Summary
The active form of vitamin D, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], has been reported to influence the functioning of the immune system by targeting the activities of cellular signaling pathways, in addition to its direct genomic effects. One of the signaling pathways reported to be targeted by vitamin D is the NF-κB pathway, which is highly active in most immune cell types, including T cells. However, the effects of vitamin D on the NF-κB pathway in T cells are not fully understood. Therefore, we examined the effects of 1α,25(OH)2D3 on the NF-κB pathway in the Jurkat cell line, a human T cell line that constitutively expresses endogenous vitamin D receptor. We found that 1α,25(OH)2D3 does not inhibit the induction of IκBα degradation and the expression of an NF-κB-dependent reporter gene in Jurkat cells following treatment with PMA/ionomycin. Also, 1α,25(OH)2D3 did not suppress the activation of NF-κB by TNFα or PHA. Furthermore, we demonstrate that 1α,25(OH)2D3 does not block the induction of CD69, which is an NF-κB target gene and an early T cell activation marker. Therefore, we conclude that vitamin D does not modulate the activity of the NF-κB pathway in Jurkat cells.
Keywords: Vitamin D, NF-κB, Jurkat cells, T cells, CD69
1. Introduction
The physiologically active form of vitamin D, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] is, apart from its established role in the maintenance of bone metabolism and the homeostasis of calcium and phosphorus, involved in the regulation of certain functions of the immune system [1]. The first observations of the possible roles of vitamin D in immunoregulation were made early in the 1980’s with the appearance of reports on the expression of the receptor for vitamin D (VDR) in human peripheral blood monocytes [2], in activated human peripheral T lymphocytes [2] and in the bovine thymus [3]. Additional evidence came from the demonstration that 1α,25(OH)2D3 can induce the differentiation of precursor cells into monocytes and macrophages [4, 5]. Subsequently, 1α,25(OH)2D3 has been shown to influence the functions of all of the major cellular components of the immune system, namely T cells, B cells, monocytes/macrophages, dendritic cells, and natural killer cells, with its sphere of influence spanning both adaptive immunity and innate immunity [1, 6].
Although the effects of 1α,25(OH)2D3 at the cellular level are primarily exerted via its binding to the VDR which then directly interacts with vitamin D response elements (VDRE) in the genome or with other transcriptional regulators [7, 8], 1α,25(OH)2D3 has been shown to regulate the functioning of immune cells by also targeting the activities of cellular signaling pathways [1, 6]. One of the major signaling targets of vitamin D is the Nuclear Factor-κB (NF-κB) pathway, which is highly active in most immune cell types, including T cells, and which plays a key role in the immune response and in inflammation [1, 6]. In T cells, the two major stimuli that cause the activation of the NF-κB pathway are the triggering the T cell receptor (TCR) by an MHC-II-bound antigen peptide and the ligation of the Tumor Necrosis Factor Receptor 1 (TNFR1) by its ligand, Tumor Necrosis Factor-α (TNFα) [9, 10]. These stimuli activate intracellular signaling cascades that lead to the proteasomal degradation of the Inhibitor of κB alpha (IκBα). The degradation of IκBα releases the NF-κB proteins (a set of five proteins known as p65, p50, p52, c-Rel and RelB) which normally exist sequestered in the cytoplasm due to their binding to IκBα [11]. The released NF-κB proteins then form homodimers and heterodimers, translocate to the nucleus and modulate the expression of target genes [11].
The reported effects of 1α,25(OH)2D3 on the NF-κB pathway include the inhibition of the induction of NF-κB binding to DNA and the repression of the induction of an NF-κB reporter gene by TNFα in human monocytes [12] and by lipopolysaccharide (LPS) in murine macrophages [13], the reduction of the expression of RelB in murine dendritic cells [14], the suppression of the PMA/ionomycin-induced phosphorylation and the LPS-induced nuclear translocation of p65 in myeloid dendritic cells [15], and the inhibition of the TNFα-induced IκBα degradation in murine embryonic fibroblasts [16]. In other instances, 1α,25(OH)2D3 has on the contrary been reported to stimulate the activity of the NF-κB pathway in some immune and non-immune cell types [17–19].
The effects of 1α,25(OH)2D3 on the NF-κB pathway in T cells have not been fully established. According to two previous studies, 1α,25(OH)2D3 inhibited the induction of an NF-κB reporter gene by PMA/ionomycin in the presence of transfected VDR in Jurkat cells [20, 21]. Also, in naïve mouse T cells, in the presence of dexamethasone co-treatment, 1α,25(OH)2D3, inhibited NF-κB binding to DNA following the triggering of the TCR [22]. In those circumstances, the effect of 1α,25(OH)2D3 on NF-κB activity was examined either in the presence of a co-treatment with dexamethasone, which is known to modulate NF-κB activity [23, 24], or in the presence of an overexpression of VDR, which is known to interact with the NF-κB subunit p65 [16, 25], making it difficult to determine the effects that were purely due to vitamin D. Yu et al had examined the effects of 1α,25(OH)2D3 on NF-κB activation by phytohemagglutinin (PHA) in Jurkat cells that expressed only endogenous VDR and they had reported that 1α,25(OH)2D3 suppresses the activation of NF-κB in Jurkat cells [26]. However, PHA by itself is a weaker inducer of NF-κB activity and is usually used in a combined treatment with phorbol esters to activate NF-κB [27, 28].
