Abstract
Organophosphorus (OP) compounds cause toxic symptoms, including convulsions, coma, and death, as the result of irreversible inhibition of acetylcholinesterase (AChE). The development of effective treatments to block these effects and attenuate long-term cognitive and motor disabilities that result from OP intoxication is hampered by a limited understanding of the CNS pathways responsible for these actions. We employed a candidate method (called CNSProfile™) to identify changes in the phosphorylation state of key neuronal phosphoproteins evoked by the OP compound, diisopropyl fluorophosphate (DFP). Focused microwave fixation was used to preserve the phosphorylation state of phosphoproteins in brains of DFP-treated mice; hippocampus and striatum were analyzed by immunoblotting with a panel of phospho-specific antibodies. DFP exposure elicited comparable effects on phosphorylation of brain phosphoproteins in both C57BL/6 and FVB mice. DFP treatment significantly altered phosphorylation at regulatory residues on glutamate receptors, including Serine897 (S897) of the NR1 NMDA receptor. NR1 phosphorylation was bi-directionally regulated after DFP in striatum versus hippocampus. NR1 phosphorylation was reduced in striatum, but elevated in hippocampus, compared with controls. DARPP-32 phosphorylation in striatum was selectively increased at the Cdk5 kinase substrate, Threonine75 (T75). Phencynonate hydrochloride, a muscarinic cholinergic antagonist, prevented seizure-like behaviors and the observed changes in phosphorylation induced by DFP. The data reveal region-specific effects of nerve agent exposure on intracellular signaling pathways that correlate with seizure-like behavior and which are reversed by the muscarinic receptor blockade. This approach identifies specific targets for nerve agents, including substrates for Cdk5 kinase, which may be the basis for new anti-convulsant therapies.
Keywords: AChE Inhibitor, DARPP-32, NR1, phospho-specific antibody, muscarinic receptor, phencynonate hydrochloride (PCH)
1. Introduction
Organophosphorus (OP) cholinesterase inhibitors, including sarin, soman, and the insecticide diisopropylfluorophosphate (DFP), irreversibly inhibit the primary metabolic enzyme acetylcholinesterase (AChE), blocking the breakdown of the neurotransmitter acetylcholine (ACh). The resulting overactivity of cholinergic neurotransmission at peripheral nerve endings and throughout the central nervous system (CNS), leads to toxic symptoms, including convulsions, coma, and death (Taylor, 1985). Further, individuals who survive the acute phase of high-level nerve agent exposure (e.g., survivors of the Tokyo sarin attack) often experience persistent impairments in complex behaviors, including cognition (Suzuki et al., 1995; Nozarki et al., 1995; Miyaki et al., 2005). Individuals sustaining sub-chronic or low-level exposure to OP agents (e.g., Persian Gulf War veterans; agricultural workers) also display persistent learning and memory impairments (Rohlman et al., 2007; Golomb, 2008). Experimental animals exposed to sub-lethal doses of soman nerve gas display spatial learning and sensorimotor processing deficits that can be measured months after exposure (Shih et al., 2003).
The biological changes that lead to persistent impairments in cognition and sensorimotor function after nerve agent exposure are poorly understood. Nerve agents evoke massive and sustained elevations in brain levels of ACh (Lallement et al., 1992), which activate muscarinic-type and nicotinic-type cholinergic receptors throughout the brain, and ultimately flood intracellular signaling responses and open voltage-gated ion channels (Felder, 1995; Hogg et al., 2003). The resultant long-term deficit of cholinergic neurotransmission is one likely contributor to persistent cognitive deficits. Loss or dysregulation of ACh-containing neurons in the basal forebrain, which includes cholinergic inputs to the hippocampus, would impact spatial memory function. Degeneration of cholinergic neurons in the hippocampus and cortex is associated with the loss of cognitive function in Alzheimer’s disease (Mesulam, 1996). Additionally, cholinergic interneurons within the striatum contribute to complex forms of learning that require behavioral flexibility (e.g., reversal learning) (Ragozzino et al., 2009). However, simple loss of cholinergic neurons does not entirely explain the persistent effects of nerve agent exposure. Non-cholinergic neurotransmitter systems are also impacted by nerve agent exposure and may contribute to continuing cognitive and motor dysfunction. The initial phase of cholinergic hyperactivity following nerve agent exposure in animals predictably leads to delayed phases of hyperactive glutamatergic and GABAergic neurotransmission that are independent of continued cholinergic receptor activation (Lallement et al., 1991; Lallement et al., 1992; Shih et al., 2003). It is hypothesized that this sequential activation of cholinergic neurotransmission, followed by non-cholinergic neurotransmission persisting for hours after the initial exposure, results in cognitive and motor deficits in animals (Shih et al., 2003; Phillippens et al., 1992). In support of this hypothesis, actions of nerve agents in the CNS rapidly become non-responsive to reversible cholinergic receptor blocker treatments such as atropine (Shih and McDonough, 1997). Therefore, effective treatments that can prevent or reverse the effect of OP intoxication after the initial cholinergic activation phase are needed to attenuate persistent cognitive and motor disabilities. However, development of these agents has been hampered by our limited understanding of the non-cholinergic substrates for OP compounds.
Since multiple neurotransmitter systems are recruited by nerve agent exposure, points of signal integration might be exploited to develop effective treatments able to prevent the long-term consequences of nerve gas exposure. For example, the second messenger pathways that regulate glutamatergic neurotransmission via protein phosphorylation are potential signal transduction targets for the persistent effects of nerve agents on cognition and motor performance. The present study is designed to determine whether nerve agents and other compounds that elevate brain cholinergic neurotransmission leave a distinct neuronal imprint in the form of the phosphorylation of critical signaling molecules. For this purpose we have analyzed the effects of DFP on protein phosphorylation in the mouse brain using CNSProfile, a candidate method that characterizes the intracellular actions of drugs by focusing upon representative phosphorylation sites/phosphoproteins that are involved in the regulation of neuronal excitability. These studies provide insight into the molecular mechanisms underlying the biological effects of nerve agents.
2. Results
2.1. DFP Treatment of Female C57BL/6 Mice Elicits Robust Seizure-Like Behavior
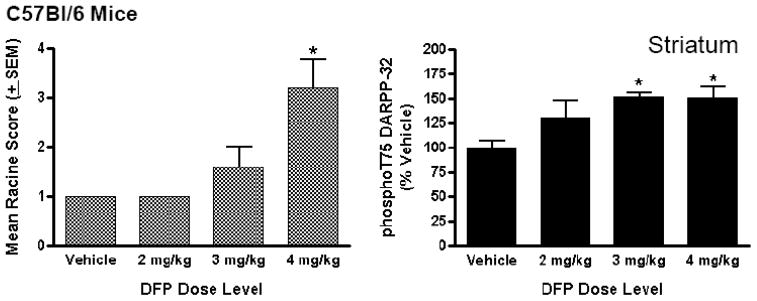
Administration of DFP to female C57BL/6 mice consistently generally induced robust seizure-like behaviors within minutes following intraperitoneal (i.p.) injection, often leading to death. The effects of DFP were dose-dependent over a narrow dose range; a 2 mg/kg dose of DFP did not elicit seizure-like behavior, whereas 3 mg/kg and 4 mg/kg doses did elicit seizure-like behavior within 5 min post-injection. The mean seizure scores for animals in each DFP treatment group are shown in Figure 1.
