Abstract
Glial derived tumors, gliomas, are highly invasive cancers that invade normal brain through the extracellular space. To navigate the tortuous extracellular spaces cells undergo dynamic changes in cell volume, which entails water flux across the membrane through aquaporins (AQPs). Two members of this family, AQP1 and AQP4 are highly expressed in primary brain tumor biopsies and both have a consensus phosphorylation site for PKC, which is a known regulator of glioma cell invasion. AQP4 colocalizes with PKC to the leading edge of invading processes and clustered with ClC2 and KCC1, believed to provide the pathways for Cl- and K+ secretion to accomplish volume changes. Using D54MG glioma cells stably transfected with either AQP1 or AQP4, we show that PKC activity regulates water permeability through phosphorylation of AQP4. Activation of PKC with either phorbol 12-myristate 13-acetate, or thrombin enhanced AQP4 phosphorylation, reduced water permeability and significantly decreased cell invasion. Conversely inhibition of PKC activity with Chelerythrine reduced AQP4 phosphorylation, enhanced water permeability and significantly enhanced tumor invasion. PKC regulation of AQP4 was lost after mutational inactivation of the consensus PKC phosphorylation site S180A. Interestingly, AQP1 expressing glioma cells, by contrast, were completely unaffected by changes in PKC activity. To demonstrate a role for AQPs in glioma invasion in vivo, cells selectively expressing AQP1, AQP4 or the mutated S180A-AQP4 were implanted intracranially into SCID mice. AQP4 expressing glioma cells showed significantly reduced invasion compared to AQP1 and S180 expressing tumors as determined by quantitative stereology, consistent with a differential role for AQP1 and AQP4 in this process.
Keywords: migration, edema, volume regulation, tumor, signaling
Introduction
Under physiological conditions, the brain and spinal cord rarely encounter significant changes in the osmolarity of the extracellular fluid. Primary brain tumors often disrupt the blood-brain barrier leading to an increase in brain water content and swelling, or edema 13. Surprisingly gliomas thrive and expand rapidly in this edematous environment. Glioma cells are able to regulate their cell volume through the release of osmotically active anions, primarily Cl-, and likely utilizes water channels called aquaporins (AQP) for the redistribution of water 8. Aquaporins form a superfamily of 13 members and are the principle pathway for water movement across most cellular membranes. Of these, AQP1 and AQP4 proteins are highly expressed in the most malignant gliomas called glioblastomas 27,28. Functional studies using recombinant over expression delineate differential roles for these AQPs. AQP1 confers enhanced cell migration whereas AQP4 enhanced cell adhesion actually reducing cell migration 20. Since both AQPs are prominently expressed across human gliomas, we hypothesize that their relative contribution to the cell's biology may be regulated to support a particular biological state. More specifically, during tumor dissemination, the activity of AQP4 may be suppressed to enhance invasiveness, whereas during times of growth and tumor expansion, adhesion to neighboring cells may be enhanced hence displaying enhanced AQP4 activity.
Protein kinase C is known to regulate tumor invasion and has recently been shown to regulate aquaporins in other systems. PKCs constitute a family of serine/threonine kinases with 9 isoforms showing distinct cellular and subcellular localization in the brain and spinal cord. Generally, PKC is recruited to the cellular membrane in response to a rise in Ca2+ and diacylglycerol. PKC has been shown to be an important modulator of tumor proliferation and migration 5. PKC activation causes enhanced tumor invasion in U87 5 and increases proliferation of U138 tumor cell lines 24.
Here we set out to examine whether PKC regulates AQP4 in glioma cells in ways that enhance two important biological states of these tumors, growth and invasion. Using D54-MG glioma cells stably transfected with AQP4, we show that the enhanced PKC activity, activated via phorbol 12-myristate 13-acetate (PMA), causes a phosphorylation of AQP4 resulting in reduced water permeability and inhibition of cell migration. By contrast, chelerythrine, a PKC inhibitor, reduces AQP4 phosphorylation leading to enhanced water permeability and tumor invasion. AQP4 localizes to the leading edge of the invadipodia of migrating cells and colocalizes with ClC2 and KCC1, which are believed to provide the pathways for Cl- and K+ release in the context of cell migration 21 providing further support for a role for KCl-water extrusion as an enhancer of cell invasion.
Materials and Methods
Cell Culture
D54-MG (WHO grade IV) were a gift from Dr. D.D. Bigner (Duke University, Durham, NC). Cells were grown in Dulbecco's modified Eagle medium (DMEM/F12; Media Tech, University of Alabama at Birmingham Media Preparation Facility) and supplemented with 2 mM glutamine (Media Tech) and 7% heat-inactivated fetal bovine serum (Hyclone, Logan, UT) at 37°C and 90% O2/10% CO2 humidified environment. AQP4 and AQP1 stable cells lines were made as described previously 20 from D54 glioma cell lines that lack AQP1 and AQP4 expression.
Western Blot Analysis
Western blot procedure has been described previously 20. All antibodies were obtained from Chemicon (Temecula, CA) and used following manufacturer's instructions. Images were taken on Kodak Imager (Rochester, NY) and analyzed using Kodak Imager software.
Immunoprecipitation
Cells were grown to confluency in a 10 cm2 dish. Cells were washed 2× in ice cold PBS. Cells were scraped and collected in 500μl ice cold PBS and centrifuged for 5 min at 10,000 rpm. Supernatant was removed and pellet resuspended in 500 μl RIPA buffer [(50 mM TrisCl, pH 7.5, 150 mM NaCl, 1% Nondet P-40 (NP-40), 0.5% sodium deoxycholate, 1% sodium dodecyl sulfate (SDS)] containing protease and phosphatase inhibitors. Lysates were rotated for 30 min at 4°C followed by a brief sonication. Protein quantification was performed using a DC protein assay kit (BioRad, Hercules, CA) and lysates diluted to 1 mg/ml. Lysates were cleared with 50 μl agarose-conjugated protein A beads (Roche, Indianapolis, IN) for 30 min at 4°C and centrifuged briefly to pellet beads. Supernatant was transferred to a new tube and incubated for 1 hr with mouse anti-phosphoserine (Sigma) at 4°C. Protein A beads (50 μl) were added and allowed to rotate overnight. On the following day, immunoprecipitates were pelleted at low speed at 4°C and supernatant removed as unbound fraction. Immunoprecipitates were rinsed 3× with ice cold RIPA and released from beads using 25 μl of 100 mM glycine and incubated for 3 min. Five μl of 6× sample buffer was added and spun down to pellet beads. Supernatant was removed and ran onto 10% gel as described in previously 20.