Therefore, we wanted to examine the effects of 1α,25(OH)2D3 on the NF-κB pathway in the presence of endogenous VDR only, in order to determine the possible effects of vitamin D on NF-κB activity in T cells following exposure to the two major NF-κB-inducing stimuli, namely the triggering of the TCR or the ligation of the TNFR1. Our experiments were carried out in the human Jurkat T cell line, which was found to highly express VDR in the resting state. We used a combined treatment with phorbol 12-myrsitate 13-acetate (PMA) and ionomycin to mimic the triggering of the TCR and we exposed cells to TNFα to activate the TNFR1. We found that 1α,25(OH)2D3 did not inhibit the activation of NF-κB in Jurkat cells by PMA/ionomycin or by TNFα. Also, we did not find an inhibitory effect of 1α,25(OH)2D3 on NF-κB activation by PHA, which for that matter caused only a slight activation of NF-κB. In addition, 1α,25(OH)2D3 by itself did not alter basal NF-κB activity. Finally, 1α,25(OH)2D3 treatment failed to block the induction of CD69, which is an NF-κB target gene.
2. Materials and Methods
2.1 Cells
A Jurkat cell line that stably expresses CD14 as a reporter gene under eight copies an NF-κB binding site [29] was obtained from Dr. Shao-Cong Sun (Pennsylvania State University, Hershey, PA) [30, 31]. The Jurkat cell line is a T cell line that was originally established from the peripheral blood of a 14-year-old boy with acute lymphoblastic leukemia. Subsequently, different sublines have been derived by various laboratories from the original clone. The cell line used in this study was derived from the J77 subline [29], which is a variant of the E6-1 clone [32]. The NF-κB binding sites in front of the CD14 reporter gene expressed in the cell line used in our experiments consist of four concatamers of the sequence 5′-ctagTGGGGACTTTCCACcTGGGGACTTTCCACct-3′, each of which contains two NF-κB binding sites derived from the SV40 virus [29]. The cells were maintained in RPMI-1640 medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen), 100 U/ml penicillin (Mediatech), 100 μg/ml streptomycin (Mediatech) and 50 μM 2-mercaptoethanol (Sigma). For cell treatment, PMA was used at 50 ng/ml, ionomycin at 1 μM, TNFα at 10 ng/ml, PHA at 1 μg/ml and MG132 at 10 μM. When 1α,25(OH)2D3 was used to pre-treat or treat the cells, control samples were exposed to 0.1% v/v ethanol, which was the same concentration as the ethanol concentration in the samples to which 1α,25(OH)2D3 was added. HeLa cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were grown in the incubator at 37°C and 5% CO2 in a humidified atmosphere.
2.2 Reagents
Chemicals were obtained as follows: PMA (dissolved in DMSO) and 1α,25(OH)2D3 (dissolved in 100% ethanol) from Sigma; PHA (dissolved in DMEM) from Sigma; ionomycin (dissolved in ethanol) from Calbiochem; TNFα (resuspended in phosphate buffered-saline, PBS) from R&D Systems; and MG132 (dissolved in DMSO) from Biomol. Non-conjugated antibodies were obtained as follows: VDR and IκBα rabbit polyclonal antibodies from Santa Cruz Biotechnology; β-actin mouse monoclonal antibody (Ab) from Abcam. Horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary Ab was from GE Healthcare and HRP-conjugated anti-rabbit IgG Ab was from Invitrogen. Anti-CD14 Ab (PE-mouse anti-human CD14 monoclonal Ab) and anti-CD69 (PE-mouse anti-human CD69 monoclonal Ab) were both from BD Biosciences.
2.3 Immunoblotting
Jurkat cells were seeded on six-well plates at a density of 1,000,000 cells per well on the day before harvesting and then exposed to pre-treatment and treatment conditions as appropriate. HeLa cells were seeded on six-well plates at a density of 500,000 cells per well and cultured for 24 hours. After the appropriate duration of culture, the Jurkat and HeLa cells were harvested. The cells were lysed in 60 μl of Cell Extraction Buffer (Invitrogen) to which Complete Protease Inhibitors Cocktail tablets (Roche) were added and then incubated on ice for 30 minutes. Total protein concentration was normalized across the cell lysates and SDS-PAGE followed by immunoblotting was carried out, as described elsewhere, on cell lysates containing 10 μg of total protein [33].
2.4 Luciferase assay
For CYP24A1 luciferase assay, Jurkat cells were grown to the mid-log phase and then for each condition 12 million cells were electroporated with 10 μg of CYP24A1-luciferase plasmid (generously provided by Dr. Sylvia Christakos) and 1 μg of pRL-TK plasmid (Promega) diluted in Opti-MEM reduced serum medium (Invitrogen). Electroporation was carried out at 300 V and 1000 μF in 0.4 cm cuvettes using the Gene Pulser (Bio-Rad). After transfection, the cells were transferred to 10-cm dishes containing complete medium and the appropriate treatment was added. The cells were harvested 36 hours after the addition of treatment and were lysed in 250 μl of Passive Lysis Buffer (Promega). Measurement of luciferase activities was carried out as described before on 100 μl of cell lysate for each of the samples [33].
