Abstract
Positive behavioral responses to attractive faces have led neuroscientists to investigate underlying neural mechanisms in a ‘reward circuit’ that includes brain regions innervated by dopamine pathways. Using male faces ranging from attractive to extremely unattractive, disfigured ones, this study is the first to demonstrate heightened responses to both rewarding and aversive faces in numerous areas of this putative reward circuit. Parametric analyses employing orthogonal linear and nonlinear regressors revealed positive nonlinear effects in anterior cingulate cortex (ACC), lateral orbitofrontal cortex (LOFC), striatum (nucleus accumbens (NAC), caudate, putamen), and ventral tegmental area (VTA), in addition to replicating previously documented linear effects in MOFC and LOFC and nonlinear effects in AMY and MOFC. The widespread nonlinear responses are consistent both with single cell recordings in animals showing responses to both rewarding and aversive stimuli and some human fMRI investigations of non-face stimuli. They indicate that the reward circuit does not process face valence with any simple dissociation of function across structures. Perceiver gender modulated some responses to our male faces: women showed stronger linear effects, and men showed stronger nonlinear effects, which may have functional implications. Our discovery of nonlinear responses to attractiveness throughout the reward circuit echoes the history of amygdala research: early work indicated a linear response to threatening stimuli, including faces; later work also revealed a nonlinear response with heightened activation to affectively salient stimuli regardless of valence. The challenge remains to determine how such dual coding influences feelings, like pleasure and pain, and guides goal-related behavioral responses, like approach and avoidance.
Keywords: facial attractiveness, fMRI, reward circuit
Introduction
The face is a highly significant vehicle for social communication across many species, and responsiveness to facial attractiveness has been of considerable interest to biologists, neuroscientists, and psychologists (Grammer, Fink, Møller, & Thornhill, 2003; Winston, O'Doherty, Kilner, Perrett, & Dolan, 2007; Rhodes & Zebrowitz, 2002). Psychological research demonstrates an ‘attractiveness halo’, whereby people with more attractive faces are judged more positively on a host of dimensions (Berscheid & Walster, 1974; Eagly, Ashmore, Makhijani, & Longo, 1991; Feingold, 1992) as well as given preferential treatment in many life domains (Langlois, Kalakanis, Rubenstein, Larson, Hallam et al., 2000). These effects appear to reflect some universal mechanism rather than arbitrary cultural inferences, since they are shown by people from diverse cultures as well as by infants and young children (Cunningham, Roberts, Barbee, & Druen, 1995; Dion, 2002; Kramer, Zebrowitz, San Giovanni, & Sherak, 1995; Langlois, Ritter, Roggman, & Vaughn, 1991; Ramsey, Langlois, Hoss, Rubenstein, & Griffin, 2004).
One explanation for reactions to variations in facial attractiveness is that they derive from variations in similarity to anomalous faces. On this account, adaptive responses to individuals with diseases or bad genes are overgeneralized to normal individuals whose faces resemble those who are unfit (Zebrowitz, 1997; Zebrowitz, Bronstad, & Montepare, in press). Consistent with this hypothesis, an artificial neural network trained to recognize the facial metrics of anomalous, disfigured faces was subsequently activated more by the metrics of normal unattractive faces than attractive faces (Zebrowitz, Fellous, Mignault, & Andreoletti, 2003). In addition, greater similarity of normal faces to disfigured ones predicted lower attractiveness ratings as well as impressions of more unfit traits - poorer health, lower intelligence, and lower sociability - the attractiveness halo effect (Zebrowitz, et al., 2003; Zebrowitz & Rhodes, 2004). Also consistent with the suggestion that disfigured faces anchor the low end of the attractiveness continuum is evidence that the attractiveness halo effect is driven more by the perception that ‘ugly is bad’ than by the perception that ‘beautiful is good’ (Griffin & Langlois, 2006).
The positive behavioral response to attractive faces has led neuroscientists to investigate underlying neural mechanisms in a reward circuit that includes brain regions innervated by dopamine pathways, including amygdala (AMY), anterior cingulate cortex (ACC), lateral and medial orbital frontal cortex (LOFC, and MOFC), striatum (caudate, nucleus accumbens (NAC) putamen), and ventral tegmental area (VTA) (Gurevich & Joyce, 1999; Martinez, Slifstein, Broft, Mawlawi, Hwang, Huang et al., 2003; Mawlawi, Martinez, Slifstein, Broft, Chatterjee, Hwang et al., 2001). The function of this reward system is to guide goal-related behavioral responses, such as approach and avoidance (Delgado, 2007). Animal research has shown that many of these brain regions may serve that function by processing both the appetitive and the aversive qualities of stimuli. For example, single cell recording in monkey caudate nucleus and putamen showed neurons within each region that responded to anticipation of reward, punishment, and no outcome. Some neurons responded to all events, some responded to two, some responded to one, and others responded to none (Yamada, Matsumoto, & Kimura, 2004). Similar results have been reported for neuronal responsiveness in monkey amygdala (Nishijo, Ono, & Nishino, 1988), monkey ACC (Koyama, Kato, Tanaka, & Mikami, 2001), the lateral part of monkey OFC (Hosokawa, Kato, Inoue, & Mikami, 2007), and in dopamine neurons within the VTA (Horvitz, 2000).
Although single cell recording is not feasible in human research, examining nonlinear responses to facial attractiveness in fMRI would indicate whether a particular brain region is activated more by both rewarding and aversive stimuli than by neutral ones. However, fMRI research investigating human responses to facial attractiveness has typically examined only linear responses within the various reward regions. Activation in LOFC and putamen increases with decreasing attractiveness (Cloutier, Heatherton, Whalen, & Kelley, 2008), activation in MOFC increases with increasing attractiveness (Cloutier et al., 2008; Kawataba & Zeki, 2007; O'Doherty, Winston, Critchley, Perrett, Burt et al., 2003), as does activation in striatum, including NAC (Aharon, Etcoff, Ariely, Chabris, O'Connor et al., 2001; Cloutier, Heatherton, Whalen, & Kelley, 2008; Kampe, Frith, Dolan, & Frith, 2001), although the NAC effect was not replicated in a study that intermixed faces of both sexes (O'Doherty, et al., 2003). The opposite linear effects in LOFC and putamen as compared with MOFC and striatum could be construed as reflecting a dissociation between processing of ‘punishment’ in the former and ‘reward’ in the latter. However, with the exception of MOFC and AMY, investigators have rarely examined nonlinear responses to attractiveness. Moreover, the relatively narrow range of attractiveness in previous research may have obscured nonlinear effects.
One region that has shown nonlinear effects is AMY, with higher activation to faces high or low in attractiveness than those of medium attractiveness (Winston et al., 2007; Krendl, Macrae, Kelley, Fugelsang, & Heatherton, 2006), an effect that is consistent with evidence that AMY response reflects affective salience rather than positive or negative valence (Fitzgerald, Angstadt, Jelsone, Nathan, & Phan, 2006; Phelps, 2006; Said, Baron, & Todorov, 2008). There also is some evidence for a positive non-linear effect of attractiveness in MOFC (Winston et al., 2007). Other fMRI research examining human responses to stimuli other than facial attractiveness also has demonstrated a non-linear effect of monetary reward in MOFC and LOFC, with either high or low rewards eliciting higher activation than moderate outcomes (Elliott, Newman, Longe, & Deakin, 2003). In addition, both rewarding and aversive outcomes elicit activation in the striatum (Delgado, Locke, Stenger, & Fiez, 2003; Jensen et al., 2003, 2007; Seymour et al., 2004). These results coupled with the animal research suggest that many regions in the human reward circuit may be activated by both rewarding and aversive faces. To investigate this possibility, we examined linear and nonlinear activation to variations in facial attractiveness that included high attractive, medium attractive, and unattractive disfigured faces, taken from an atlas of genetic anomalies. Examining the neural response to attractiveness when disfigured faces anchor the low end of the continuum not only provides a better opportunity to observe nonlinear effects in regions heretofore reported as showing only a positive linear effect, but also is consistent with psychological theory and research, as discussed above.
