1. Summary
Protease-Activated Receptors (PARs), Nucleotide-binding Oligomerization Domain (NOD) receptors and Toll-like receptors (TLRs) play a role in innate immunity, but little is known about interaction between these receptors. The goal of this study was to investigate how silencing one receptor affects the expression of other receptors and downstream innate immune markers in response to bacteria. Human gingival epithelial cells (GECs) were transfected with siRNA specific for PAR1 or PAR2, then stimulated with periopathogen Porphyromonas gingivalis, bridging organism between pathogens and non-pathogens Fusobacterium nucleatum, or non-pathogen Streptococcus gordonii. PAR1 or PAR2 knock-down resulted in up-regulated NOD1 and NOD2 expression with P. gingivalis or F. nucleatum stimulation (p<0.01), as well as enhanced TLR2 and TLR4 expression when cells were stimulated by bacteria that utilize TLR2 or TLR4, respectively. Involvement of PARs for induction of CC chemokine ligand 20 (CCL20), a cytokine with antimicrobial properties, was observed following stimulation of the three bacterial species. Furthermore, results from multiple cytokine ELISA array showed receptors utilized in the induction of various innate immune markers are tailored to individual bacterium tested. Our data suggest complex interplay of several receptors is required for appropriate innate immune responses to the different types of bacteria present within the oral cavity and that receptor expression itself is altered depending on which organism the cell encounters.
Keywords: PAR, NOD, Innate immunity, Oral epithelia
2. Introduction
Epithelial tissues function as the first line of defense between the host and the outside environment, which includes multiple bacteria of the oral cavity. Various epithelia of the body are populated with distinct populations of bacteria, and recent conceptual advances suggest that Pattern Recognition Receptors (PRRs) continually monitor microbial colonization and detect conserved microbial structures, such as lipopolysaccharides (LPS), leading to host innate immune responses [1,2]. Nevertheless, it is not clear how these receptors work together in epithelial responses to various bacteria to provide appropriate innate immune reactions which differ for individual bacterial species. In the oral cavity, gingival epithelial cells (GECs) are one of the first host cell types that encounter colonizing bacteria. As a consequence, epithelial cells respond to the presence of bacteria through an elaborate signaling network, producing antimicrobial peptides and cytokines, and at times stimulating apoptotic cell death. This bacterial-host communication takes place via a number of signal transduction pathways, but responses to different bacteria induce different signals from the host [3–5]. Conversely, various host immune responses may interfere with the way commensals and pathogens communicate to form biofilm (a complex multi-species aggregation of microorganisms), although this means of defense is poorly understood.
GECs utilize different cell surface or cytoplasmic receptors in the induction of innate immune responses to various bacteria. These include TLRs, Protease-Activated Receptors (PARs), and Nucleotide-binding Oligomerization Domain receptors (NODs). PARs are a family of the seven transmembrane domain G-protein-coupled receptors that mediate cellular responses to extracellular proteases [6]. PARs are expressed and function on GECs as a part of immuno-surveillance system [7]. Proteolytic cleavage of N-terminal domain activates PARs, and a new N-terminal receptor generated after cleavage serves as a tethered ligand that binds to one of the extracellular loops of the receptor [6,8]. PARs are widely expressed, and four PARs, each with a distinct N-terminal cleavage site, have been identified so far. PAR1, PAR3 and PAR4 can be activated by thrombin, a serine protease and a member of coagulation cascade; PAR2 is activated by trypsin-like enzymes such as trypsin, mast cell tryptase, neutrophil proteinase 3 as well as Porphyromonas gingivalis proteases [6,9,10]. PAR1 and PAR2 are particularly prominent in gastrointestinal tract [11] and gingiva [10,12].
NOD proteins are cytosolic pattern recognition molecules, recognizing peptidoglycan (PGN), a component of bacterial cell walls. They belong to the family of NOD-like receptors (NLRs), which also includes NACHT-LRR (leucine-rich repeat)- and pyrin-domain-containing proteins (NALPs), neuronal apoptosis inhibitor factors (NAIPs), and ICE-protease activating factor (IPAF) [13–15]. NOD1 and NOD2 ligands are PGN-derived γ-D-glutamyl-mesodiaminopimelic acid (iE-DAP) and muramyl dipeptide (MDP), respectively [16,17]. MDP is found in both Gram-negative and Gram-positive bacterial PGN, while iE-DAP is found only in Gram-negative bacterial PGN and in PGN of a certain Gram-positive bacteria such as Bacillus subtilis and Listeria monocytogenes [18]. Thus, NOD1 is mainly involved in sensing products from Gram-negative bacteria, while NOD2 can sense both [19,20].
Since bacteria have multiple Microbial-Associated Molecular Patterns (MAMPs) and epithelial cells express multiple receptors, it is reasonable to expect that there would be cross-communication between these receptors in epithelial responses to bacteria in order to induce appropriate innate immune responses. The hypothesis to be tested in this study is that cell surface and intracellular receptors cooperate in responding to various oral bacteria and that this interaction contributes to the epithelial innate immune response. The goal of this study was to investigate how expression of receptors changes with bacterial exposure and how silencing one receptor affects the expression of other receptors and downstream innate immune markers in response to bacteria. We used small interfering RNA (siRNA) to block PAR1 or PAR2 and subsequently stimulated GECs with various oral bacteria. We show in this study that suppressing a receptor increases the expression of other receptors in epithelial response to oral bacteria. Additionally, we show that PARs play a role in the induction of antimicrobial cytokines following bacterial stimulation, and that different receptors utilized in the induction of various innate immune markers are tailored to individual bacterium tested.