Figure 1. Dose-dependent effects of DFP on seizure-like behaviors and the phosphorylation state of DARPP-32 at T75 in C57BL/6 mice.
Female, adult C57BL/6 mice were injected (i.p.) with vehicle (50% DMSO) or DFP in vehicle solution at specific dose levels (2, 3, or 4 mg/kg). Mouse behavior was characterized using a Modified Racine Seizure Inventory every 3 min for 15 min and recorded. The mice were sacrificed by focused microwave irradiation of the head at 15 min post-injection and brain tissue collected for phosphorylation state analysis. (Left panel) Mean seizure ratings are shown for each treatment group (N=5 mice/treatment condition). (Right panel) Mean levels of phospho-T75 DARPP-32 in mouse striatum are shown as a function of DFP treatment. Phospho-T75 DARPP-32 and total DARPP-32 levels were detected in striatal homogenates from individual mice by immunoblotting and antibody binding revealed using dual-channel fluorescence imaging using Li-Cor Odyssey software then normalized for total levels. Data are expressed as a percent ± SEM of levels in the brains of vehicle-treated control mice. (*p<0.05 compared with vehicle-injected controls, ANOVA with Newman-Keuls post-hoc test, n=5 mice/group).
2.1.1. DFP Exposure Elevates DARPP-32 Phosphorylation Selectively at the Cdk5 Substrate, T75
DFP treatment significantly increased the state of phosphorylation of DARPP-32 in striatum of female C57BL/6 mice. The increase was detected as a selective increase at T75, a residue phosphorylated by the cyclin-dependent kinase-5 (Cdk5). DARPP-32, phosphorylated at T75, has been shown to function as an inhibitor of protein kinase A (PKA) (Bibb et al., 1999). The increase in phospho-T75 DARPP-32 (to a maximum value of 152 ± 3.8% of control) was found to be dose-dependent with respect to DFP treatment; phospho-DARPP-32 levels were significantly increased only after exposure to DFP dose levels that led to seizure-like behaviors (i.e., 3 mg/kg and 4mg/kg)(Figure 1). In contrast, phosphorylation of DARPP-32 at T34, a PKA-dependent residue that confers potency as an inhibitor of protein phosphatase-1 (PP-1) (Hemmings et al., 1984), was unaffected by DFP exposure, as was the phosphorylation state of other DARPP-32 phosphorylation sites.
2.1.2. DFP Treatment Elicits Region-Specific Effects on NMDA Receptor Phosphorylation in C57BL/6 Mice
Striatum
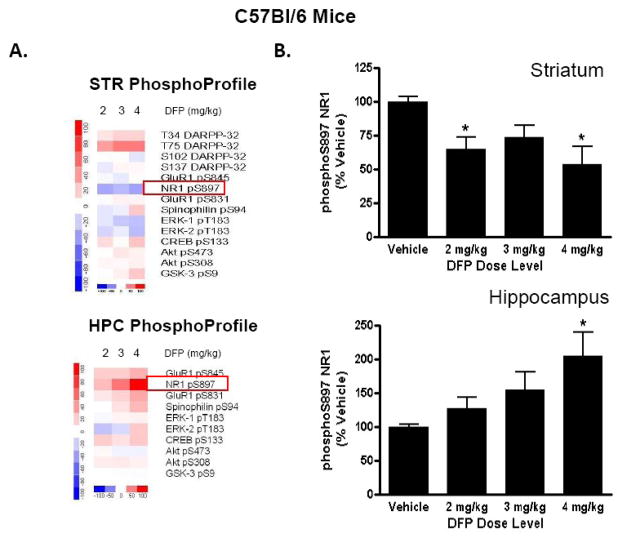
DFP exposure resulted in a reduced phosphorylation of the NMDA receptor, NR1 in striatum of female C57BL/6 mice. Levels of the receptor phosphorylated at S897, a PKA substrate, were significantly decreased (to 54 ±12% of control, respectively) in striatal homogenates from mice treated with 2 mg/kg DFP, compared with vehicle-treated mice (Figure 2). It is noteworthy that phospho-S897 levels were significantly reduced after doses of DFP (2 mg/kg) that were insufficient to elicit seizure-like behaviors; the decreases in NR1 phosphorylation levels were similar in mice treated with DFP at all three dose levels tested. In contrast to NR1 phosphorylation, effects of DFP on striatal AMPA receptor phosphorylation were modest and did not reach statistical significance. Phosphorylation of the AMPA-type glutamate receptor GluR1 was measured after DFP treatment at two C-terminal residues, S831, a CaMKII/PKC site that regulates channel conductance (Barria et al., 1997), and S845, a PKA-dependent residue that increases AMPA channel open time probability (Roche et al., 1996). AMPA receptor phosphorylation was unaffected by DFP at any dose level tested (data not shown).
Figure 2. Dose-dependent effect of DFP on the phosphorylation state of the NR1-type NMDA receptor in striatum and hippocampus of C57BL/6 mice.
Female, adult C57BL/6 mice were injected (i.p.) with vehicle (50% DMSO) or specific dose levels of DFP (2, 3, or 4 mg/kg) in DMSO vehicle, then sacrificed by focused microwave irradiation of the head 15 min later. (A) Changes in phosphorylation at a panel of phosphorylation sites comprising the CNSProfile platform were measured vehicle-treated and drug-treated mice. Levels of phosphorylation in drug-treated samples minus levels in vehicle-treated samples were plotted using dChip 1.3 software and compiled into heat map representations for striatum (top) and hippocampus (bottom). Increases in phosphorylation due to the drug treatment condition are depicted in red; decreases in phosphorylation due to drug are depicted in blue. (B) Levels of phosphorylated NR1 (S897) were detected by immunoblotting in striatum (top) and hippocampus (bottom). Levels for phosphorylated NR1 were quantitated using Li-Cor Odyssey software then normalized for total levels of the phosphoprotein of interest. Data are expressed as a percent ± SEM of levels in the brains of vehicle-treated control mice. (*p<0.05 compared with vehicle-injected controls, ANOVA with Newman-Keuls post-hoc test, n=5–11 mice/group).
Hippocampus
In striking contrast to striatum, the phosphorylation state of NR1-type NMDA receptors in the hippocampus was significantly increased (to a maximum of 205 ± 35% of control) following exposure to DFP. Levels of phospho-S897 NR1 increased in the hippocampus of mice treated with a 4 mg/kg dose level of DFP, relative to vehicle-injected control (Figure 2). The increase in NR1 phosphorylation appears to be dose-dependently related to the appearance of seizure-like behaviors, as levels were not significantly increased in mice treated with 2 mg/kg or 3mg/kg doses of DFP. As in striatum, effects on GluR1-type AMPA receptors in hippocampus, were modest and did not reach statistical significance.