Immunocytochemistry
Cells plated on coverslips and grown to a confluent monolayer and were scratched using a 200 μl pipette tip. Cells were allowed to recover for 5 hr, washed with PBS and fixed in 4% paraformaldehyde for 10min. Staining procedure has been described previously 20. Images of migrating cells were acquired using an inverted Olympus IX-81 spinning disk confocal microscope (Olympus, Center Valley, PA).
Site-Directed Mutagenesis and Transfections
AQP4 was excised from DsRed plasmid using restriction enzymes Apa1 and EcoRI (New England Biolabs). Two sets of primers were created. 5′-CGA ATT CTG ATG GTG GCT TTC AAA GGG G -3′ and 5′-AAC ATC AGT CCG TTT GGC ATC ACA GCT GGC-3′ and 5′-GCC AGC TGT GAT GCC AAA CGG ACT GAT GTT-3′ and 5′-CCG GGC CCG TAC AGA AGA TAA TAC CTC TCC-3′ were used in two separate PCR reactions with the mutation site (bolded). A final PCR reaction was done using primers 5′-CGA ATT CTG ATG GTG GCT TTC AAA GGG G-3′ and 5′-CCG GGC CCG TAC AGA AGA TAA TAC CTC TCC-3′ with PCR products from the previous reactions with annealing occurring at the mutation site. PCR reactions used Phusion High-Fidelity Polymerase (New England Biolabs) and were done as follows: denaturation at 98°C for 2 min followed by 35 cycles of 98°C for 10 s, 60°C for 10 s, 72°C for 45 s and a final extension at 72°C for 7 min. PCR product was purified and inserted into the DsRed-Monomer-N1 (Clontech) plasmid using T4 ligase. Stable cell lines were made as described previously 20.
Volume Regulation
Cell volume measurements were performed using a Coulter Counter Multisizer 3 (Beckman-Coulter, Miami, FL) as described previously 23 with minor modifications 20. Data were collected by Multisizer 3 software, and 5000 pulse listings were exported to EXCEL as the average of 40-50 cells for each 20 ms time point. Data were collected as mean diameter and were converted to mean cell volume. Mean cell volumes were normalized to baseline values. Data were plotted in Origin 7.0 (MicroCal, Northhampton, MA) ± se with (n) experiments performed. Each time point graphed is an average of the mean cell volume for 40-50 cells per 20 ms.
Cell Migration
Migration was assessed using a modified Boyden Chamber and described previously 20. Images of five random fields were taken using Zeiss Axiovert 200M (München, Germany). All experiments were performed in triplicate.
Cell Adhesion Assay
Coverslips were coated overnight at 4°C with various matrices: 10 μg/ml collagen I (Sigma), 10 μg/ml fibronectin III (Sigma), 20 μg/ml laminin (Sigma), 20 μg/ml vitronectin (Sigma) or 1% BSA (Sigma). 200,000 cells were seeded per well and allowed to adhere for 1 hr at 37°C. The non-adherent cells were washed away gently using PBS and cells were fixed using 4% paraformaldehyde. Images of five random fields were taken using Zeiss Axiovert 200M (München, Germany) at 20× magnification.
Tumor Implantation
We obtained 6 wk old CB17 SCID mice. Animals were anesthetized using isofluorane. An incision was made along the midline and a hole was drilled ∼2 mm post bregma and 2 mm to the right of the midline where a 30-gauge syringe was stereotactically inserted 2 mm. 500,000 cells were injected and animals were sutured and allowed to recover for 2 weeks. Mice were transcardially perfused with 4% paraformaldehyde and incubated overnight at 4°C. Brains were placed in 10% sucrose for 1hr at 4°C followed by an overnight incubation with 30% sucrose. Brains were fixed in OCT and place in -80°C then serial sectioned in cryostat at 20 μm. For imaging cell migration, sections were dried overnight at 37°C, washed with 0.1 M PB for 45 s followed by 4× 10 s in dH2O and incubated overnight at 37°C. Sections were rehydrated by incubating for 5 min in each ethanol: 100%, 95% and 70% followed by 2× washes in dH2O, mounted and imaged on Zeiss Axiovert 200M. To determine cell migration, we used the Axiovert software and drew a line from the edge of the tumor mass to the cell that had migrated away and recorded the distance.
Results
PKC phosphorylates AQP4
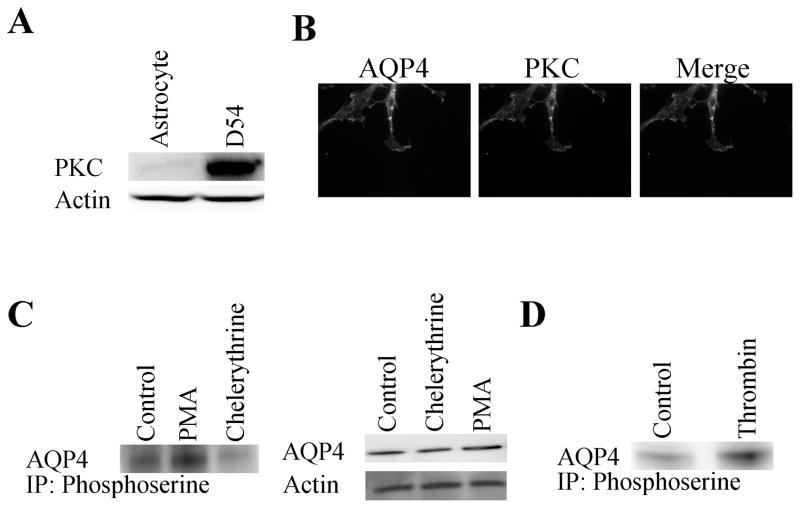
The primary objective of this study was to determine whether regulation of AQP4 function may alter the degree of tumor migration or invasiveness. This objective is predicated on our previous observation that overexpression of AQP4 in D54 glioma cells causes a marked reduction in cell migration 20. Furthermore, astrocytes treated with dopamine 38 and thrombin 34 show a reduction in AQP4 water permeability as a direct result of PKC phosphorylation. Therefore, it appears to be a logical first step to examine whether PKC phosphorylates AQP4 and whether this in turn may affect the degree to which these cells migrate. To achieve this goal we first examined the level of endogenous PKC expression in AQP4 expressing glioma cells (AQP4-D54) as compared to nonmalignant astrocytes (Fig. 1A). Consistent with previous studies 5,6, we found much enhanced expression of PKC in tumor cells by Western blot. Importantly, AQP4 and PKC were found colocalized at the leading edge of migrating cells (Fig. 1B). To examine whether the relative activity of PKC alters the degree of AQP4 phosphorylation, we used immunoprecipitation experiments in which anti-phosphoserine antibodies were used to precipitate proteins that show phosphoserine phosphorylation and then probed these with antibodies to AQP4. These experiments were done prior to (control) and following activation [phorbol 12-myristate 13-acetate (PMA)] or inhibition (chelerythrine) of PKC. As illustrated in Fig. 1C, AQP4 showed constitutive phosphorylation which could be further enhanced by exposure of cells for 45 min to 1 μM PMA, a PKC activator. Conversely, 45 min exposure to 1 μM chelerythrine, a broad spectrum PKC inhibitor, resulted in significantly decreased AQP4 phosphorylation. Importantly, neither treatment altered the total protein level for AQP4 as illustrated in Fig. 1C (right). To examine whether a more physiological activator of PKC could similarly enhance AQP4 phosphorylation we used the known PKC activator thrombin. Thirty minute exposure of glioma cells to 0.5 U thrombin showed significantly enhanced AQP4 phosphorylation (Fig. 1D). Taken together this data shows that AQP4 in gliomas is the target of PKC phosphorylation, and albeit significantly phosphorylated at rest, phosphorylation can be further enhanced by PKC activation.
