Abstract
Neurogenesis occurs continually throughout life in all mammals and the extent of neurogenesis is influenced by many factors including gonadal hormones. Most research regarding hormones and neurogenesis has been performed on non-primate species. To determine whether gonadal hormones can modulate endogenous neurogenesis in the dentate gyrus (DG) of the hippocampus in non-human primates, ovariectomized (OVX) female rhesus monkeys received continuous, unopposed β-estradiol (OVX-E-Con), cyclic unopposed β-estradiol (OVX-E-Cyc), continuous β-estradiol + cyclic progesterone (OVX-E-Con+P-Cyc), or control (OVX-Veh) treatments. At week 29, all monkeys received BrdU injections for four consecutive days, in addition to the ongoing treatment. Twenty days after the last BrdU injection, all animals were sacrificed for tissue collection. In DG of hippocampus, scattered BrdU-ir cells were observed mainly in the subgranular zone (SGZ) and in the granule cell layer and occasionally these BrdU-ir cells in the SGZ formed clusters containing between 2–5 cells. In the granule cell layer and SGZ, virtually none of the BrdU-ir cells were either Dcx, a marker of immature neurons, or GFAP positive. However, an occasional BrdU-ir cell was positive for both neuronal marker NeuN or β III-tubulin. Unbiased stereological analysis of BrdU-ir cells within the SGZ and the granule cell layer of DG revealed that among the experimental groups, there was no significant difference in number of BrdU-ir cells within the SGZ and the granule cell layer of the DG: OVX-E-Con (1801+218.7), OVX-E-Cyc (1783+415.6), OVX-E-Con+P-Cyc (1721+229.6), and OVX-Veh (1263+106.3), but a trend towards increased BrdU-ir cells was observed in all the experimental groups.
Keywords: monkey, hippocampal neurogenesis, dentate gyrus, estrogen
Introduction
Neurogenesis, a process through which new neurons are generated and become integrated within neuronal circuits, consists of multiple processes that include cell proliferation, migration, and integration. Neurogenesis in the adult mammal brain occurs throughout life and has been found in all mammals studied to date, including non-human primates (Gould et al., 1998; Gould et al., 1999; Kornack and Rakic, 2001) and humans (Shingo et al., 2003). Adult neurogenesis has been clearly demonstrated at two locations under normal conditions: the subventricular zone of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus (see review (Zhao et al., 2008)). However, the nature of these newly generated cells and the role they play in brain function still raises many questions. Many studies have suggested that adult neurogenesis has a significant impact on learning and memory (see reviews (Leuner, et al., 2006, Li, ea al., 2009; Brinton, 2009)). Several factors influence the capacity of the adult neurogenesis, including growth factors (Gustafsson et al., 2003; Okada et al., 2008), endogenous neuronal activity (Arvidsson et al., 2001), environmental enrichment (Wang and Zhu, 2008), as well as pathological conditions such as Alzheimer's disease (Jin et al., 2004), Huntington's disease (Curtis et al., 2003), and stroke (Jin et al., 2006).
In addition to well established reproductive functions, gonadal hormones exert a wide variety of effects in a non-reproductive context. For instance gonadal hormones seem to have an influence in the development, growth, differentiation, maturation, and function of the central nervous system (Martinez-Cerdeno et al., 2006). Specifically estrogen, one of major female hormones, plays an essential role in the development of the central nervous system as well as in maintaining normal brain function in adulthood.
