Abstract
Aim
TNF-α is known to cause adverse myocardial remodeling. While we have previously shown a role for cardiac mast cells in mediating myocardial TNF-α, matrix metalloproteinases (MMP) activation of TNF-α may also be contributory. We sought to determine the relative roles of MMPs and cardiac mast cells in the activation of TNF-α in the hearts of rats subjected to chronic volume overload.
Methods
Interventions with the broad spectrum MMP inhibitor, GM6001, or the mast cell stabilizer, nedocromil, were performed in the rat aortocaval fistula (ACF) model of volume overload.
Results
Myocardial TNF-α levels were significantly increased in the ACF. This increase was prevented by MMP inhibition with GM6001 (p ≤ 0.001 vs. ACF). Conversely, myocardial TNF-α levels were increased in the ACF + nedocromil treated fistula groups (p ≤ 0.001 vs. sham). The degradation of interstitial collagen volume fraction seen in the untreated ACF group was prevented in both the GM6001 and nedocromil treated hearts. Significant increases in LV myocardial ET-1 levels also occurred in the ACF group at 3 days post-fistula. Whereas administration of GM6001 significantly attenuated this increase, mast cell stabilization with nedocromil markedly exacerbated the increase, producing ET-1 levels 6.5 fold and 2 fold greater than that in the sham-operated control and ACF group, respectively.
Conclusion
The efficacy of the MMP inhibitor, GM6001, to prevent increased levels of myocardial TNF-α is indicative of MMP-mediated cleavage of latent extracellular membrane bound TNF-α protein as the primary source of bioactive TNF-α in the myocardium of the volume-overload heart.
Keywords: MMP, TNF-α, ventricular remodeling, aorto-caval fistula, cytokines, GM6001
Introduction
The induction of heart failure, regardless of etiology (i.e., chronic pressure overload, sustained volume overload, or myocardial infarction), is associated with increased expression of multiple pro-inflammatory cytokines.(1–5) TNF-α is chief among these inflammatory mediators and undergoes acute elevations in the remodeling heart, with TNF-α having been shown to mediate pathologic events.(6–11) Cardiac mast cells have been identified as the predominant source of TNF-α in the unstressed myocardium,(12–14) while Reil et al.(15) and others(13) demonstrated a biphasic pattern in which the initial release of myocardial TNF-α in response to ischemia and reperfusion was mast cell mediated, whereas, the subsequent increase in TNF-α protein expression was mast cell independent. Furthermore, we recently found that TNF-α was undetectable by ELISA and there were no volume overload-induced increases in TNF-α in the hearts of mast cell deficient rats.(16) However, subsequent findings have indicated other sources are also important.(17), and Diwan et al.(9) implicated enzymatic processing of TNF-α as a critical event initiating adverse ventricular remodeling. The A Disintegrin And Metalloproteinase (ADAM) family of enzymes encompassing ADAM 17, otherwise known as TNF-α converting enzyme (TACE),(18–20) is capable of generating soluble TNF-α by proteolytically cleaving membrane-bound pro-TNF-α (21;22) Therefore, the purpose of this study was to determine the predominant mechanism of the pathologic increases in myocardial TNF-α secondary to chronic cardiac volume overload. To this end, TNF-α levels were determined following interventions with the broad spectrum matrix metalloproteinase inhibitor, GM6001 or the mast cell stabilizer, nedocromil, three days after creating an aortocaval fistula (ACF) in the male rat.
Methods
Animal Welfare
All experiments were performed using 9 week (200–250 g) male Sprague-Dawley rats housed under standard environmental conditions and maintained on commercial rat chow and tap water ad libitum. All studies conformed to the principles of the National Institutes of Health “Guide for the Care and Use of Laboratory Animals,” and were approved by University of South Carolina School of Medicine Animal Care and Use Committee. The anesthetic agent used for all experimental procedures was sodium pentobarbital (50 mg/kg, i.p.).
Experimental Design
In order to determine if mast cell stabilization or inhibition of MMPs would limit TNF-α release, in vivo experiments utilized either: 1) the mast cell stabilizer, nedocromil sodium, administered three days prior to fistula surgery via a 21 day time release pellet (Innovative Research of America, FL) placed subcutaneously to achieve a delivered dosage of 10mg/kg/day as previously published.(23;24); or 2) the broad spectrum matrix metalloproteinase inhibitor, GM6001, (5 mg/kg/day, Calbiochem®), dissolved in ethanol and phosphate buffered saline and delivered via a once daily subcutaneous injection initiated three days prior to surgery and continued for the duration of the experiment.(25) The dissociation constant for the binding of GM6001 to collagenases (MMP-1, MMP-8, MMP-3) and gelatinases (MMP-2, MMP-9) has been extensively characterized in vitro and in vivo.(19;26;27)
Animal Groups
Four separate in vivo study groups were analyzed at 3 days post-surgery as follows: sham-operated control (Sham, n=6), untreated aortocaval fistula (ACF, n=5), nedocromil treated ACF (ACF + Nedocromil, n=5), and MMP inhibitor treated ACF (ACF + GM6001, n=6). Nedocromil and GM6001 were administered as described above and continued until the experimental endpoint. This time point was chosen based on extensive prior characterization performed by our laboratory demonstrating a significant increase in cardiac mast cell density and concomitant MMP activation that resulted in marked collagen degradation and changes in ventricular morphology.(20;24;28) Drug treated sham operated controls were also performed, however, no differences between the treated and untreated sham-operated control groups were noted (data not shown).