Guided by the hypothesis that the reward value of facial attractiveness may vary with the sex of the perceiver, several studies have investigated whether the neural response to attractiveness is modulated by gender. No gender modulation of the response to attractiveness has been shown in the striatum (Kampe et al., 2001), AMY (Winston et al, 2007), NAC, or LOFC (Cloutier et al. 2008). On the other hand, only men showed an enhanced ACC response to attractive male and female faces (Winston et al. (2007), and Cloutier et al., (2008) found that greater facial attractiveness in opposite sex faces elicited greater MOFC activation in male but not female perceivers. In addition, stronger MOFC activation has been found for sexually preferred faces either when the faces included a range of attractiveness (Kranz & Ishai, 2006) or when they were highly attractive (Ishai, 2007). These findings, coupled with the fact that all the faces in the present study were male, led us to investigate whether the nonlinear and linear neural responses to faces that range from attractive to disfigured are modulated by gender.
Materials and Methods
Subjects
fMRI data were provided by 17 healthy, right handed (self-reported), Caucasian participants (9 females), 21-36 years (M=26.5), each paid $80. Subjects with neurological or psychiatric problems were excluded. Attractive ratings on 7-point scales were provided by a different group of 17 Caucasian raters (9 females) 18-21 years old (M = 18.8), who received credit toward an Introductory Psychology course requirement. The experimental protocol was approved by IRBs at MGH/MIT/HST/Martinos Center and Brandeis University, and conformed to all regulatory standards, including the ethical standards laid down in the 1975 Declaration of Helsinki as revised in 1983. All participants signed an informed consent form prior to their inclusion in the study.
Stimuli
120 grayscale faces with direct gaze formed twelve equal a priori categories: high attractive, low attractive, disfigured, babies, babyfaced, maturefaced, elderly, female, angry, disgust, fear and happy. Except for the female and baby categories, all were adult males. Except for the emotion faces, all had neutral expressions with closed mouths. Mean luminance and contrast of faces was equated using the Michelson contrast, eyes were at the same height, and middle of the eyes was at the same plane location. A small fixation cross was superimposed on the mid-point of the eyes. All neutral expression faces had been used in a previous study (Zebrowitz et al., 2003).
The current study focused on faces in the first three categories, which were all male: high attractive, low attractive, disfigured.1 Faces in the disfigured category were adult men from atlases depicting birth defects and syndromes characterized by face deformity (sources and a summary table of anomalies is available from the second author). The high and low attractive men were drawn from a sample of 185 adolescent men from the Intergenerational Studies (IGS) archive, a longitudinal study of representative samples of individuals born in Berkeley California or attending school in Oakland, CA (Eichorn, 1981). All 185 men had previously been rated on a 7-point scale of attractiveness (Zebrowitz, Olson, & Hoffman, 1993). Faces in the high attractive category had scored at or above the 90th percentile in rated attractiveness; those in the low attractive category had scored in the bottom 20th percentile. When the faces were re-rated on the same scale in the context of the 12 face categories used in the present study, the designation of the high attractive category was sustained, with a mean rating higher than all other categories except for the babies. However, the low attractive faces were judged as medium in attractiveness within this new context that included disfigured faces as well as negative emotion expressions, receiving a mean z score close to zero (z = -.07). We therefore refer to our three face categories as attractive, medium, and disfigured.
Experimental paradigm
Faces were presented in a six-run block design. All 120 faces were shown in each run with the sequence of the 12 categories randomized across runs.2 Within each run, a 20 s block of 10 faces from the same category was alternated with a 20 s block of 10 fixation crosses. Both faces and fixation crosses were presented for 0.2s each, followed by a blank grey screen for 1.8s.
Data acquisition
MRI scanning was conducted at MGH/MIT/HST/Martinos Center. Stimuli were presented by E-Prime with a back-projected device and mirrors mounted on the head coil. Participants passively viewed faces with instructions to maintain central fixation and to press a button whenever they saw a big fixation cross. The latter task was included to maintain alertness, and fixation crosses were equally distributed between each of the face blocks.
235 volumes were acquired on a head coil Siemens 3T scanner. Scanning included T2*-weighted functional images followed by (1) T1-weighted structural images for co-registration purposes, and (2) Two T1, 128 sagittal images (1×1×1.33mm) for anatomy. Functional images included 25 contiguous axial oblique (AC-PC line) 6 mm slices (TR=2000 ms; TE=30 ms; FOV=40×20cm; 25 slices; voxel resolution= 3.1×3.1×6mm).
fMRI image analysis
fMRI data processing was performed using FEAT5.43 (www.fmrib.ac.ox.uk/fsl). We applied the following pre-statistics processing: motion correction using MCFLIRT (Jenkinson et al., 2002); non-brain removal using BET; spatial smoothing (FWHM=10mm); high-pass filtering (sigma=50s). Statistical analysis was performed using FILM with local autocorrelation correction.
We used a parametric design (Buchel et al., 1998) to investigate linear and nonlinear BOLD responses to facial attractiveness in the whole brain with a focus on a priori ROIs in the reward circuitry: ACC amygdala, caudate, MOFC, LOFC, NAC, putamen, and VTA. These were all anatomical ROIs, defined by coordinates in Montreal Neurological Institute (MNI) space using MARINA software (Walter. et al., 2003). To create the regressor for face perception, representing response to faces relative to baseline, the presentation of each face was modeled by convolving a delta function at each event onset with a canonical hemodynamic response function and its temporal derivative. In addition to that regressor, we also included first-order linear and second-order nonlinear regressors that were parametrically adjusted by facial attractiveness ratings. All the regressors were orthogonal. In addition to identifying regions that showed effects across all subjects, we performed subtraction contrasts to determine whether the effects differed between genders: male – female linear; female – male linear; male – female nonlinear; female – male nonlinear.
We performed whole brain first- level analysis on each subject to generate a single statistical map corresponding to the 3 regressors (face, linear, nonlinear) using GLM within FEAT. We then normalized the results to the MNI-152 template using FSL's FLIRT (Jenkinson et al., 2002). Mixed-effects group analyses were performed using FSL's FLAME. To test our a priori predictions concerning activation in reward regions, Z (Gaussianised T/F) statistical maps of activation within the ROIs were thresholded using a voxel-level threshold of p<.05. Because we had a lenient voxel-level threshold, we used Alphasim in AFNI (Ward 2000) to correct for false positives. Alphasim is a Monte Carlo simulation that determined the minimum cluster size in each ROI needed for an overall alpha of p < .05 taking into account both height and spatial extent. When we failed to show previously documented gender modulation of the linear effect in MOFC using the foregoing threshold, we relaxed the cluster size threshold to achieve an overall alpha of p < 0.10 when taking into account both height and cluster size. Z (Gaussianised T/F) statistical maps of gender differences in activation were thresholded and corrected for false positives using the same criteria.