It has recently been established that gonadal hormones also influence adult brain neurogenesis. One decade ago, Tanapat and his coworkers (Tanapat et al., 1999) revealed a role for estrogen in adult neurogenesis. They found that female rats had significantly more newly generated cells in the DG than did male rats suggesting that estrogen may have an influence on neurogenesis. Since then, an emerging data base supports estrogen's influence on both cell proliferation and cell survival in the DG of the adult animals (see reviews: (Galea, 2008; Galea et al., 2008). These influences varied depending on the time and amount of exposure to estrogen. Short-term exposure (2–4 h) to estradiol in ovariectomized (OVX) adult female rodents (Tanapat et al., 1999; Banasr et al., 2001; Ormerod et al., 2003) and female meadow voles (Ormerod and Galea, 2001) resulted in enhanced cell proliferation, but the enhancement of cell proliferation was subsequently suppressed with a 48 hours exposure to estradiol in OVX adult female rodents (Ormerod et al., 2003) and female meadow voles (Ormerod and Galea, 2001). The augmented cell proliferation in the DG induced by estrogen was dose-dependent (Tanapat et al., 1999; Tanapat et al., 2005). Estrogen's ability to enhance cell proliferation also depends on the amount of time the rodent has been deprived of its normal circulating levels of estradiol and on the length of time the rodent is exposed to estrogen. Estradiol administration does not enhance cell proliferation if administered 4 weeks after OVX (Tanapat et al., 2005). There were conflicting results with long-term exposure (over two weeks) to estrogen in OVX adult female rodents. Continuous replacement with estradiol for 3 weeks does not persistently increase the rate of cell proliferation in the DG of OVX rats (Tanapat et al., 2005). By contrast, repeated estradiol administration for 15 days shows an increase in cell proliferation and a decrease in cell survival in OVX adult female rats (Barker and Galea, 2008). Furthermore, motherhood alters the cellular response to estrogens in the hippocampus later in life and previous reproductive experience may make the older brain more responsive to estrogens later in life (Barha and Galea, 2009). Estrogen influence neurogenesis is estrogen receptor (ER) dependent. There are two identified estrogen ER subtypes—α and β. Both ERα and ERβ have been localized in the rat dentate gyrus indicating that they could directly mediate estragen on neurogenesis (see review:(Galea, 2008). A recently study on the human neural progenitor cells (hNPCs) demonstrated that ERβ appears to play a major role in brain development and neurogenesis (Wang et al., 2008).
Progesterone has also been shown to have an effect on adult neurogenesis, albeit a more limited one. Tanapat and coworkers established that when OVX rats are treated with estradiol and then subsequently treated with progesterone, abolished the estradiol- induced enhancement of cell proliferation (Tanapat et al., 2005). Wang and coworkers demonstrated that allopregnanolone, the neuroactive progesterone metabolite, significantly increases the proliferation of neuroprogenitor cells derived from the rat hippocampus and human neural stem cells derived from the cerebral cortex in a dose-dependent manner (Wang et al., 2005). Progesterone mediates neural progenitor cell proliferation and concomitant regulation of mitotic cell cycle genes via a PGRMC/ERK pathway mechanism (Liu, et al., 2009). A recent study found that treatment with progesterone after focal cerebral ischemia suppresses proliferation of progenitor cells but enhances survival of newborn neurons in adult male mice (Zhang, et al., 2010). To date, most investigations examining the influence of gonadal hormones on adult neurogenesis in DG has been done on non-primate species. Insight regarding implications for human neurogenesis can be gained through research on long-term hormonal treatment in primates, analogous to human clinical trials. The purpose of the present study is to investigate whether long-term female hormones replacement can affect the endogenous neurogenesis in the DG of the hippocampus in non-human primates.