At the experimental endpoint, aortocaval fistula was confirmed visually at sacrifice based upon the pulsatile flow of oxygenated blood into the vena cava. Additionally, a marked increase of ~50% or greater in cardiac output, as determined by measuring aortic flow (Aortic flow probe, Transonic Systems Inc®, Itacha NY), was indicative of a patent fistula. Under deep anesthesia, the heart was excised; the atria and great vessels removed, and the LV (including septum) and right ventricle were separated and weighed. A complete transmural section of the LV at the midventricular level was placed in 4% paraformaldehyde, and the remaining tissue was minced into 1 mm cubes and snap-frozen in liquid nitrogen for storage at −80° C.
Infrarenal Abdominal Aorta-Inferior Vena Cava (AV) Fistula
Infrarenal AV fistula was created in rats as described previously.(20;28) Briefly, a ventral abdominal laparotomy was performed to expose the aorta and caudal vena cava approximately 1.5 cm below the renal arteries. Both vessels were temporarily occluded, and an 18 gauge needle was inserted into the exposed abdominal aorta and advanced through the medial wall into the vena cava. The needle was withdrawn and the puncture site sealed with surgical glue.
Protein extraction from LV tissue
100mg of LN2 frozen LV tissue from each heart was maintained on ice and minced. Tissue was placed into homogenization tubes with 800 µl PBS/protease inhibitor cocktail solution. Tissues were homogenized on ice prior to sonication. 50µl of 10% Triton-X 100 was added to each sample and vortexed. Samples were then incubated on ice for 30 min and vortexed after the first 15 min. Samples were then centrifuged @ 16,000 rpm (4°C) for 30min and the supernatant collected and frozen at −80°C.
Assessment of Mast Cell Density and Fibrillar Collagen Concentration
At the end of the experimental period, a transmural section of LV was taken from the mid ventricle, formalin-fixed and processed for routine histopathology. A 5 micron paraffin-embedded section was stained with pinacyanol erthrosinate for the visualization of mast cell morphology. Mast cell density was determined from the total number of mast cells in the LV cross section normalized for tissue area determined from a digitized image (ImageQuant, Molecular Dynamics). The percentage of mast cell degranulation was determined based upon 400× microscopic examination of the entire LV slide section of four randomly selected hearts from control and treated groups. Degranulation was defined as the presence of disseminated granules external to the cell membrane and/or loss of membrane integrity as previously described.(29)
LV interstitial collagen volume fraction (CVF) was determined by analysis of picrosirius red-stained sections as previously described.(20;29) Sections were incubated with 0.2% phosphomolybdic acid (2 min) to reduce background staining before staining with picrosirius red (0.1% Sirius Red F3BA in picric acid). Twenty randomly-chosen fields per LV section were analyzed in a blinded fashion using a Biorad MRC-1024 confocal laser-scanning microscope under 400× magnification. Image analysis was performed using Scion-image software. For each field, the percent of collagen was obtained by dividing the pixel count of fluorescent interstitial collagen fibers in the field by the total number of pixels present in the field. The individual collagen fraction values were then averaged to obtain the representative CVF for the LV.
Enzyme-Linked ImmunoSorbent Assay
Myocardial tissue levels of ET-1 and TNF-α were analyzed using commercially available kits (Alpco© and R&D Systems Quantikine, respectively). Briefly, precoated wells were incubated with 100µl of extracted protein sample with assay buffer for one hour, followed by washing 5 times with wash buffer. Wells were then incubated with appropriate monoclonal antibody and washed again before incubation with HRP conjugated secondary antibody. The plates were then read by spectrophotometry at 450nm, in accordance with manufacturer’s specifications.
Flow Cytometry
Cardiac mast cells were isolated as previously described.(20;30) Isolated cells were washed and blocked against non-specific staining with PBS/1% BSA for 30 min before incubation for 1 hr at 37°C with AR32AA4 (mast cell; mouse monoclonal, 1:100; BD Pharmingen) followed by conjugation for 1 hr at 37°C with PE secondary antibody (rabbit, 1:500; Abcam). After washing, the cells were fixed with 4% paraformaldehyde and permeabalized using saponin (0.1%) for 20 min at 4°C. The cells were then incubated with anti-TNF-α antibody (rabbit monoclonal, 1:500; Abcam) and conjugated with Alexa Fluor 488 secondary antibody. Cell preparations were analyzed using an Epics XL FACS system equipped with a 15mW argon ion laser operating at 488nm and data analyzed with EXPO 32 software.
Statistical analysis
Statistical analyses were performed with Graphpad Prism 5.0 (GraphPad Software, Inc. San Diego, CA). All grouped data are expressed as means ± SEM unless otherwise noted. Grouped data comparisons were made by one-way analysis of variance with intergroup comparisons analyzed using Bonferroni post-hoc testing. Statistical significance was taken to be p ≤ 0.05.