3. Materials and Methods
Human epithelial cells and bacterial culture conditions
Healthy gingival samples were obtained from patients undergoing third-molar extraction at the Department of Oral Surgery, School of Dentistry, University of Washington. Tissue was prepared as described earlier, and subsequently isolated GECs were grown in Keratinocyte Basal Media supplemented with Keratinocyte Growth Media (Cambrex, Walkersville, MD) [21]. Cells were grown in media containing 0.15 mM calcium (Ca++). P. gingivalis (ATCC 33277) cells were cultured in anaerobic conditions (85% N2, 10% H2, 5% CO2) at 37°C in Trypticase soy broth (BBL, Sparks, MD) supplemented with 1 g of yeast extract, 5 mg of hemin and 1 mg of menadione per liter. Streptococcus gordonii (Challis DL1) was grown in Trypticase soy broth at 37°C under static conditions. Fusobacterium nucleatum (ATCC 25586) was grown in Todd-Hewitt broth supplemented with 1 g of yeast extract per 100 ml at 37°C in anaerobic conditions. All bacterial species used in this study are from our laboratory stock. Bacterial numbers were estimated by density in a GENios Multi-detection Reader (Phenix, Hayward, CA).
Transfection of keratinocytes with siRNA
Specific siRNAs were custom-synthesized by Qiagen (Valencia, CA). Cultured GECs were seeded in a 24-well plate for determining expression of mRNA and a 12-well plate for protein 1 h prior to adding siRNA. Initial experiments to evaluate appropriate siRNA concentration to give effective knock-down without off-target effects were performed. siRNA oligos were diluted to 5 – 20 nM in appropriate buffer and medium according to the manufacturer’s suggestions, and added to each well. Unstimulated cells and cells transfected with non-silencing siRNA were used as controls. 5 nM concentration was enough for most siRNAs to give greater than 75 % knock-down. The cells were subsequently stimulated with appropriate bacteria 48 h after transfection, and total RNA and whole cell proteins were extracted after 16 h. Successful knock-down of targeted genes was determined by Quantitative RT-PCR (QRT-PCR) and Western blot analyses as described below.
Western blot analyses
Whole cell proteins were isolated 72 h post transfection using RIPA buffer (Pierce, Rockford, IL) and protease inhibitor cocktails, and the concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA). Cell lysates were separated by NuPage 4–12% Bis-Tris gradient gel electrophoresis (Invitrogen, Carlsbad, CA) and transferred to a Westran S membrane (Schleicher and Schuell, Keene, NH). The membrane was blocked with 5% non-fat milk in TBST (50 mM Tris, 150 mM NaCl, 0.1% Tween 20, pH 7.6) for 90 min at room temperature and was incubated with each primary antibody in 0.5% non-fat milk in TBST overnight at 4°C. The blotting antibodies used were goat anti-PAR1 (R&D Systems, Minneapolis, MN), mouse anti-PAR-2 (SAM11, Santa Cruz Biotechnology, Santa Cruz, CA) and beta-actin (Santa Cruz). The membrane was washed and incubated with either HRP-conjugated sheep anti-mouse (Amersham, Piscataway, NJ) or donkey anti-goat (Jackson ImmunoResearch, West Grove, PA) immunoglobin for 1 h at room temperature and then visualized using enhanced chemiluminescence using superSignal West Dura Extended Duration Substrate and protocol of Thermo Scientific (Rockford, IL).
Conditions for real-time QRT-PCR
Total RNA was extracted from keratinocytes using RNeasy kit (Qiagen) according to the manufacturer’s suggestion. Reverse transcription was performed with 500 ng of total RNA, 1X RT buffer, 250 nM of oligo dT primer, 10 mM deoxynucleoside triphosphate (dNTP) mix, 50 U of reverse transcriptase (RT), and 13 U of RNase inhibitor. Initial denaturation of secondary RNA structure was carried out at 72°C for 2 min, followed by annealing of the primer and template at 42°C for 1 h. The temperature was subsequently raised to 95°C for 10 min in order to inactivate RT. Controls without RT were included in each experiment. Quantitative analyses of the resulting cDNA were carried out with each 25 µl of PCR mixture containing 1 µl cDNA, and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) according to the manufacturer’s suggestion. Ribosomal phosphoprotein (RPO) was used as a housekeeping control gene to determine the total RNA level. The primers for PAR1, PAR2, CCL20, hBD-2 and RPO have been previously described [7,22]. The primer sequences for NOD1, NOD2, TLR2 and TLR4 are as follow. NOD1-5’: CAG AGC AAA GTC GTG GTC AA; NOD1-3’: GAT GGT CTC ACC CTG CTC AT; NOD2-5’: AGC CAT TGT CAG GAG GCT C; NOD2-3’: CGT CTC TGC TCC ATC ATA GG; TLR2-5’: TGA TGC TGC CAT TCT CAT TC; TLR2-3’: CGC AGC TCT CAG ATT TAC CC; TLR4-5’: TGA GCA GTC GTG CTG GTA TC; TLR4-3’: CAG GGC TTT TCT GAG TCG TC. At the end of each QRT-PCR, melting curve analysis was performed to confirm that there was no spurious product. All reactions were carried out in duplicate, and average threshold cycle values were calculated. After normalization with the housekeeping gene control, the relative expression was calculated using the MyiQ 5 software (Bio-Rad), and statistical analyses were performed using the paired two-tailed t test.