DFP exposure, then, evoked opposing regulation of NMDA receptors in striatum and hippocampus of female C57BL/6 mice. The regulation of NMDA receptor phosphorylation in striatum after DFP was found to be independent of dose level, whereas hippocampal effects occurred only at dose levels of the nerve agent that elicited seizure-like behaviors. DFP exposure in female C57BL/6 mice was selective for regulation of NMDA-type, as opposed to AMPA-type glutamate receptors, as both PKA-dependent and PKC-dependent residues on the GluR1 receptors were unaltered by DFP treatment.
2.1.2.1. Region-Specific Effects of DFP on NMDA Receptor Phosphorylation in C57BL/6 Mice are mimicked by Cholinergic Agonists
In female C57BL/6 mice, significant alterations in striatal NR1 phosphorylation state were seen after exposure to dose levels of DFP (e.g., 2 mg/kg) that did not result in overt convulsive behaviors. These data suggest that the observed changes in NMDA receptor phosphorylation could occur as the result of pharmacological stimulation of cholinergic receptors, rather than as a consequence of seizure activity elicited by the nerve agent. We hypothesized that if the former interpretation was correct, then the pattern of NR1 phosphorylation changes seen after low-level DFP exposure should be mimicked by non-convulsant dose levels of other cholinergic receptor agonists. We tested the effect of a therapeutic inhibitor of AChE, donepezil, and a non-selective agonist for muscarinic cholinergic receptors, oxotremorine, for their effects on NR1 phosphorylation state after dosing to C57BL/6 mice, in vivo. As shown in Table 2, donepezil, which would be expected to increase extracellular availability of ACh, elicited a significant reduction in S897 phosphorylation in striatum of C57BL/6 mice, and a concomitant increase in S897 phosphorylation in hippocampus. Oxotremorine also increased hippocampal phospho-S897 levels; a trend toward reduced S897 phosphorylation in striatum after oxotremorine treatment failed to reach statistical significance (p=.06). These data support the idea that increased cholinergic neurotransmission in the brain, secondary to inhibition of AChE by DFP or donepezil, results in a predictable pattern of NR1 phosphorylation changes in striatum and hippocampus; these changes may be due to muscarinic receptor effects.
Table 2.
Effect of the acetylcholinesterase inhibitor, Donepezil, and the non-selective muscarinic receptor agonist, Oxotremorine, on the phosphorylation state of NR1-type NMDA receptor at S897 measured 1h after drug treatment
| Drug Treatment | STRIATUM | N | p value | HIPPOCAMPUS | N | p value | ||
|---|---|---|---|---|---|---|---|---|
| Veh | Drug | Veh | Drug | |||||
| Donepezil (5 mg/kg I.P.) | 100 ± 6.0 | 65.6 ± 6.2 | 6 | 0.0027 | 100 ± 6.1 | 149 ± 14.7 | 6 | 0.0113 |
| Oxotremorine (1 mg/kg SC) | 100 ± 3.6 | 85.9 ± 5.5 | 11 | 0.0603 | 100 ± 5.9 | 130 ± 10.0 | 11 | 0.0156 |
2.2. DFP Treatment of FVB Mice Elicits Persistent Seizure-Like Behaviors
In order to establish a model to study persistent (hrs) effects of DFP on brain signaling and behavior we sought to use a mouse strain that would be more resistant to the lethal effect of DFP. Male FVB mice were injected (i.p.) with 4 mg/kg DFP and visually monitored for up to 2 h post-injection. DFP treatment elicited seizure-like behaviors, including loss of locomotor activity, trembling, rigidity, and tail curling within 5 min of injection (Table 3). The mean seizure score of mice in the DFP-treated group increased within 15 min of treatment and was maintained throughout the 2h observation period, with visual scoring performed every 15 min during this period. Since behaviors elicited by DFP treatment were stable over the experiment, data for only the final (120 min or 2h) time point are shown in Table 3. DFP treatment also resulted in mortality (3/7 mice died) as early by 30 min after drug injection. Vehicle injected mice displayed no significant seizure-like behaviors or mortality during the 2h observation period.
Table 3.
Effect of pretreatment with muscarinic and nicotinic cholinergic ligands on Racine Seizure Score and mortality after treatment of FVB mice with DFP.
| Treatment | Racine Score | Racine Score | N | Mortality |
|---|---|---|---|---|
| 15 min | 2h | |||
| Mean ±SEM | Mean ± SEM | |||
| Vehicle | 1 | 1 | 5 | 0/5 |
| DFP (4 mg/kg) | 2.6 ± 0.42* | 3.0 ± 0.50* | 7 | 3/7 |
| DFP (4mg/kg)+PCH|| (25mg/kg) | 1.3 ± 0.18§§ | 1.1 ± 0.14§ | 7 | 0/7 |
| DFP (4mg/kg)+Varenicline (25 mg/kg)# | 2.4 ± 0.64** | 3.3 ± 0.28** | 7 | 0/7 |
| DFP (4 mg/kg) + AC-260584 (25 mg/kg)† | 1.2 ± 0.14§§ | 2.6 ± 0.48* | 7 | 0/7 |
Ki=46.49nM at muscarinic cholinergic receptors; Wang et al., (2005) Acta Pharmacologica Sinica, 26,527.
Ki=0.06nM (α4β2); 322nM (α7); J. Med Chem (2005) 38, 3774.
Ki=32 nM (muscarinic); Behavioral Neuroscience (2008), 122, 570.
p<.05 vs Vehicle;
p<.01 vs Vehicle;
p<.05 vs DFP alone,
p<.01 vs DFP alone, ANOVA with Newman-Keuls post-test.
2.2.1. Comparison of Muscarinic and Nicotinic Receptor Ligands for Attenuation of DFP-Evoked Seizure-Like Behaviors in FVB Mice
Various chemical ligands with known high affinity binding to either muscarinic or nicotinic receptor sub-classes were tested for their ability to interfere with DFP-evoked seizure-like behaviors in mice. Male FVB mice were pretreated with vehicle solution or one of the following cholinergic compounds, each at a 25 mg/kg dose level, given (via i.p. injection) 30 min prior to treatment with DFP (4 mg/kg, i.p.): phencynonate hydrochloride (PCH), a non-specific muscarinic receptor antagonist (Wang et al., 2005a) (Table 4); Varenicline, a partial agonist at α4β2 and weaker α7 nicotinic receptors (Coe et al., 2005); and AC-260584, an agonist preferential at M1-receptors with weaker activity against M4-type muscarinic receptors (Vanover et al., 2008). Mice were visually rated for seizure-like behaviors and mortality every 15 min for 2h after DFP injection. As shown in Table 3, a DFP treatment that elicited an increase in mean seizure score and animal mortality was attenuated by pretreatment with the non-selective muscarinic receptor antagonist PCH (see also Table 4). Mice pretreated with PCH prior to DFP injection all survived a 2h treatment period and displayed significantly lower average seizure scores in addition to enhanced survival. In contrast, mice pretreated with either the nicotinic partial agonist Varenicline displayed seizure-like behaviors throughout the 2h period after DFP. Mice pretreated with AC-260584, a partial allosteric modulator with partial agonist activity at M1-type muscarinic receptors, showed reduced seizure activity at 15 min, but not at 2 h, compared with mice treated with DFP alone, indicating a delayed seizure response to the nerve agent exposure. Both Varenicline and AC-260584 pretreatments appeared to attenuate mortality, as no deaths were reported in these experimental groups (Table 3).
