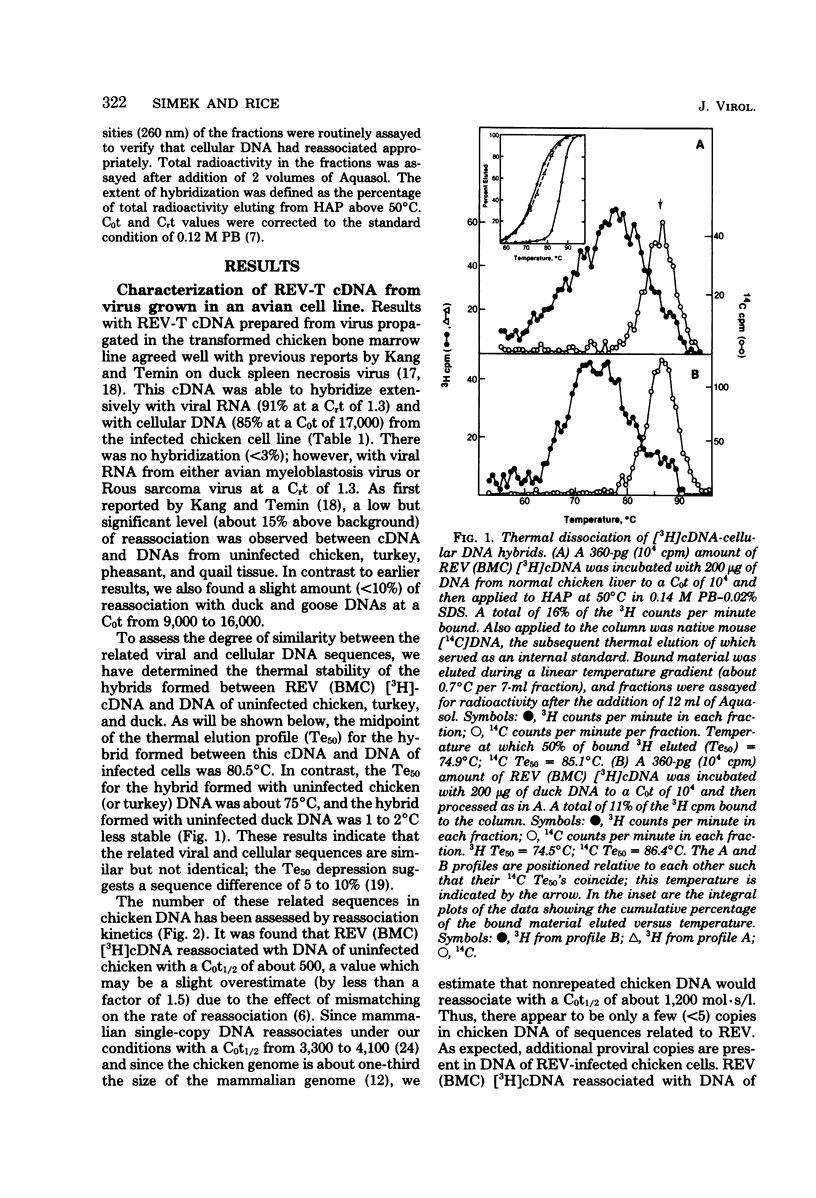
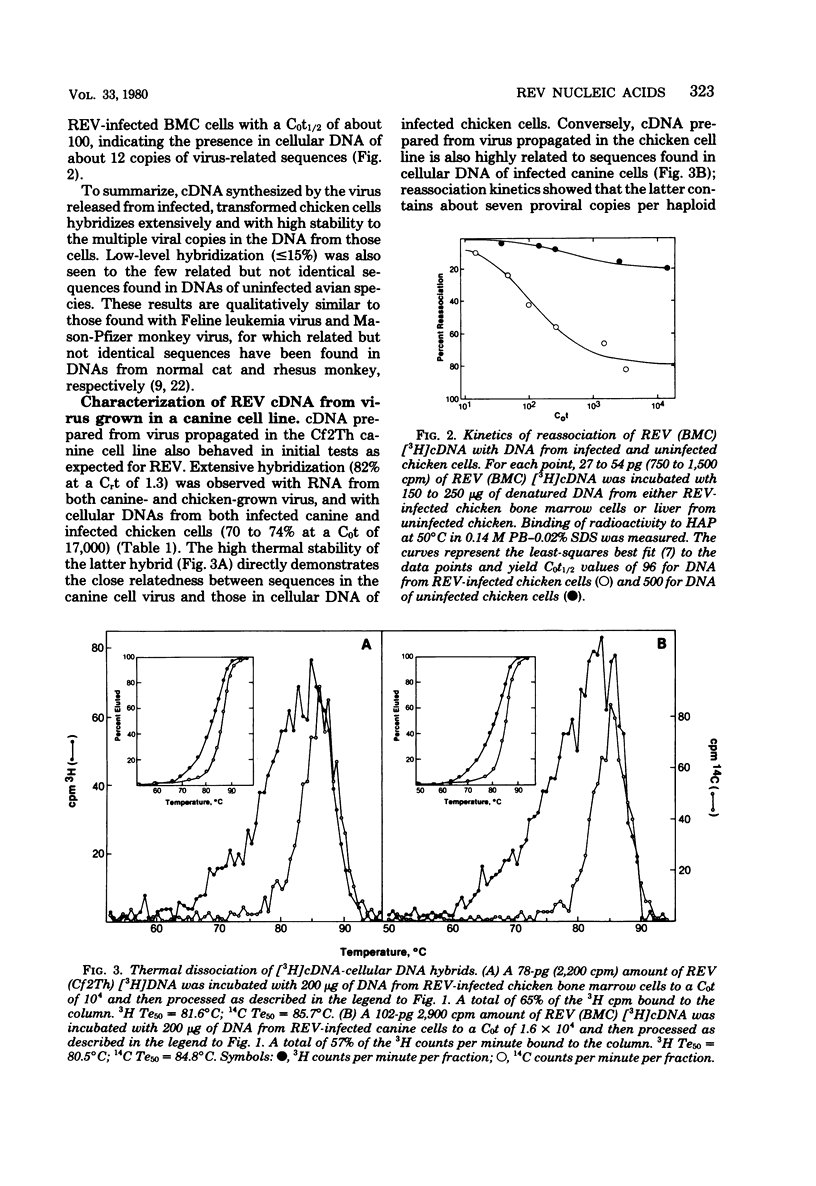
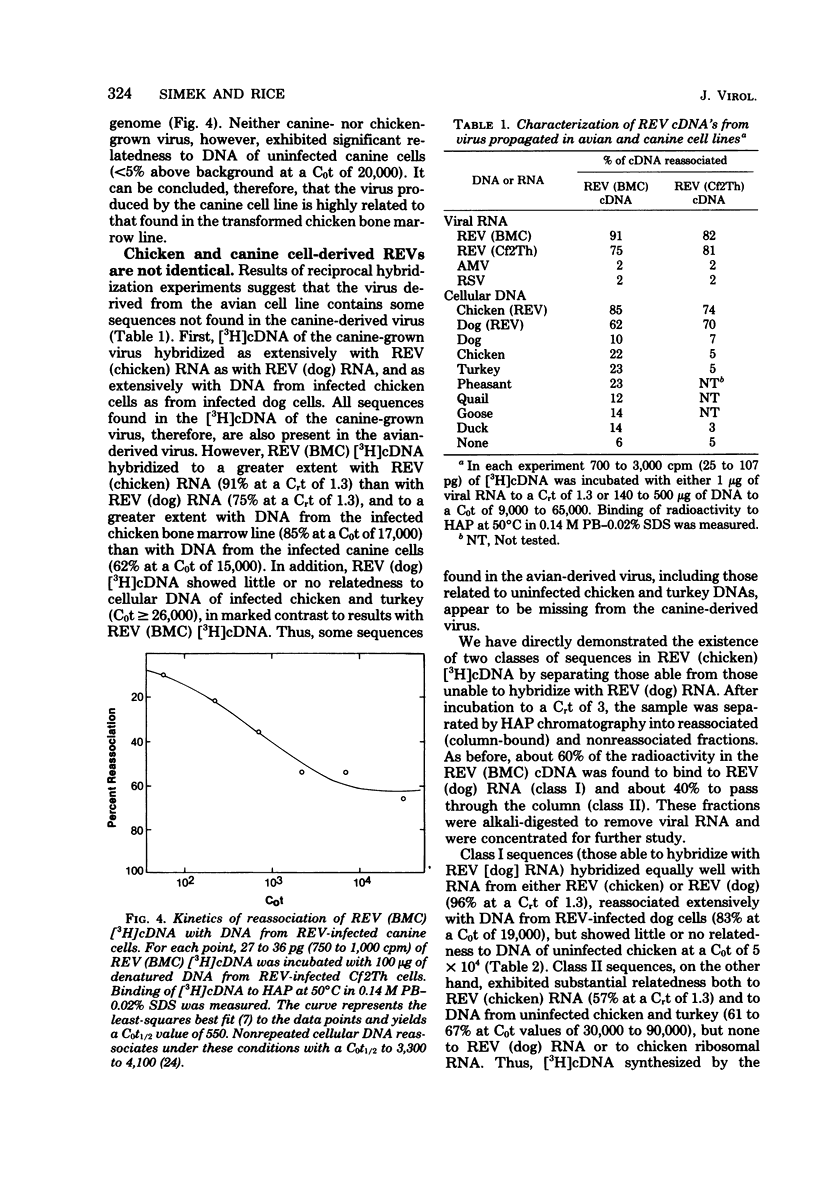
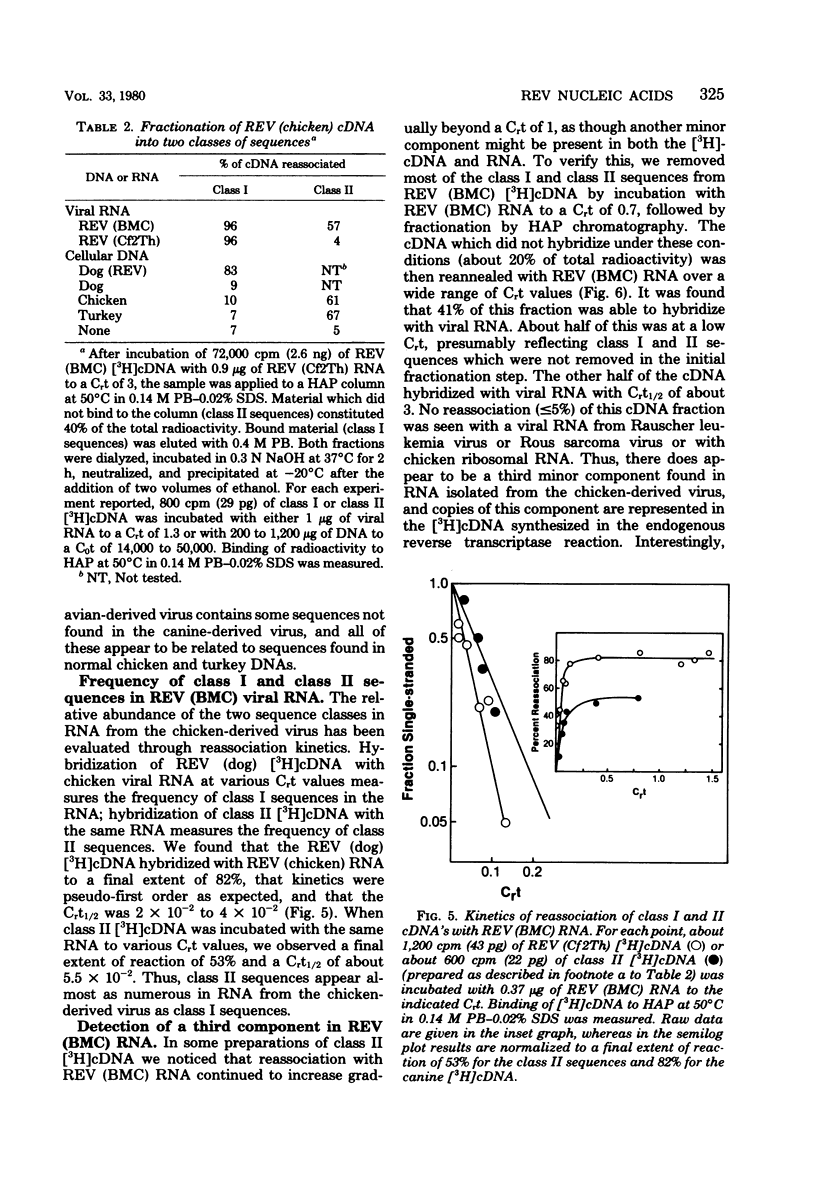
Abstract
Reticuloendotheliosis virus (REV) is known to be capable of transforming chicken bone marrow cells in vivo and embryo fibroblasts in vitro. As with spleen necrosis virus, we have found that sequences related to REV are found in DNA of several uninfected avian species. For example, about 15% of the [3H]cDNA synthesized in the endogenous reverse transcriptase reaction reassociated with DNA of uninfected chickens. Kinetic analysis revealed only a few (less than five) such sequences per haploid genome, and the thermal stability of the reassociated duplex indicated less than perfect complementarity. Comparison of REV propagated in an avian cell line with REV grown in a canine line has revealed clear differences between the two isolates. Viral RNA and [3H]cDNA of REV isolated from the transformed chicken bone marrow cell line appear to consist of at least three sequence classes. The most numerous of these classes is highly related to REV propagated in canine cells. Only slightly less abundant is a class unrelated to RNA isolated from the canine virus but highly related to sequences found in normal uninfected avian cellular DNA. A third component is present at about 1% the level of the most numerous class. Although REV appears to be unrelated to the other known avian retroviruses, distant relatedness between p30's of REV and