2.5 Flow cytometry
For flow cytometry experiments, 1,000,000 Jurkat cells were seeded per well on six-well plates. After the appropriate duration of treatment, the cells were harvested and were washed in Stain Buffer (2% FBS in PBS). The cells were then incubated for 15 minutes in the appropriate fluorescent-labeled Ab diluted in Stain Buffer. Next, the cells were washed twice in Stain Buffer. The stained cells were resuspended in 500 μl of 2% formaldehyde in PBS and flow cytometry was carried out. Flow cytometry data was collected on a FACS Calibur Flow Cytometer (BD Biosciences) using CellQuest software (BD Biosciences). For each sample, data was collected from 10,000 cells gated to identify the live cell population based on the light scatter profiles of the cells. The data was analyzed using the WinMDI software (The Scripps Institute).
3. Results
3.1 The active form of vitamin D does not inhibit the degradation of IκBα in Jurkat cells
The stimulus-induced degradation of IκBα is a key step in the activation of the NF-κB pathway by various stimuli, including PMA/ionomycin [34]. So, we wanted to determine if 1α,25(OH)2D3 interferes with the degradation of IκBα in Jurkat cells treated with PMA/ionomycin. Jurkat cells were cultured without pre-treatment or were pre-treated with 1α,25(OH)2D3 for 24 hours and were then treated with PMA/ionomycin for 15 minutes. The levels of total IκBα were determined by immunoblotting. The results presented in Fig. 1A showed that PMA/ionomycin treatment induced a nearly complete degradation of IκBα (second lane) as compared to the non-treated sample (first lane). In addition, pre-treatment with either 10 nM (third lane) or 100 nM (fourth lane) 1α,25(OH)2D3 did not have any inhibitory effect on the PMA/ionomycin-induced degradation of IκBα.
Fig. 1.
Vitamin D does not inhibit the degradation of IκBα induced by PMA/ionomycin. (A) Jurkat cells were left without pre-treatment or were pre-treated with 1α,25(OH)2D3 (Vit D) as shown for 24 hours and were then treated for 15 minutes with PMA/ionomycin. Whole cell lysates were resolved by SDS-PAGE and immunoblotting was carried out for IκBα and β-actin. (B) Whole cell lysates of HeLa cells and Jurkat cells were resolved by SDS-PAGE and immunoblotting was carried out for VDR and β-actin. (C) Jurkat cells seeded on six-well plates were exposed to 1α,25(OH)2D3 (Vit D) for the indicated amount of time before harvesting 24 hours after the initial seeding. Whole cell lysates were prepared, resolved by SDS-PAGE and immunoblotting was carried out for VDR and β-actin. (D) Jurkat cells were electroporated with a plasmid coding for firefly luciferase driven by the human CYP24A1 promoter and with the pRL-TK plasmid and shortly after transfection 1α,25(OH)2D3 was added as shown. The cells were harvested 36 hours after the addition of 1α,25(OH)2D3 and luciferase assays were performed. The firefly luciferase relative luminescence units (RLU) were normalized to those of Renilla luciferase. For the immunoblotting experiments the data shown are representative of 2–3 independent experiments with similar results. For the luciferase experiment the data shown is the mean and standard deviation of three independent experiments. Statistical comparisons were carried out using Student’s t test. Vehicle (ethanol) was added to a concentration of 0.1% to the control samples labeled 0 nM.
Since previous reports have indicated that the expression of VDR in resting T cells is low [2], a condition which could limit the ability of vitamin D to regulate NF-κB activity, we examined the level of VDR in Jurkat cells by immunoblotting. As shown in Fig. 1B, there is a high degree of expression of VDR in resting Jurkat cells. The expression of VDR in non-activated Jurkat cells has also been reported previously [26, 35, 36]. For purposes of comparison, we also show the degree of VDR expression in HeLa cells, a cell line that has been previously reported to express VDR [37]. The level of expression of VDR in Jurkat cells was found not to be affected by treatment with 10 nM or 100 nM 1α,25(OH)2D3 for 6, 18 or 24 hours (Fig. 1C). This is in contrast to previous reports of 1α,25(OH)2D3 increasing the expression of VDR in other cell types, including Schwann cells [38] and myeloid leukemia cells [39, 40]. To ascertain that the 1α,25(OH)2D3 preparation used in our experiments did not suffer from loss of activity, we examined the effect of the 1α,25(OH)2D3 preparation on the activity of the human CYP24A1 (25-hydroxyvitamin D3 24-hydroxylase) promoter, which is highly sensitive to the active form of vitamin D [41, 42], by conducting a CYP24A1 luciferase assay. As shown in Fig. 1D, 1α,25(OH)2D3 treatment caused a marked induction of the CYP24A1 promoter in Jurkat cells, confirming that the preparation used in our studies did not suffer from loss of activity. Therefore, based on the results described above, we conclude that vitamin D pre-treatment does not block the degradation of IκBα induced by PMA/ionomycin in Jurkat cells.
