 1997;38:653–61. doi: 10.1002/mrm.1910380420. [DOI] [PubMed] [Google Scholar]
- 2.Jansen MA, Van Emous JG, Nederhoff MG, Van Echteld CJ. Assessment of myocardial viability by intracellular 23Na magnetic resonance imaging. Circulation. 2004;110:3457–64. doi: 10.1161/01.CIR.0000148132.15105.0E. [DOI] [PubMed] [Google Scholar]
- 3.Horn M, Weidensteiner C, Scheffer H, Meininger M, de Groot M, Remkes H, Dienesch C, Przyklenk K, von Kienlin M, Neubauer S. Detection of myocardial viability based on measurement of sodium content: A (23)Na-NMR study. Magn Reson Med. 2001;45:756–64. doi: 10.1002/mrm.1103. [DOI] [PubMed] [Google Scholar]
- 4.Kim RJ, Lima JA, Chen EL, Reeder SB, Klocke FJ, Zerhouni EA, Judd RM. Fast 23Na magnetic resonance imaging of acute reperfused myocardial infarction. Potential to assess myocardial viability. Circulation. 1997;95:1877–85. doi: 10.1161/01.cir.95.7.1877. [DOI] [PubMed] [Google Scholar]
- 5.Pogwizd SM, Sipido KR, Verdonck F, Bers DM. Intracellular Na in animal models of hypertrophy and heart failure: contractile function and arrhythmogenesis. Cardiovascular Research. 2003;57:887–96. doi: 10.1016/s0008-6363(02)00735-6. [DOI] [PubMed] [Google Scholar]
- 6.Thulborn KR, Gindin TS, Davis D, Erb P. Comprehensive MR imaging protocol for stroke management: tissue sodium concentration as a measure of tissue viability in nonhuman primate studies and in clinical studies. Radiology. 1999;213:156–66. doi: 10.1148/radiology.213.1.r99se15156. [DOI] [PubMed] [Google Scholar]
- 7.Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA. Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology. 2003;227:529–37. doi: 10.1148/radiol.2272020483. [DOI] [PubMed] [Google Scholar]
- 8.Sharma R, Kline RP, Wu EX, Katz JK. Rapid in vivo Taxotere quantitative chemosensitivity response by 4.23 Tesla sodium MRI and histo-immunostaining features in N-Methyl-N-Nitrosourea induced breast tumors in rats. Cancer Cell Int. 2005;5:26. doi: 10.1186/1475-2867-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pike MM, Springer CS. Aqueous shift reagents for high-resolution cationic nuclear magnetic resonance. Journal of Magnetic Resonance. 1982;46:348–353. [Google Scholar]
- 10.Pike MM, Simon SR, Balschi JA, Springer CS., Jr High-resolution NMR studies of transmembrane cation transport: use of an aqueous shift reagent for 23Na. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:810–4. doi: 10.1073/pnas.79.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta RK, Gupta P. Direct observation of resolved resonances from intra- and extracellular sodium-23 ions in NMR studies of intact cells and tissues using dysprosium (III) tripolyphosphate as paramagnetic shift reagent. Journal of Magnetic Resonance. 1982;47:344–349. [Google Scholar]
- 12.Chu SC, Pike MM, Fossel ET, Smith TW, Balschi JA, Springer CS., Jr Aqueous Shift Reagents for High-Resolution Cationic Nuclear Magnetic Resonance. III. Dy(TTHA)3−, Tm(TTHA)3−, and Tm(PPP)27−. Journal of Magnetic Resonance. 1984;56:33–47. [Google Scholar]
- 13.Balschi JA, Cirillo VP, Springer CS., Jr Direct high-resolution nuclear magnetic resonance studies of cation transport in vivo, Na+ transport in yeast cells. Biophysical Journal. 1982;38:323–6. doi: 10.1016/S0006-3495(82)84566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springer CS, Jr, Pike MM, Balschi JA, Chu SC, Frazier JC, Ingwall JS, Smith TW. Use of shift reagents for nuclear magnetic resonance studies of the kinetics of ion transfer in cells and perfused hearts. Circulation. 1985;72:IV89–93. [PubMed] [Google Scholar]