Figure 1.
PKC expression and phosphorylation of AQP4. Cell lysates were separated using 10% SDS-PAGE gels and transferred onto PVDF paper and probed with antibodies Western blot analysis was used to examine PKC expression levels in AQP4-D54 cells as compared to primary astrocyte cultures (A) and localization in migrating tumor cells (B) using PKC antibodies as described in Materials and Methods. (C) Immunoprecipitation showed that pretreatment with either chelerythrine or PMA modulated AQP4 phosphorylation levels but did not alter basal AQP4 expression levels (D).
PKC modulators alter AQP4 water permeability
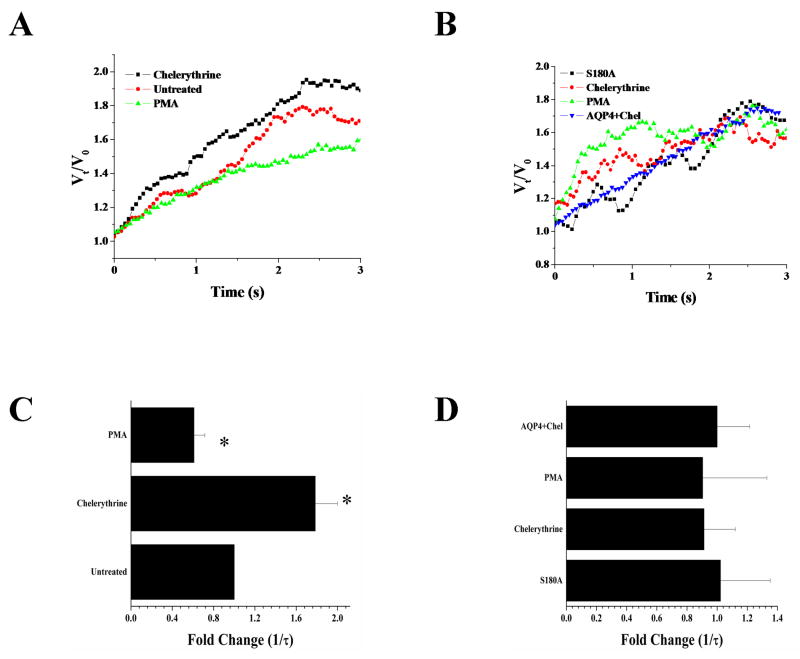
Previous work using astrocytes has shown that activation of PKC leads to a reduction in water permeability via AQP4, and this occurs through direct phosphorylation of AQP4 at the S180 site 38. Hence the expectation would be that exposure to PKC activators would reduce while PKC inhibitors should enhance water permeability. To examine water transport directly, we measured the rate of cell swelling in response to a hypo osmotic challenge using a Coulter-Counter Cell Sizer as previously described 20. This instrument allowed us to size populations of cells in real time with a 20 msec time resolution. Recordings were obtained over a 3 min period during which a 60s baseline volume was acquired followed by the addition of a 50% hypo osmotic challenge for an additional 2 min (see methods for detail). The average cell volume +/- S.E.M. for 100 cells each was plotted every 20 msec. Figure 2A shows representative examples of volume changes caused by water influx into AQP4 expressing D54 glioma cells treated with either chelerythrine or PMA as compared to untreated controls. From this data, we derived the time constant (τ) of the rise in volume and plotted the reciprocal, which is directly proportional to the water permeability. Relative changes compared to untreated control AQP4-D54 cells are given in (Fig 2C). This data suggests that inhibition of PKC using chelerythrine results in a ∼2-fold enhancement of water permeability when exposed to a 50% hypo osmotic challenge. By contrast, cells treated with PMA showed ∼2-fold decrease. Comparable results to PMA were obtained with thrombin (data not shown). To rule out non-specific effects of these drugs we next mutated the phosphorylation site, S180A, of AQP4 and created sister cells that expressed the mutated form of AQP4, which should now be insensitive to PKC. Indeed, as illustrated in Fig. 2B/C, these cells showed no difference in water permeability when treated with either chelerythrine or PMA as compared to wildtype AQP4 expressing cells treated with chelerythrine (AQP4+Chel). This data suggests that in gliomas water permeability through AQP4 is under the regulation of PKC via phosphorylation of S180.
Figure 2.
Functional regulation of AQP4 by PKC. Mean cell volume was measured for 3 min in AQP4-D54 cells (A; untreated n=7, Chel n=6, PMA n=4) and S180 mutant AQP4 (B; n=6). Cells were treated with either chelerythrine or PMA for 45 min before being given a 50% hyposmotic challenge. Representative graphs show water permeability over the first few seconds following hyposmotic challenge. Reciprocal exponential time constant (τ−1) is proportional to osmotic water permeability (C). AQP4+Chel are gliomas expressing WT AQP4 that were treated with chelrythrine. (Significance was assessed using an ANOVA *p<0.05 as compared to control.)