various mammalian type C viruses has recently been reported. We have asked whether REV-related sequences can be detected in various mammalian DNAs and viral RNAs. Hybridization experiments performed at low stringency have revealed no such sequences.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G., Friis R. R., Mattersberger H., Hofschneider P. H. Controlled release of particle-associated RNA-dependent DNA polymerase by primary chick embryo cell cultures. Exp Cell Res. 1978 Dec;117(2):383–392. doi: 10.1016/0014-4827(78)90151-9. [DOI] [PubMed] [Google Scholar]
- Bauer G., Temin H. M. RNA-directed DNA polymerase from particles released by normal goose cells. J Virol. 1979 Mar;29(3):1006–1013. doi: 10.1128/jvi.29.3.1006-1013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton C. V., Hodge H. M., Fine D. L. Comparative large-scale propagation of retroviruses from Old World (Mason-Pfizer monkey virus) and New World (squirrel monkey virus) primates. In Vitro. 1978 Feb;14(2):192–199. doi: 10.1007/BF02618222. [DOI] [PubMed] [Google Scholar]
- Besmer P., Olshevsky U., Baltimore D., Dolberg D., Fan H. Virus-like 30S RNA in mouse cells. J Virol. 1979 Mar;29(3):1168–1176. doi: 10.1128/jvi.29.3.1168-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Charman H. P., Gilden R. V., Oroszlan S. Reticuloendotheliosis virus: detection of immunological relationship to mammalian type C retroviruses. J Virol. 