IL-8 ELISA
Cell culture supernatants after siRNA transfection and bacterial stimulation were collected, and the level of IL-8 secretion was measured with an ELISA kit (R&D Systems) in accordance with the manufacturer's protocol. A standard curve was generated with each set of samples assayed. The linear region of the standard curve is obtainable in a series of two-fold dilutions in reagent diluents ranging from 2000 to 31.2 pg/ml. The optical density (OD) value of blank control (reagent diluents) was subtracted from the measured OD of the different standards and samples. Final concentrations in each sample were calculated as the mean of the results at the proper sample dilution yielding ODs in the linear parts of the standard curves. Supernatants of cells from three different donors were tested, with duplicate samples from each donor.
Multiple Cytokine ELISA Array
After appropriate siRNA transfection and subsequent bacterial stimulation as described above, supernatants from GECs were collected and centrifuged for 10 min at 1000 × g to remove any particulate material. Studies on the expression of multiple inflammatory and innate immune markers were performed using Multi-analyte ELISArray kits from SABiosciences (Fredericks, MD) according to the manufacturer’s suggestions. The cytokines represented by these arrays include: pro-inflammatory cytokines IL-1a & b, IL-2, IL-6, IL-12, IL-17a and GM-CSF; anti-inflammatory cytokines IL-4, IL-10 and IL-13; IFNγ and TNFα. Positive and negative controls are also included in each kit. Initially, antigen standard and sample dilutions were tested. The OD was measured at 450-nm absorbance with a 570-nm correction wavelength. Values greater than the positive control for each antigen were not counted. Also, absorbance values less than two times the negative control absorbance values for each antigen were not interpreted. For each antigen, we subtracted the observed absorbance by the absorbance of the negative control to obtain the corrected absorbance values.
Statistical analyses
Statistical significance was determined by the two-tailed Student’s t-test, compared to the non-silencing and unstimulated controls.
4. Results
Successful knock-down of specific genes determined at both mRNA and protein levels
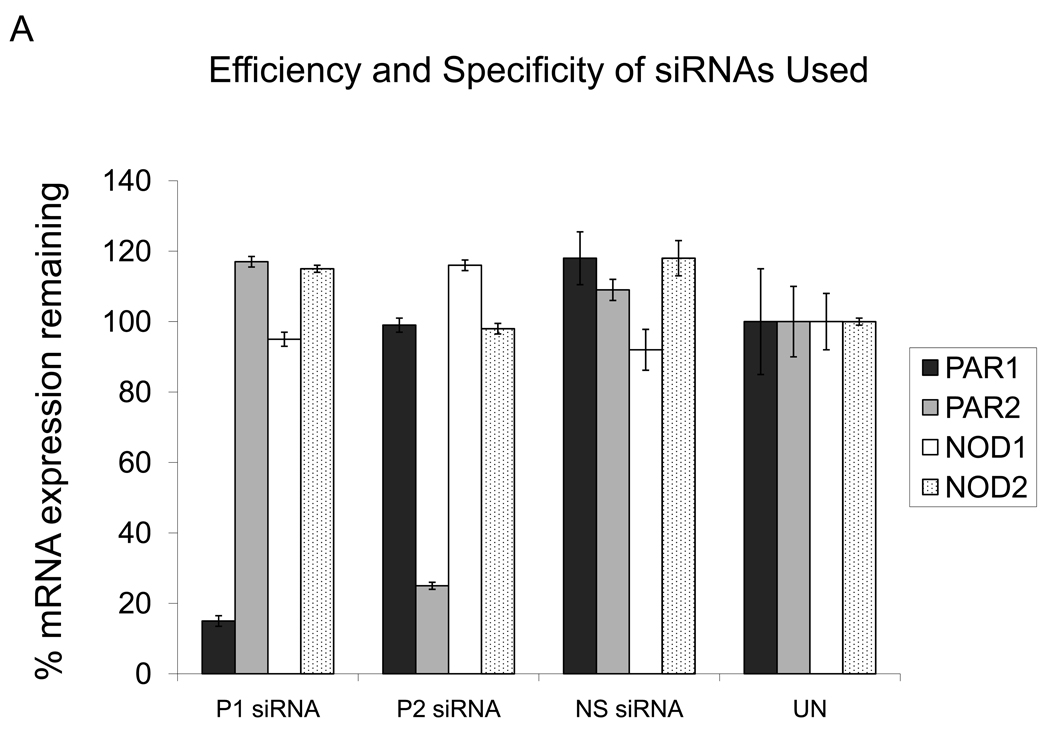
The siRNA-mediated RNAi technique is a highly specific post-transcriptional gene silencing system. In order to determine that specific targeted genes were successfully knocked down, both mRNA and protein expression levels of the targeted genes were determined. Messenger RNA levels for transcripts of interest were determined by QRT-PCR. The experiments were performed in duplicate with GECs from three different donors, and the results reported here were very consistent. All of the siRNAs used in this study are able to knock-down 75 – 98 % of the respective gene at the mRNA level compared to the non-transfected control (Figure 1A). Figure 1A also shows that the siRNAs do not have off-target effects, not significantly altering expression of the other receptors tested. Knock-down of each specific targeted gene was also determined at the protein level. When the whole cell protein from GECs transfected with siRNA specific for PAR1 was incubated with primary antibody for PAR1, decreased protein expression level is seen, compared to the non-silencing and unstimulated controls and to GECs transfected with PAR2 siRNA (Figure 1B). A parallel study conducted using proteins from PAR2 siRNA-transfection showed similar results (Figure 1B). Thus, these data confirm that the siRNAs utilized in our study are highly specific and have effectively blocked a gene function at both mRNA and protein levels.