PKC regulates tumor migration in AQP4 expressing cells
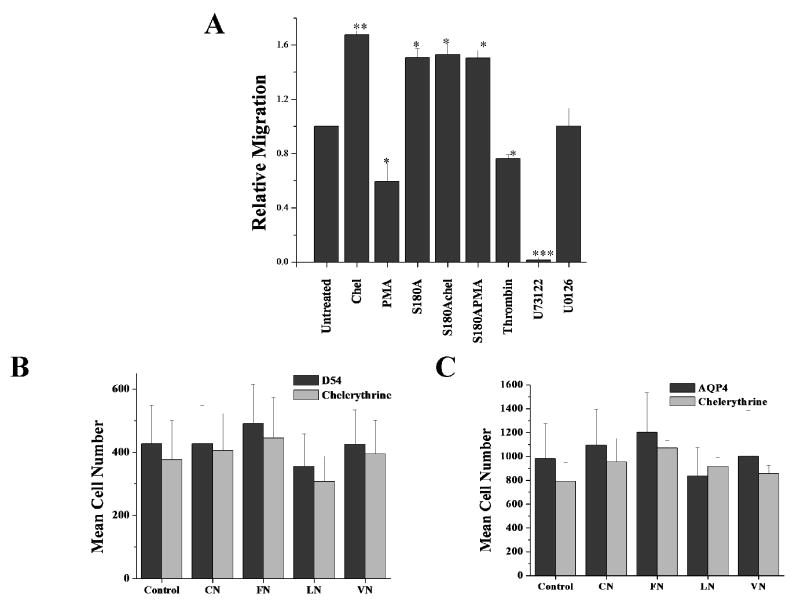
It has been hypothesized that water channels aid cell volume changes required by tumor cells as they invade brain tissue 21. To examine whether PKC regulation of AQP4 may alter the ability of cells to undergo volume changes as they invade, we used Transwell assays which mimic the spatial constraints of the extracellular space. We plated 40,000 cells into the top compartment of a Transwell insert containing 8 μm pores and cells were allowed to migrate for 5 hrs at which time the cells were fixed and images taken for analysis. Cells were continuously treated with specific inhibitors for defined signaling steps associated with PKC signaling. These included the PKC inhibitor chelerythrine, a PLC inhibitor, U73122, and a MEK1/2 inhibitor, U0126. As illustrated in Fig. 3A, a dephosphorylation of AQP4 with chelerythrine not only enhanced water permeability but also showed ∼75% enhancement in tumor cell migration as compared to untreated AQP4 cells (Fig 3A). Conversely, PMA, which reduced water permeability in AQP4-D54 cells, also reduced tumor migration by ∼40%. Once again, the specificity of these effects for AQP4 mediated phosphorylation was validated by the fact that glioma cells containing the mutated serine residue that eliminated PKC phosphorylation, S180A, showed migration rates similar to those of chelerythrine treated cells and were insensitive to treatment with PMA or chelerythrine. Also, MAPK signaling appeared to have little effect since inhibition of MEK1/2 activation with U0126 did not affect the migration of AQP4-D54 glioma cells. By contrast, in all cell types tested, the PLC inhibition by U73122 completely eliminated cell migration (Fig 3A). This finding is consistent with previous studies showing an absolute requirement for PLC in chemotactic migration since PLC is important for increasing the Ca2+ concentration necessary for inducing migration 2,4 and cytoskeletal reorganization 3. These effects were of course unrelated to AQP4 as the drug also inhibited the migration of glioma cells that lack AQP4 expression (data not shown). Taken together, the above data suggests that AQP4-D54 cell migration is effectively modulated by PKC signaling through altering the cells water permeability and in turn the cells ability to adjust their shape as they migrate.
Figure 3.
Role of PKC in modulating migration and adhesion in AQP4-expressing tumor cells. (A) Transwell migration assay comparing AQP4-D54 cells treated with inhibitors of various signaling molecules. 40,000 cells were allowed to migrate for 5 hrs through 8 μm FluorBlok filters. Cells were treated with PKC modulators, chelerythrine and PMA, U73122, a PLC inhibitor and U0126 as a MAPK inhibitor. To measure adherence, 100,000 cells were plated onto various matrices [1% BSA (Control), 10 μg/ml collagen I (CN), 10 μg/ml fibronectin III (FN), 20 μg/ml laminin (LN), 20 μg/ml vitronectin (VN)] for 1 hr. Cells were gently washed away and fixed. Five random images were taken and counted. There was no difference in cell adhesion between D54 cells lacking AQP4 expression (B) nor AQP4-expressing cells (C) when treated with chelerythrine.
AQP4 modulation does not alter cell adhesion
Although the above data are consistent with the hypothesized role of aquaporins in volume changes, it is possible that PKC effects may be explained by other mechanism. For example, PKC has been shown to modulate cellular adhesion through phosphorylation of focal adhesion kinase 18. This is important in light of previous findings demonstrating that AQP4 cells expressing the M23 isoform show enhanced cellular adhesion 12. To rule out changes in adhesiveness as mediators of the observed changes in cell migration, we examined this alternative possibility directly. Therefore, we conducted traditional cell adhesion studies in which we coated coverslips with several common extracellular matrices (collagen, fibronectin, laminin, and vitronectin), allowing cells to adhere for 1 hr and gently washing away any non-adherent cells. Adherent cells were quantified and compared to controls. We found that although expression of AQP4 per se enhanced cell adhesion compared to that of glioma cells lacking AQP4, there was no change in the cellular adhesion when AQP4 expressing cells were treated with chelerythrine and this was true for all matrices examined (Fig 3C) as compared to D54 control cells with no AQP4 (B). This data suggests that the observed changes in AQP4-D54 migration following PKC modulation, is not due to an alteration in the cell's ability to adhere to various matrix molecules.
AQP1 is not regulated by PKC
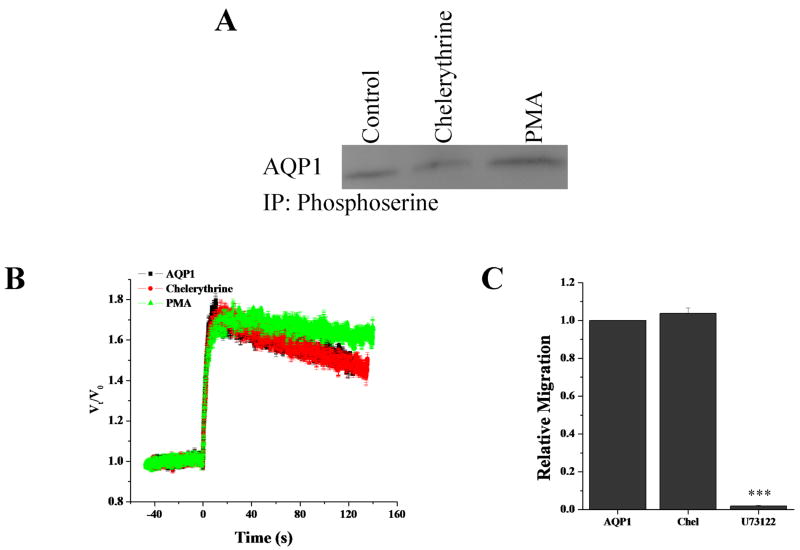
Previous work suggested that PKC regulates AQP1 whereby increasing PKC activity increases AQP1 mediated water permeability 41. Since glioma in vivo express both AQP1 and AQP4, we wanted to determine if AQP1 was also under regulation by PKC. We examined AQP1-D54 cells in regards to phosphorylation, volume regulation and migration as performed on AQP4-D54. Immunoprecipitations revealed little change in phosphorylation of AQP1 by PKC modulators PMA and chelerythrine (Fig. 4A). Further, neither cell swelling nor cell migration were altered by chelerythrine in AQP1 expressing tumors (Fig 4B/C). This data suggests that AQP1 is not functionally regulated by PKC activity, which is in stark contrast to AQP4.