Additional analyses using 3 discrete face categories (attractive, medium, disfigured) were performed to explicate the precise pattern of linear and nonlinear effects and gender modulation shown in the parametric analyses. Specifically, we performed one way analyses of variance (ANOVA) on percent signal change (PSC) in the three a priori face categories (attractive, medium, disfigured) as well as 2 (participant gender) × 3 (face category) ANOVAs on PSC in regions showing gender modulation. Regressors corresponding to each face category convolved with HRF were used to model the time series. With fixation as baseline, we set up the contrasts: attractive vs. fixation, medium vs. fixation, disfigured vs. fixation. To extract PSC for attractive, medium, and disfigured faces, 6-mm spherical ROIs were defined on the coordinates of the peaks determined in whole brain parametric analyses for the category contrasts and for the gender subtraction contrasts defined above. To extract PSC, we used the software PEATE (http://www.jonaskaplan.com/peate/) with the time resolution of 1s, yielding 20 time points in the time courses for each face category.
Results and Discussion
Face category attractiveness
A face category (3) analysis of variance performed on the mean attractiveness ratings for the attractive (M = 4.53, SD = .49), medium (M = 3.67, SD = .78), and disfigured face (M = 1.50, SD = .20) categories confirmed the decreasing attractiveness of these a priori groups, F(2,27) = 82.40, p < .0001. Post-hoc LSD tests showed that each category differed significantly in attractiveness from the others, all ps ≤ .001. The correlation between ratings of these male faces by male and female judges was r (28) = .96, p < .0001, and we therefore collapsed the ratings across judge sex. The parametric analyses ordered the faces according to their attractiveness ratings within the fMRI face set (Figure 1).
Figure 1.
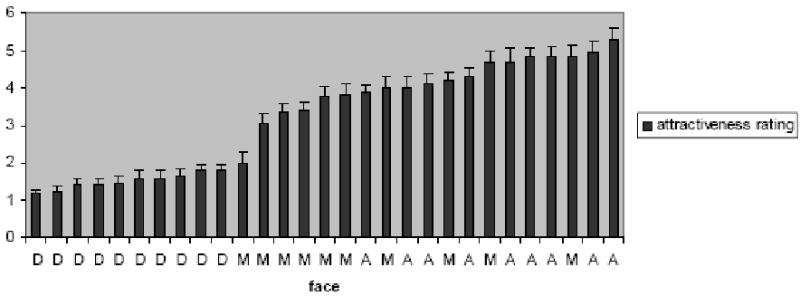
Attractiveness ratings of all 30 faces, with the error bar showing standard errors. A: attractive faces; M: medium attractive faces; D: disfigured faces.
Linear and Nonlinear Activation to Variations in Attractiveness
Replication of previous findings
Our results replicate previously documented linear and nonlinear effects of attractiveness on neural activation with positive linear effects in ACC and left MOFC, negative linear effects in left and right LOFC (Aharon et al., 2001; Cloutier, 2008; Kawabata & Zeki, 2004, O'Doherty et al., 2003, Winston et al., 2007), and a positive nonlinear effect in right amygdala (Winston et al., (2007). The latter effect is consistent with other evidence that amygdala activation tracks the affective salience of a stimulus rather than positive or negative valence both in human fMRI research (Fitzgerald et al., 2006; Phelps, 2006; Said, Baron, & Todorov, 2008), and in single cell recording in monkeys (Nishijo et al., 1988). Although we failed to replicate previously documented positive nonlinear responses to variations in attractiveness in left and right MOFC (Krendl et al., 2007; Winston, et al., 2007), we did find a positive nonlinear effect that was modulated by gender as described below. The significant effects are shown in significant linear and nonlinear contrasts summarized in Table 1 and depicted in Figures 2 and 3B as well as in PSC for the three face categories plotted in Figures 4 and 5B and analyzed below.
Table 1.
Brain regions in which parametric analyses of BOLD signal revealed linear or nonlinear effects with respect to facial attractiveness ratings.
| Brain region | Z value | x | y | z | cluster size | minimum cluster size |
|---|---|---|---|---|---|---|
| Positive linear effect | ||||||
| ACC | 2.2 | 0 | 32 | 18 | 1306 | 275 |
| Left MOFC | 2.3 | -1 | 52 | -6 | 118 | 110 |
| Negative linear effect | ||||||
| Left LOFC | 2.2 | -40 | 22 | -12 | 250 | 194 |
| Right LOFC | 2.8 | 42 | 3 | -12 | 811 | 197 |
| Positive nonlinear effect | ||||||
| ACC | 2.3 | -2 | 16 | 30 | 612 | 275 |
| Right Amygdala | 2.6 | 18 | -8 | -12 | 79 | 74 |
| Left Caudate | 2.3 | -10 | 16 | 8 | 276a | 129a |
| Right Caudate | 2.1 | 10 | 6 | 2 | 120 | 90 |
| Left LOFC | 2.5 | -28 | 24 | -8 | 368 | 194 |
| Right LOFC | 2.8 | 32 | 26 | -4 | 332 | 197 |
| Left NAC | 2.5 | -6 | 10 | -4 | 276a | 129a |
| Left Putamen | 2.3 | -28 | 10 | 2 | 246 | 144 |
| Right Putamen | 2.3 | 28 | 12 | 4 | 474 | 143 |
| VTA | 2.3 | 0 | -24 | -16 | 159 | 107 |
Note. Shown for each ROI are: the maximum Z score, location of peak (MN1 space), number of voxels activated, and minimum cluster size needed for an overall alpha of p < .05 (Alphasim, Ward, 2000).
This cluster size considered the activated voxels in both left caudate and left NAC as a whole.
Figure 2.
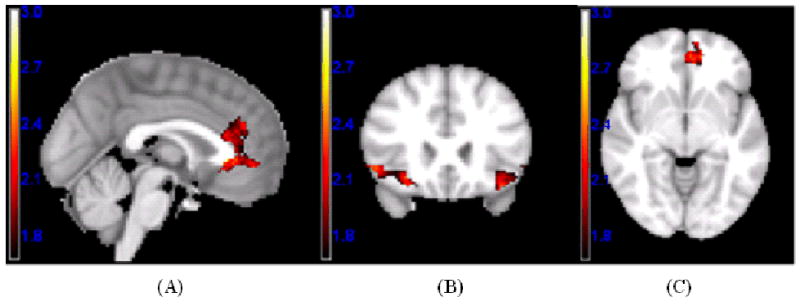
Significant linear responses to variations in facial attractiveness using a voxel-level threshold p < .05 and a cluster-extent threshold derived from Alphasim (Ward, 2000) to achieve an overall alpha of p < .05, Z-maps are illustrated in the following regions: (A) ACC (Positive linear effect); (B) left and right LOFC (Negative linear effect); (C) left MOFC (Positive linear effect).
Figure 3.