3.2 The active form of vitamin D does not block the induction of an NF-κB reporter gene by PMA/ionomycin in Jurkat cells
We examined the effect of 1α,25(OH)2D3 treatment on the PMA/ionomycin-induction of the CD14 NF-κB reporter gene stably expressed in the Jurkat cell line used in our experiments. In the absence of any pre-treatment, treatment of Jurkat cells with PMA/ionomycin for 6 h caused a large induction of CD14 expression in the majority of the cells (Fig. 2A). Pre-treatment for 1 h with MG132, a substance that inhibits the activation of NF-κB by blocking the proteasomal degradation of IκBα [43], caused a marked suppression of the induction of CD14 by PMA/ionomycin (Fig. 2B). However, pre-treatment of Jurkat cells with 10 nM 1α,25(OH)2D3 for 2 h or for 24 h, or with 100 nM or 500 nM for 24 h did not cause any significant inhibition of the induction of CD14 by PMA/ionomycin (Fig. 2C to F). Since the induction of NF-κB-dependent promoters is the end-result of the activation of the NF-κB pathway and since 1α,25(OH)2D3 did not block the activation of such a promoter (in this instance, the NF-κB-binding promoter in the CD14 reporter construct) by PMA/ionomycin, we conclude that vitamin D pre-treatment does not inhibit the activation of NF-κB signal transduction pathway by PMA/ionomycin in Jurkat cells.
Fig. 2.
Vitamin D does not inhibit the induction of an NF-κB reporter gene in Jurkat cells by PMA/ionomycin. Jurkat cells stably expressing surface-localizing CD14 under a promoter that contains NF-κB binding sites were left without pre-treatment (A) or were pre-treated with MG132 (B) or with 10 nM (C and D), 100 nM (E) or 500 nM (F) 1α,25(OH)2D3 (Vit D) for the indicated duration of time. After the appropriate duration of pre-treatment, the samples were treated with PMA/ionomycin for 6 hours. At the end of the treatment period, the cells were harvested and stained with PE-anti-CD14 and flow cytometry was carried out. Shaded histograms represent samples that were not exposed to pre-treatment and not treated with PMA/ionomycin. Histograms shown by continuous lines represent samples that were not exposed to pre-treatment but were treated with PMA/ionomycin. Histograms shown by broken lines represent samples that were exposed to the indicated pre-treatment and were then treated with PMA/ionomycin. Vehicle (DMSO for the MG132 samples and ethanol for the Vit D samples) was added to a concentration of 0.1% to the control samples labeled Pre-Rx (pre-treatment). Data shown is representative of three independent experiments.
3.3 The active form of vitamin D does not block the induction of an NF-κB reporter gene by TNFα or PHA in Jurkat cells
We then examined the effects of 1α,25(OH)2D3 on the induction of the CD14 NF-κB reporter gene by TNFα or PHA. Jurkat cells were pre-treated with 10 nM 1α,25(OH)2D3 and were then exposed to TNFα or to PHA for 6 h. TNFα caused a marked induction of CD14 and this induction was completely blocked by pre-treatment with MG132 for 1 h (Fig. 3A). However, treatment with 10 nM 1α,25(OH)2D3 either for 2 h or for 24 h did not have any effect on the induction of CD14 by TNFα (Fig. 3B and C). PHA induced only a slight induction of CD14 and this induction was not suppressed by MG132 (Fig. 3D). Also, pre-treatment with 10 nM 1α,25(OH)2D3 for 2 h or for 24 h did not have any effect on the induction of CD14 by PHA (Fig. 3E and F). Thus, we conclude that vitamin D does not suppress the activation of NF-κB by TNFα or by PHA in Jurkat cells.
Fig. 3.
Vitamin D does not inhibit the induction of an NF-κB reporter gene in Jurkat cells by TNFα or PHA. Jurkat cells stably expressing surface-localizing CD14 under a promoter that contains NF-κB binding sites were left without pre-treatment or were pre-treated with MG132 (A and D) or with 10 nM (B and E) or 100 nM (C and F) 1α,25(OH)2D3 (Vit D) for the indicated duration of time. After the appropriate duration of pre-treatment, the samples were treated with TNFα (A, B and C) or PHA (D, E, and F) for 6 hours. At the end of the treatment period, the cells were harvested and stained with PE-anti-CD14 and flow cytometry was carried out. Shaded histograms represent samples that were not exposed to pre-treatment and not treated with TNFα or PHA. Histograms shown by continuous lines represent samples that were not exposed to pre-treatment but were treated with TNFα or PHA. Histograms shown by broken lines represent samples that were exposed to the indicated pre-treatment and were then treated with TNFα or PHA. Vehicle (DMSO for the MG132 samples and ethanol for the Vit D samples) was added to a concentration of 0.1% to the control samples labeled Pre-Rx (pre-treatment). Data shown is representative of three independent experiments.
3.4 The active form of vitamin D does not induce an NF-κB reporter gene in Jurkat cells
Vitamin D has been reported to stimulate NF-κB activity in certain cell types [17, 18]. So, we tested if 1α,25(OH)2D3 treatment by itself has any effect on the expression of the CD14 NF-κB reporter gene in Jurkat cells. As shown in Fig. 4, while PMA/ionomycin treatment for 6 h caused a marked induction of CD14 (Fig. 4A), treatment with 100 nM 1α,25(OH)2D3 for 6 h or for 30 h (Fig. 4B and C) did not cause any induction of CD14 expression. In addition, treatment with 10 nM 1α,25(OH)2D3 for 8 h or for 24 h, with 250 nM 1α,25(OH)2D3 for 6 h or 30 h, or with 500 nM 1α,25(OH)2D3 for 6 h or 30 h did not induce CD14 expression (data not shown). Hence, we conclude that vitamin D does not have an activating effect on the NF-κB pathway in Jurkat cells.