Figure 4.
PKC does not regulate AQP1. Using immunoprecipitation as described in Fig. 1, chelerythrine and PMA treatment did not alter AQP1 phosphorylation (A). Neither water permeability (B) nor cell migration (C) was altered by PKC activity.
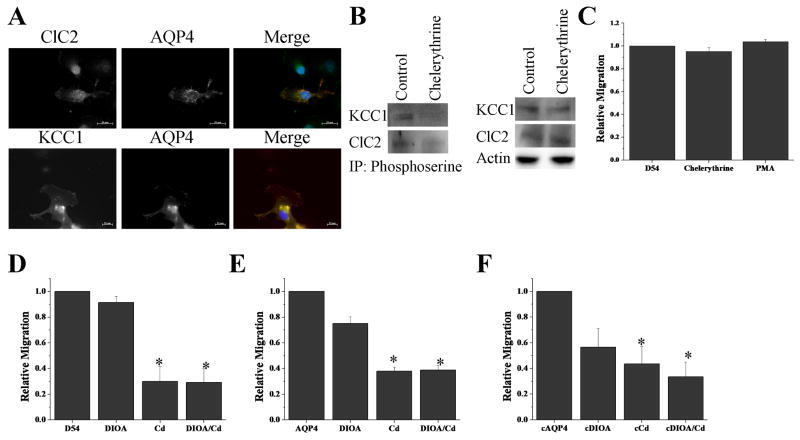
AQP4 colocalizes with KCC1 and ClC2 in migrating cells
While water permeability is required for cell shape changes, the directionality of the water movement as well as the energetic driving force is the consequence of salt movements. In this context it is thought that the efflux of Cl- and K+, i.e. KCl, provide the driving force for cell shrinkage at the leading invading edges of the cells. Important candidates that have been implicated in the release of Cl- are the K+-Cl- cotransporter 1 (KCC1) and the chloride channel (ClC2). Interestingly, phosphorylation by PKC regulates these proteins in a similar fashion as AQP4 and with the same directionality. Specifically, phosphorylation by PKC reduces the outward Cl- movement of both the transporter and the channel 9,15 and reduces water permeability. This would work hand-in-hand to reduce the overall release of cytoplasm and hence reduce shrinkage, whereas a decrease in phosphorylation affects each protein in the opposite manner hence enhancing cytoplasmic release. In light of this, we examined the possibility that these proteins may colocalize, and in order to address this question, we wished to examine cells that were in the process of extending a leading process and were actually migration. This can be conveniently accomplished using an in vitro wound assay. Therefore using a sterile 200μl pipette tip a confluent monolayer of glioma cells was scratched, and cells were allowed to migrate for 5hrs into the bare regions of the coverslip. Following fixation images of cells that appeared to be migrating into the lesion were triple labeled with antibodies to ClC-2, KCC1, AQP4 and cell nuclei were labeled with DAPI. Representative examples of individual and merged images are shown in Fig. 5A each showing that AQP4 colocalized with both ClC2 and KCC1 along the leading edge of the cell.
Figure 5.
PKC modulates phosphorylation of ClC2 and KCC1 but not migration. Using immunfluorescence (A) and immunoprecipitation as described in Fig. 1, chelerythrine reduced PKC mediated ClC2 and KCC1 phosphorylation without altering the basal expression levels of either (B). PKC does not modulate migration in D54 control cells lacking AQP4 (C). Migrating cells were treated acutely with 40 μM DIOA, 12.5 μM CdCl2, or both to block ClC2 and/or KCC1. Inhibition was examined in D54 control cells (D), AQP4-expressing cells (E), and in AQP4 cells treated with chelerythrine (F). (Significance was determined using ANOVA; *p<0.05)
Chelerythrine reduces phosphorylation of ClC2 and KCC1
We next examined if ClC2 and KCC1 were indeed also subject to regulation via PKC phosphorylation in glioma. We performed immunoprecipitation experiments to determine the degree of phosphorylation of ClC2 and KCC1 in AQP4-D54 cells treated with chelerythrine. As illustrated in Fig. 5B, cells treated with the PKC inhibitor, chelerythrine, showed reduced phosphorylation for both KCC1 and ClC2; chelerythrine did not alter expression of total protein of either KCC1 or ClC2 (Fig. 5B) similar to what we demonstrated for AQP4 in Fig. 1C.
PKC modulation of chloride channels and transporters does not alter migration
Unlike other cancers that spread hematogenously, gliomas invade the brain by active cell migration. As alluded to above, and more extensively discussed in the literature 21,32,33, migrating tumor cells exude K+ and Cl- along with obligated water in order to undergo the shape changes required of migrating cells. Since de-phosphorylation enhances migration while also enhancing water permeability and K+ and Cl- secretion, we were interested to delineate whether these phosphorylation effects required all of these constituents to be simultaneously regulated or whether water permeability may be the rate limiting step. To do so, we used D54MG glioma cells that lack expression of all aquaporins while maintaining expression of ClC2 and KCC1 to ask whether altering the phosphorylation status of ClC2 and KCC1 via a PKC inhibitor would suffice to change the rate of migration. Using Transwell assays, we show in Fig. 5C that in the presence of chelerythrine or PMA glioma migration was unaltered suggesting that PKC modulation of Cl- movement alone does not alter tumor migration. However, we further tested whether AQP4 cells, with enhanced water permeability, were more sensitive to chloride channel and transporter inhibitors than control cells. We then seeded D54 cells and AQP4 cells, as well as AQP4 cells treated with chelerythrine (cAQP4), onto a Transwell migration assay filter in the presence of a KCC inhibitor (40 μM DIOA), a ClC inhibitor (12.5 μM CdCl2) or both and allowed the cells to migrate for 5 hrs. We found that both D54 cells and AQP4-expressing D54 cells were inhibited to comparable levels under all conditions (Fig 5D,E,F). In addition, AQP4 cells treated with chelerythrine showed no enhanced sensitivity to the Cl- channel/transporter inhibitors. This data implies that enhanced water permeability does not increase the tumor cells sensitivity to inhibitors of Cl- movement.
Finally, we examined whether AQP4-D54 tumor cells in which chelerythrine was used to maximize water permeability through AQP4 would now show an enhanced susceptibility to Cl- channel/transporter inhibitors with regards to their rate of Transwell migration. As illustrated in Fig. 5D, this was not the case as there was only a marginal and non-significant enhancement in tumor migration in chelerythrine treated cells compared to control cells. Taking this data together suggests that, while Cl- and K+ release is essential for cell volume changes to support glioma migration/invasion, expression levels of the KCC1 and ClC channels that mediate electrolyte release are not rate limiting but instead, water permeability is ultimately rate limiting with regards to the rate of tumor migration.