Figure 1.
Successful gene knock-down after siRNA transfection was determined at the mRNA and protein levels. A: QRT-PCR analyses show siRNAs used in this study knocked down specific genes of interest effectively without off-target effects. P1: PAR1; P2: PAR2; NS: non-silencing control. GECs from three different donors were tested in duplicate, and the average value with standard deviation is shown. B: The Western blot analysis shows whole cell protein extract from GECs after no transfection, transfection with PAR2 siRNA (P2) or non-silencing (NS) siRNA has strong protein expression of PAR1, while decreased protein expression is seen after transfection with siRNA specific for PAR1 (P1). Parallel studies conducted using proteins from PAR2 siRNA-transfection and PAR2 antibodies show similar results. Western blots of beta-actin, used as a control for equal gel load of cell lysate protein, show comparable levels between different samples.
NOD1 and NOD2 compensate for PAR1 and PAR2 knock-down in epithelial response to bacteria
NOD1 and NOD2 are cytosolic receptors sensing the iE-DAP and MDP components of the bacterial PGN, respectively. For this study, we chose three bacteria with different disease implications in oral epithelia: P. gingivalis, a Gram-negative pathogenic bacterium strongly implied as an etiological agent of severe adult periodontitis; F. nucleatum, a Gram-negative species that works as a bridging organism between pathogens and non-pathogens in biofilm formation; and S. gordonii, a Gram-positive non-pathogenic bacterium. In order to investigate if silencing cell surface receptors PAR1 or PAR2 affects the expression of cytosolic receptors NOD1 or NOD2 upon exposure to these bacteria, we transfected GECs with siRNAs specific for PAR1 or PAR2 followed by bacterial stimulation. A significant increase in NOD1 mRNA expression is seen in GECs stimulated for 16 h with F. nucleatum when PAR1 is knocked down (5.4 fold), and when GECs are stimulated with P. gingivalis after PAR2 knock-down (4.1 fold) (Figure 2A). Next, we investigated whether NOD2 mRNA expression level is similarly affected by the absence of PAR1 or PAR2. When GECs were transfected with siRNA for PAR1, no significant changes in NOD2 mRNA induction was observed compared to the unstimulated control (Figure 2B). In contrast, when PAR2 was blocked and GECs were stimulated with F. nucleatum, the level of NOD2 induction was increased 4.2 fold compared to the controls with no transfection or non-silencing siRNA transfection (Figure 2B). The expression of NOD2 was also increased in GECs stimulated with F. nucleatum without any siRNA transfection (2.1 fold) (Figure 2B). Each experiment was repeated using GECs from three different donors with consistent results. Our data suggest that NOD1 and NOD2 can compensate for the lack of cell surface receptors PAR1 or PAR2 in epithelial response to bacteria.
Figure 2.
QRT-PCR analyses of the effect of silencing PAR1 or PAR2 on NOD1 (A) and NOD2 (B) mRNA induction by various oral bacteria. Cells were transfected with a specific siRNA for 48 h, then subsequently stimulated with bacteria for 16 h. GECs transfected with non-silencing (NS) siRNA and unstimulated cells (UN) are used as controls in each experiment. Data from duplicates with cells from three different donors were normalized to the housekeeping gene control ribosomal phosphoprotein, and the average with standard deviation is shown. PG: P. gingivalis; FN: F. nucleatum; SG: S. gordonii.
TLR2 and TLR4 compensate for absence of PARs in epithelial response to bacteria
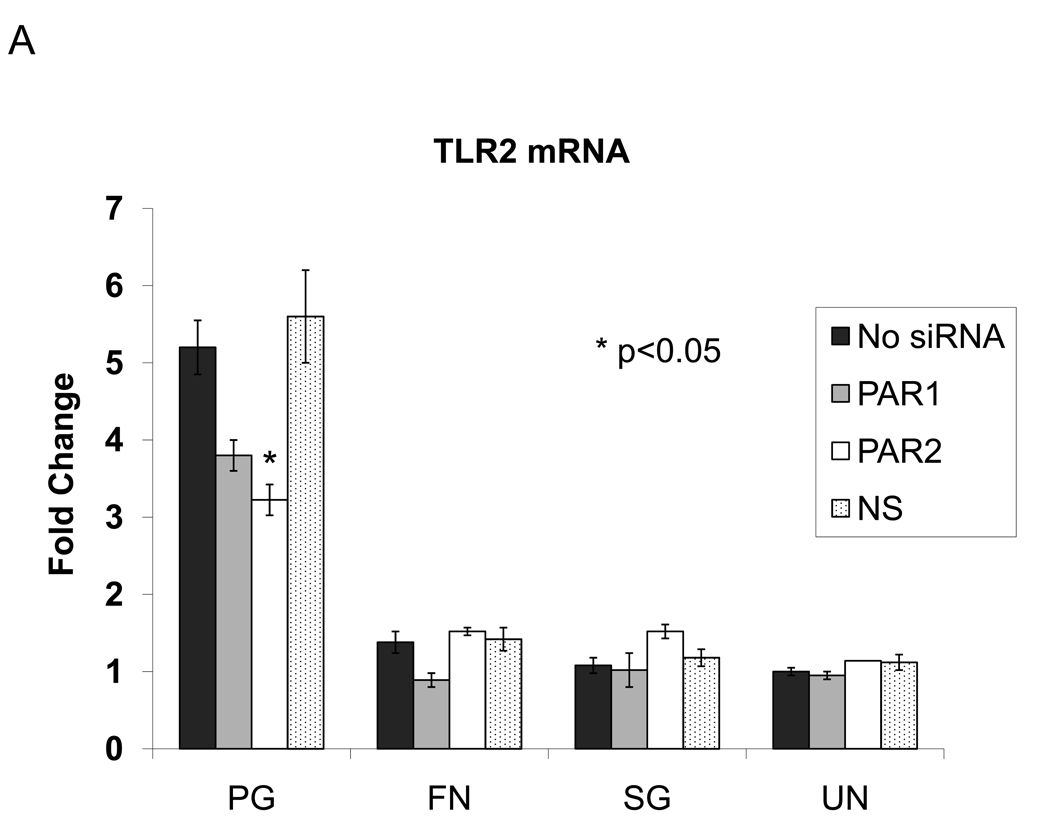