2. Materials and Mathods
2.1. Female hormone treatment and BrdU treatments
Twenty adult female rhesus monkeys (Macaca mulatta) (7-14.7 years old, average 9.9 years old (equivalent to about 30 human years old)) were used in this study. The animals were bilaterally OVX to simulate menopause. The animals were under post-OVX hormone assessment for at least one and half months (42-58 days), and then were randomly divided into four groups: continuous, unopposed estrogen (OVX-E-Con), cyclic unopposed estrogen (OVX-E-Cyc), continuous estrogen + cyclic progesterone (OVX-E-Con+P-Cyc), or vehicle (OVX-Veh). Hormone replacement was by injections of estradiol cypionate (100 μg) in 1ml vehicle (peanut oil) for a delayed slow release effect (OVX-E-Cyc), by release from subcutaneous implanted silastic capsules filled with estradiol in vehicle (OVX-E-Con and OVX-E-Con+P-Cyc), or by giving 100mg capsules of Prometrium (progesterone)/day given orally (OVX-E-Con+P-Cyc). A single dose every four weeks of estradiol was selected for OVX-E-Cyc in order to simulate the natural cyclicity of estrogen release. The chronic estradiol replacement regimen was delivered using silastic implants. Briefly, two silastic implants (3 cm long and 0.46 i.d containing estradiol) were used. Since each of these implants results in circulating levels of approximately 75-80 pg/ml (early follicular phase levels) two implants were used to achieve serum levels of approximately 150 pg/ml. The implants could retain levels near to 150pg/ml for at least 3 months, and the implants were replaced if the serum levels approach to 100pg/ml, or every 6 months at most. The 100mg capsule of Prometrium was given daily started at every 11th day and end at 20th day of every cycle. The hormone replacement continued for 8 cycles (28 days/cycle). OVX-Veh animals were given empty silastic implants as the OVX-E-Con animals. On the 1st day of week 29, monkeys received BrdU (75 mg/kg, i.v.; Sigma, St. Louis, MO) daily injection for four consecutive days, in addition to the ongoing treatment. The animals were sacrificed for tissue collection 20 days after the last BrdU injection. Blood was drawn to test both estradiol and progesterone levels. To assess baseline hormone levels, blood was drawn one day before OVX surgery. Blood was drawn again one day before treatment to determine if OVX surgery was successful. A total of 18 blood draws were taken during the treatment phase, beginning 28 days from the start of treatment and ending the day before sacrifice.
2.2. Tissue preparation
Animals were deeply anesthetized with ketamine (10mg/kg) and pentobarbital (60 mg/kg). They were then injected with 1ml of heparin (20,000 IU) into the left ventricle and perfused transcardially with 0.9% saline followed by fixation with a 4% paraformaldehyde in 0.1M sodium phosphate buffer (PB, pH 7.4). Brains were removed from the calvaria, postfixed for 6 hours in the same fixative and cryoprotected through graded sucrose (10, 20 and 30%) in phosphate-buffered saline (PBS, pH 7.2) at 4 °C. Serial sections (40μm, six series) throughout the brain were cut frozen on a sliding knife microtome and stored at -20 °C in cryoprotectant.
2.3. BrdU immunocytochemistry with Hematoxylin counter staining
A 1 in 12 series of sections from each monkey were used for stereological analysis and were processed for immunohistochemistry using a rat anti- BrdU polycolonal antibody via the biotin-labeled antibody procedure. Briefly, following several washes in the Tris-buffered saline (TBS, pH7.4) solution containing 0.05% Triton X-100 (TBS-Tx), sections were incubated for 20 minutes in a TBS solution containing 0.1 M sodium periodate to eliminate endogenous peroxidase activity. After three washes in TBS-Tx, sections were denatured with 2M HCl at 37°C for 30 min and then neutralized with 0.1M borate buffer (pH 8.5) for 10 min. After three washes in TBS-Tx, sections were blocked for 1 h in TBS-Tx containing 5% normal goat serum (NGS). The primary antibody (1:500, Accurate, Westbury, MA) was applied overnight at room temperature (RT) in a 0.1 M sodium phosphate buffer saline solution (PBS, pH 7.4) containing 0.4% Triton X-100 and 3% NGS. After six washes in TBS-Tx, sections were sequentially incubated at RT with biotinylated goat anti-rat IgG (Vector labs, Burlingame, CA; 1:200) for 1h and the “Elite” avidin–biotin complex (ABC,Vector labs; 1:500) for 75 min separated by six washes in TBS-Tx. Sections were then reacted with 0.05% 3′3-diaminobenzidine (DAB), 0.005% H2O2 and 2.5% nickel II sulfate, yielding a black reaction product for the BrdU-labeled cells. Sections were mounted on gelatin-coated slides. The sections were counterstained by hematoxylin and dehydrated through graded alcohol (50%, 70%, 95%, 99%), cleared in xylene, and coverslipped with Cytoseal.