- 15.Buster DC, Castro MM, Geraldes CF, Malloy CR, Sherry AD, Siemers TC. Tm(DOTP)5−: a 23Na+ shift agent for perfused rat hearts. Magnetic Resonance in Medicine. 1990;15:25–32. doi: 10.1002/mrm.1910150104. [DOI] [PubMed] [Google Scholar]
- 16.Balschi JA, Bittl JA, Springer CS, Jr, Ingwall JS. 31P and 23Na NMR spectroscopy of normal and ischemic rat skeletal muscle. Use of a shift reagent in vivo. NMR in Biomedicine. 1990;3:47–58. doi: 10.1002/nbm.1940030202. [DOI] [PubMed] [Google Scholar]
- 17.Albert MS, Huang W, Lee JH, Balschi JA, Springer CS., Jr Aqueous shift reagents for high-resolution cation NMR. VI. Titration curves for in vivo 23Na and 1H2O MRS obtained from rat blood. NMR in Biomedicine. 1993;6:7–20. doi: 10.1002/nbm.1940060103. [DOI] [PubMed] [Google Scholar]
- 18.Bansal N, Germann MJ, Lazar I, Malloy CR, Sherry AD. In vivo Na-23 MR imaging and spectroscopy of rat brain during TmDOTP5- infusion. Journal of Magnetic Resonance Imaging. 1992;2:385–91. doi: 10.1002/jmri.1880020405. [DOI] [PubMed] [Google Scholar]
- 19.Balschi JA. Na-23 NMR demonstrates prolonged increase of intracellular sodium following transient regional ischemia in the in situ pig heart. Basic Research in Cardiology. 1999;94:60–69. doi: 10.1007/s003950050127. [DOI] [PubMed] [Google Scholar]
- 20.Seo Y, Murakami M, Suzuki E, Watari H. A new method to discriminate intracellular and extracellular K by 39K NMR without chemical-shift reagents) Journal of Magnetic Resonance. 1987;75:529–533. [Google Scholar]
- 21.Kuki S, Suzuki E, Watari H, Takami H, Matsuda H, Kawashima Y. K-39 Nuclear Magnetic Resonance Observation of Intracellular Potassium without Chemical Shift Reagents during Metabolic Inhibition in the Isolated Perfused Rat Heart. Circulation Research. 1990;67:401–405. doi: 10.1161/01.res.67.2.401. [DOI] [PubMed] [Google Scholar]
- 22.Pekar J, Renshaw PF, Leigh JS. Selective Detection of Intracellular Sodium by Coherence-Transfer NMR. Journal of Magnetic Resonance. 1987;72:159–161. [Google Scholar]
- 23.Degani H, Elgavish GA. Ionic permeabilities of membranes. Na and Li NMR studies of ion transport across the membrane of phosphatidylcholine vesicles. FEBS Letters. 1978;90:357–60. doi: 10.1016/0014-5793(78)80404-9. [DOI] [PubMed] [Google Scholar]
- 24.Hofeler H, Jensen D, Pike MM, Delayre JL, Cirillo VP, Springer CS, Jr, Fossel ET, Balschi JA. Sodium transport and phosphorus metabolism in sodium-loaded yeast: simultaneous observation with sodium-23 and phosphorus-31 NMR spectroscopy in vivo. Biochemistry. 1987;26:4953–62. doi: 10.1021/bi00390a011. [DOI] [PubMed] [Google Scholar]
- 25.Labadie C, Lee JH, Vetek G, Springer CS., Jr Relaxographic imaging. Journal of Magnetic Resonance Series B. 1994;105:99–112. doi: 10.1006/jmrb.1994.1109. [DOI] [PubMed] [Google Scholar]
- 26.Silva MD, Helmer KG, Lee JH, Han SS, Springer CS, Jr, Sotak CH. Deconvolution of compartmental water diffusion coefficients in yeast-cell suspensions using combined T(1) and diffusion measurements. Journal of Magnetic Resonance. 2002;156:52–63. doi: 10.1006/jmre.2002.2527. [DOI] [PubMed] [Google Scholar]
- 27.Kroeker RM, Stewart CA, Bronskill MJ, Henkelman RM. Continuous distributions of NMR relaxation times applied to tumors before and after therapy with X-rays and cyclophosphamide. Magn Reson Med. 1988;6:24–36. doi: 10.1002/mrm.1910060104. [DOI] [PubMed] [Google Scholar]