Fig. 4.
Vitamin D treatment does not alter the basal activity of an NF-κB reporter gene in Jurkat cells. Jurkat cells stably expressing surface-localizing CD14 under a promoter that contains NF-κB binding sites were treated with PMA/ionomycin for 6 h (A) or with 100 nM 1α,25(OH)2D3 (Vit D) for 6 h (B) or for 30 h (C). At the end of the treatment period, the cells were harvested and stained with PE-anti-CD14 and flow cytometry was carried out. Shaded histograms represent samples that were not exposed to any treatment. Histograms shown by continuous lines represent samples that were treated with the indicated reagent. Vehicle (ethanol) was added to a concentration of 0.1% to the non-treated samples. Data shown is representative of three independent experiments.
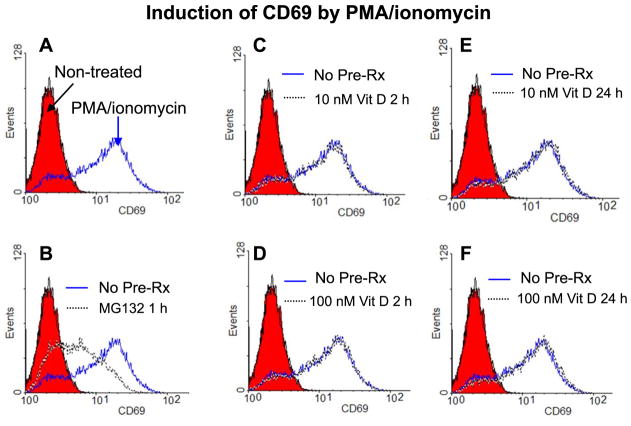
3.5 The active form of vitamin D does not block the induction of CD69 by PMA/ionomycin in Jurkat cells
We examined the effect of 1α,25(OH)2D3 on the induction of CD69 to confirm our observations made on the NF-κB reporter gene. CD69 is an early T cell activation marker that has NF-κB binding sites within its promoter [44] and inhibition of NF-κB has been shown to reduce the induction of CD69 by PMA/ionomycin [45]. Treatment of Jurkat cells with PMA/ionomycin for 6 h caused a marked induction of CD69 expression (Fig. 5A) which was partially suppressed by pre-treatment with MG132 for 1 h (Fig. 5B). However, pre-treatment with 10 nM 1α,25(OH)2D3 for 2 h or 24 h (Fig. 5C and E) or with 100 nM 1α,25(OH)2D3 for 2 h or 24 h (Fig. 5D and F) did not cause any suppression of CD69 induction by PMA/ionomycin. Therefore, we conclude that vitamin D does not block the induction of the NF-κB target gene CD69 in Jurkat cells, supporting our observations described above regarding the lack of effect of vitamin D on NF-κB activity in Jurkat cells.
Fig. 5.
Vitamin D does not inhibit the induction of CD69 in Jurkat cells by PMA/ionomycin. Jurkat cells were left without pre-treatment (A) or were pre-treated with MG132 (B) or with 10 nM (C and E) or 100 nM (D and F) 1α,25(OH)2D3 (Vit D) for the indicated duration of time. After the appropriate duration of pre-treatment, the samples were treated with PMA/ionomycin for 6 hours. At the end of the treatment period, the cells were harvested and stained with PE-anti-CD69 and flow cytometry was carried out. Shaded histograms represent samples that were not exposed to pre-treatment and not treated with PMA/ionomycin. Histograms shown by continuous lines represent samples that were not exposed to pre-treatment but were treated with PMA/ionomycin. Histograms shown by broken lines represent samples that were exposed to the indicated pre-treatment and were then treated with PMA/ionomycin. Vehicle (DMSO for the MG132 samples and ethanol for the Vit D samples) was added to a concentration of 0.1% to the control samples labeled Pre-Rx (pre-treatment). Data shown is representative of three independent experiments.
4. Discussion
The active form of vitamin D, 1α,25(OH)2D3, has been shown to inhibit the functioning of the NF-κB pathway in some cell types [12, 14] while stimulating NF-κB activity in other cell types [17, 18]. In this article, we presented data that demonstrate that in Jurkat cells, which express endogenous VDR, 1α,25(OH)2D3 does not inhibit the activation of the NF-κB pathway by various stimuli, namely, PMA/ionomycin, TNFα and PHA. We also showed that 1α,25(OH)2D3 does not inhibit the induction of CD69, an early T cell activation marker, which is an NF-κB target gene. Our findings are supported by a study carried out by Takeuchi and colleagues who demonstrated that treatment of human tonsillar T cells with 50 nM 1α,25(OH)2D3 does not block the PMA/ionomycin-induction of NF-κB binding to DNA [46]. We did not test the effect of 1α,25(OH)2D3 on the activation of NF-κB induced by a combined treatment with anti-CD3 and anti-CD28 antibodies since the Jurkat cell subline used in our studies does not respond to those stimuli [31].