2.4. Double-Label Immunofluoresence for BrdU and one of several cell-specific markers
To assess whether BrdU-immunoreactivity was localized to one of several cell-specific markers, we employed a double-label immunofluorescence protocol. Since the first primary antibody, the rat anti-BrdU antibody, was a rat IgG, and the second primary antibodies were either rabbit IgG, mouse IgG or goat IgG. Briefly, after pretreating for BrdU staining, sections were incubated for rat anti-BrdU antibody (1:100, Accurate, Westbury, MA) overnight at room temperature. After several washes in TBS-Tx, the anti-rat IgG coupled to Dylight 549 (1:500, Jackson Immuno Research Laboratories, Inc.) were applied. Following several washes in PBS-Tx, BrdU-labeled sections were incubated in one of the second primary antibodies at appropriate dilution (immature neurons marker doublecortin (Dcx) (goat anti-Dcx, 1:200, Santa Cruz Biotechnology), both immature and mature neuronal marker β III-tubulin (rabbit anti- β III-tubulin, 1:100, Abcam); neuronal marker neuronal nuclei (NeuN) (mouse anti-NeuN, 1:200, Chemicon), and the astroctye marker glial fibrillary acid protein (GFAP) (rabbit anti-GFAP, 1:500, DAKO, Denmark)) overnight at room temperature. After several washes in TBS-Tx, the anti-second primary antibodies IgG coupled to Dylight 488 (1:500, Jackson Immuno Research Laboratories, Inc.) were applied for visualization of cell-specific markers. Following several washes in PBS, sections were mounted on gelatin-coated slides, dehydrated through graded alcohol (50%, 70%, 95%, 99%), cleared in xylene, and coverslipped with DPX. Immunofluoresence double-labeled sections were examined by a Fluoview Laser Confocal system equipped with an Olympus microscope.
2.5. Stereological Assessment of BrdU-ir cell number within dentate gyrus
Estimation of the total number of BrdU-ir containing cells within the SGZ and the granule cell layer of DG was achieved using the optical fractionator procedure (West, 1993; Peterson et al., 1999; Kordower et al., 1999). For every 12th section through the DG, the number of labeled cells was determined by using an Olympus BX-60 light microscope. The StereoInvestigator 2000 software (Micro-BrightField, Colchester, VT) performed all analyses. Rough boundaries to delimit the optical fractionator area sampling fraction were drawn by using a 1.25× objective. 100% of the outlined region was measured with a systematic random design of disector counting frames (70×70 μm=4900 μm2) using a 60× planapo objective. Brfore the stereological count, the average section thickness from each case was roughly determined. The rough section thickness of each case is between 14-20μm. Since this method allowed for a 2 μm top guard zones and at least a 3μm bottom guard zones, so the disector height was 8μm in this study. Briefly, after initially focusing on the top of the section which served as the forbidden plane, a guard focus height of 2 μm was set in the software, which would then focus 8 μm through each sample region. The accurate section thickness was determined by measuring ever 5th counting sites. By calculating the total number of raw counts (Q), section sampling fraction (ssf), area sampling fraction (asf), and thickness sampling fraction (h/t), the total number of BrdU-ir cells within SGZ and the granule cell layer of DG can be calculated from N=Q × 1/ssf × 1/asf × t/h.
2.6. Data analysis
Single staining for BrdU was developed by using immunoperoxidase procedures and examined under standard light microscopy. All immunofluoresence double-labeled sections were examined by a Fluoview Laser Confocal system equipped with an Olympus microscope with 2 channel argon/krypton lasers using appropriate excitation and emission filters for Delight 549 and Delight 488. Data obtained from the unbiased stereological analysis of BrdU-ir cell number within DG were evaluated using a one-way analysis of variance. Following a significant (p<.05) interaction effect, An ANOVA test that controls for multiple comparisons was employed for specific comparisons.