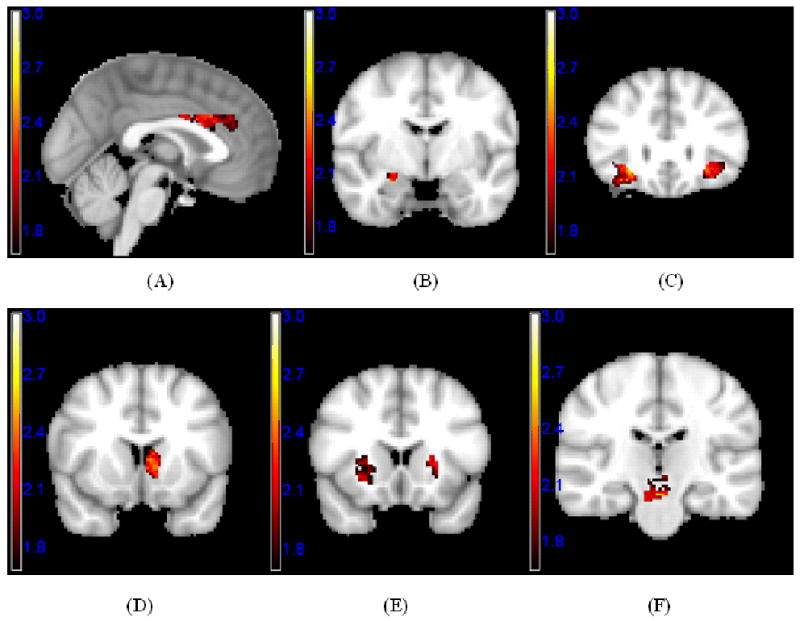
Significant positive nonlinear responses to variations in facial attractiveness using a voxel-level threshold p < .05 and a cluster-extent threshold derived from Alphasim (Ward, 2000) to achieve an overall alpha of p < .05, Z-maps are illustrated in the following regions: (A) ACC; (B) right amygdala; (C) left and right LOFC; (D) left caudate and NAC; (E) left and right putamen; (F) VTA.
Figure 4.
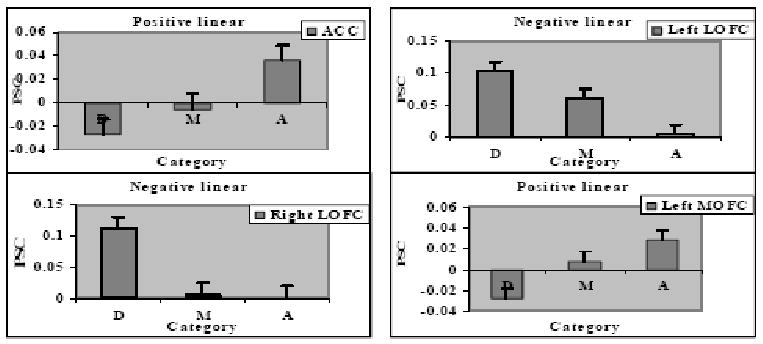
Linear effect of attractiveness shown in percent signal change (PSC) to disfigured faces, D, medium attractive faces, M, and attractive faces, A, in 4 ROIs: (A) ACC; (B) Left LOFC; (C) Right LOFC; (D) Left MOFC.
Figure 5.
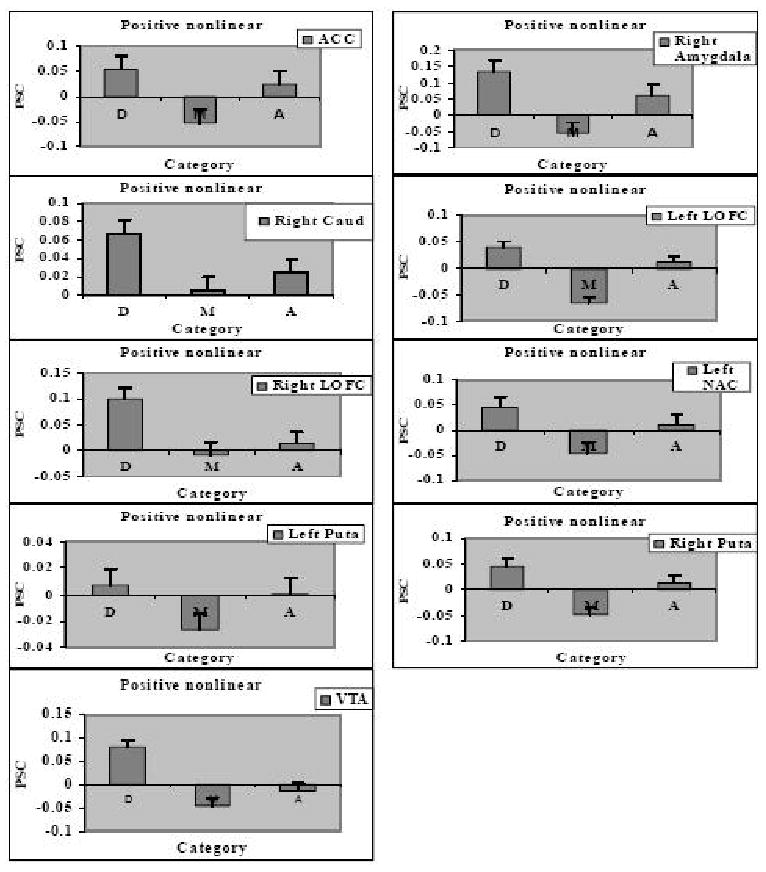
Nonlinear effect of attractiveness shown in percent signal change (PSC) to disfigured faces, D, medium attractive faces, M, and attractive faces, A, in 9 ROIs: (A) ACC; (B) Right amygdala; (C) Right Caudate; (D) Left LOFC; (E) Right LOFC; (F) Left NAC; (G) Left putamen; (H) Right putamen; (I) VTA.
Analyses of variance on PSC corroborated the significant positive linear effects in the parametric analyses in ACC, F(1,57)= 11.08, p= .002, and left MOFC, F(1,57)= 16.64, p< .001, both reflecting greater activation to attractive than disfigured faces. Pairwise comparisons further revealed greater activation in ACC to attractive than medium faces, p = .03, with no difference between medium and disfigured faces, p = .27, and greater activation in left MOFC to medium than disfigured faces, p = .02, but no difference between attractive and medium faces, p = .12,. Significant negative linear effects in left LOFC, F(1, 57)= 21.89, p< .001 and right LOFC, F(1, 57)= 16.82, p< .001, both reflected greater activation to disfigured than attractive faces. Pairwise comparisons further revealed greater activation to disfigured than medium faces in both left and right LOFC, respective ps < .05 and 001, and greater activation to medium than attractive faces in left LOFC, p = .01, but not right LOFC, p = .84. The positive nonlinear effect in right amygdala, F (1,57)= 13.66, p<.001, revealed greater activation to attractive than medium faces, p = .02, as well as greater activation to disfigured than medium faces, p < .001, with no significant difference between attractive and disfigured faces, p = .13.
Additional non-linear effects
In addition to replicating previously reported linear and nonlinear effects as summarized above, our research is the first to demonstrate positive nonlinear responses to attractiveness in other regions of the ‘reward circuit’: ACC, caudate, LOFC, NAC, putamen, and VTA. We found no significant negative nonlinear responses to attractiveness. The significant effects are shown in significant nonlinear contrasts summarized in Table 1 and depicted in Figures 3 and 5B as well as in PSC for the three face categories plotted in Figure 5 and analyzed below.
Analyses of variance on PSC corroborated the significant positive nonlinear effects in the parametric analyses: ACC, F(1,57)= 7.77, p< .01; right caudate, F (1,57)= 5.76, p = .02,, left and right LOFC, respective Fs (1,57)= 48.24 and 5.18, ps < .001 and .05; left NAC, F(1,57) = 8.69, p= .005; left and right putamen, respective Fs (1,57) = 4.25 and 20.14, ps < .05 and .001; and VTA, F(1,57)= 17.00; p < .05. Pairwise comparisons revealed significantly higher activation to disfigured than medium faces in all regions, all ps < .01 except left putamen, p = .05. There also was significantly higher activation to attractive than medium faces in ACC, left LOFC, left NAC, and right putamen, all ps < .05 except left NAC, p = .06., but no significant difference in activation to attractive vs. medium faces in right caudate, left putamen, right LOFC and VTA. Finally, there was no significant difference in activation to attractive and disfigured faces in ACC, left NAC, or left and right putamen, all ps > .10, while disfigured faces elicited greater activation than attractive ones in right caudate, right LOFC, and VTA, ps < .05, with a marginally significant trend in left LOFC, p = .08.