We used 1α,25(OH)2D3 at concentrations of 10 nM and 100 nM in most of our experiments. These levels were chosen based on published reports [20–22, 26]. In addition, 1α,25(OH)2D3 concentrations in the same range were recently reported to be optimal for modulating T cell responses [47]. Watson and colleagues had measured the serum levels of 1α,25(OH)2D3 in a group of human subjects and found that the mean level was 39.4±12.6 pg/ml during the winter months and 40.7±12.1 pg/ml during the summer months [48]; these levels correspond to 94.6±30.2 pM and 97.7±29.0 pM, respectively. Even though these levels are much lower than the concentrations used in our study, local production of 1α,25(OH)2D3 by macrophages and dendritic cells is believed to generate tissue levels of the active form of vitamin D that are much higher than are normally found in the serum and that could modulate the functions of T cells in a paracrine manner [1].
There are a few previous studies that have examined the effect of vitamin D in Jurkat cells and other T cell types. According to a study carried out by Yu et al, in Jurkat cells pre-treatment with 10 nM 1α,25(OH)2D3 for 40 h caused a 39% reduction in the PHA-induction of a chloramphenicol acetyl transferase reporter gene [26]. The reporter gene used in that experiment had in its promoter four copies of the NF-κB binding sequence (5′-gatcCAGAGGGGACTTTTCCGAGA-3′) derived from the immunoglobulin κ light chain [26], which is more or less similar to the sequence in the CD14 reporter gene used in our studies. The discrepancy between the findings of Yu et al and those of ours could partly be due to the differences in experimental conditions. An additional reason for the disparity of the findings could be the differences in the number of copies of NF-κB binding sites in the reporter genes used, with the lower number of binding sites in the construct used by Yu et al limiting the amount of NF-κB dimers bound and hence increasing the efficacy of a potential inhibitor. Moreover, it is worth noting that in our experiments PHA treatment induced the NF-κB reporter gene to a much lower degree as compared to that attained following treatment with PMA/ionomycin or TNFα.
Komine and colleagues had reported that in Jurkat cells 100 nM of 1α,25(OH)2D3 or 10 nM or 100 nM of oxacalcitriol, a vitamin D analog, partially inhibited the PMA/ionomycin-induction of an NF-κB luciferase reporter gene (that had similar NF-κB binding sequence as the one used by Yu et al) [20]. However, those experiments were done in the presence of a transfected plasmid expressing VDR. In another study, van Etten and colleagues showed that, in Jurkat cells, pre-treatment with 10 nM or 100 nM 1α,25(OH)2D3 partially inhibited the PMA/ionomycin-induction of an NF-κB luciferase reporter gene. But, again, those experiments were carried out in the presence of transfected plasmids expressing VDR and RXR [21]. The presence of an overexpression of VDR could by itself affect the activity of the NF-κB pathway since VDR is known to interact with the NF-κB subunit p65 [16, 25]. Also, overexpressed VDR has been shown to inhibit the expression of another NF-κB subunit, namely RelB, in macrophages and dendritic cells [49, 50]. Barrat et al had reported that in mouse primary T cells cultured in the presence of vitamin D, there was decreased NF-κB binding to DNA [22]; however, those experiments were conducted in the presence of a co-treatment with dexamethasone, making it difficult to attribute the observed effect to vitamin D, since dexamethasone, which is a potent immunosuppressant, has been shown to inhibit NF-κB activity by itself [23, 24].
A number of studies have established that the active form of vitamin D inhibits the production of certain cytokines, such as IL-2, IFN-γ and Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF), by T cells [1, 36, 47]. The activation of NF-κB is involved in the induction of IL-2 [31], IFN-γ [51] and GM-CSF [52]. Since, according to our findings, vitamin D does not inhibit the activation of NF-κB in T cells, the inhibitory effect of vitamin D on the induction of these cytokines is likely to be mediated by other mechanisms. The most likely mechanism mediating the effect of vitamin D on the production of these cytokines is through VDR interacting with VRDE regions or regulating the activities of other transcriptional regulators, as reported before for the GM-CSF gene [53]. Another possible mechanism is the interference of vitamin D with other signaling pathways, such as the Nuclear Factor of Activated T-cells (NFAT) pathway [46], that are required for the induction of these cytokines.