AQP1 expression leads to a more aggressive tumor in vivo
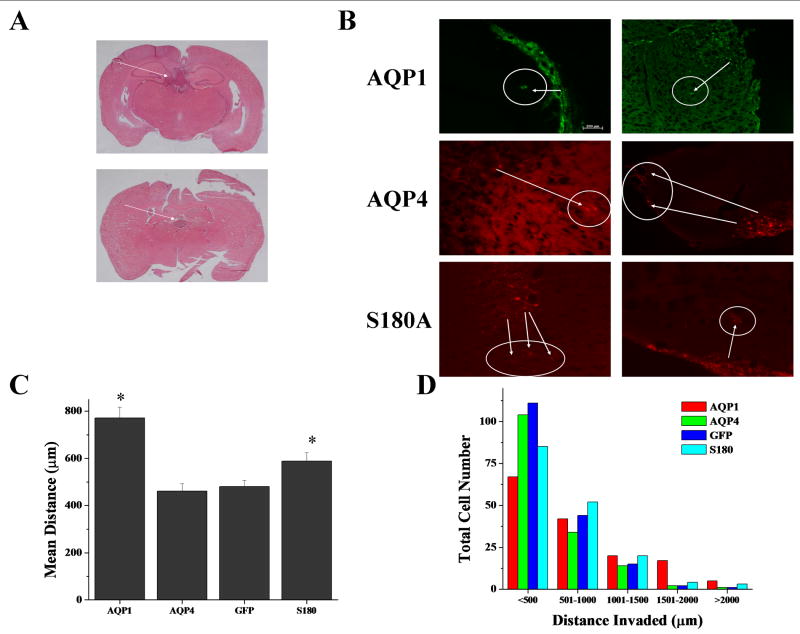
While previous reports have shown AQP1 and AQP4 expression in vivo in gliomas 19,27,28, our lab has shown individual functions for both AQP1 and AQP4 in vitro20, specifically that AQP1 is more migratory than AQP4. We aimed to determine if a similar result could be found in vivo, and if so then we would hypothesize that the reduced rate of migration found in AQP4 tumor cells would be dependent on its regulation by PKC activity since primary gliomas express high levels of PKC as compared to astrocytes. In order to accomplish this, we intracranially injected 500,000 cells expressing AQP1, AQP4, S180A-AQP4 or control cells and allowed the tumors to grow for 2 weeks in 6 week old SCID mice and then examined cell invasion by quantitative stereology. Representative images in Figure 6 demonstrate the tumor mass (Fig 6A) and individual cells that have invaded away from the main tumor mass (Fig 6B). Using the endogenous fluorescence associated with the AQP1-GFP and the AQP4-DsRed, we measured the distance each migrated away from the primary tumor mass. Upon examination, AQP1 expressing tumor cells invaded ∼2 fold further than either D54-GFP control cells or AQP4 tumors cells (Fig 6C). AQP1 tumor cells showed increased cell numbers for distances >1000 μm (Fig 6D), and these tumors formed more distant satellite colonies (data not shown), which was in contrast to AQP4-expressing tumor and control cells where there were increased cell numbers migrating distances <500 μm (being close to the tumor). When we examined the mutant AQP4 tumor cells, we discovered that, consistent with the in vitro data, S180A-AQP4-DsRed mutants had increased invasion as compared to the AQP4 expressing gliomas (Fig 6C). Additionally, S180A-AQP4 tumors showed enhanced tumor migration for distance greater than 1000 μm, though not as robust as exhibited by AQP1-expressing tumors (Fig 6D). Taken together, the data suggests that AQP1 tumors display a more invasive phenotype and suggests that these tumor cells are forming the satellite colonies characteristic of gliomas. Further, AQP4 mediated invasion is regulated by PKC and its invasion is limited in vivo due to an increase in PKC activity upregulated in gliomas.
Figure 6.
AQP1 expression enhances tumor invasion. AQP1 (n=9), AQP4 (n=8) and S180A-AQP4 (n=8) expressing D54MG were injected intracranially into 6 weeks old mice. Representative images of tumor growth (A). Arrows indicate location of main tumor mass. Fluorescent images demonstrate tumor cell invasion away from the major tumor mass for D54 glioma cells expressing AQP1-GFP, AQP4-DsRed, or AQP4-S180A-DsRed (B). Arrows indicate invading cells. D54 cells expressing AQP1-GFP invaded ∼2 fold over D54-GFP and AQP4-DsRed expressing D54 cells (C). We measured invasion over 500 μm increments and discovered that AQP1 tumors were able to invade greater distances than either D54 or AQP4 cells (D). There were significantly more cells that had migrated distances greater than 1500 μm. Most D54 and AQP4 tumors invaded short distances, while S180A-AQP4 mutants invaded intermediate distances.
Discussion
In this study, we were able to demonstrate that PKC regulates AQP4 function in glioma cells through phosphorylation on S180 leading to reduced water permeability and enhanced water permeability when de-phosphorylated. Our findings agree with previous work in astrocytes where exposure to dopamine 38 or thrombin 34 activates PKC and results in a reduction in water permeability. Our studies mutating S180 on AQP4 in gliomas unequivocally suggest that PKC signaling is biologically important for this regulation. Interestingly, our immunoprecipitation and Western blotting experiments suggest a significant degree of constitutive phosphorylation at this site in unstimulated gliomas. This leads us to conclude that water permeability is maintained at a reduced level in resting cells. Experimentally, we were able to greatly enhance water permeability and the rate at which cell changes their cell volume using chelerythrine to reduce AQP4 phosphorylation. This may be important in invading cells that are exposed to environmental signals that cause a reduction in PKC activity.