Table 4.
Percent inhibition by PCH (racemic; 100 nM) of binding at muscarinic and nicotinic acetylcholine receptor subclasses.
| Muscarinic Receptor Subclasses % Inhibition @ 100 nM | Nicotinic Receptor Subclasses % Inhibition @ 100 nM | |||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | α4β2 | α7 |
| 99 | 94 | 93 | 89 | 94 | 1 | −1 |
2.2.2. DFP Exposure Results in Bi-Directional Regulation of NMDA Receptor Phosphorylation in Striatum and Hippocampus in FVB Mice
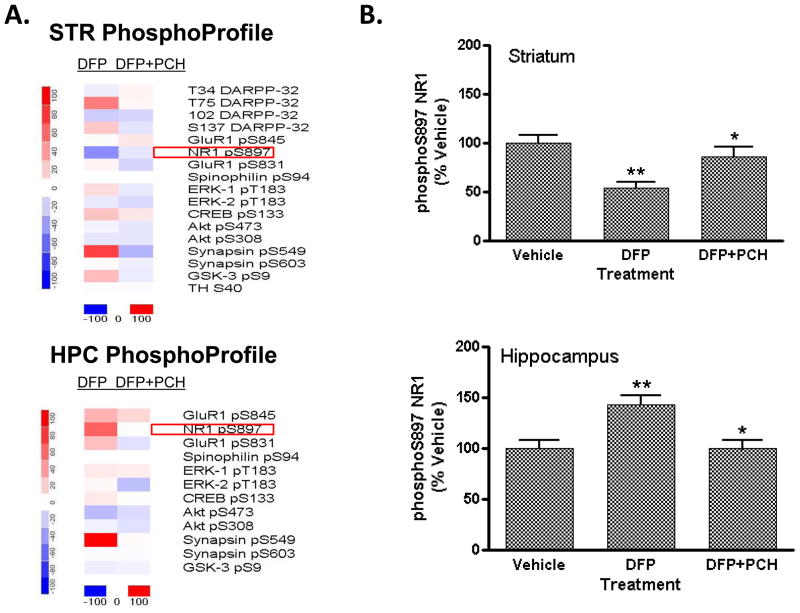
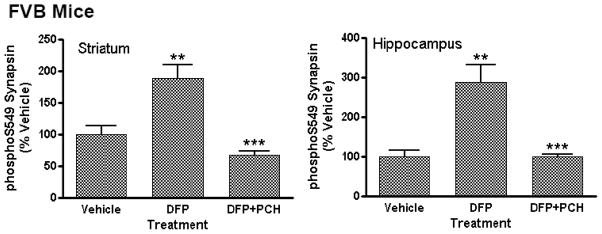
FVB mice treated with DFP displayed site-specific and regionally-restricted changes in protein phosphorylation (Figure 3) that were comparable to those seen in C57BL/6 mice receiving this treatment (Figure 1). A heat map representation shown in Figure 3A depicts the effect of specific dose levels of DFP on the state of phosphorylation of a panel of phospho-sites comprising CNSProfile. Changes in phosphorylation in both striatum (Figure 3A, top) and hippocampus (Figure 3A, bottom) are shown at 2h after DFP treatment. Notably, NR1 phosphorylation at S897 was bi-directionally regulated in striatum and hippocampus (Figure 3B); phosphoS897 levels were significantly decreased in striatum (to 56 ± 6% of control at 2h) and markedly increased in hippocampus (to 147 ± 8% of control at 2h). The effect of DFP on NR1 phosphorylation in striatum was persistent; phosphorylation of the receptor was also reduced at other time points measured (30 min, 1h; data not shown). In contrast to the rapid effects on hippocampal NR1 phosphorylation in the C57BL/6 mouse, significant changes in phospho-S897-NR1 in hippocampus of the FVB mouse were late-developing. Significant effects were only noted at 2h after DFP injection and did not reach significance at 30 min or 1h after treatment. In addition, FVB mice displayed robust changes in the phosphorylation state of synapsin I/II, presynaptic vesicle-associated proteins (Figure 3A; Figure 4). DFP treatment selectively increased phosphorylation at S549, a Cdk5 kinase-dependent residue (Matsubara et al., 1996) and not at S603, a CaMKII-dependent residue (Jovanovic et al., 2001). Phospho-S549 synapsin levels were markedly increased in both striatum and in hippocampus in response to DFP treatment (Table 5); phospho-S603 levels were unaffected by DFP exposure (data not shown) in both brain regions. The effect of DFP intoxication on synapsin phosphorylation state was mimicked by the systemic administration of pharmacological doses of both the AChE inhibitor, donepezil, and the muscarinic cholinergic agonist, oxotremorine, to C57BL/6 mice (Table 5).
Figure 3. Effect of DFP treatment on brain protein phosphorylation in FVB mice: reversal of effects on NR1 phosphorylation by the muscarinic antagonist, PCH.
Male, adult FVB mice were injected (s.c.) with vehicle solution (50% DMSO) or PCH (25 mg/kg). Thirty min later mice received either vehicle (50% DMSO) or DFP (4 mg/kg i.p.). The mice were then sacrificed by focused microwave irradiation of the head 120 min later. Levels of each phospho-site were detected in striatal and hippocampal tissue by immunoblotting and antibody binding revealed using dual-channel fluorescent detection methods (LiCor Odyssey). Levels of immunoreactivity detected for each phospho-site were normalized for total levels of the phosphoprotein. (A) Heat map representations showing changes in phosphorylation in striatum (top) and hippocampus (bottom) of mice treated with DFP alone or DFP after PCH pre-treatment. Data were plotted using dChip 1.3 software, as described in the legend to Figure 2 above. (B) Effect of DFP and PCH pretreatment on NR1 phosphorylation in mouse striatum (top) and hippocampus (bottom). Levels of S897-phosphorylated NR1 and total NR1 were detected, revealed and quantitated, as described above. Data are expressed as a percent ± SEM of levels in the striatum or hippocampus of vehicle-treated control mice. (*p<0.01 compared with DFP alone; **p<0.001 compared with vehicle-injected controls, ANOVA with Newman-Keuls post-hoc test, n=5–6 mice/group).
Figure 4. Effect of the muscarinic antagonist, PCH, on phosphorylation of Synapsin I/II on S549 detected after DFP treatment of FVB mice.
Male, adult FVB mice were injected (i.p.) with vehicle solution (50% DMSO) or a 25 mg/kg dose of PCH. Thirty min later mice received an injection (i.p.) or either a vehicle solution or a 4 mg/kg dose of DFP. The mice were then sacrificed by focused microwave irradiation of the head 120 min later. Levels of S549-phosphorylated synapsin I/II and total synapsin I/II were detected were detected by immunoblotting in striatum (left) and hippocampus (right). Antibody binding was revealed using dual-channel fluorescent detection methods. Levels for each phosphoprotein were quantitated using Li-Cor Odyssey software then normalized for total levels. Data are expressed as a percent ± SEM of levels in the striatum of vehicle-treated control mice. (**p<0.01 compared with vehicle-injected controls; ***p<.001 compared with DFP alone, ANOVA with Newman-Keuls post-hoc test, n=5–6 mice/group).