Results
In Vivo TNF-α Elaboration and Neurohormone Cytokine Interaction
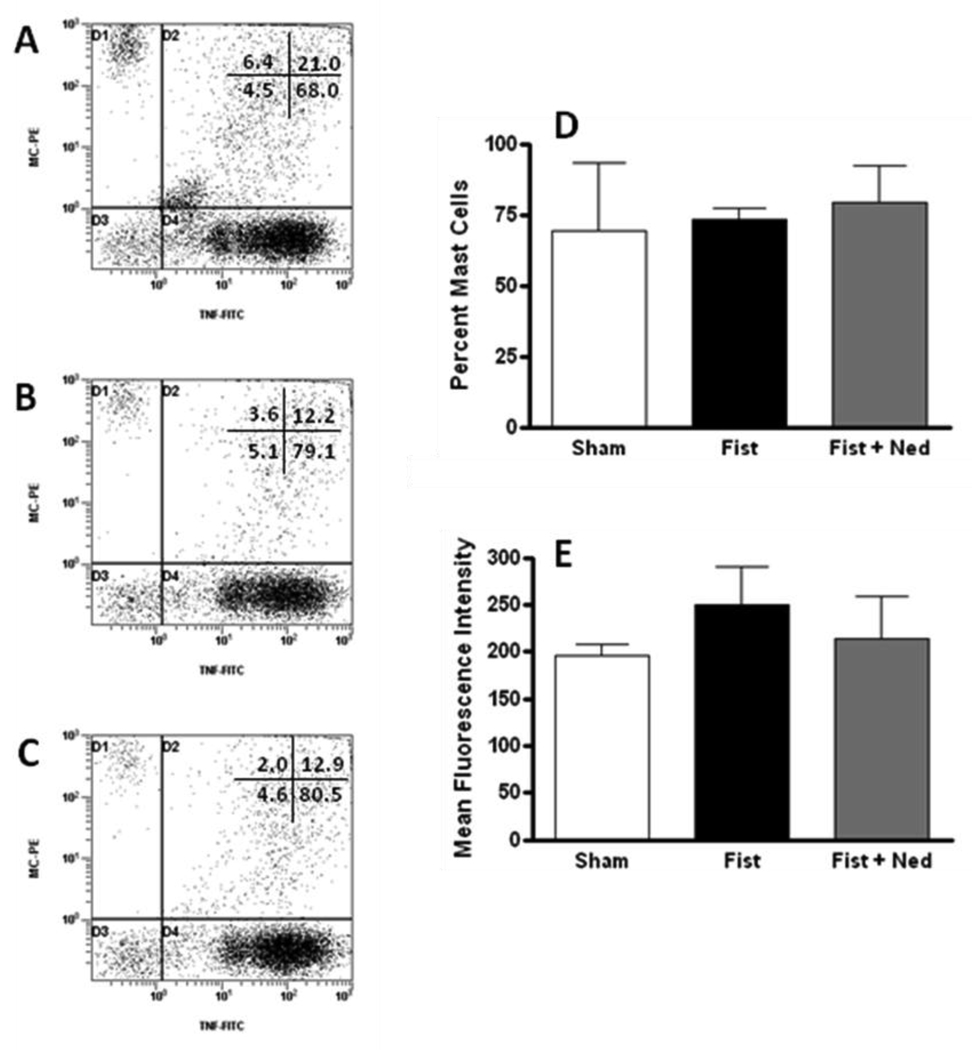
A substantial 5 fold elevation in myocardial TNF-α levels relative to that in the sham-operated group occurred in the untreated rats at 3 days post-fistula (Table 1). GM6001 treatment prevented the volume overload induced increase in TNF-α levels post-fistula (p≤ 0.01 vs. ACF). In contrast, mast cell stabilization with nedocromil failed to attenuate the significant increase in LV TNF-α levels. In figure 1, flow cytometry of mast cells stained for TNF-α shows that the percentage of mast cells containing TNF-α was unchanged between sham, fistula and fistula treated with nedocromil. Additionally, mean fluorescent intensity indicated that there was no difference in the amount of TNF-α in mast cells isolated from sham, fistula or fistula treated with nedocromil.
Table 1.
Analysis of Tumor Necrosis Factor- α (TNF-α), Endothelin-1 (ET-1), mast cell density, and interstitial collagen in left ventricular tissue in sham-operated, drug treated controls, untreated aortocaval fistula (ACF), ACF + Nedocromil and ACF + GM6001 treated fistula hearts.
| TNF-α (pg/ml) |
ET-1 (fmol/ml) |
Mast Cell Density (LV mast cell /mm2) |
Collagen (% myocardial area) |
|
|---|---|---|---|---|
| Sham | 5 ± 0.6 | 1 ± 0.2 | 2.2 ± 0.3 | 1.8 ± 0.1 |
| Sham+ Nedo | 4 ± 0.1 | 1 ± 0.1 | 1.4 ± 0.2 | 1.7 ± 0.1 |
| Sham+ GM6001 | 6± 1 | 0.8 ± 0.03 | 2.9 ± 0.4 * | 1.8 ± 0.05 |
| ACF | 27 ± 3 * | 3 ± 0.6 * | 3.5 ± 0.3 * | 1.4 ± 0.1 * |
| ACF+ Nedoc | 21 ± 2 * | 6.5 ± 1.7 * | 1.3 ± 0.1 † | 2.2 ± 0.1 † |
| ACF+GM6001 | 5 ± 2 † | 1.7 ± 0.2 † | 3.8 ± 0.2 * | 1.9 ± 0.05 † |
Values are reported as average ± SEM.
p ≤ 0.01 vs. sham.
denotes p ≤ 0.01 vs. untreated ACF.
Figure 1.