3. Results
The base line of estradial level was 74.51 pg/ml. After OVX, the estradiol level was below 10pg/ml. Among the treatment groups, the estradiol levels are: OVX-E-Con (137.31 pg/ml), OVX-E-Cyc (29.92 pg/ml), OVX-E-Con+P-Cyc (126.73pg/ml), and OVX-Veh (<10 pg/ml). The base line of progesterone level was 2.19 ng/ml. After OVX, the progesterone level was 1.00 ng/ml. Among the treatment groups, the progesterone levels are: OVX-E-Con (0.74 ng/ml), OVX-E-Cyc (1.08 ng/ml), OVX-E-Con+P-Cyc (1.96 ng/ml), and OVX-Veh (1.07ng/ml). BrdU-ir cells were observed throughout the neuraxis in all the monkeys examined. In the DG, BrdU-ir cells were present in the hilus, in the SGZ and in the granule cell layer of the DG. Occasionally these BrdU-ir cells in the SGZ were seen forming clusters containing 2–5 cells (Figure 1). To examine whether the long-term gonadal steroid replacement had an effect in cell proliferation in DG of OVX female monkeys, unbiased stereological analysis was used to estimate the total number of BrdU-ir cells within the SGZ and the granule cell layer of DG. The statistical analysis of the data of BrdU-ir cells within the SGZ and the granule cell layer of DG revealed that among the experimental groups, although there was no statistically significant difference in number of BrdU-ir cells within the SGZ and the granule cell layer of the DG in comparison to OVX-Veh animals (0.24≥P≥0.17) (Figure 2), there was a strong trend towards an increase in BrdU-ir cells (at least 36% increased of BrdU-ir cells compare to the control animals): OVX-E-Con (1801±218.7), OVX-E-Cyc (1783±415.6), OVX-E-Con+P-Cyc (1721±229.6), and OVX-Veh (1263±106.3).
Figure 1.
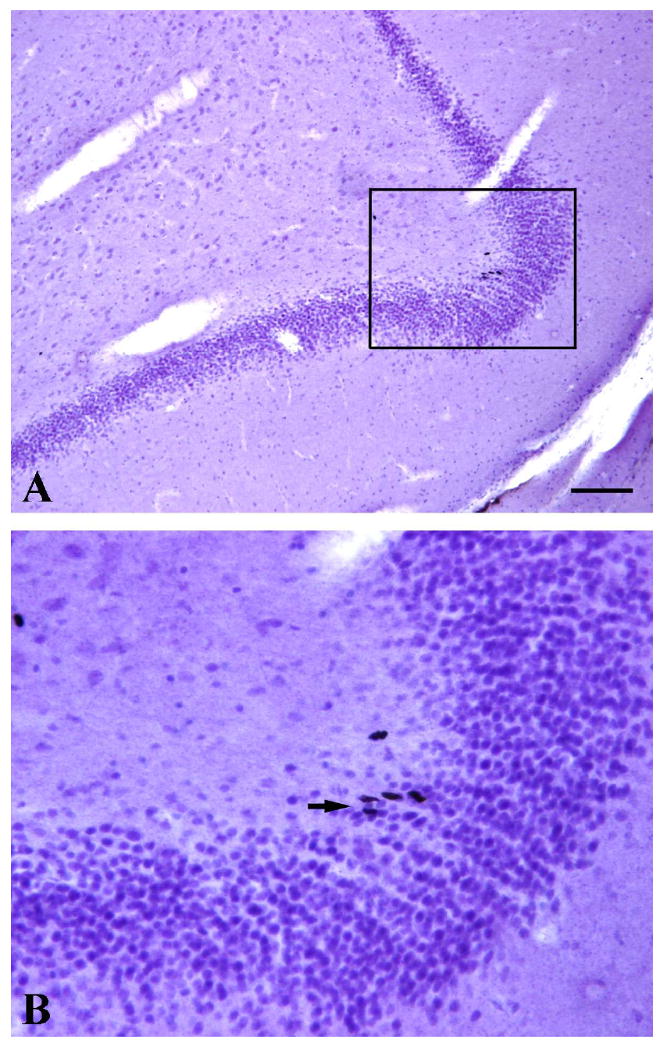
Images from BrdU-ir staining (Black) and hematoxylin counter staining were from an OVX-E-Con monkey. Higher magnification image (B) marked by the frame in A. Note that: Occasionally these BrdU-ir cells in the subgranular zone or granular zone of the DG were seen forming clusters containing between 2–5 cells (arrows pointed in B). Scale bar in A represents the following magnifications: A=100 μm and B=31 μm.
Figure 2.