In order to investigate if TLR expression is also affected by the absence of another cell surface receptors (PAR1 and PAR2), we compared the mRNA expression levels of TLR2 and TLR4 in response to bacterial stimulation when a PAR is knocked down. TLR2 expression was decreased in response to P. gingivalis when PAR2 was knocked down (Figure 3A). TLR2 mRNA expression was not altered in knocked-down cells stimulated with F. nucleatum, while TLR4 mRNA expression was increased when cells were transfected with PAR2 siRNA and subsequently stimulated with F. nucleatum (Figure 3B). This Gram-negative bacterium utilizes TLR4, while Gram-negative P. gingivalis has a unique LPS structure and is able to utilize TLR2. Thus, our data suggest in the absence of PAR receptors, expression of TLRs is altered in response to bacteria consistent with their utilization. We have shown that different PRRs can compensate for absence of one another in epithelial response to bacteria and that these responses vary for different bacteria.
Figure 3.
QRT-PCR analyses of the effect of silencing PAR1 or PAR2 on TLR2 (A) and TLR4 (B) mRNA induction by various oral bacteria. Cells were transfected with a specific siRNA for 48 h, then subsequently stimulated with bacteria for 16 h. GECs transfected with non-silencing (NS) siRNA and unstimulated cells (UN) are used as controls in each experiment. Data from duplicates with cells from three different donors were normalized to the housekeeping gene control ribosomal phosphoprotein, and the average with standard deviation is shown. PG: P. gingivalis; FN: F. nucleatum; SG: S. gordonii.
mRNA expression of downstream innate immune markers are differentially affected by PARs
In order to investigate the role of PARs in the epithelial expression of innate immune and inflammatory markers, QRT-PCR for human beta-defensin-2 (hBD-2) and CC chemokine ligand 20 (CCL20) were utilized. hBD-2 is an antimicrobial peptide expressed in all epithelial cells, including gingival epithelia. CCL20 is a chemokine and antimicrobial protein with regions that are structurally related to hBD-2 [23]. Both CCL20 and hBD-2 link the innate and adaptive immunities [24,25]. We have previously reported that CCL20 follows the expression pattern of hBD-2, and the up-regulation of CCL20 and hBD-2 mRNA expression by P. gingivalis supernatant and purified P. gingivalis RgpB protease was mediated via PAR2, but not via PAR1 [22]. In this study, Figure 4A shows both PAR1 and PAR2 play a role in CCL20 mRNA expression induced by F. nucleatum and S. gordonii. We also observed decrease in CCL20 expression in GECs transfected with PAR2 siRNA and subsequently stimulated with P. gingivalis, but this decrease was statistically not significant (p=0.09) (Figure 4A), perhaps due to different experimental conditions. Our data suggest that PARs are also involved in the induction of CCL20 in epithelial innate immune responses to both Gram-negative and Gram-positive bacteria. However, no statistically significant changes in the induction of hBD-2 mRNA level are seen in cells stimulated with F. nucleatum or S. gordonii after PAR1 or PAR2 knock-down (Figure 4B).
Figure 4.
QRT-PCR analyses of the effect of silencing PAR1 or PAR2 on CCL20 (A) and hBD-2 (B) mRNA induction by various oral bacteria. Cells were transfected with a specific siRNA for 48 h, then subsequently stimulated with bacteria for 16 h. GECs transfected with non-silencing (NS) siRNA and unstimulated cells (UN) are used as controls in each experiment. Data from duplicates with cells from three different donors were normalized to the housekeeping gene control ribosomal phosphoprotein, and the average with standard deviation is shown. PG: P. gingivalis; FN: F. nucleatum; SG: S. gordonii.