Representative scatter plots of isolated cardiac mast cells containing TNF-α from (A) shams; (B) untreated fistula; and (C) fistula treated with nedocromil. The grouped data are presented as mean ± SD. (D) Percent of cardiac mast cells containing TNF-α; (E) mean intensity fluorescence for cardiac mast cells containing TNF-α.
Significant increases in LV myocardial ET-1 levels also occurred in the ACF group at 3 days post-fistula (Table 1). Whereas administration of GM6001 significantly attenuated this increase in ET-1 levels post-fistula, mast cell stabilization with nedocromil markedly exacerbated the increase, producing ET-1 levels 6.5 fold and 2 fold greater than that in the sham-operated control and ACF group, respectively.
In Vivo Mast Cell Density and Percent Degranulation
The influence of in vivo mast cell stabilization and MMP inhibition on volume overload induced increases in LV mast cell density was also determined at 3 days post-fistula. As can be seen in Table 1, volume overload induced a significant increase in LV mast cell density in both the untreated and GM6001 treated fistula groups (79% and 98%, respectively, relative to control). Conversely, administration of nedocromil prevented the AV fistula-induced increase in myocardial mast cell density. Consistent with the increased mast cell density, the percentage of activated cells was significantly greater in the untreated fistula group (19 ± 4% degranulated, p≤ 0.05), relative to the small number of degranulated cells seen in the sham (10 ± 2%) and nedocromil (11 ± 4%) groups. MMP inhibition with GM6001 resulted in substantially greater mast cell activation above that in the untreated fistula group (32 ± 5% degranulated, p≤ 0.05 vs. ACF).
Extracellular Matrix Content and Composition
Compared to control, untreated fistula hearts underwent a marked loss of ventricular collagen at 3 days post-fistula (1.8 ± 0.1% vs. 1.3 ± 0.09% percent of myocardial tissue; Sham vs. ACF, p≤ 0.05, Table 1). However, interventions with nedocromil or GM6001 were equally efficacious in preventing the fistula associated decrease in myocardial CVF.
Discussion
The increase in TNF-α in the stressed or injured myocardium has been well documented.(2;12–15) We recently found significant elevations in myocardial TNF-α after 5 days of chronic LV volume overload.(28) Bozkurt et al.(31) found that chronic administration of TNF-α in normal rats produced significant LV dilatation along with a striking reduction in interstitial collagen. In this study we utilized a broad spectrum matrix metalloproteinase inhibitor, GM6001 and treated male adult rats for three days after creating an ACF. GM6001 possesses a broad spectrum MMP inhibitory capacity which includes the A Disintegrin And Metalloproteinase (ADAM) family of enzymes encompassing ADAM 17, otherwise known as TNF-α converting enzyme (TACE).(27;32) ADAM 17 is capable of generating soluble TNF-α by proteolytically cleaving membrane-bound pro-TNF-α.(21;22) However, it must be noted that redundant mechanisms for generating myocardial TNF-α have also been described.(15;17;33;34) Specifically, other MMPs have also been shown to mediate cleavage of pro-TNF.(35;36) In the chronic in vivo scenario, MMP inhibition with GM6001 was efficacious in preventing the increase in myocardial TNF-α in the ACF. GM6001 also prevented ACF-induced collagen degradation. The significantly decreased level of total myocardial collagen in the untreated fistula group (28% less than that in sham-operated control), reflecting degradation by MMP activation is consistent with previous reports.(29;37) Of further interest is the observation that GM6001 inhibition of TNF-α cleavage resulted in similar results to those observed using the TNF-α inhibitor, etanercept, where collagen degradation was also prevented in the same model of volume overload.(38)
While our results with GM6001 clearly show the importance of MMP cleavage of TNF-α in volume overload-induced remodeling, previous histological analysis of LV sections from normal hearts demonstrated concentrated localization of TNF-α within cardiac mast cells.(13;14;39) Consistent with mast cells regulating myocardial TNF-α, levels of TNF-α were undetectable by ELISA in mast cell deficient rats post-fistula.(16) However, in that same study we also observed diffuse labeling of TNF-α dispersed throughout the myocardium of the wild type control, indicating the possibility of cardiomyocyte membrane-bound TNF-α in normal rats. Further, a recent study by Reil et al. (15) indicates that, although the initial release of TNF-α following ischemia-reperfusion is cardiac mast cell-dependent, subsequent increases are derived from other sources. In fact, in a similar volume overload model of heart failure to that herein, Chen Y and coworkers(17) identified isolated myocytes as a potential source of TNF-α. These observations, together with our previous findings that mast cells activate MMPs in the ACF model, raise the possibility that increased levels of TNF-α in stressed or injured myocardium are the result of mast cell mediated activation of MMPs cleaving latent extracellular membrane bound TNF-α.
The confounding factor in this study is that TNF-α levels in the myocardium remained significantly elevated after treatment with nedocromil. One limitation of this study is the inability to differentiate interstitial TNF-α from the cytokine content of cardiac mast cells in whole tissue homogenate, thus, flow cytometry was used to determine whether the increased levels of myocardial TNF-α following treatment with nedocromil was the result of retention and/or increases of TNF-α in mast cells due to membrane stabilization. Our results indicate that the percentage of mast cells containing TNF-α was unchanged between sham, fistula and fistula treated with nedocromil. Additionally, mean fluorescent intensity indicated that there was no difference in the amount of TNF-α in mast cells isolated from sham, fistula or fistula treated with nedocromil. Therefore, at this stage we are unable to explain the increased myocardial TNF-α in the ACF+nedocromil group. It is possible though that nedocromil prevents release of mast cell products that activate MMPs, thus, preventing the cleavage of cardiomyocyte-bound TNF-α. Since cardiomyocytes are known to upregulate TNF-α in the ACF (17) this may lead to an accumulation of membrane bound TNF-α in the ACF+nedocromil group.