We hypothesized that the invasive migration of glioma cells requires cells to undergo coordinated cell volume changes, most notably shrinkage as cells invade into narrow extracellular spaces in brain 33. Previously, we showed localization of AQP4 to the leading edge of migrating tumor cells 20 and our immunohistochemical data reveals localization of Cl- channels/transporters to the leading edge of migrating cells. Furthermore, we showed that secretion of Cl- and K+ is required for cell invasion 21;25. Although, Cl- and K+ channels provide the pathways for KCl secretion, water release is ultimately required to achieve any volume change in the context of cell migration with AQP4 in the leading edge of an invading cell possibly serving this role. Intuitively, we had assumed that water permeability would not be limiting in this context but our experimental data refute this. Our data suggests that modulating the phosphorylation state of chloride channels or KCC transporters, which are known to be enhanced in their activity following dephosphorylation, does not alter tumor migration. Yet, inhibiting chloride movement also inhibits invasion. Hence, the data suggest that while Cl- movement is necessary for tumor invasion, an enhancement of water permeability also correlated with enhanced tumor invasion leads us to conclude that water permeability does become the rate limiting step. A recent study suggests that AQP1 is also regulated by PKC but with opposite effects, namely PKC inhibition would lead to a reduction in water permeability 41. This would lead us to conclude that since tumor cells exhibit high protein levels of PKC as compared to astrocytes, AQP4 water permeability would be highly depressed, while tumor cells expressing high levels of AQP1 would be able to utilize rapid water flux to invade into the surrounding tissue. However, we saw only small if any changes in water permeability in AQP1-D54 cells treated with chelerythrine and no difference in tumor migration. Since PKC is highly expressed in tumor cells, we may not see an additional increase in AQP1 water permeability with PMA treatment as this protein may be maximally phosphorylated. However, this data is in contrast with previous work 41. One possibility is that we are using two different model systems that express different PKC isoforms. It is likely that the isoform is not found in our tumor cells or that it is being expressed at low levels. In addition, several studies have demonstrated a role for AQP4 mediated migration 1,16,29,30. However, these cell types all express low levels of PKC in comparison to primary glioblastomas. If PKC is indeed reducing AQP4-mediated tumor migration, then cells may simply not expressed it at high enough levels to inhibit the channel to prohibit migration. It is also quite possible that the PKC isoform expressed in our tumor cells is not the isoform shown to regulate AQP4 activity in other cell types. Clearly, further studies are needed to further elucidate the role of PKC in regulating AQP1.
The importance of regulating tumor migration is demonstrated by understanding the effect thrombin has on PKC activity. Since AQP4-expressing tumors demonstrate an increase in the phosphorylation state of AQP4 and a reduction in migration following exposure to thrombin, we would conjecture that exposure of a tumor to thrombin would alter the invasiveness of the tumor. Thrombin is found in high levels in the blood and is an endogenous PKC activator. However, it is expressed at very low levels in the brain. Since tumor cells disrupt the BBB and cause peritumoral edema, thrombin would enter the surrounding brain tissue and activate PKC in tumor cells, making it possible that PKC activity is preventing the tumor from invading into regions of high edema formation.
PKC activity promotes migration in several cell types. Specifically, PKC activity enhances migration in endothelial cells 11, CHO cell spreading 35, and vascular smooth muscle cell migration 22. In addition, other PKC isoforms have been implicated in regulating cell migration. PKC reduces chemotaxic migration in monocytes 10, PDGF-stimulated migration of smooth muscle cells 17 and EGF-stimulated migration of fibroblasts 14. PKC activity also plays a role in regulating cell invasiveness in several different tumor systems, i.e. breast carcinoma 31, mammary carcinoma 7 and colon carcinoma 26. This is consistent in primary glioblastomas where glioma invasion is modulated by PKC 40. However, this study suggests that while PKC modulates tumor migration and invasion, water movement is the limiting factor for regulating migration/invasion. Based on previous studies showing that PKC modulates AQP4 water permeability 37,38, we hypothesize that reducing water permeability should slow glioma migration. AQP4 KO mice show reduced astrocyte migration in vitro as well as in vivo following injury 30, which leads to a reduction in the formation of the glial scar. This suggests that altering water permeability by eliminating the channel alone can prevent migration. Therefore, reducing water permeability should also reduce cell migration. Further, Warth et al (2007) showed that while AQP4 is upregulated in primary glioblastomas obtained from patient biopsies, the cells located in the infiltration zone contain very low levels of AQP4 36. Our in vivo data is consistent with these results where by AQP4 cells are less invasive than AQP1 expressing gliomas. This suggests that while AQP4 is highly expressed in primary glioblastomas, it is not the primary AQP necessary for tumor migration. Instead, AQP1 mediated water permeability may be the more important pathway for cell volume changes as glioma cells infiltrating the surrounding brain.
Acknowledgments
The authors thank the UAB Neuroscience NINDS Protein Core-C for creation of site mutation and UAB Neuroscience Blueprint Cellular and Molecular Neuropathology Core-C for use of stereotactic microscope and cryostat. This work was supported by NIH Neuroscience Blueprint Core Grant NS57098, NINDS Core Grant P30 NS47466, and NIH 5R01NS031234 and NIH 2R01NS036692.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, Verkman AS. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- 2.Chen P, Gupta K, Wells A. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol. 1994;124:547–555. doi: 10.1083/jcb.124.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen P, Murphy-Ullrich JE, Wells A. A role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J Cell Biol. 1996;134:689–698. doi: 10.1083/jcb.134.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P, Xie H, Sekar MC, Gupta K, Wells A. Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho KK, Mikkelsen T, Lee YJ, Jiang F, Chopp M, Rosenblum ML. The role of protein kinase Calpha in U-87 glioma invasion. Int J Dev Neurosci. 1999;17:447–461. doi: 10.1016/s0736-5748(99)00054-4. [DOI] [PubMed] [Google Scholar]
- 6.Couldwell WT, Antel JP, Apuzzo ML, Yong VW. Inhibition of growth of established human glioma cell lines by modulators of the protein kinase-C system. J Neurosurg. 1990;73:594–600. doi: 10.3171/jns.1990.73.4.0594. [DOI] [PubMed] [Google Scholar]