PARs play a role in secretion of cytokines
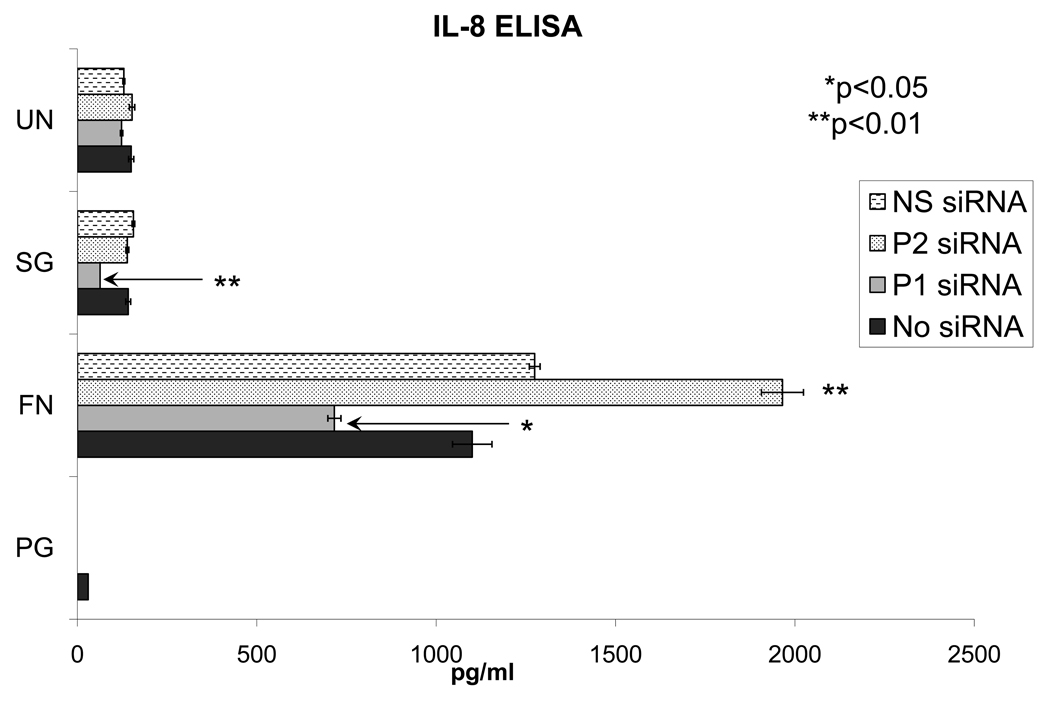
In order to investigate which receptors are involved in the downstream protein expression of cytokines, we first evaluated IL-8 secretion by ELISA (Figure 5). Controls with non-silencing siRNA and without siRNA were similar for each bacteria tested. Stimulation with F. nucleatum resulted in the greatest level of IL-8 secretion. This was significantly reduced in PAR1 knock-down samples. However, IL-8 secretion was enhanced in PAR2 knock-down samples. Suppression of IL-8 secretion was also shown with S. gordonii for PAR1 knock-down samples but the values were close to levels in epithelial cells without bacterial stimulation. The data show that IL-8 secretion in response to F. nucleatum may be via PAR1. On the other hand, PAR2 may have suppressive effect on IL-8 secretion (Figure 5). The results of IL-8 ELISA follow a pattern similar to the changes in IL-8 mRNA expression level tested via QRT-PCR (data not shown). The level of IL-8 secretion in response to P. gingivalis was under the minimal detection level (below 31.2 pg/ml), which corresponds with earlier findings that P. gingivalis may inhibit IL-8 secretion in GECs as well as in endothelial cells [26,27]. In order to investigate if other cytokines are similarly regulated by PARs, we next evaluated the changes in protein expression of multiple cytokines. PAR1 knock-down resulted in increased IL-1a secretion after P. gingivalis stimulation (Figure 6A). Figures 6B and 6C show a similar pattern of IL-1a secretion between F. nucleatum and S. gordoni with increase in secretion level after knock-down of PAR1 and PAR2. However, utilization of PAR1 or PAR2 was tailored to individual bacterium tested for the expression of IL-1b, IL-6, IL-8 and GM-CSF (Figure 6). No significant induction of the other 7 markers was detected (data not shown).
Figure 5.
The effect of silencing PAR1 (P1) or PAR2 (P2) on IL-8 induction in response to various oral bacteria is evaluated by ELISA. Cells were transfected with a specific siRNA for 48 h, then subsequently stimulated with bacteria for 16 h. Cell-free supernatant was collected and the amount of IL-8 secreted is shown in pg/ml. Unstimulated cells (UN) are used as controls in each experiment. Data from duplicates with cells from three different donors are shown. PG: P. gingivalis; FN: F. nucleatum; SG: S. gordonii.
Figure 6.
The effect of silencing PAR1 (P1) or PAR2 (P2) on the induction of multiple cytokines in response to various oral bacteria is evaluated by ELISA. Cells were transfected with a specific siRNA for 48 h, then subsequently stimulated with bacteria for 16 h. Cell-free supernatant was collected and the amount of cytokines secreted is compared in fold changes relative to bacteria only controls. Data from two different donor cells are shown. PG: P. gingivalis; FN: F. nucleatum; SG: S. gordonii.
5. Discussion
The oral cavity is a complex environment in which epithelial soft tissue is associated with hundreds of microorganisms, thus host response needs to be appropriate for different bacteria present. In this study we report that oral epithelial cells balance receptor expression for specific bacterial recognition and subsequent innate immune responses. Two recent studies report synergism of different receptors in cytokine induction [28,29], but this is the first study to demonstrate the possible interplay between NODs, PARs and TLRs in epithelia and illustrates how balancing multiple receptors may lead to appropriate host epithelial innate immune responses to different bacteria. In this study utilizing siRNA-mediated RNA interference to post-transcriptionally knock-down various receptors, we show evidence that epithelial cells alter expression of NODs, PARs or TLRs in response to various bacteria when one receptor is knocked down. These data suggest in the absence of one receptor, the epithelial cells can up-regulate expression of other receptors to compensate for the absent receptor in responding to different bacteria regardless of whether the receptors are located on cell surface or are intracellular. Our data also suggest that complex interplay of several receptors leads to appropriate innate immune responses to the different types of bacteria present within the oral cavity and that receptor expression itself is altered depending on which organism the cell encounters. Since epithelial cells express multiple receptors in responding to various MAMPs present on different bacteria, it is reasonable to expect these three classes of receptors should all be functional in order to produce appropriate innate immune responses to individual bacterial species. By using whole live bacteria with multiple MAMPs, we have observed that the cells can balance receptors expression, in some cases quite dramatically, in an apparently logical way. This new observation provides deeper understanding of the bacteria-host interaction.