Table 5.
Comparison of the effects of DFP, the acetylcholinesterase inhibitor, Donepezil, and the non-selective muscarinic receptor agonist, Oxotremorine, on the phosphorylation state of synapsin at S549 measured 2h (DFP) or 1h (Donepezil and after drug treatment.
| Drug Treatment | STRIATUM | N | p value | HIPPOCAMPUS | N | p value | ||
|---|---|---|---|---|---|---|---|---|
| Veh | Drug(s) | Veh | Drug(s) | |||||
| DFP (4 mg/kg I.P.) | 100 ± 13 | 252 ± 46 | 5 | p<.01 | 100 ± 15 | 162 ± 20 | 5 | p<.01 |
| Donepezil (5 mg/kg I.P.) | 100 ± 6.0 | 149 ± 7.8 | 6 | p=0.0005 | 100 ± 6.1 | 113 ± 4.3 | 6 | p=0.02 |
| Oxotremorine (1 mg/kg SC) | 100 ± 9.0 | 150 ± 17 | 6 | p=0.02 | 100 ± 8.6 | 170 ± 14 | 5 | p=0.006 |
| DFP (4 mg/kg) + PCH (25mg/kg) | 100 ± 13 | 104 ± 14 | 5 | NS | 100 ± 17 | 94 ± 6.0 | 5 | NS |
| DFP (4 mg/kg) + AC-260584 (25 mg/kg) | 100 ± 17 | 314 ± 73 | 5 | p<.05 | 100 ± 11 | 185 ± 31 | 5 | p<.05 |
2.2.3. Comparison of Striatal and Hippocampal Effects of DFP in FVB Mice
As shown above, DFP exposure elicited region-specific effects on the state of phosphorylation of NMDA receptors and T75 DARPP-32 in both female C57BL/6 and male FVB mice. In addition to these sites, a panel of other neuronal protein phosphorylation sites, comprising the CNSProfile technology platform, was also monitored for regulation by DFP in striatum and hippocampus of male FVB mice 2h after DFP treatment. Some sites, such as the regulatory sites on DARPP-32, were not monitored in hippocampus, due to the low level of expression of DARPP-32 in this brain region. As shown in Table 6, these additional sites were generally not significantly altered by DFP exposure, indicating that the overall effects of DFP exposure were relatively restricted to particular phosphoproteins.
Table 6.
Effect of DFP on a panel of neuronal phosphorylation sites comprising the CNSProfile technology platform as measured in striatum and hippocampus of male FVB mice.
| Target protein | Phospho-Site | Mean Change (% Control) | SEM | Mean Change(% Control) | SEM |
|---|---|---|---|---|---|
| STRIATUM | HIPPOCAMPUS | ||||
| TH | S40 | 99.0 | 5.9 | ND | |
| CREB | S133 | 128 | 22 | 109 | 6.6 |
| ERK1 | T183 | 114 | 11 | 109 | 6.7 |
| ERK2 | T183 | 91.6 | 11 | 103 | 9.6 |
| DARPP-32 | T34 | 93.7 | 4.8 | ND | |
| S97 | 80.7 | 4.1 | ND | ||
| S130 | 122 | 26 | ND | ||
| GluR1 | S845 | 102 | 5.7 | 130 | 13 |
| S831 | 105 | 7.7 | 124 | 18 | |
| Spinophilin | S94 | ND | ND | ||
| Synapsin | S603 | 102 | 9.6 | 98.1 | 4.9 |
| GSK3β | S9 | 127 | 9.1 | 93.9 | 2.5 |
| Akt | S473 | 95.5 | 5.8 | 77.1 | 8.3 |
| T308 | 90.5 | 6.0 | 94.2 | 5.0 | |
ND=Not detected
2.3. Reversal of Effect of DFP on Phosphorylation in Striatum and Hippocampus by the Muscarinic Cholinergic Ligand, PCH, But Not the Muscarinic Agonist, AC-260584, in FVB Mice
Pre-treatment of FVB mice with a 25 mg/kg (i.p.)dose of PCH, a compound with high affinity for preferential binding to all classes of muscarinic receptors (Wang et al., 2005a), fully blocked the effects of a 4 mg/kg dose of DFP on seizure-like behaviors and all changes in protein phosphorylation in both striatum and hippocampus when measured 2h after DFP treatment. Notably, the effects of DFP on phospho-S897 NR1 levels (Figure 3) and phospho-S549 synapsin levels (Figure 4) in both striatum and hippocampus were both completely blocked in mice receiving PCH pre-treatment. In contrast, the muscarinic cholinergic agonist, AC-260584, which provided no apparent attenuation of DFP-evoked seizure-like behavior measured 2 h after DFP exposure (Table 3), did not significantly attenuate DFP-induced changes in protein phosphorylation (Table 5).
3. Discussion
Nerve agents have profound effects on brain neurotransmission, mediated via direct effects on ligand-gated receptors and voltage-gated ion channels that are controlled by muscarinic-type and nicotinic-type cholinergic receptors and by indirect modulation of multiple downstream intracellular neuronal signaling pathways (Shih & McDonough, 1997). Here, we have examined the coordinated effects of the nerve agent DFP on multiple signaling pathways in mouse hippocampus and striatum in vivo using CNSProfile to monitor the state of phosphorylation of neuronal signaling proteins. Common patterns of protein phosphorylation changes were evident after DFP treatment of two different mouse strains (C57BL/6 and FVB) with different sensitivities to the nerve agent. Because these changes correlate with onset of CNS symptoms of nerve agent toxicity they represent important signaling targets for nerve agents that will be useful for the development of more effective treatments to block or attenuate short-term and long-term nerve agent effects.
Female C57BL/6 mice displayed dose-dependent increases in seizure-like behavior in response to DFP within 5 min after nerve agent administration, often culminating in death within 20–30 min. Male FVB mice of a similar age and body weight also developed seizure-like behaviors rapidly (within 5–10 min) after DFP injections, but exhibited sustained seizure-like symptoms for several hours with lower overall lethality. In both mouse strains, DFP exposure elicited comparable site- and region-specific effects on phosphorylation of several signaling phosphoproteins in the brain that correlated with the onset of the most severe seizure-related behaviors. Phosphorylation site changes were typically observed by 15 min in the female C57BL/6 mouse brains, whereas most phosphorylation changes in the brains of male FVB were most pronounced at 2h after nerve agent exposure.