The foregoing positive nonlinear responses are consistent with animal research demonstrating that neurons in each of these regions do not respond exclusively to stimuli of a single valence, but rather to rewarding and aversive ones (Horvitz, et al., 2000, VTA; Hosokawa et al., 2007, LOFC; Koyama et al., 2001, ACC; Yamada et al., 2004, (striatum -- caudate, putamen). They also are consistent with human fMRI research showing a nonlinear response to monetary rewards in LOFC (Elliott et al., 2003), and activation of putative reward regions in striatum by both rewarding and aversive stimuli (Jensen et al., 2007; O'Doherty, Buchanan, Seymour, & Dolan, 2006; Seymour et al., 2004). The most obvious reason for our discovery of novel positive nonlinear responses to attractiveness in the reward circuitry is that previous investigators have rarely looked for nonlinear effects in these regions. In addition, anchoring the attractiveness continuum with disfigured faces may increase the likelihood of finding nonlinearity in regions previously shown to increase with increasing attractiveness when there is no low anchor, such as NAC and VTA (Aharon et al., 2001; Cloutier; Kawabata & Zeki, 2004, O'Doherty et al., 2003, Winston et al., 2007). Although including unattractive, disfigured faces may strengthen nonlinear effects, this does not negate our evidence that the reward circuitry responds both to positively and negatively valenced facial structures. Moreover, there is both theoretical and empirical basis for the claim that these faces fall on a parametric continuum of attractiveness (Zebrowitz et al., 2003, 2008; Zebrowitz & Rhodes, 2004).
Gender modulation
The nonlinear responses to variations in attractiveness in amygdala, left and right LOFC, left and right putamen, and VTA were not modulated by gender and neither were the linear responses in ACC, left MOFC, and left LOFC. However, several other effects did vary with participant sex, with women showing stronger linear effects and men showing stronger nonlinear effects. This gender modulation is shown in significant gender contrasts within various ROIs in the parametric analyses that are summarized in Table 2 and depicted in Figures 6 and 7, as well as in PSC for the three face categories for men and women, plotted in Figures 8 and 9, and analyzed below.
Table 2.
Brain regions in which there were gender differences in the linear or nonlinear effects of facial attractiveness identified by parametric analyses
| Brain regions | Z value | x | y | z | cluster size | minimum cluster size |
|---|---|---|---|---|---|---|
| Linear effect | ||||||
| Females-males | ||||||
| Right LOFC | 2.9 | 36 | 54 | -12 | 305 | 197 |
| Right MOFC | 2.2 | 12 | 54 | -6 | 112 | 129(97a) |
| Males-females | ||||||
| No Effects | ||||||
| Nonlinear effect | ||||||
| Females-males | ||||||
| No effects | ||||||
| Males-females | ||||||
| ACC | 3.4 | -2 | 36 | 0 | 1059 | 257 |
| Left Caudate | 2.5 | -10 | 16 | 2 | 239b | 129b |
| Right Caudate | 2.5 | 12 | 18 | -2 | 247c | 143c |
| Left MOFC | 3.1 | -4 | 52 | -4 | 463 | 110 |
| Right MOFC | 3.7 | 10 | 44 | -8 | 507 | 129 |
| Left NAC | 2.5 | -12 | 18 | -4 | 239b | 129b |
| Right NAC | 2.5 | 10 | 18 | -2 | 247c | 143c |
Note. Shown for each ROI are: the maximum Z score, location of peak (MNI space), number of voxels activated, and minimum cluster size needed for an overall alpha of p < .05 (Alphasim. Ward, 2000).
This minimum cluster size needed for an overall alpha of p < .10 (Alphasim Ward, 2000).
This cluster size considered the activated voxels in both left caudate and left NAC as a whole.
This cluster size considered the activated voxels in both right caudate and right NAC as a whole.
Figure 6.
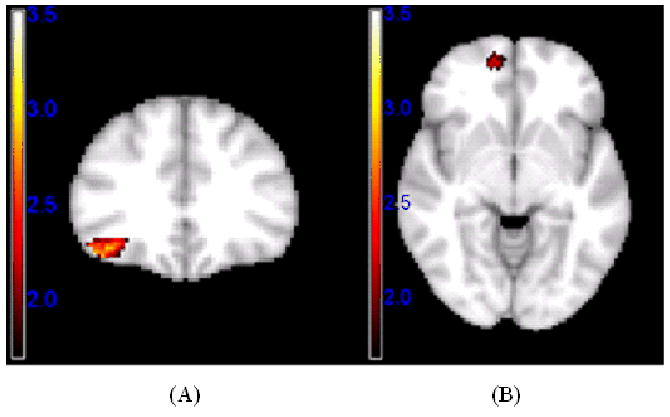
Significant gender modulation of linear responses to variations in facial attractiveness using a voxel-level threshold p < .05 and a cluster-extent threshold derived from Alphasim (Ward, 2000) to achieve an overall alpha of p < .05, Z-maps are illustrated in the following regions: (A) right LOFC for female - male contrast; and an overall alpha of p < .10 for (B) Right MOFC for female-male contrast.
Figure 7.
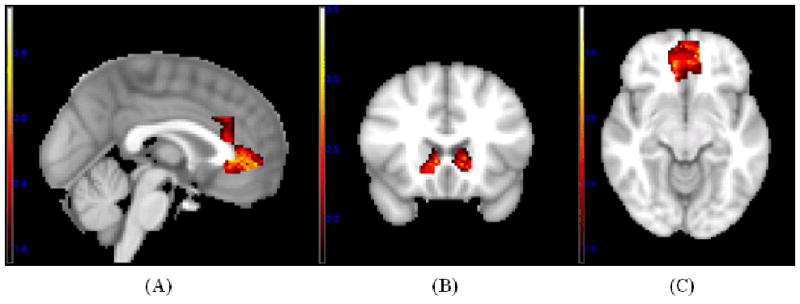
Significant gender modulation of nonlinear responses to variations in facial attractiveness using a voxel-level threshold p < .05 and a cluster-extent threshold derived from Alphasim (Ward, 2000) to achieve an overall alpha of p < .05, Z-maps are illustrated in the following regions: (A) ACC for male - female contrast; (B) left and right caudate and NAC (striatum) for male - female contrast; (C) MOFC for male - female contrast.
Figure 8.
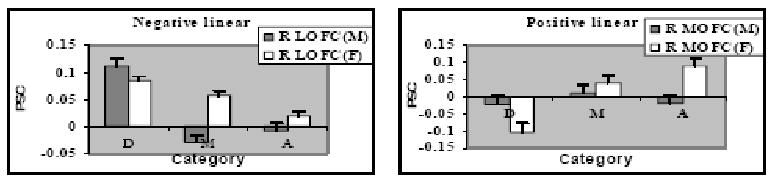
Gender differences in linear effect of attractiveness shown in percent signal change (PSC) to disfigured faces, D, medium attractive faces, M, and attractive faces, A, in 2 ROIs: (A) Right LOFC; (B) Right MOFC.
Figure 9.