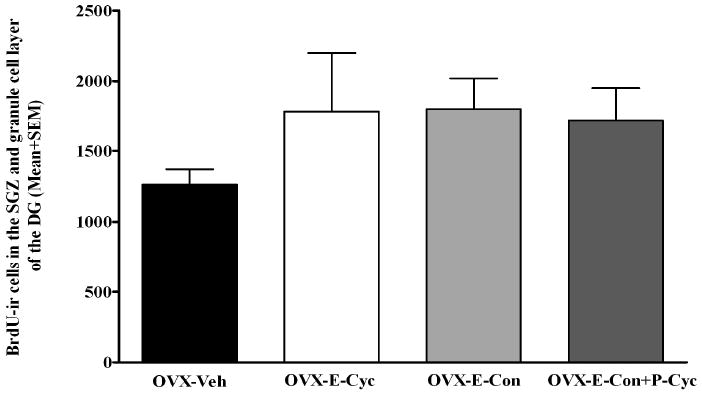
Stereological estimates reveal that among the experimental groups, although there was no statistically significant difference in number of BrdU-ir cells within the SGZ and the granule cell layer of the DG in comparison to OVX-Veh animals (0.24≥P≥0.17), there was a definite strong trend towards an increase in BrdU-ir cells (at least 36% increased of BrdU-ir cells increased compare to the control animals).
Because of the relatively low number of double-labeled cells, it was impossible to stereologically assess the percentage of cells expressing specific markers that colabeled with BrdU-ir within the SGZ and the granule cell layer of the DG between different groups of the animals. In the granule cell layer and SGZ, virtually none of the BrdU-ir cells were either Dcx, a marker of immature neurons, or GFAP positive (Figure 3). However, an occasional BrdU-ir cell was positive for both neuronal marker NeuN (Figure 3) or β III-tubulin. Since these animals were sacrificed for tissue collection 20 days after the last BrdU injection, the BrdU-ir cells in this study are surviving cells rather than newly generated cells. The lack of Dcx or GFAP positive cells is not surprising given that these cells would have undergone phenotypic differentiation.
Figure 3.
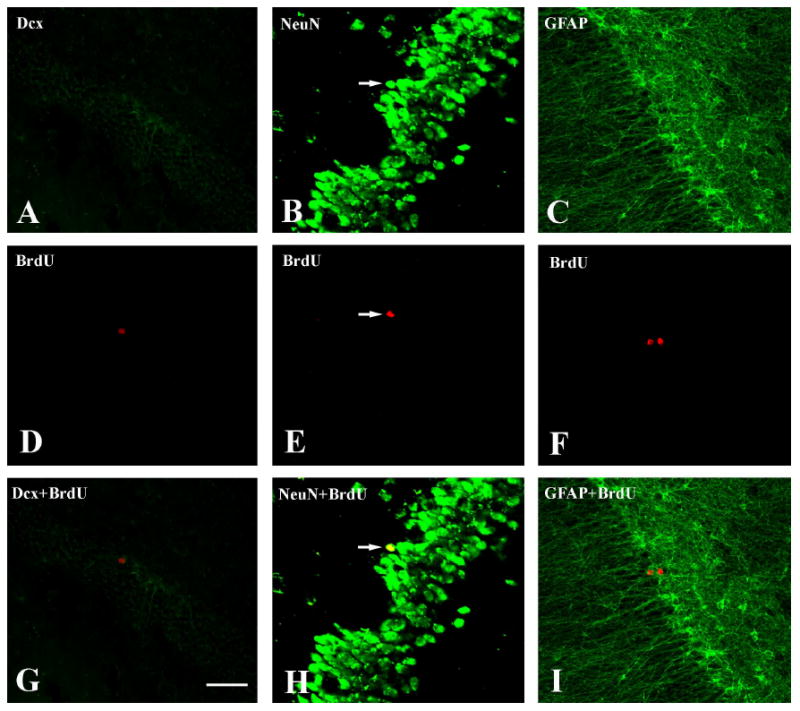
Confocal laser scanning microscopy from the section illustrating colocalization of BrdU with Dcx (A.D.G), NeuN (B,E,H), and GFAP (C,F,I) within within DG from the OVX-E-Con monkey. Upper column shows Delight 488-labeled different cell-specific markers including Dcx (A), NeuN (B), and GFAP (C). Middle column shows Delight 549-labeled BrdU-ir cells within the same sections as the upper column. Lower column shows the colocalization of upper column and the middle column. Note that: Some BrdU-ir cells were NeuN-ir positive (arrow pointed). No BrdU-ir cell was Dcx-ir or GFAP-ir positive. Scale bar in G represents 100μm for all images.