We have also previously demonstrated that cardiac mast cells are responsive to the endogenous endothelin system.(28;40) Cardiac mast cell activation herein was also positively correlated with myocardial ET-1 levels. A similar relationship has been postulated between ET-1 levels and MMP activation,(41–44) Previous work by Podesser et al.(44) and Ozdemir and coworkers(45) indicated that MMP induction following myocardial infarction was an ETA receptor specific phenomenon, yet no particular cell type was implicated. Few have thus far considered that cardiac mast cells may broker the interaction.(28;29) A recent study by Deschamps et al.(43) found selective ETA receptor antagonism attenuated the ET-1 mediated increase in membrane type MMP (MT1-MMP) activity following ischemia/reperfusion and MT1-MMP is involved in the processing of bioactive cytokines.(46) Similarly, studies have documented that ET-1 is capable of inducing TNF-α expression in mucosal and connective tissue type mast cells.(47;48)
The marked increase in ET-1 levels in the ACF and ACF + Nedocromil groups may have been the outcome of an endogenous stimulus to mediate MMP activation. However, the substantially greater increase in the ACF + Nedocromil group could also be related to mast cell-derived chymase being essential for localized degradation of ET- 1.(49;50) The reduction in myocardial ET-1 levels by GM6001 was in stark contrast to the increases seen in the ACF and ACF + Nedocromil group. However, Fernandez-Patron et al. recently reported that MMP-2 cleavage of big endothelin 1 yielded the vasoactive peptide ET-1,(51) therefore, this observation may reflect impaired MMP mediated generation of ET-1. Further, GM6001 would not be expected to alter mast cell activation (i.e. release of preformed serine proteases) which would still allow degradation of ET- 1 via mast cell-derived chymase.
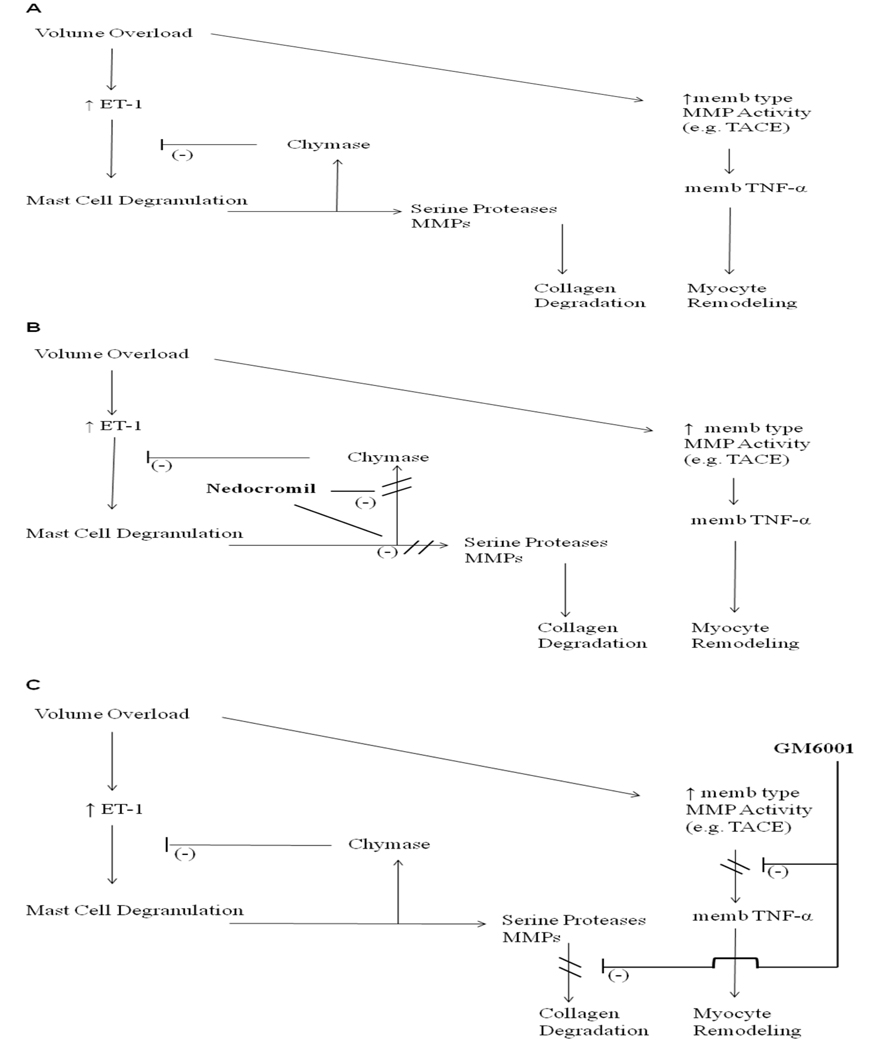
In summary, the efficacy of the MMP inhibitor, GM6001, to prevent increased levels of myocardial TNF-α and subsequent myocardial remodeling is indicative of MMP-mediated cleavage of latent extracellular membrane bound TNF-α protein as the source of bioactive TNF-α in the volume-stressed myocardium. The results presented in Table 1 demonstrate that stabilization of mast cells failed to attenuate the local generation of TNF and ET-1, but that MMP inhibition significantly reduced TNF and ET-1 levels following acute AV fistula. The schematics depicted in Figure 2 further illustrate the involved pathways. In panel A, volume overload results in an increase in ET-1 which in turn causes mast cell degranulation and activation of membrane (memb) type MMPs. Mast cell-derived chymase feeds back to degrade ET-1 while other mast cell-released substances activate interstitial MMPs. These activated MMPs are then responsible for interstitial collagen degradation and possibly contribute to additional activation of memb type MMPs. The activated memb type MMPs are responsible for the release of membrane bound TNF-α which is known to cause myocyte remodeling. The results of pretreatment with nedocromil are depicted in Panel B. By preventing mast cell degranulation, the negative feedback associated with chymase release is no longer present and serine proteinase activation of MMPs does not occur. As a result, ET-1 is further elevated, membrane type MMPs are activated and membrane bound TNF-α released. Thus, while interstitial collagen remains intact, myocyte remodeling will occur. As can be seen in Panel C, GM6001 inhibits the memb type MMPs despite the fact that mast cells are degranulated. As a result collagen degradation and myocyte remodeling do not occur. Accordingly, the local source and conversion of TNF following the volume overload stimulus at this time point is not the mast cell per se, but that this induction is an MMP dependent process.