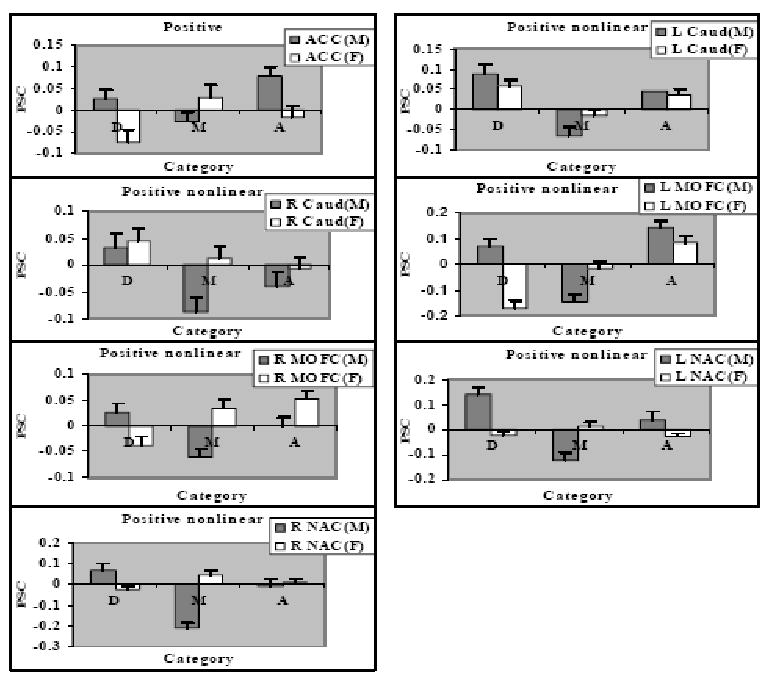
Gender differences in the positive nonlinear effect of attractiveness shown in percent signal change (PSC) to disfigured faces, D, medium attractive faces, M, and attractive faces, A, in 7 ROIs: (A) ACC; (B) Left Caudate; (C) Right Caudate; (D) Left MOFC; (E) Right MOFC; (F) Left NAC; (G) Right NAC. Table 1. Brain regions in which parametric analyses of BOLD signal revealed linear or nonlinear effects with respect to facial attractiveness ratings.
In the case of linear effects, the male-female contrast showed no differences, but the female-male contrast revealed sex differences in the linear response to attractiveness in right LOFC and right MOFC (Table 2, Figures 6 and 8). Analyses of variance performed on PSC revealed significant gender × face category effects in right LOFC, F (2,57) = 17.99, p < .001, and right MOFC, F (2, 57) = 6.18, p =.004. Analyses within each gender revealed that the negative linear effect in right LOFC was significant for both sexes, ps < .001, but only women showed a significant decrease in response with each level of increasing attractiveness, both ps < .05, while men showed a significantly stronger response to disfigured faces than the other two categories, both ps = .001, which did not differ, p > .25 (Fig. 8). The positive linear effect in right MOFC was significant for women, p < .001, but not for men, p > .95, and women showed lower activation to disfigured faces than to high or medium attractive faces, ps< .001, which did not differ, p> .15.
Since the faces were all male, gender differences in the linear effects of attractiveness are consistent with previous evidence for a stronger response to attractiveness in MOFC when people view sexually preferred faces (Kranz & Ishai, 2006), and they suggest that the linear response in MOFC reflects the valence of potential sexual partners more than that of facial aesthetics. On the other hand, the restriction of the linear trend to women contrasts with other evidence that MOFC response to more attractive opposite sex faces was limited to men (Cloutier et al., 2008). Our inclusion of extremely low attractive disfigured male faces may account for the differing results. Indeed, women's positive linear response to facial attractiveness in MOFC reflected a significantly weaker response to disfigured faces than to medium or attractive faces, which did not differ. This is an interesting contrast to women's more fine-grained response to facial attractiveness in right LOFC.
In the case of non-linear effects, the female-male contrast showed no differences, but the male-female contrast showed stronger nonlinear responses to attractiveness for men in ACC, left and right caudate, left and right MOFC, and left and right NAC (Table 2, Figures 7 and 9). Analyses of variance on PSC yielded gender × face category effects in each of the foregoing regions: F (2, 57) = 6.49, p = .003 for ACC; Fs (2,57) = 2.80 and 3.44 for left and right caudate, ps = .07 and .04, respectively; Fs (2,57) = 18.95 and 9.42 for left and right MOFC, ps < .001; Fs (2,57) = 25.24 and 45.75, for left and right NAC, ps < .001. Analyses within each gender revealed that men showed significant positive nonlinear responses in all of these regions, ps ≤ .01, whereas women showed a significant positive nonlinear response only in left caudate, p < .001.
Pairwise comparisons for men revealed that the positive nonlinear effects reflected significantly stronger activation to both disfigured and attractive faces than to medium ones, all ps < .01, except for ACC with disfigured vs. medium p = .08, and right caudate with attractive vs medium p = .22. Pairwise comparisons for women revealed that the nonlinear effect in left caudate reflected significantly stronger activation to both disfigured and attractive faces than to medium ones, both ps ≤.01.
Ours is the first evidence for gender modulation of nonlinear neural responses to attractiveness in the reward circuit. Because all of our faces were male, a full explanation for these effects requires additional research investigating sex differences in the nonlinear response to similar variations in female attractiveness as well to other types of highly polarized stimuli. However, some possible explanations can be considered. One is that variations in the attractiveness of male faces are more likely to engage the neural response to stimulus salience in male than female perceivers, with the latter responding more to stimulus valence, as discussed above. Perhaps variations in the attractiveness of female faces would engage the neural response to stimulus salience in female perceivers, with males responding more to valence. Evidence that own-sex faces are more salient is provided by research demonstrating better recognition of own-than other-sex faces (Shaw & Skolnick, 1994; Wright, 2003), although this result may be more reliable for females (Rehnman & Herlitz, 2007). Research has not yet explained the own-sex advantage in face recognition, but the effect suggests greater attention to own-sex faces, a tendency that may be heightened when they have the intrinsic salience of high or low attractiveness.
Another possible explanation for mens' stronger nonlinear response to attractiveness is that men experience greater arousal than women do, not only when viewing more attractive faces, as Winston et al. (2007) observed, but also when viewing extremely unattractive, disfigured faces. An indication of greater arousal in male perceivers reported by Winston et al. (2007) was a stronger positive linear response to attractiveness of male and female faces in ACC that was correlated with physiological indicators of arousal. Although we had no direct measures of arousal in the present study, our male-female contrasts also showed gender modulation of the nonlinear response to attractiveness in ACC which, as noted above, was significant for men but not for women. Since Winston et al. (2007) found that men showed greater arousal to more attractive faces of either sex, the arousal explanation for gender modulation of nonlinear effects would predict similar effects for female faces. The overall pattern of sex differences in neural activation to our male faces is consistent with both of the foregoing explanations. Men showed lower activation than women to medium attractive faces and higher activation to disfigured faces, consistent with either a stronger salience or arousal effect for men. Men also showed equal or lower activation than women to attractive faces, consistent with a salience or arousal effect for men that was matched or exceeded by a positive valence effect for women.