Acknowledgments
Research in the laboratory of J.A.H. is supported by a grant from the Alliance for Lupus Research. A.S. was a Research Fellow supported by an NIAMS Rheumatology Training Grant, T32 AR050947. We thank Dr. Laiping Xie for help in the conduct of our studies. We express our gratitude to Dr. Shao-Cong Sun (Pennsylvania State University) for the Jurkat cell line and to Dr. Sylvia Christakos (University of Medicine and Dentistry of New Jersey) for the CYP24A1-luciferase plasmid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–45. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 2.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 3.Reinhardt TA, Horst RL, Littledike ET, Beitz DC. 1,25-Dihydroxyvitamin D3 receptor in bovine thymus gland. Biochem Biophys Res Commun. 1982;106:1012–8. doi: 10.1016/0006-291x(82)91812-5. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Shavit Z, Teitelbaum SL, Reitsma P, Hall A, Pegg LE, Trial J, et al. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1983;80:5907–11. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990–4. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van EE, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Carlberg C, Polly P. Gene regulation by vitamin D3. Crit Rev Eukaryot Gene Expr. 1998;8:19–42. doi: 10.1615/critreveukargeneexpr.v8.i1.20. [DOI] [PubMed] [Google Scholar]
- 8.Jones G, Strugnell SA, Deluca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Wange RL. T cell receptor signaling: beyond complex complexes. J Biol Chem. 2004;279:28827–30. doi: 10.1074/jbc.R400012200. [DOI] [PubMed] [Google Scholar]
- 10.Kruppa G, Thoma B, Machleidt T, Wiegmann K, Kronke M. Inhibition of tumor necrosis factor (TNF)-mediated NF-kappa B activation by selective blockade of the human 55-kDa TNF receptor. J Immunol. 1992;148:3152–7. [PubMed] [Google Scholar]
- 11.Li X, Stark GR. NFkappaB-dependent signaling pathways. Exp Hematol. 2002;30:285–96. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- 12.Chung J, Koyama T, Ohsawa M, Shibamiya A, Hoshi A, Hirosawa S. 1,25(OH)(2)D(3) blocks TNF-induced monocytic tissue factor expression by inhibition of transcription factors AP-1 and NF-kappaB. Lab Invest. 2007;87:540–7. doi: 10.1038/labinvest.3700550. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol Dial Transplant. 2006;21:889–97. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 14.Xing N, MLLM, Bachman LA, McKean DJ, Kumar R, Griffin MD. Distinctive dendritic cell modulation by vitamin D(3) and glucocorticoid pathways. Biochem Biophys Res Commun. 2002;297:645–52. doi: 10.1016/s0006-291x(02)02262-3. [DOI] [PubMed] [Google Scholar]
- 15.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–53. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, et al. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–E322. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 17.Berry DM, Clark CS, Meckling-Gill KA. 1alpha,25-dihydroxyvitamin D3 stimulates phosphorylation of IkappaBalpha and synergizes with TPA to induce nuclear translocation of NFkappaB during monocytic differentiation of NB4 leukemia cells. Exp Cell Res. 2002;272:176–84. doi: 10.1006/excr.2001.5410. [DOI] [PubMed] [Google Scholar]
- 18.Adams LS, Teegarden D. 1,25-dihydroxycholecalciferol inhibits apoptosis in C3H10T1/2 murine fibroblast cells through activation of nuclear factor kappaB. J Nutr. 2004;134:2948–52. doi: 10.1093/jn/134.11.2948. [DOI] [PubMed] [Google Scholar]
- 19.Mathiasen IS, Hansen CM, Foghsgaard L, Jaattela M. Sensitization to TNF-induced apoptosis by 1,25-dihydroxy vitamin D(3) involves up-regulation of the TNF receptor 1 and cathepsin B. Int J Cancer. 2001;93:224–31. doi: 10.1002/ijc.1325. [DOI] [PubMed] [Google Scholar]
- 20.Komine M, Watabe Y, Shimaoka S, Sato F, Kake K, Nishina H, et al. The action of a novel vitamin D3 analogue, OCT, on immunomodulatory function of keratinocytes and lymphocytes. Arch Dermatol Res. 1999;291:500–6. doi: 10.1007/s004030050444. [DOI] [PubMed] [Google Scholar]
- 21.van EE, Verlinden L, Giulietti A, Ramos-Lopez E, Branisteanu DD, Ferreira GB, et al. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol. 2007;37:395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 22.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumura M, Kakishita H, Suzuki M, Banba N, Hattori Y. Dexamethasone suppresses iNOS gene expression by inhibiting NF-kappaB in vascular smooth muscle cells. Life Sci. 2001;69:1067–77. doi: 10.1016/s0024-3205(01)01196-1. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki T, Tukiyama T, Tokiwa T. Effect of dexamethasone on binding activity of transcription factors nuclear factor-kappaB and activator protein-1 in SW982 human synovial sarcoma cells. In Vitro Cell Dev Biol Anim. 2005;41:80–2. doi: 10.1290/0502011.1. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Farmer P, Rubin J, Nanes MS. Integration of the NfkappaB p65 subunit into the vitamin D receptor transcriptional complex: identification of p65 domains that inhibit 1,25-dihydroxyvitamin D3-stimulated transcription. J Cell Biochem. 2004;92:833–48. doi: 10.1002/jcb.20143. [DOI] [PubMed] [Google Scholar]
- 26.Yu XP, Bellido T, Manolagas SC. Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1995;92:10990–4. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13:3301–10. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginn-Pease ME, Whisler RL. Optimal NF kappa B mediated transcriptional responses in Jurkat T cells exposed to oxidative stress are dependent on intracellular glutathione and costimulatory signals. Biochem Biophys Res Commun. 1996;226:695–702. doi: 10.1006/bbrc.1996.1416. [DOI] [PubMed] [Google Scholar]
- 29.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–96. [PMC free article] [PubMed] [Google Scholar]