Figure 2.
(A) Schematic diagram of resident cardiac mast cell mediated ventricular remodeling secondary to chronic volume overload. Panel B depicts the potential effects of mast cell stabilization (Nedocromil) on preformed product release from mast cells and how this would play a role in ET-1 and TNF-α levels. (C) Illustrates the possible effects of the broad spectrum inhibition of matrix metalloproteinases (GM6001) and subsequent attenuation of adverse myocardial remodeling.
Acknowledgments
We are grateful to Christin Carpenter and Purnima Jani for their expert technical assistance.
Funding Sources: This study was supported by National Heart, Lung and Blood Institute Grants RO1-HL-62228 (JSJ) and R01-HL-073990 (JSJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: We have no conflicts of interest to declare in regards to relationships (inclusive of but not limited to, employment by an industrial concern, ownership of stock, membership on a standing advisory council or committee, being on the board of directors, or being publicly associated with the company or its products) with pharmaceutical companies, biomedical device manufacturers, or other corporations whose products or services related to the subject matter of this article.
Reference List
- 1.Hausenloy DJ, Yellon DM. Clinical translation of cardioprotective strategies : report and recommendations of the Hatter Institute 5th International Workshop on Cardioprotection. Basic Res Cardiol. 2008 Sep;103(5):493–500. doi: 10.1007/s00395-008-0736-x. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Zhong S, Zeng K, Luo Y, Zhang F, Sun X, et al. Blockade of NF-kappaB by pyrrolidine dithiocarbamate attenuates myocardial inflammatory response and ventricular dysfunction following coronary microembolization induced by homologous microthrombi in rats. Basic Res Cardiol. 2010 Jan;105(1):139–150. doi: 10.1007/s00395-009-0067-6. [DOI] [PubMed] [Google Scholar]
- 3.Chorianopoulos E, Heger T, Lutz M, Frank D, Bea F, Katus HA, et al. FGF-inducible 14-kDa protein (Fn14) is regulated via the RhoA/ROCK kinase pathway in cardiomyocytes and mediates nuclear factor-kappaB activation by TWEAK. Basic Res Cardiol. 2010 Mar;105(2):301–313. doi: 10.1007/s00395-009-0046-y. [DOI] [PubMed] [Google Scholar]
- 4.Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, et al. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008 Sep;103(5):485–492. doi: 10.1007/s00395-008-0739-7. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher G, Menzie S, Huang Y, Jackson C, Hunyor SN. Regional cardiac dysfunction is associated with specific alterations in inflammatory cytokines and matrix metalloproteinases after acute myocardial infarction in sheep. Basic Res Cardiol. 2007 Jan;102(1):63–72. doi: 10.1007/s00395-006-0610-7. [DOI] [PubMed] [Google Scholar]
- 6.Bozkurt B, Torre-Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, et al. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001 Feb 27;103(8):1044–1047. doi: 10.1161/01.cir.103.8.1044. [DOI] [PubMed] [Google Scholar]
- 7.Conraads VM, Vrints CJ, Rodrigus IE, Hoymans VY, Van Craenenbroeck EM, Bosmans J, et al. Depressed expression of MuRF1 and MAFbx in areas remote of recent myocardial infarction: a mechanism contributing to myocardial remodeling? Basic Res Cardiol. 2010 Mar;105(2):219–226. doi: 10.1007/s00395-009-0068-5. [DOI] [PubMed] [Google Scholar]
- 8.Dorge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, et al. Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol. 2002 Jan;34(1):51–62. doi: 10.1006/jmcc.2001.1489. [DOI] [PubMed] [Google Scholar]
- 9.Diwan A, Dibbs Z, Nemoto S, DeFreitas G, Carabello BA, Sivasubramanian N, et al. Targeted overexpression of noncleavable and secreted forms of tumor necrosis factor provokes disparate cardiac phenotypes. Circulation. 2004 Jan 20;109(2):262–268. doi: 10.1161/01.CIR.0000109642.27985.FA. [DOI] [PubMed] [Google Scholar]
- 10.Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, et al. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007 Jan 5;100(1):140–146. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 11.Thielmann M, Dorge H, Martin C, Belosjorow S, Schwanke U, van De SA, et al. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res. 2002 Apr 19;90(7):807–813. doi: 10.1161/01.res.0000014451.75415.36. [DOI] [PubMed] [Google Scholar]
- 12.Frangogiannis NG, Burns AR, Michael LH, Entman ML. Histochemical and morphological characteristics of canine cardiac mast cells. Histochem J. 1999 Apr;31(4):221–229. doi: 10.1023/a:1003541332070. [DOI] [PubMed] [Google Scholar]