Alternative Explanations
Some possible alternative explanations for the effects of facial attractiveness warrant consideration. Like aversive stimuli, novel stimuli have also been shown to activate putative reward regions, both in animal research (Horvitz, 2000), and in human research (Bunzeck & Düzel, 2006). This is noteworthy because the disfigured faces that elicited high activation in these regions in our study were both aversive to look at and relatively novel. Whereas the only type of novelty that elicited higher VTA activation in the Bunzeck & Duzel (2006) study was the absolute novelty of being seen less often in the experiment, all of our faces were seen equally often. Thus, the disfigured faces were novel only in the sense of being farther removed from a typical face. This is actually an inherent feature of unattractive faces, even if not disfigured. Less attractive faces are farther from the population average and thus more novel than high attractive faces, which are closer to the population average and more prototypical (Langlois & Roggman, 1990; Potter, Corneille, Ruys, & Rhodes, 2007; Rhodes & Tremewan, 1996). Whereas one might suggest that the observed responses to disfigured faces in the reward circuit reflect their novelty rather than their negative valence, it is less plausible to suggest that the responses to attractive faces reflect their typicality rather than their positive valence, and neural responses to novelty cannot account for the positive nonlinear effects in which disfigured and attractive faces elicited greater activation than medium faces. Variations in attention to the three face categories also cannot readily account for the pattern of results, since a particular category of faces did not uniformly elicit lower levels of activation, which is what variations in attention would predict. Two other facial qualities that might provide alternative explanations for our results are variations in smiling, which has been shown to modulate the neural response to attractiveness in previous research (O'Doherty et al., 2003), or babyfaceness, which has been shown to influence AMY activation (Zebrowitz, Luevano, & Aharon, 2009). However, ratings of the faces on seven-point scale with endpoints labeled no smile/big smile and maturefaced/babyfaced revealed no category differences in smiling, F=.09, MSE = .25, p = .92, and all faces were rated close to the bottom of the scale consistent with our selection of neutral expression faces (Mattractive = 1.33, SD = .37, Mmedium = 1.42, SD = .58, Mdisfigured = 1.38, SD = .52). There were also no category differenes in babyfaceness, F=.36, MSE = .42, p = .70, (Mattractive = 3.71, SD = .35, Mmedium = 3.52, SD = .48, Mdisfigured = 3.48, SD = .95).
Limitations
Some limitations of the present research should be noted. As discussed above, one limitation is that we have data only for male faces. In addition, one of the possible explanations that we offer for gender modulation presumes that our participants were heterosexual, something that we did not confirm. Another limitation is that our Block design cannot differentiate the neural response to anticipating a rewarding/aversive stimulus from the response to experiencing the stimulus, and it would be desirable to replicate our findings using an event-related design. Our modest statistical threshold also calls for replication of our results. In addition, our passive viewing paradigm does not elucidate how reward regions respond when people are making behavioral responses that may lead to rewarding or aversive outcomes. The imprecision of the BOLD response is also a limitation, leaving open the possibility that it is different neurons within the same brain region that respond to rewarding and aversive faces. However, even if this were true, the fact remains that there is no clear division of labor across brain regions in the coding of stimulus valence. Moreover, single cell recordings in the animal literature suggest that some neurons do respond to both rewarding and aversive stimuli. The absence of temporal data is an additional limitation. There may be temporal dissociations to attractive and disfigured faces within brain regions that respond to both, with some regions responding more quickly to rewarding faces and others more quickly to aversive ones, as shown in response to monetary rewards and punishments by Delgado et al., (2003). Also, although we have shown that many regions in the reward circuit respond to both attractive and disfigured faces, the functional connectivity among these regions remains to be determined. This connectivity may be modulated by the valence of the face, and it is likely to be important for predicting emotional and behavioral responses. Finally, the fact that we assessed neural responses to variations in facial attractiveness within a larger set of face categories might be viewed as limiting the generalizability of our results. However, this design could be viewed as a strength rather than a weakness. More specifically, it demonstrates that systematic variations in linear and nonlinear responses to facial attractiveness in the reward circuit are sufficiently robust to be evident even in a noisy context that includes other affectively significant face categories.
Conclusion
Although it is well accepted that the ‘reward circuit’ functions to process subjective feelings of pleasure and pain and to guide goal-related behavioral responses, such as approach and avoidance, the specifics of how this is accomplished remains an active area of inquiry (Delgado, 2007; Fareri, Martin, & Delgado, 2008; Grahn, Parkinson, & Owen, 2008). Our findings have some significant implications for this endeavor. Two issues that have received considerable attention involve the processing of stimulus valence and salience. It has been suggested that some regions process rewarding events, either in relative or absolute terms, while others process aversive ones (Kringelbach & Rolls,2004; Jensen et al., 2007). There also is evidence that some regions process motivationally salient stimuli, regardless of valence (Jensen et al., 2007; Phelps, 2006). Our evidence for widespread nonlinear responses to variations in facial attractiveness suggests that the reward circuit does not process valence with any simple dissociation of function across structures. At the same time, these results should not be taken as evidence that all regions in which we found nonlinear effects process salience to the exclusion of valence. Rather, the nonlinear responses to facial attractiveness should be an impetus for more sophisticated analyses to better determine how valence is processed. Temporal information may be important in this undertaking, as may functional connectivity. Differences in gender modulation across regions in the reward circuitry also may provide some guidance in sorting out their functions. For example, regions that showed no gender modulation may not function to guide goal-related behavioral responses that are unique to same vs. opposite sex faces, such as mate selection. In contrast, those that did show gender modulation may be involved in such responses, although research that uses female faces and ascertains the sexual orientation of participants is needed to support this interpretation. In sum, our findings echo research on amygdala, where early work indicated a heightened response to negative, threatening faces (Morris, Frith, Perrett, Rowland, Young et al., 1996), and later work also revealed an effect of stimulus salience, shown in a nonlinear response to affectively significant faces whether they are positively or negatively valenced (Fitzgerald et al., 2006). Like the nonlinear response to attractiveness in amygdala, the nonlinear responses we have documented in other brain regions are consistent with single cell recordings in animals demonstrating that these regions process both appetitive and aversive stimuli. The challenge remains to determine how that dual coding influences feelings, such as pleasure and pain, and guides goal-related behavioral responses, such as approach and avoidance.
Acknowledgments
This work was supported by the National Institutes of Mental Health Grants [grant numbers MH066836 and K02MH72603] and National Science Foundation Grant [grant number 0315307] to L.A.Z.
Footnotes
The other face categories were included to examine the neural substrate for babyface overgeneralization in behavioral responses to faces that vary in their resemblance to babies (Zebrowitz, Luevano, & Aharon, 2009), and to examine the neural substrate for the categorical and dimensional differentiation of emotion expressions (Liang, Zebrowitz, & Aharon, 2009).
Randomizing the order of face categories across runs resulted in the blocks of attractive and average faces and attractive and disfigured faces preceding and following each other an equal number of times (3 runs each). The block of unattractive faces preceded disfigured faces in 4 runs and followed in 2 runs.
In whole-brain level analysis, using minimum cluster-size threshold determined by Alphasim with voxel level threshold of p<0.05, we also found gender modulation of positive nonlinear effects of attractiveness in the following regions (both left and right) for which we had no a prior predictions: lingual gyrus, precentral gyrus, precuneus, and superior temporal gyrus.