1979 Mar;29(3):1221–1225. doi: 10.1128/jvi.29.3.1221-1225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W., Colcher D., Schochetman G., Schlom J. Distribution of Mason-Pfizer virus-specific sequences in the DNA of primates. J Virol. 1977 Jul;23(1):36–43. doi: 10.1128/jvi.23.1.36-43.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. B., Kang C. Y., Min-Min Wan K., Bose H. R., Jr Transformation of chick embryo fibroblasts by reticuloendotheliosis virus. Virology. 1977 Dec;83(2):313–321. doi: 10.1016/0042-6822(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Franklin R. B., Maldonado R. L., Bose H. R. Isolation and characterization of reticuloendotheliosis virus transformed bone marrow cells. Intervirology. 1974;3(5-6):342–352. doi: 10.1159/000149771. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Franklin R. B., Bose H. R., Jr Transformation by reticuloendotheliosis virus: development of a focus assay and isolation of a nontransforming virus. Virology. 1979 Feb;93(1):20–30. doi: 10.1016/0042-6822(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Howk R. S., Troxler D. H., Lowy D., Duesberg P. H., Scolnick E. M. Identification of a 30S RNA with properties of a defective type C virus in murine cells. J Virol. 1978 Jan;25(1):115–123. doi: 10.1128/jvi.25.1.115-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Bhown A. S., Bennett J. C. Amino-terminal amino acid sequence of the major structural polypeptides of avian retroviruses: sequence homology between reticuloendotheliosis virus p30 and p30s of mammalian retroviruses. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2708–2712. doi: 10.1073/pnas.75.6.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird C. D., McConaughy B. L., McCarthy B. J. Rate of fixation of nucleotide substitutions in evolution. Nature. 1969 Oct 11;224(5215):149–154. doi: 10.1038/224149a0. [DOI] [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R., Jr Group-specific antigen shared by the members of the reticuloendotheliosis virus complex. J Virol. 1976 Mar;17(3):983–990. doi: 10.1128/jvi.17.3.983-990.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Lack of serological relationship among DNA polymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken cells. J Virol. 1973 Sep;12(3):440–448. doi: 10.1128/jvi.12.3.440-448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe H., DuBuy J., Gilden R. V., Gardner M. B. A portion of the feline leukaemia virus genome is not endogenous in cat cells. Int J Cancer. 1978 Jul 15;22(1):70–78. doi: 10.1002/ijc.2910220114. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Simek S., Ryder O. A., Coggins L. Detection of proviral DNA in horse cells infected with equine infectious anemia virus. J Virol. 1978 Jun;26(3):577–583. doi: 10.1128/jvi.26.3.577-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sherwin S. A., Rapp U. R., Benveniste R. E., Sen A., Todaro G. J. Rescue of endogenous 30S retroviral sequences from mouse cells by baboon type C virus. J Virol. 1978 May;26(2):257–264. doi: 10.1128/jvi.26.2.257-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