- 30.Harhaj EW, Good L, Xiao G, Uhlik M, Cvijic ME, Rivera-Walsh I, et al. Somatic mutagenesis studies of NF-kappa B signaling in human T cells: evidence for an essential role of IKK gamma in NF-kappa B activation by T-cell costimulatory signals and HTLV-I Tax protein. Oncogene. 2000;19:1448–56. doi: 10.1038/sj.onc.1203445. [DOI] [PubMed] [Google Scholar]
- 31.Shifera AS, Horwitz MS. Mutations in the zinc finger domain of IKK gamma block the activation of NF-kappa B and the induction of IL-2 in stimulated T lymphocytes. Mol Immunol. 2008;45:1633–45. doi: 10.1016/j.molimm.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 32.Jin YJ, Friedman J, Burakoff SJ. Regulation of tyrosine phosphorylation in isolated T cell membrane by inhibition of protein tyrosine phosphatases. J Immunol. 1998;161:1743–50. [PubMed] [Google Scholar]
- 33.Shifera AS, Hardin JA. PMA induces expression from the herpes simplex virus thymidine kinase promoter via the activation of JNK and ERK in the presence of adenoviral E1A proteins. Arch Biochem Biophys. 2009;490:145–57. doi: 10.1016/j.abb.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y, Ichiyama T, Ohsaki A, Hasegawa S, Shiraishi M, Furukawa S. Anti-inflammatory effect of 1alpha,25-dihydroxyvitamin D(3) in human coronary arterial endothelial cells: Implication for the treatment of Kawasaki disease. J Steroid Biochem Mol Biol. 2009;113:134–8. doi: 10.1016/j.jsbmb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Towers TL, Freedman LP. Granulocyte-macrophage colony-stimulating factor gene transcription is directly repressed by the vitamin D3 receptor. Implications for allosteric influences on nuclear receptor structure and function by a DNA element. J Biol Chem. 1998;273:10338–48. doi: 10.1074/jbc.273.17.10338. [DOI] [PubMed] [Google Scholar]
- 37.Wagner KD, Wagner N, Sukhatme VP, Scholz H. Activation of vitamin D receptor by the Wilms’ tumor gene product mediates apoptosis of renal cells. J Am Soc Nephrol. 2001;12:1188–96. doi: 10.1681/ASN.V1261188. [DOI] [PubMed] [Google Scholar]
- 38.Cornet A, Baudet C, Neveu I, Baron-Van EA, Brachet P, Naveilhan P. 1,25-Dihydroxyvitamin D3 regulates the expression of VDR and NGF gene in Schwann cells in vitro. J Neurosci Res. 1998;53:742–6. doi: 10.1002/(SICI)1097-4547(19980915)53:6<742::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Gocek E, Kielbinski M, Marcinkowska E. Activation of intracellular signaling pathways is necessary for an increase in VDR expression and its nuclear translocation. FEBS Lett. 2007;581:1751–7. doi: 10.1016/j.febslet.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 40.Pramanik R, Asplin JR, Lindeman C, Favus MJ, Bai S, Coe FL. Lipopolysaccharide negatively modulates vitamin D action by down-regulating expression of vitamin D-induced VDR in human monocytic THP-1 cells. Cell Immunol. 2004;232:137–43. doi: 10.1016/j.cellimm.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Chen KS, Deluca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta. 1995;1263:1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- 42.Barletta F, Dhawan P, Christakos S. Integration of hormone signaling in the regulation of human 25(OH)D3 24-hydroxylase transcription. Am J Physiol Endocrinol Metab. 2004;286:E598–E608. doi: 10.1152/ajpendo.00214.2003. [DOI] [PubMed] [Google Scholar]
- 43.Fiedler MA, Wernke-Dollries K, Stark JM. Inhibition of TNF-alpha-induced NF-kappaB activation and IL-8 release in A549 cells with the proteasome inhibitor MG-132. Am J Respir Cell Mol Biol. 1998;19:259–68. doi: 10.1165/ajrcmb.19.2.3149. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Cabrera M, Munoz E, Blazquez MV, Ursa MA, Santis AG, Sanchez-Madrid F. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-alpha-responsive elements. J Biol Chem. 1995;270:21545–51. doi: 10.1074/jbc.270.37.21545. [DOI] [PubMed] [Google Scholar]
- 45.Castellanos MC, Munoz C, Montoya MC, Lara-Pezzi E, Lopez-Cabrera M, de Landazuri MO. Expression of the leukocyte early activation antigen CD69 is regulated by the transcription factor AP-1. J Immunol. 1997;159:5463–73. [PubMed] [Google Scholar]
- 46.Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S. Nuclear factor of activated T cells (NFAT) as a molecular target for 1alpha,25-dihydroxyvitamin D3-mediated effects. J Immunol. 1998;160:209–18. [PubMed] [Google Scholar]
- 47.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–60. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 49.Griffin MD, Dong X, Kumar R. Vitamin D receptor-mediated suppression of RelB in antigen presenting cells: a paradigm for ligand-augmented negative transcriptional regulation. Arch Biochem Biophys. 2007;460:218–26. doi: 10.1016/j.abb.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong X, Lutz W, Schroeder TM, Bachman LA, Westendorf JJ, Kumar R, et al. Regulation of relB in dendritic cells by means of modulated association of vitamin D receptor and histone deacetylase 3 with the promoter. Proc Natl Acad Sci U S A. 2005;102:16007–12. doi: 10.1073/pnas.0506516102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, et al. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272:30412–20. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- 52.Thomas RS, Tymms MJ, McKinlay LH, Shannon MF, Seth A, Kola I. ETS1, NFkappaB and AP1 synergistically transactivate the human GM-CSF promoter. Oncogene. 1997;14:2845–55. doi: 10.1038/sj.onc.1201125. [DOI] [PubMed] [Google Scholar]
- 53.Towers TL, Staeva TP, Freedman LP. A two-hit mechanism for vitamin D3-mediated transcriptional repression of the granulocyte-macrophage colony-stimulating factor gene: vitamin D receptor competes for DNA binding with NFAT1 and stabilizes c-Jun. Mol Cell Biol. 1999;19:4191–9. doi: 10.1128/mcb.19.6.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]