- 7.Doll F, Pfeilschifter J, Huwiler A. The epidermal growth factor stimulates sphingosine kinase-1 expression and activity in the human mammary carcinoma cell line MCF7. Biochim Biophys Acta. 2005;1738:72–81. doi: 10.1016/j.bbalip.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW. Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol. 2005;288:C1451–C1460. doi: 10.1152/ajpcell.00503.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrell CM, Lauf PK, Wilson BA, Adragna NC. Lithium and protein kinase C modulators regulate swelling-activated K-Cl cotransport and reveal a complete phosphatidylinositol cycle in low K sheep erythrocytes. J Membr Biol. 2000;177:81–93. doi: 10.1007/s002320001101. [DOI] [PubMed] [Google Scholar]
- 10.Fine JS, Byrnes HD, Zavodny PJ, Hipkin RW. Evaluation of signal transduction pathways in chemoattractant-induced human monocyte chemotaxis. Inflammation. 2001;25:61–67. doi: 10.1023/a:1007152903135. [DOI] [PubMed] [Google Scholar]
- 11.Harrington EO, Loffler J, Nelson PR, Kent KC, Simons M, Ware JA. Enhancement of migration by protein kinase Calpha and inhibition of proliferation and cell cycle progression by protein kinase Cdelta in capillary endothelial cells. J Biol Chem. 1997;272:7390–7397. doi: 10.1074/jbc.272.11.7390. [DOI] [PubMed] [Google Scholar]
- 12.Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, Walz T, Sasaki S, Mitsuoka K, Kimura K, Mizoguchi A, Fujiyoshi Y. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol. 2006;355:628–639. doi: 10.1016/j.jmb.2005.10.081. [DOI] [PubMed] [Google Scholar]
- 13.Hossman KA, Bloink M. Blood flow and regulation of blood flow in experimental peritumoral edema. Stroke. 1981;12:211–217. doi: 10.1161/01.str.12.2.211. [DOI] [PubMed] [Google Scholar]
- 14.Iwabu A, Smith K, Allen FD, Lauffenburger DA, Wells A. Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C delta-dependent pathway. J Biol Chem. 2004;279:14551–14560. doi: 10.1074/jbc.M311981200. [DOI] [PubMed] [Google Scholar]
- 15.Kajita H, Whitwell C, Brown PD. Pflugers Arch. Vol. 440. 2000. Properties of the inward-rectifying Cl- channel in rat choroid plexus: regulation by intracellular messengers and inhibition by divalent cations; pp. 933–940. [DOI] [PubMed] [Google Scholar]
- 16.Kong H, Fan Y, Xie J, Ding J, Sha L, Shi X, Sun X, Hu G. AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J Cell Sci. 2008;121:24–36. doi: 10.1242/jcs.035758. [DOI] [PubMed] [Google Scholar]
- 17.Leng L, Du B, Consigli S, McCaffrey TA. Translocation of protein kinase C-delta by PDGF in cultured vascular smooth muscle cells: inhibition by TGF-beta 1. Artery. 1996;22:140–154. [PubMed] [Google Scholar]
- 18.Lewis JM, Cheresh DA, Schwartz MA. Protein kinase C regulates alpha v beta 5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markert JM, Fuller CM, Gillespie GY, Bubien JK, McLean LA, Hong RL, Lee K, Gullans SR, Mapstone TB, Benos DJ. Differential gene expression profiling in human brain tumors. Physiol Genomics. 2001;5:21–33. doi: 10.1152/physiolgenomics.2001.5.1.21. [DOI] [PubMed] [Google Scholar]
- 20.McCoy E, Sontheimer H. Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia. 2007;55:1034–1043. doi: 10.1002/glia.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFerrin MB, Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glial Biology. 2006;2:39–49. doi: 10.1017/S17440925X06000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okazaki J, Mawatari K, Liu B, Kent KC. The effect of protein kinase C and its alpha subtype on human vascular smooth muscle cell proliferation, migration and fibronectin production. Surgery. 2000;128:192–197. doi: 10.1067/msy.2000.108062. [DOI] [PubMed] [Google Scholar]
- 23.Parkerson KA, Sontheimer H. Contribution of chloride channels to volume regulation of cortical astrocytes. Am J Physiol Cell Physiol. 2003;284:C1460–C1467. doi: 10.1152/ajpcell.00603.2002. [DOI] [PubMed] [Google Scholar]
- 24.Patel R, Win H, Desai S, Patel K, Matthews JA, Acevedo-Duncan M. Involvement of PKC-iota in glioma proliferation. Cell Prolif. 2008;41:122–135. doi: 10.1111/j.1365-2184.2007.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ransom CB, O'Neal JT, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci. 2001;21:7674–7683. doi: 10.1523/JNEUROSCI.21-19-07674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigot V, Lehmann M, Andre F, Daemi N, Marvaldi J, Luis J. Integrin ligation and PKC activation are required for migration of colon carcinoma cells. J Cell Sci. 1998;111:3119–3127. doi: 10.1242/jcs.111.20.3119. [DOI] [PubMed] [Google Scholar]
- 27.Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87:621–623. doi: 10.1038/sj.bjc.6600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 30.Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:24–28. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 31.Sliva D, English D, Lyons D, Lloyd FP., Jr Protein kinase C induces motility of breast cancers by upregulating secretion of urokinase-type plasminogen activator through activation of AP-1 and NF-kappaB. Biochem Biophys Res Commun. 2002;290:552–557. doi: 10.1006/bbrc.2001.6225. [DOI] [PubMed] [Google Scholar]
- 32.Sontheimer H. Malignant gliomas: perverting glutamate and ion homeostasis for selective advantage. Trends Neurosci. 2003;26:543–549. doi: 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Sontheimer H. Ion channels and amino acid transporters support the growth and invasion of primary brain tumors. Mol Neurobiol. 2004;29:61–71. doi: 10.1385/MN:29:1:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y, Cai D, Chen Y. Thrombin inhibits aquaporin 4 expression through protein kinase C-dependent pathway in cultured astrocytes. J Mol Neurosci. 2007;31:83–93. doi: 10.1007/BF02686120. [DOI] [PubMed] [Google Scholar]
- 35.Thodeti CK, Albrechtsen R, Grauslund M, Asmar M, Larsson C, Takada Y, Mercurio AM, Couchman JR, Wewer UM. ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA. J Biol Chem. 2003;278:9576–9584. doi: 10.1074/jbc.M208937200. [DOI] [PubMed] [Google Scholar]
- 36.Warth A, Simon P, Capper D, Goeppert B, Tabatabai G, Herzog H, Dietz K, Stubenvoll F, Ajaaj R, Becker R, Weller M, Meyermann R, Wolburg H, Mittelbronn M. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood-brain barrier disturbance but not with patient survival. J Neurosci Res. 2007;35 doi: 10.1002/jnr.21224. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto N, Sobue K, Miyachi T, Inagaki M, Miura Y, Katsuya H, Asai K. Differential regulation of aquaporin expression in astrocytes by protein kinase C. Brain Res Mol Brain Res. 2001;95:110–116. doi: 10.1016/s0169-328x(01)00254-6. [DOI] [PubMed] [Google Scholar]
- 38.Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A. Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. Am J Physiol Renal Physiol. 2002;283:F309–F318. doi: 10.1152/ajprenal.00260.2001. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Verkman AS. Evidence against involvement of aquaporin-4 in cell-cell adhesion. J Mol Biol. 2008;382:1136–1143. doi: 10.1016/j.jmb.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, Law RE, Hinton DR, Couldwell WT. Inhibition of human malignant glioma cell motility and invasion in vitro by hypericin, a potent protein kinase C inhibitor. Cancer Lett. 1997;120:31–38. doi: 10.1016/s0304-3835(97)00287-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Zitron E, Homme M, Kihm L, Morath C, Scherer D, Hegge S, Thomas D, Schmitt CP, Zeier M, Katus H, Karle C, Schwenger V. Aquaporin-1 channel function is positively regulated by protein kinase C. J Biol Chem. 2007;282:20933–20940. doi: 10.1074/jbc.M703858200. [DOI] [PubMed] [Google Scholar]