- 13.Gilles S, Zahler S, Welsch U, Sommerhoff CP, Becker BF. Release of TNF-alpha during myocardial reperfusion depends on oxidative stress and is prevented by mast cell stabilizers. Cardiovasc Res. 2003 Dec 1;60(3):608–616. doi: 10.1016/j.cardiores.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, et al. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998 Aug 18;98(7):699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 15.Reil JC, Gilles S, Zahler S, Brandl A, Drexler H, Hultner L, et al. Insights from knock-out models concerning postischemic release of TNFalpha from isolated mouse hearts. J Mol Cell Cardiol. 2007 Jan;42(1):133–141. doi: 10.1016/j.yjmcc.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Levick SP, Gardner JD, Holland M, Hauer-Jensen M, Janicki JS, Brower GL. Protection from adverse myocardial remodeling secondary to chronic volume overload in mast cell deficient rats. J Mol Cell Cardiol. 2008 Jul;45(1):56–61. doi: 10.1016/j.yjmcc.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Pat B, Zheng J, Cain L, Powell P, Shi K, et al. Tumor necrosis factor-alpha produced in cardiomyocytes mediates a predominant myocardial inflammatory response to stretch in early volume overload. J Mol Cell Cardiol. 2010 Jan 4; doi: 10.1016/j.yjmcc.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss ML, Bartsch JW. Therapeutic benefits from targeting of ADAM family members. Biochemistry. 2004 Jun 15;43(23):7227–7235. doi: 10.1021/bi049677f. [DOI] [PubMed] [Google Scholar]
- 19.Levy DE, Lapierre F, Liang W, Ye W, Lange CW, Li X, et al. Matrix metalloproteinase inhibitors: a structure-activity study. J Med Chem. 1998 Jan 15;41(2):199–223. doi: 10.1021/jm970494j. [DOI] [PubMed] [Google Scholar]
- 20.Murray DB, Gardner JD, Levick SP, Brower GL, Morgan LG, Janicki JS. Response of cardiac mast cells to atrial natriuretic peptide. Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H1216–H1222. doi: 10.1152/ajpheart.01388.2006. [DOI] [PubMed] [Google Scholar]
- 21.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997 Feb 20;385(6618):733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 22.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997 Feb 20;385(6618):729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 23.Brower GL, Janicki JS. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Card Fail. 2005 Sep;11(7):548–556. doi: 10.1016/j.cardfail.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Brower GL, Chancey AL, Thanigaraj S, Matsubara BB, Janicki JS. Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol. 2002 Aug;283(2):H518–H525. doi: 10.1152/ajpheart.00218.2000. [DOI] [PubMed] [Google Scholar]
- 25.Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res. 2004 Oct 1;299(2):465–475. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Lu L, Gunja-Smith Z, Woessner JF, Ursell PC, Nissen T, Galardy RE, et al. Matrix metalloproteinases and collagen ultrastructure in moderate myocardial ischemia and reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2000 Aug;279(2):H601–H609. doi: 10.1152/ajpheart.2000.279.2.H601. [DOI] [PubMed] [Google Scholar]
- 27.Moss ML, Rasmussen FH. Fluorescent substrates for the proteinases ADAM17, ADAM10, ADAM8, and ADAM12 useful for high-throughput inhibitor screening. Anal Biochem. 2007 Jul 15;366(2):144–148. doi: 10.1016/j.ab.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 28.Murray DB, Gardner JD, Brower GL, Janicki JS. Effects of nonselective endothelin-1 receptor antagonism on cardiac mast cell-mediated ventricular remodeling in rats. Am J Physiol Heart Circ Physiol. 2008 Mar;294(3):H1251–H1257. doi: 10.1152/ajpheart.00622.2007. [DOI] [PubMed] [Google Scholar]
- 29.Murray DB, Gardner JD, Brower GL, Janicki JS. Endothelin-1 mediates cardiac mast cell degranulation, matrix metalloproteinase activation, and myocardial remodeling in rats. Am J Physiol Heart Circ Physiol. 2004 Nov;287(5):H2295–H2299. doi: 10.1152/ajpheart.00048.2004. [DOI] [PubMed] [Google Scholar]
- 30.Morgan LG, Levick SP, Voloshenyuk TG, Murray DB, Forman MF, Brower GL, et al. A novel technique for isolating functional mast cells from the heart. Inflamm Res. 2008 May;57(5):241–246. doi: 10.1007/s00011-007-7059-5. [DOI] [PubMed] [Google Scholar]
- 31.Bozkurt B, Kribbs SB, Clubb FJ, Jr, Michael LH, Didenko VV, Hornsby PJ, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998 Apr 14;97(14):1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto H, Koga H, Iida M, Tarumi K, Fujita M, Haruma K. Blockade of tumor necrosis factor-alpha-converting enzyme improves experimental small intestinal damage by decreasing matrix metalloproteinase-3 production in rats. Scand J Gastroenterol. 2006 Nov;41(11):1320–1329. doi: 10.1080/00365520600684571. [DOI] [PubMed] [Google Scholar]