4. Discussion
The purpose of the present study was to investigate whether long-term (28 weeks) estrogen and estrogen plus progesterone treatments could modulate endogenous neurogenesis in the DG of the hippocampus of non-human primates. In this study, we used different chronic ovarian hormone replacement regimens including either estrogen alone (continuous or cyclic) or estrogen (continuous) plus progesterone (cyclic). Our results revealed that although there was no significant difference in the number of BrdU-ir cells within the SGZ and the granule cell layer of the DG among all the experimental groups in comparison to the control animals (0.24≥p≥0.17), there was a strong trend of increased BrdU-ir cells observed in all the experimental groups (at least 36% greater increase of BrdU-ir cells in comparison to the control animals). Our inability to discern a statistically significant difference may be due to the small sample size of animals in each group. Since these animals were sacrificed for tissue collection 20 days after the last BrdU injection, the BrdU-ir cells in this study were surviving cells rather than newly generated cells. While one must be extremely cautious when interpreting trends, this study suggests long-term female hormones treatment may affect endogenous neurogenesis especially for cell survival in the DG of the hippocampus in adult female monkey (Figures 1 and 2).
Interestingly, these findings are in discord with a recent report in rats, in which OVX rats that were given subcutaneous injections of estrogen daily for 15 days demonstrated decreased new cell survival in the DG (Barker and Galea, 2008). The disparity in results between rat and monkey (current study) may be due to differential estrogen response between species, experimental conditions and/or methodological differences. Previous studies have also indicated that estrogen has differing effects on the capacity for spine induction among rats and monkeys. In this regard, estrogen increases the density of dendritic spines (Gould et al., 1990; Woolley and McEwen, 1993) and synapses (Woolley et al., 1996) on CA1 pyramidal cells of young OVX female rats, but the same effects of estrogen were lost on the aged OVX female rats (Adams et al., 2001; Morrison et al., 2006). However, unlike the aged female rats, aged female monkeys retain the capacity for such an increase in spines. Estrogen led to a 35% increase in total spines within CA1 stratum radiatum of both young and aged OVX female rhesus monkey (Hao et al., 2003). The incongruity of the capacity for spine induction in response to estrogen between rat and monkey would indicate that relative to the rodent brain the nonhuman primate brain has an extended period of response to estrogen after deprived of ovarian hormones. There could be an extended window of estrogen response in morphogenesis during neurogenesis in monkey when compared to neurogenesis in rats.
Long-term female hormone replacement increased the survival of newly generated cells which is consistent with estrogen increases in the density of dendritic spines of CA1 pyramidal cells both in adult non-aged rat (Gould et al., 1990; Woolley and McEwen, 1993) and in monkey (Hao et al., 2003). Increasing morphological differentiation of newly generated neurons would increase their integration into neural circuits and hence their survival. Furthermore, many studies in rodents have demonstrated that the neurogenic effects of estrogen is vulnerable to the duration of ovarian hormone deprivation and regimen of hormone replacement (see review (Galea, 2008). Although it has been shown that OVX suppresses cell proliferation in the short-term (Tanapat et al., 1999), long-term OVX does not result in a significant suppression of cell proliferation in mice (Lagace et al., 2007) or rats (Tanapat et al., 2005), suggesting that perhaps the duration of the treatment may be an important variable as well. Since it is well known that the hippocampus has endogenous production of estradiol that could change depending on hormonal status (Barker and Galea, 2009), we cannot rule out the possibility that the increase of neurogenesis in the DG of female rhesus monkey could be a reflection of estrogen local production in the hippocampus as a compensatory mechanism after long term-OVX (Fester et al., 2006). Finally, short term exposure (2–4 h) to estradiol in OVX adult female rodents (Tanapat et al., 1999; Banasr et al., 2001; Ormerod et al., 2003) and female meadow voles (Ormerod and Galea, 2001) resulted in enhanced cell proliferation. This may be the case in the female rhesus monkey, however, short-term treatment was not tested in the current study. If an opportunity arises further studies should include variations in treatment time period.