A major effect of DFP exposure in mice is the alteration of the state of phosphorylation of regulatory residues on glutamate receptors, including S897 of the NR1 NMDA receptor subunit (Tingley et al., 1997). These data are consistent with reports that nerve agents induce a sequential activation of distinct neurochemical systems in the brain resulting in a delayed recruitment of glutamatergic neurons (Shih & McDonough; 1997; Shih et al., 2003). A rapid reduction was seen in the level of NR1 phosphorylated at the S897 residue in mouse striatum at the earliest time point monitored after DFP exposure (15 min in female C57BL/6 mice and 30 min in male FVB mice). Previous work from our laboratory (Snyder et al., 1998) has shown that the phosphorylation state of S897 on NR1 in striatum is under the control of a PKA-dependent signaling cascade that is reciprocally regulated by both dopamine and glutamate neurotransmission. Phosphorylation of NR1 S897 accentuates NMDA receptor signaling, increasing gene transcription involving CREB (Dudman et al., 2003) and reducing receptor removal from the plasma membrane (Scott et al., 2003). We interpret the profound dephosphorylation of striatal S897 NR1 observed after DFP exposure as a signal subsequent to elevated glutamatergic activity which occurs as the delayed response to the nerve agent. Dephosphorylation of this site in response to glutamate overactivity could be anticipated to dampen glutamate effects by attenuating gene expression effects via CREB, and reducing receptors in the plasma membrane.
In contrast, NR1 phosphorylation in hippocampus was upregulated after DFP exposure. S897 phosphorylation was elevated by 75% in hippocampus, relative to vehicle-treated control mice. The biochemical basis for the bi-directional regulation of S897 phosphorylation in these two brain regions is unclear. The increase in NR1 phosphorylation state was delayed until 2 h after DFP treatments, compared to striatal NR1, which was significantly dephosphorylated at 30 min after DFP exposure. One plausible hypothesis for the difference in NMDA receptor phosphorylation in these two brain regions is that rapid activation of protein phosphatase-1, governed by the DARPP-32 cascade, leads to striatal dephosphorylation of NR1. Since DARPP-32 is enriched in striatal medium spiny-type neurons, but is essentially undetectable in hippocampus, this would explain the distinct hippocampal pattern (Ouimet et al., 1984) Future studies comparing NR1 phosphorylation in response to DFP in wildtype versus DARPP-32 knockout mice will be useful in testing this hypothesis. In addition to the effects of NR1 phosphorylation, increased phosphorylation of GluR1 residues is believed to enhance AMPA receptor activity (Derkach et al., 1997). A trend toward an increase in phosphorylation at S831 and S845 of the GluR1 AMPA receptor subunit (Barria et al., 1997; Derkach et al., 1997), but did not reach statistical significance.
DARPP-32 phosphorylation in striatum was selectively increased at a single regulatory site in DFP-exposed mice. Phosphorylation of DARPP-32 at T75 was significantly increased in the striatum in both C57BL/6 and FVB mice exposed to DFP. Cdk5, a member of the cyclin-dependent kinase family that exists in neurons as a neuron-specific Cdk5/p35 complex, is the key kinase phosphorylating this site. Cdk5 has been implicated as a mediator with diverse biochemical and cell biological roles in models of drug addiction, learning and memory and neurodegenerative disease (Fienberg et al., 1998; Greengard, 2001). Whether the elevation of T75 DARPP-32 phosphorylation is due to an increase in Cdk5 activity or a reduction in activity of the phosphatase (PP2A) that dephosphorylates T75, the increase in phospho-T75 DARPP-32 levels is also seen with other OP nerve agents, including sarin (i.e., G.L. Snyder, T.M. Shih, and J. McDonough, unpublished observations and G.L. Snyder and H. van Helden, unpublished observations). The induction of Cdk5-mediated phosphorylation after DFP (and sarin) represents an early potentially causative factor in long-term neuronal damage. Damage to neurons leads to calpain-induced increases in activated Cdk5, and results in tau phosphorylation reminiscent of Alzheimer’s disease pathology (Ahlijanian et al., 2000). However, DFP exposure in the present study did not lead to increased expression of well-characterized markers of neuronal damage, including the glial fibrillary acidic protein (GFAP) (J. P. O’Callaghan and D.B. Miller, unpublished observations). The observed increases in T75 DARPP-32 are apparently indicative of early-steps toward neuronal damage. Increases in T75 DARPP-32 phosphorylation levels of similar magnitude to those seen in this study have been found to result from exposure to drugs of abuse (Bibb et al., 1999; Norrholm et al., 2003), and appear to accompany a process of synaptic re-organization that occurs after drug exposure. For example, repeated exposure to cocaine, which can lead to long-lasting drug craving, also results in the remodeling of synaptic spines, a process which is dependent upon enhanced activity in Cdk5-dependent signaling pathways (Norrholm et al., 2003). In fact, excessive stimulation of NMDA receptors (such as that evoked by exposure to nerve agents) has been shown to increase brain levels of the constitutively-active Cdk5 cofactor, p25, and to result in persistent motor in-coordination and impaired learning in mice (Meyer et al., 2008). It would be tempting to speculate, then, that increased Cdk5 activity, as monitored by elevated phospho-T75-DARPP-32 levels, may be responsible for initiating synaptic changes responsible for the persistent deficits in motor behavior and cognitive performance characteristic of animals and humans surviving nerve agent-related seizures (Shih et al., 2003; Miyaki et al., 2005; Suzuki et al., 1997).
In addition to DARPP-32, exposure of mice to DFP resulted in a robust increase in phosphorylation of synapsin I, a presynaptic vesicle-associated protein, in both striatum and hippocampus. Synapsin I is enriched in nerve terminals throughout the brain (DeCamilli et al., 1983). Different functional properties of the protein appear to be mediated via distinct phosphorylation sites that are controlled by different protein kinases/protein phosphatases (Matsubara et al., 1996; Jovanovic et al., 1996; Jovanovic et al., 2001). DFP exposure selectively increased phosphorylation of synapsin I at S549, a Cdk5 substrate (Matsubara et al., 1996; Yamagata et al., 2002) that has been shown to affect interactions of the protein with the cytoskeleton mediated via F actin (Jovanovic et al., 2001) while having no effect on phosphorylation state of a CaMKII-dependent residue, S603 (Yamagata et al., 2002), which may control interactions of synapsin with synaptic vehicles. Though direct measures of Cdk5 activity were beyond the scope of the present study, the data support the idea that nerve agent exposure may promote increases in Ckd5 in multiple brain regions (e.g., striatum and hippocampus) and in both presynaptic nerve terminals and in specific post-synaptic neuron populations (e.g., striatal medium spiny neurons containing DARPP-32).
The seizure-like behaviors and protein phosphorylation changes evoked by DFP treatment are differentially affected by distinct classes of cholinergic ligands; both outcomes are substantially reversed by a non-selective muscarinic cholinergic antagonist. Pre-treatment of mice with phencynonate hydrochloride (PCH), a muscarinic cholinergic antagonist (Wang et al., 2005a) reduced seizure-like behaviors and reversed DFP-induced changes in phosphorylation. These data complement and extend a previous study reporting that PCH, at similar dose levels used in the present study, effectively reduces seizure activity in rats elicited by the nerve agent, soman (Wang et al., 2005b). The salient biochemical effects of DFP exposure, including the striatal and hippocampal changes in the phosphorylation state of S897 NR1 and S549 synapsin, are effectively mimicked in vivo by treatment of mice with pharmacological doses of an AChE inhibitor therapeutic agent used for Alzheimer’s disease, donepezil, and by a non-selective muscarinic receptor agonist, oxotremorine. The data support the idea that these phosphorylation changes reflect a cellular biochemical response to increased muscarinic receptor activation that is a secondary response to the increased synaptic levels of acetylcholine occurring after DFP exposure. Thus, increased neurotransmission via muscarinic cholinergic receptors appears sufficient to mediate many of the biochemical and behavioral effects of DFP.