- 28.Song YQ, Venkataramanan L, Hurlimann MD, Flaum M, Frulla P, Straley C. T-1-T-2 correlation spectra obtained using a fast two-dimensional Laplace inversion. Journal of Magnetic Resonance. 2002;154:261–268. doi: 10.1006/jmre.2001.2474. [DOI] [PubMed] [Google Scholar]
- 29.Seshan V, Sherry AD, Bansal N. Evaluation of triple quantum-filtered 23Na NMR spectroscopy in the in situ rat liver. Magnetic Resonance in Medicine. 1997;38:821–7. doi: 10.1002/mrm.1910380519. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Huang W, Morris EA, Tudorica LA, Seshan VE, Rooney WD, Tagge I, Wang Y, Xu J, Springer CS., Jr Dynamic NMR effects in breast cancer dynamic-contrast-enhanced MRI. Proc Natl Acad Sci U S A. 2008;105:17937–42. doi: 10.1073/pnas.0804224105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rooney WD, Springer CS., Jr A comprehensive approach to the analysis and interpretation of the resonances of spins 3/2 from living systems. NMR in Biomedicine. 1991;4:209–26. doi: 10.1002/nbm.1940040502. [DOI] [PubMed] [Google Scholar]
- 32.Kroeker RM, Mark Henkelman R. Analysis of biological NMR relaxation data with continuous distributions of relaxation times. Journal of Magnetic Resonance. 1986;69:218–235. [Google Scholar]
- 33.Landis CS, Li X, Telang FW, Molina PE, Palyka I, Vetek G, Springer CS., Jr Equilibrium transcytolemmal water-exchange kinetics in skeletal muscle in vivo. Magnetic Resonance in Medicine. 1999;42:467–78. doi: 10.1002/(sici)1522-2594(199909)42:3<467::aid-mrm9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Bansal N, Germann MJ, Seshan V, Shires GTd, Malloy CR, Sherry AD. Thulium 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(methylene phosphonate) as a 23Na shift reagent for the in vivo rat liver. Biochemistry. 1993;32:5638–43. doi: 10.1021/bi00072a020. [DOI] [PubMed] [Google Scholar]
- 35.Gupta RK, Kostellow AB, Morrill GA. NMR studies of intracellular sodium ions in amphibian oocytes, ovulated eggs, and early embryos. J Biol Chem. 1985;260:9203–8. [PubMed] [Google Scholar]
- 36.Hutchison RB, Malhotra D, Hendrick RE, Chan L, Shapiro JI. Evaluation of the double-quantum filter for the measurement of intracellular sodium concentration. Journal of Biological Chemistry. 1990;265:15506–10. [PubMed] [Google Scholar]
- 37.Rooney WD, Springer CS., Jr The molecular environment of intracellular sodium: 23Na NMR relaxation. NMR in Biomedicine. 1991;4:227–45. doi: 10.1002/nbm.1940040503. [DOI] [PubMed] [Google Scholar]
- 38.Tauskela JS, Dizon JM, Whang J, Katz J. Evaluation of multiple-quantum-filtered 23Na NMR in monitoring intracellular Na content in the isolated perfused rat heart in the absence of a chemical-shift reagent. Journal of Magnetic Resonance. 1997;127:115–27. doi: 10.1006/jmre.1997.1181. [DOI] [PubMed] [Google Scholar]
- 39.Bretthorst GL, Kotyk JJ, Ackerman JJ. 31P NMR Bayesian spectral analysis of rat brain in vivo. Magn Reson Med. 1989;9:282–7. doi: 10.1002/mrm.1910090214. [DOI] [PubMed] [Google Scholar]
- 40.Seland JG, Bruvold M, Brurok H, Jynge P, Krane J. Analyzing equilibrium water exchange between myocardial tissue compartments using dynamical two-dimensional correlation experiments combined with manganese-enhanced relaxography. Magnetic Resonance in Medicine. 2007;58:674–686. doi: 10.1002/mrm.21323. [DOI] [PubMed] [Google Scholar]