We also report in this study that knock-down of these receptors has effects on the downstream induction of innate immune markers, and that different receptors are utilized in the induction of these markers depending on each bacterium epithelia are exposed to. Previous studies reported that LPS from P. gingivalis, E. coli and F. nucleatum was a poor stimulant of hBD-2 in GECs, suggesting involvement of signaling pathways other than LPS activation of TLR4 signaling [30]. This was in contrast to tracheal epithelia, in which bacterial LPS up-regulates hBD-2 mRNA transcription, and antibodies against CD14, a cell-surface receptor for LPS, inhibit this transcription [31,32]. These findings indicate that different epithelial cell types utilize different pathways for stimulation of hBD-2 induction in response to bacteria. Utilizing both direct methods and specific siRNAs, our laboratory reported that oral epithelial cells recognize and utilize PARs in addition to (or in contrast to) LPS-TLR signaling in distinguishing pathogens from commensals for hBD-2 and CCL20 induction or alternatively, that there is some cross-talk between various components in these complex pathways [22,33–35]. Here we report the apparent interplay of NODs, PARs and TLRs in the induction of CCL20, hBD-2 and IL-8 in response to various bacteria. The induction of CCL20 and hBD-2 by cell-free supernatant of P. gingivalis or purified P. gingivalis protease RgpB occurs via PAR2 but not PAR1 [22], consistent with the activation of PAR2 by gingipains [10]. In addition, the induction of hBD-2 and CCL20 by whole live P. gingivalis involves MAPK and NFκB [21,33,35]. In our current study using whole cell live P. gingivalis, we observed decrease in CCL20 and hBD-2 mRNA expression in GECs transfected with PAR2 siRNA, but this decrease was statistically not significant (p=0.09 and p=0.12, respectively). Thus, this difference may reflect different signaling pathways GECs utilize in inducing hBD-2; purified P. gingivalis protease works through PAR2 in inducing hBD-2, while the induction by whole P. gingivalis utilizes MAPK and/or NFκB signaling in the absence of PAR2. Surprisingly, our results also suggest a role for PAR1 and/or PAR2 in induction of hBD-2, CCL20, and IL-8 for F. nucleatum, a bacterium that has not previously been suggested to activate PARs, and suggest divergent effects of PAR1 and PAR2 on innate immune responses.
It has been widely known that cell-surface receptors, such as TLRs and PARs, are activated when appropriate bacterial ligands or proteases are present on cell surface, and activation of TLRs and PARs eventually leads to activation of NFκB and/or MAPK pathways [36–39]. However, since NODs are expressed intracellularly, whether the presence of non-invasive bacteria would lead to NOD activation was uncertain. Different studies showed that infection by non-invasive bacteria Pseudomonas aeruginosa and H. pylori can trigger the induction of inflammatory cytokines via NOD1 by injecting PGN fragments into the host through type IV secretion system [40,41]. Extracellular bacteria can also be recognized by and activate NOD2 through a plasma membrane transporter hPepT1 [42,43]. Our data suggest NODs are involved in cross-communicating with PARs during epithelial responses to P. gingivalis and F. nucleatum, both of which are able to internalize in GECs.
In this study we report that a complex interplay of several receptors is required for appropriate innate immune responses to the different types of bacteria present within the oral cavity and that receptor expression itself is altered depending on the requirements of the cell. Understanding how critical receptors are activated in a coordinated way in response to each bacterium, leading to appropriate innate immune responses to pathogenic and non-pathogenic bacteria, will give us insights into the host responses to bacteria and help find suitable targets for new therapeutics.