- 33.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998 Mar;274(3 Pt 2):R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 34.Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor-alpha gene and protein expression in adult feline myocardium after endotoxin administration. J Clin Invest. 1995 Aug;96(2):1042–1052. doi: 10.1172/JCI118090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, et al. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997 Dec 15;250(3):751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- 36.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements JM, Crimmin M, et al. Matrix metalloproteinases and processing of pro-TNF-alpha. J Leukoc Biol. 1995 May;57(5):774–777. doi: 10.1002/jlb.57.5.774. [DOI] [PubMed] [Google Scholar]
- 37.Chancey AL, Brower GL, Janicki JS. Cardiac mast cell-mediated activation of gelatinase and alteration of ventricular diastolic function. Am J Physiol Heart Circ Physiol. 2002 Jun;282(6):H2152–H2158. doi: 10.1152/ajpheart.00777.2001. [DOI] [PubMed] [Google Scholar]
- 38.Jobe LJ, Melendez GC, Levick SP, Du Y, Brower GL, Janicki JS. TNF-alpha inhibition attenuates adverse myocardial remodeling in a rat model of volume overload. Am J Physiol Heart Circ Physiol. 2009 Oct;297(4):H1462–H1468. doi: 10.1152/ajpheart.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levick SP, Gardner JD, Holland M, Hauer-Jensen M, Janicki JS, Brower GL. Protection from adverse myocardial remodeling secondary to chronic volume overload in mast cell deficient rats. J Mol Cell Cardiol. 2008 Jul;45(1):56–61. doi: 10.1016/j.yjmcc.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray DB, McMillan R, Brower GL, Janicki JS. ETA selective receptor antagonism prevents ventricular remodeling in volume-overloaded rats. Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H109–H116. doi: 10.1152/ajpheart.00968.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan AT, Yan RT, Spinale FG, Afzal R, Gunasinghe HR, Stroud RE, et al. Relationships between plasma levels of matrix metalloproteinases and neurohormonal profile in patients with heart failure. Eur J Heart Fail. 2008 Feb;10(2):125–128. doi: 10.1016/j.ejheart.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang XY, Liu Q, Dai DZ, Dai Y. CPU0213, a novel endothelin receptor antagonist, suppresses the upregulation of matrix metalloproteinases and connexin 43 in hyperthyroid myocardium. Pharmacol Rep. 2008 Jul;60(4):524–531. [PubMed] [Google Scholar]
- 43.Deschamps AM, Zavadzkas J, Murphy RL, Koval CN, McLean JE, Jeffords L, et al. Interruption of endothelin signaling modifies membrane type 1 matrix metalloproteinase activity during ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H875–H883. doi: 10.1152/ajpheart.00918.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Podesser BK, Siwik DA, Eberli FR, Sam F, Ngoy S, Lambert J, et al. ET(A)-receptor blockade prevents matrix metalloproteinase activation late postmyocardial infarction in the rat. Am J Physiol Heart Circ Physiol. 2001 Mar;280(3):H984–H991. doi: 10.1152/ajpheart.2001.280.3.H984. [DOI] [PubMed] [Google Scholar]
- 45.Ozdemir R, Parlakpinar H, Polat A, Colak C, Ermis N, Acet A. Selective endothelin a (ET(A)) receptor antagonist (BQ-123) reduces both myocardial infarct size and oxidant injury. Toxicology. 2006 Feb 15;219(1–3):142–149. doi: 10.1016/j.tox.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Spruill LS, Lowry AS, Stroud RE, Squires CE, Mains IM, Flack EC, et al. Membrane-type-1 matrix metalloproteinase transcription and translation in myocardial fibroblasts from patients with normal left ventricular function and from patients with cardiomyopathy. Am J Physiol Cell Physiol. 2007 Oct;293(4):C1362–C1373. doi: 10.1152/ajpcell.00545.2006. [DOI] [PubMed] [Google Scholar]
- 47.Matsushima H, Yamada N, Matsue H, Shimada S. The effects of endothelin-1 on degranulation, cytokine, and growth factor production by skin-derived mast cells. Eur J Immunol. 2004 Jul;34(7):1910–1919. doi: 10.1002/eji.200424912. [DOI] [PubMed] [Google Scholar]
- 48.Coulombe M, Battistini B, Stankova J, Pouliot P, Bissonnette EY. Endothelins regulate mediator production of rat tissue-cultured mucosal mast cells. Up-regulation of Th1 and inhibition of Th2 cytokines. J Leukoc Biol. 2002 May;71(5):829–836. [PubMed] [Google Scholar]
- 49.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004 Nov 25;432(7016):512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 50.Metsarinne KP, Vehmaan-Kreula P, Kovanen PT, Saijonmaa O, Baumann M, Wang Y, et al. Activated mast cells increase the level of endothelin-1 mRNA in cocultured endothelial cells and degrade the secreted Peptide. Arterioscler Thromb Vasc Biol. 2002 Feb 1;22(2):268–273. doi: 10.1161/hq0202.103994. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999 Nov 12;85(10):906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]