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Berscheid E, Walster E. Physical Attractiveness. In: Berkowitz L, editor. Advances in experimental social psychology. New York: Academic Press; 1974. pp. 157–215. [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Düzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. Journal of Cognitive Neuroscience. 2008;20:941–951. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MR, Roberts AR, Barbee AP, Druen PB. ‘Their ideas of beauty are, on the whole, the same as ours’: Consistency and variability in the cross-cultural perception of female physical attractiveness. Journal of Personality and Social Psychology. 1995;68:261–279. [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. In: Balleine BW, Doya K, Odoherty J, Sakagami M, editors. Reward and Decision Making in Corticobasal Ganglia Networks. Vol. 1104. New York: New York Academy of Sciences; 2007. pp. 70–88. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Dion KK. Cultural perspectives on facial attractiveness. In: Rhodes C, Zebrowitz LA, editors. Facial attractiveness: Evolutionary, cognitive, and social perspectives. Westport, CT: Ablex; 2002. pp. 239–259. [Google Scholar]
- Eagly AH, Ashmore RD, Makhijani MG, Longo LC. What Is Beautiful Is Good, but : A Meta-analytic Review of Research on the Physical Attractiveness Stereotype. Psychological Bulletin. 1991;110:109–128. [Google Scholar]
- Eichorn DH. Samples and procedures. In: C JA, Eichorn DH, Haan N, Honzik MP, Mussen PH, editors. Present and past in middle life. New York: Academic Press; 1981. pp. 33–51. [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JFW. Differential Response Patterns in the Striatum and Orbitofrontal Cortex to Financial Reward in Humans: A Parametric Functional Magnetic Resonance Imaging Study. Journal of Neuroscience. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A. Good-looking people are not what we think. Psychological Bulletin. 1992;111:304–341. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Grammer K, Fink B, Moller AP, Thornhill R. Darwinian aesthetics: sexual selection and the biology of beauty. Biological Reviews. 2003;78:385–407. doi: 10.1017/s1464793102006085. [DOI] [PubMed] [Google Scholar]
- Griffin AM, Langlois JH. Stereotype directionality and attractiveness stereotyping: Is beauty good or is ugly bad? Social Cognition. 2006;24:187–206. doi: 10.1521/soco.2006.24.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. Distribution of dopamine D-3 receptor expressing neurons in the human forebrain: Comparison with D-2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Kato K, Inoue M, Mikami A. Neurons in the macaque orbitofrontal cortex code relative preference of both rewarding and aversive outcomes. Neurosci Res. 2007;57:434–445. doi: 10.1016/j.neures.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Ishai A. Sex, beauty and the orbitofrontal cortex. International Journal of Psychophysiology. 2007;63:181–185. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, et al. Separate brain regions code for salience vs. valence during reward prediction in humans. Hum Brain Mapp. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe KKW, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413:589–590. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Koyama T, Kato K, Tanaka YZ, Mikami A. Anterior cingulate activity during pain-avoidance and reward tasks in monkeys. Neuroscience Research. 2001;39:421–430. doi: 10.1016/s0168-0102(01)00197-3. [DOI] [PubMed] [Google Scholar]
- Kramer S, Zebrowitz LA, San Giovanni JP, Sherak B. Infants' preferences for attractiveness and babyfaceness. In: Bardy BG, Bootsma RJ, Guiard Y, editors. Studies in perception and action III. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.; 1995. pp. 389–392. [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Current Biology. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Macrae CN, Kelley WM, Fugelsang JA, Heatherton TF. The good, the bad, and the ugly: An fMRI investigation of the functional anatomic correlates of stigma. Social Neuroscience. 2006;1:5–15. doi: 10.1080/17470910600670579. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. [Review] Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Kalakanis L, Rubenstein AJ, Larson A, Hallam M, Smoot M. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychological Bulletin. 2000;126:390–423. doi: 10.1037/0033-2909.126.3.390. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Ritter JM, Roggman LA, Vaughn LS. Facial diversity and infant preferences for attractive faces. Developmental Psychology. 1991;27:79–84. doi: 10.1037//0012-1649.35.3.848. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Roggman LA. Attractive faces are only average. Psychological Science. 1990;1:115–121. [Google Scholar]
- Liang X, Zebrowitz LA, Aharon I. Effective connectivity between amygdala and orbitofrontal cortex differentiates the perception of facial expressions. Social Neuroscience. 2009;4:185–196. doi: 10.1080/17470910802453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. Journal of Cerebral Blood Flow Metabolism. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow Metabolism. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Nishino H. Topographic distribution of modality-specific amygdalar neurons in alert monkey. Journal of Neuroscience. 1988;8:3556–3569. doi: 10.1523/JNEUROSCI.08-10-03556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Potter T, Corneille O, Ruys KI, Rhodes G. “Just another pretty face”: a multidimensional scaling approach to face attractiveness and variability. Psychonomic Bulletin and Review. 2007;14:368–372. doi: 10.3758/bf03194079. [DOI] [PubMed] [Google Scholar]
- Ramsey JL, Langlois JH, Hoss RA, Rubenstein AJ, Griffin AM. Origins of a stereotype: Categorization of facial attractiveness by 6-month-old infants. Developmental Science. 2004;7:201–211. doi: 10.1111/j.1467-7687.2004.00339.x. [DOI] [PubMed] [Google Scholar]
- Rehnman J, Herlitz A. Women remember more faces than men do. Acta Psychologica. 2007;124:344–355. doi: 10.1016/j.actpsy.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Tremewan T. Averageness, exaggeration, and facial attractiveness. Psychological Science. 1996;7:105–110. [Google Scholar]
- Rhodes G, Zebrowitz LA, editors. Facial attrativeness: Evolutionary, cognitive, and social perspectives. Westport, CT: Ablex Publishing; 2002. [Google Scholar]
- Said CP, Baron S, Todorov A. Nonlinear amygdala response to face trustworthiness: Contributions of high and low spatial frequency information. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2009.21041. in press. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Shaw JI, Skolnick P. Sex differences, weapon focus, and eyewitness reliability. Journal of Social Psychology. 1994;134:413–420. doi: 10.1080/00224545.1994.9712191. [DOI] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, Vaitl D. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses [abstract]. Presented at the 9th International Conference on Functional Mapping of the Human Brain; June 19-22, 2003; New York, NY. 2003. Available on CD-Rom in NeuroImage. [Google Scholar]
- Winston JS, O'Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Wright DB, Sladden B. An own gender bias and the importance of hair in face recognition. Acta Psychologica. 2003;114:101–114. doi: 10.1016/s0001-6918(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Yamada H, Matsumoto N, Kimura M. Tonically active neurons in the primate caudate nucleus and putamen differentially encode instructed motivational outcomes of action. Journal of Neuroscience. 2004;24:3500–3510. doi: 10.1523/JNEUROSCI.0068-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz LA, Bronstad PM, Montepare JM. An ecological theory of face perception. In: Adams R, Ambady N, Nakayama K, Shimojo S, editors. The Science of Social Vision. Oxford University Press; in press. [Google Scholar]
- Zebrowitz LA, Fellous JM, Mignault A, Andreoletti C. Trait Impressions as Overgeneralized Responses to Adaptively Significant Facial Qualities: Evidence from Connectionist Modeling. Personality and Social Psychology Review. 2003;7:194–215. doi: 10.1207/S15327957PSPR0703_01. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Luevano V, Bronstad MP, Aharon I. Neural Activation to Babyfaced Men Matches Activation to Babies. Social Neuroscience. 2009;4:1–10. doi: 10.1080/17470910701676236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz LA, Olson K, Hoffman K. Stability of babyfaceness and attractiveness across the life span. Journal of Personality & Social Psychology. 1993;64:453–466. doi: 10.1037//0022-3514.64.3.453. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Rhodes G. Sensitivity to ‘bad genes’ and the anomalous face overgeneralization effect: Accuracy, cue validity, and cue utilization in judging intelligence and health. Journal of Nonverbal Behavior. 2004;28:167–185. [Google Scholar]