Acknowledgement
We thank Dr. Beverly A. Dale for helpful discussions and Ms. Julia H. Tracy for culturing of cells. This work was supported by NIDCR grants R01 DE16961-01 and K22 DE015812
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medzhitov R, Janeway C. Trends in Microbiology. 2000;8:452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 2.Bourhis LL, Werts C. Microbes Infect. 2007;9:629–636. doi: 10.1016/j.micinf.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Handfield M, Mans JJ, Zheng G, Lopez MC, Mao S, Progulske-Fox A, Narasimhan G, Baker HV, Lamont RJ. Cell Microbiol. 2005;7:811–823. doi: 10.1111/j.1462-5822.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa Y, Mans JJ, Mao S, Lopez MC, Baker HV, Handfield M, Lamont RJ. Infect Immun. 2007;75:2540–2547. doi: 10.1128/IAI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinane DF, Galicia JC, Gorr SU, Stathopoulou PG, Benakanakere M. Front Biosci. 2008;13:966–984. doi: 10.2741/2736. [DOI] [PubMed] [Google Scholar]
- 6.Coughlin SR, Camerer E. J Clin Invest. 2003;111:25–27. doi: 10.1172/JCI17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohani MG, Beyer RP, Hacker BM, Dommisch H, Dale BA, Chung WO. Innate Immunity. 2009 doi: 10.1177/1753425909339233. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackie EJ, Pagel CN, Smith R, de Niese MR, Song SJ, Pike RN. IUBMB Life. 2002;53:277–281. doi: 10.1080/15216540213469. [DOI] [PubMed] [Google Scholar]
- 9.Holzhausen M, Spolidorio LC, Vergnolle N. J Dent Res. 2005;84:154–159. doi: 10.1177/154405910508400209. [DOI] [PubMed] [Google Scholar]
- 10.Lourbakos A, Potempa J, Travis J, D'Andrea MR, Andrade-Gordon P, Santulli R, Mackie EJ, Pike RN. Infect Immun. 2001;69:5121–5130. doi: 10.1128/IAI.69.8.5121-5130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacNaughton WK. Mem Inst Oswaldo Cruz. 2005;100 Suppl 1:211–215. doi: 10.1590/s0074-02762005000900036. [DOI] [PubMed] [Google Scholar]
- 12.Hou L, Ravenall S, Macey MG, Harriott P, Kapas S, Howells GL. J Periodontal Res. 1998;33:205–211. doi: 10.1111/j.1600-0765.1998.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 13.Martinon F, Tschopp J. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Becker CE, O'Neill LA. Semin Immunopathol. 2007;29:239–248. doi: 10.1007/s00281-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 15.Ting JP, Kastner DL, Hoffman HM. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 16.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 17.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 18.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nunez G, Inohara N. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 19.Inohara, Chamaillard, McDonald C, Nunez G. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 20.Strober W, Murray PJ, Kitani A, Watanabe T. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 21.Chung WO, Dale BA. Infect Immun. 2004;72:352–358. doi: 10.1128/IAI.72.1.352-358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dommisch H, Chung WO, Rohani MG, Williams D, Rangarajan M, Curtis MA, Dale BA. Infect Immun. 2007;75:4326–4333. doi: 10.1128/IAI.00455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoover DM, Boulegue C, Yang D, Oppenheim JJ, Tucker K, Lu W, Lubkowski J. J Biol Chem. 2002;277:37647–37654. doi: 10.1074/jbc.M203907200. [DOI] [PubMed] [Google Scholar]
- 24.Schutyser E, Struyf S, Van Damme J. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 25.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Wang JM, Howard OM, Oppenheim JJ. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 26.Darveau RP, Belton CM, Reife RA, Lamont RJ. Infect Immun. 1998;66:1660–1665. doi: 10.1128/iai.66.4.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nassar H, Chou HH, Khlgatian M, Gibson FC, 3rd, Van Dyke TE, Genco CA. Infect Immun. 2002;70:268–276. doi: 10.1128/IAI.70.1.268-276.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eskan MA, Rose BG, Benakanakere MR, Zeng Q, Fujioka D, Martin MH, Lee MJ, Kinane DF. Eur J Immunol. 2008;38:1138–1147. doi: 10.1002/eji.200737898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uehara A, Imamura T, Potempa J, Travis J, Takada H. Cell Microbiol. 2008;10:1181–1189. doi: 10.1111/j.1462-5822.2008.01119.x. [DOI] [PubMed] [Google Scholar]
- 30.Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, Dale BA. Infect Immun. 2000;68:2907–2915. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond G, Russell JP, Bevins CL. Proc Natl Acad Sci U S A. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diamond G, Kaiser V, Rhodes J, Russell JP, Bevins CL. Infect Immun. 2000;68:113–119. doi: 10.1128/iai.68.1.113-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung WO, Dale BA. Oral Microbiol Immunol. 2008;23:119–126. doi: 10.1111/j.1399-302X.2007.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung WO, Hansen SR, Rao D, Dale BA. J Immunol. 2004;173:5165–5170. doi: 10.4049/jimmunol.173.8.5165. [DOI] [PubMed] [Google Scholar]
- 35.Dommisch H, Chung WO, Jepsen S, Hacker BM, Dale BA. Innate Immunity. 2009 doi: 10.1177/1753425909339237. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darmoul D, Gratio V, Devaud H, Laburthe M. J Biol Chem. 2004;279:20927–20934. doi: 10.1074/jbc.M401430200. [DOI] [PubMed] [Google Scholar]
- 37.Fyfe M, Bergstrom M, Aspengren S, Peterson A. Cytokine. 2005;31:358–367. doi: 10.1016/j.cyto.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Toubi E, Shoenfeld Y. Autoimmunity. 2004;37:183–188. doi: 10.1080/08916930410001704944. [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Zeng H, Lyons S, Carlson A, Merlin D, Neish AS, Gewirtz AT. Am J Physiol Gastrointest Liver Physiol. 2003;285:G282–G290. doi: 10.1152/ajpgi.00503.2002. [DOI] [PubMed] [Google Scholar]
- 40.Travassos LH, Carneiro LA, Girardin SE, Boneca IG, Lemos R, Bozza MT, Domingues RC, Coyle AJ, Bertin J, Philpott DJ, Plotkowski MC. J Biol Chem. 2005;280:36714–36718. doi: 10.1074/jbc.M501649200. [DOI] [PubMed] [Google Scholar]
- 41.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 42.Ismair MG, Vavricka SR, Kullak-Ublick GA, Fried M, Mengin-Lecreulx D, Girardin SE. Can J Physiol Pharmacol. 2006;84:1313–1319. doi: 10.1139/y06-076. [DOI] [PubMed] [Google Scholar]
- 43.Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB. Gastroenterology. 2004;127:1401–1409. doi: 10.1053/j.gastro.2004.07.024. [DOI] [PubMed] [Google Scholar]