The PCH data support a prominant role for muscarinic acetylcholine receptors in the induction of seizure-like behaviors and the associated biochemical changes seen after nerve agent exposure. The data are consistent with previous work that identified M1-subclass of receptors as the relevant muscarinic receptors mediating seizure activity resulting from cholinergic stimulation (Bymaster et al., 2003). We further explored the importance of M1 receptor-preferring agents by testing whether pretreatment with a high affinity muscarinic receptor allosteric modulator prior to DFP might protect against the effects of the nerve agent. AC-260584, a partial allosteric modulator with partial agonist properties, preferentially binds M1-type muscarinic cholinergic receptors at a binding site distinct from the binding site for acetylcholine (Vanover et al., 2008). A recent report by Conn and colleagues has demonstrated that allosteric regulators of M1 receptors can preferentially affect some signal transduction responses to M1 receptor stimulation, while having no effect on other M1 receptor pathways (Conn et al., 2008; Marlo et al., 2009). We reasoned that such agents might stabilize muscarinic receptor activities associated with seizure generation, and thus, preclude the behavioral and/or biochemical effects of DFP. Pretreatment of mice with AC-260584, however, failed to protect against both the appearance of seizure-like behaviors and the protein phosphorylation changes seen in DFP–treated mice, as measured 2h after DFP exposure. Interestingly, mice pretreated with AC-260584 did display an apparent delay in onset of seizure-like behavior (as measured at 15 min after DFP) and a reduced morbidity after DFP, compared with mice treated with DFP alone.
Varenicline, a potent partial agonist at the α4β2 subclass of nicotinic cholinergic receptors (Coe et al., 2005), which is thought to functionally desensitize and inactivate nicotinic receptors (many of them located on pre-synaptic nerve terminals), was used to test whether manipulation of (presynaptic) nicotinic receptors would attenuate the effects of DFP exposure. Varenicline did not prevent DFP-induced seizure-like behavior at 15 min or 2h after DFP, but, like AC-260584, did appear to reduce morbidity seen with DFP exposure. Taken together these data support the idea that muscarinic antagonism most effectively blocks both DFP-induced seizure-like behaviors and the biochemical cascades recruited by the nerve agent. However, novel pharmaceutical approaches, including the use of partial agonists that may displace acetylcholine from select receptor subclasses or desensitize select pre-synaptic or post-synaptic cholinergic receptor populations confer neuroprotection meriting further investigation.
In summary, these data reveal selective, region-specific, nerve agent-associated effects on intracellular signaling pathways and phosphoproteins that are reversed by the muscarinic receptor antagonism. The approach identifies specific targets for focused evaluation of novel anti-convulsant mechanisms.
4. Experimental Procedure
4.1. Drugs
DFP (diisopropyl fluorophosphate), oxotremorine, and donepezil were obtained from Sigma-Aldrich Chemical Co., St. Louis, MO. PCH, Varenicline, and AC-260584 were synthesized in the Department of Medicinal Chemistry at Intra-Cellular Therapies Inc. Compounds were dissolved in DMSO (50% v/v) immediately before administration to mice. Drugs were administered via intraperitoneal (i.p.) or subcutaneous (s.c.) injection to mice, in dose levels as indicated.
4.2. Treatment of Mice with DFP and Muscarinic Receptor Ligands
All animal experiments involving the use of DFP were performed at CDC-NIOSH. For experiments performed at CDC-NIOSH female C57BL/6 mice (8–10 weeks of age) or male FVB mice (8–10 weeks of age) were obtained from Jackson Labs (Bar Harbor, ME). They were group housed and maintained on ad libitum food and water under climate-controlled conditions and a 12 hr light/dark cycle (lights on at 6:00AM) in the Animal Facility of CDC-NIOSH. For experiments performed at ITI male C57BL/6 mice (7–8 weeks of age) were obtained from Jackson Labs (Bar Harbor, ME). The mice were group housed under climate-controlled conditions and on ad libitum food and water. They were maintained on a 12 hr light/dark cycle (lights on at 7:00AM) at the Animal Resource Facility of the Health Science Campus of the Columbia University Medical Center (CUMC), an Association for Assessment and Accreditation of Laboratory Animal Care (AALAC)-accredited facility. The experimental protocols used for all studies described here conform strictly to the guidelines of the Institutional Animal Use and Care Committee (IACUC) of CDC-NIOSH and of the CUMC. These protocols are in accordance with the Principles of Laboratory Animal Case as adopted and promulgated by the National Institutes of Health (Institute of Laboratory Animal Resources, 1996).
Protocol 1
In Experiment 1 female C57BL/6 mice were injected via the intraperitoneal (i.p.) route with DFP (2, 3, or 4 mg/kg in 0.9% saline at 0.1 ml/10 gm body wt.) or vehicle (0.9% saline). In the initial studies, mice were scored behaviorally for seizure-like activity every 5 min for 30 min period post-treatment. A modified Racine rating scale for seizure activity was used with the four-stage rating scale (Racine et al., 1972) used, as follows: Stage 0: Normal; Stage 1: Twitching, trembling and shivering occasionally with rigid posture & tail beginning to curl: Stage 2: Mild convulsions, occasionally; tail curling; Stage 3: Strong convulsions, spasms continuously; tail curling. A high mortality (>50%) of C57BL/6 mice was noted by the 30 min time point after DFP exposure. Since mice generally displayed seizure-like behaviors within the first 5 min after DFP injection we selected a time point at which these behaviors were sustained (i.e., 15 min) for sacrifice mice in order to profile phosphoprotein changes coincident with drug-induced behavioral effects. Groups of mice (N=5/treatment group) were treated with different dose levels of DFP or with vehicle, as described above, then killed at 15 min post-injection by focused microwave cranial irradiation, a technique which preserves in vivo levels of protein phosphorylation (O’Callaghan & Sriram, 2004). Hippocampus, cortex, and striatum were dissected from each mouse brain, frozen in liquid nitrogen, and stored at −80°C until analyzed for phosphoprotein levels.
Protocol 2
Other studies were conducted in male FVB mice, a strain that is more resistant to the lethal effects of the nerve agent. In these studies we first tested the effect of different dose levels of DFP in adult male FVB mice. Mice were injected (i.p.) with different dose levels of DFP (2–4 mg/kg) or vehicle (0.9% Saline) control. Behavior was rated for seizure-like behaviors using a modified Racine scale at specified times (15, 30, 45, 60, 75, 90, 105, and 120 min) over a 2h period post-injection. A 4 mg/kg dose of DFP was found to elicit reliable seizure-like behaviors in FVB mice (level 2–3) within 15 min after injection. Mortality (~20% by 2h) was generally lower in male FVB mice compared with the same dose level in female C57BL/6 mice (~100% mortality by 30 min). Groups of mice (N=5/treatment condition) were then injected (i.p.) with DFP (4 mg/kg) or vehicle (0.9% saline) and killed by focused cranial microwave irradiation at 30, 60, or 120 min post-injection. As described above for Protocol 1, hippocampus, cortex, and striatum were dissected from each mouse brain, frozen in liquid nitrogen, and stored at −80°C until they were analyzed for phosphoprotein levels.