In summary, estrogen induces a significant increase of proliferation in neural progenitor cells both in vitro (Brannvall et al., 2002; Son et al., 2003; Okada et al., 2008) and in vivo (see reviews (Galea, 2008; Galea et al., 2008). Our findings indicate that after a long period (a month) of female hormone deprivation, chronic replacement modestly enhances endogenous neurogenesis of DG in middle-aged female rhesus monkeys. These findings suggest that normal circulating level of female hormone might have important consequences for hippocampal function. Even as the role of neurogenesis in learning and memory continues to be debated, it is especially interesting to speculate on the possibility that female hormones like estrogen can affect these processes, at least in part, through neurogenesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Kokaia Z, Lindvall O. N-methyl-D-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci. 2001;14:10–18. doi: 10.1046/j.0953-816x.2001.01611.x. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- Barha CK, Galea LA. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Barker JM, Galea LA. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Barker JM, Galea LA. Sex and regional differences in estradiol content in the prefrontal cortex, amygdala and hippocampus of adult male and female rats. Gen Comp Endocrinol. 2009;164:77–84. doi: 10.1016/j.ygcen.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;4:212–22. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci U S A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester L, Ribeiro-Gouveia V, Prange-Kiel J, von Schassen C, Bottner M, Jarry H, Rune GM. Proliferation and apoptosis of hippocampal granule cells require local oestrogen synthesis. J Neurochem. 2006;97:1136–1144. doi: 10.1111/j.1471-4159.2006.03809.x. [DOI] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol. 2008;62:247–260. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson E, Lindvall O, Kokaia Z. Intraventricular infusion of TrkB-Fc fusion protein promotes ischemia-induced neurogenesis in adult rat dentate gyrus. Stroke. 2003;34:2710–2715. doi: 10.1161/01.STR.0000096025.35225.36. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Bloch J, Ma SY, Chu Y, Palfi S, Roitberg BZ, Emborg M, Hantraye P, Déglon N, Aebischer P. Lentiviral gene transfer to the nonhuman primate brain. Exp Neurol. 1999;160:1–16. doi: 10.1006/exnr.1999.7178. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci U S A. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Li Y, Mu Y, Gage FH. Development of neural circuits in the adult hippocampus. Curr Top Dev Biol. 2009;87:149–74. doi: 10.1016/S0070-2153(09)01205-8. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang J, Zhao L, Nilsen J, McClure K, Wong K, Brinton RD. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology. 2009;150:3186–3196. doi: 10.1210/en.2008-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Kriegstein AR. Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. Eur J Neurosci. 2006;24:3475–3488. doi: 10.1111/j.1460-9568.2006.05239.x. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Murase K, Makino A, Nakajima M, Kaku T, Furukawa S, Furukawa Y. Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomed Res. 2008;29:163–170. doi: 10.2220/biomedres.29.163. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience. 2001;102:369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Peterson DA, Dickinson-Anson HA, Leppert JT, Lee KF, Gage FH. Central neuronal loss and behavioral impairment in mice lacking neurotrophin receptor p75. J Comp Neurol. 1999;404:1–20. doi: 10.1002/(sici)1096-9861(19990201)404:1<1::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, Kaang BK. Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J Neurochem. 2003;85:872–881. doi: 10.1046/j.1471-4159.2003.01725.x. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Zhu KQ. Research progress--the role of astrocyte in neuronal functions. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2008;37:531–536. doi: 10.3785/j.issn.1008-9292.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Wang JM, Liu L, Brinton RD. Estradiol-17beta-induced human neural progenitor cell proliferation is mediated by an estrogen receptor beta-phosphorylated extracellularly regulated kinase pathway. Endocrinology. 2008;149(1):208–18. doi: 10.1210/en.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Wenzel HJ, Schwartzkroin PA. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J Comp Neurol. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yang R, Cai W, Bai Y, Sokabe M, Chen L. Treatment with progesterone after focal cerebral ischemia suppresses proliferation of progenitor cells but enhances survival of newborn neurons in adult male mice. Neuropharmaco. 2010:xxx, 1–10. doi: 10.1016/j.neuropharm.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]