Protocol 3
In these experiments we tested the ability of various muscarinic and nicotinic receptor ligands to block the behavioral and biochemical effects of DFP. The compounds tested included phencynonate hydrochloride (PCH) (a non-specific muscarinic receptor antagonist), Varenicline (a nicotinic receptor partial agonist), and AC-260584 (an M1-specific muscarinic receptor agonist). The effect of different dose levels of a 4 mg/kg (i.p.) dose of DFP was tested in adult male FVB mice. Three groups of mice (N=5 mice each) were injected with DFP (4 mg/kg) or vehicle (0.9% saline). Behavior was rated, using the modified Racine rating scale, at specified times (15, 30, 45, 60, 75, 90, 105, and 120 min) up to 2h period post-injection to estimate the appearance of a seizure-like behavioral state. An animal’s behavior was rated using a four stage rating scale according to the following criteria: Stage 1= Normal; Stage 2=Twitching, trembling, and shivering occasionally with rigid posture and tail beginning to curl; Stage 3=Mild convulsions, occasional, with tail taying up; Stage 4: Strong convulsions, spasms continuously with tail staying up. In parallel, other mice were treated identically with DFP and surviving mice killed by focused cranial microwave irradiation at 2h post-injection. Three additional groups of mice (N=5 mice each) were injected with a 25 mg/kg (s.c.) dose of PCH (in 50% DMSO), or Varenicline, or AC-260584 given 30 min prior to DFP treatment. These pretreatments were administered at 0.05 ml/10 gm body weight. In parallel, other groups of mice surviving DFP treatment were killed by focused cranial microwave irradiation at 2h post-injection. As described for Protocol 1, hippocampus, cortex, and striatum were dissected from each mouse brain, frozen in liquid nitrogen, and stored at −80°C until analyzed for phosphoprotein levels.
4.3. Sample Processing
Frozen samples of brain tissue from microwave-irradiated mouse brains were sonicated in an aliquot of boiling 1% (w/v) sodium dodecyl sulfate (SDS) and then boiled for an additional 10 min to prevent further phosphorylation or dephosphorylation. Small aliquots of the homogenate were retained for protein determination by the bicinchoninic acid (BCA) protein assay method (Pierce Chemical Co., Rockford, IL). Equal amounts of protein (15–50 μg) were processed by SDS/polyacrylamide gel electrophoresis (SDS-PAGE) using 10% polyacrylamide gels or 3–7% gradient Tris-acetate gels and immunoblotted as described below.
4.4. Quantitative immunoblot detection of specific phosphorylation sites
Protein phosphorylation changes in tissue from vehicle-treated and DFP-treated mouse brains were quantitated using CNSProfile, a western-blotting based technology platform developed by scientists at Intra-Cellular Therapies. The specific proteins and regulatory sites that were monitored in these studies are briefly characterized in Table 1. Equal amounts of solubilized protein were separated by SDS-PAGE, and electrophoretically transferred to nitrocellulose membranes (BioRad, Hercules, CA). The membranes were blocked in a 50:50 mix of Tris-buffered saline (TBS: 50 mM Tris HCl, 150 mM NaCl, pH 7.5)/1% (v/v) Tween 20 and LiCor Blocking Buffer (LiCor, Lincoln NE). Immunoblotting was carried out using phosphorylation state-specific antibodies (and respective phosphorylation state insensitive antibodies for measuring the total protein levels) raised against pT34, pT75, pS102 or pS137 of DARPP-32 and pS94 of spinophilin (ITI); pS133 of CREB (Upstate Biotechnology, Charlottesville, VA); pT183 of ERK1/2 (Promega, Madison, WI); pS897 of the NMDA receptor NR1 subunit (Upstate); pS831 or pS845 of the AMPA receptor GluR1 subunit (Upstate); S9 of the GSK3β kinase (BD Biosciences); S473 or T308 of the protein kinase Akt (BD Biosciences); S549 or S603 of the synaptic vesicle protein, synapsin I/II (Dr. Angus Nairn, RU and BD Biosciences); or pS40 of tyrosine hydroxylase (Chemicon, Temecula, CA). Membranes were washed 4 times for 5 min each with TBS/Tween 20 and antibody binding was detected using Alexa 680 labeled goat anti-mouse IgG (Molecular Probes, Eugene OR) or IRdye 800CW labeled goat anti-rabbit IgG (Rockland Immunochemicals, Gilbertsville, PA). Antibody binding was detected and quantitated using a LiCor Odyssey infrared fluorescent detection system.
Table 1.
Sites of phosphorylation monitored in brain after DFP treatment using CNSProfile.
| Target protein | Phospho-Site | Protein Class/Function of Phospho-Site | Kinase | Brain Region |
|---|---|---|---|---|
| GluR1 | S845 | AMPA receptor/positive modulator | PKA | STR/HPC |
| S831 | AMPA receptor/positive modulator | PKC/CaMKII | STR/HPC | |
| NR1 | S897 | NMDA receptor/positive modulator | PKA | STR/HPC |
| TH | S40 | Dopamine Synthesis/Essential for enzyme activity | PKA | STR |
| CREB | S133 | Gene Transcription/Activator | PKA | STR/HPC |
| ERK1/2 | T183 | Protein Kinase/Activator | MEK | STR/HPC |
| DARPP-32 | T34 | Protein Phosphatase Inhibitor/Activation | PKA/PKG | STR |
| T75 | Protein Kinase Inhibitor/Activator | Cdk5 | STR | |
| S97 | ModulatesT34 Phosphorylation | CK2 | STR | |
| S130 | ModulatesT34 Phosphorylation;Modulates nuclear transport | CK1 | STR | |
| Spinophilin | S94 | Cytoskeletal Protein/Anchoring Protein | PKA | STR/HPC |
| Synapsin | S549 | Synaptic vehicle protein/Modulates cytoskeleton interactions | Cdk5 | STR/HPC |
| S603 | Synaptic vesicle protein/ Modulates vesicle trafficking | CaMKII | STR/HPC | |
| GSK3β | S9 | Protein Kinase/Inhibits enzyme activity | Akt | STR/HPC |
| Akt | S473 | Protein Kinase/Promotes kinase activity | PKB | STR/HPC |
| T308 | Protein Kinase/Promotes kinase activity | PKB | STR/HPC |
Abbreviations: CaMKII, Ca2+/calmodulin-dependent protein kinase II; Cdk5, cyclin-dependent protein kinase 5; CK1, casein kinase 1; CK2, casein kinase 2; CREB, cAMP response element binding protein; DARPP-32, dopamine- and cAMP-regulated phosphoprotein, Mr=32 kDa; MEK, MAP/ERK kinase; NMDA, N-methyl-D-aspartate; AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid; PKA, cAMP-dependent protein kinase; PKC, protein kinase C; PP1, protein phosphatase 1; STR, striatum; HPC, hippocampus.
Tissue samples from hippocampus, cortex, and striatum were analyzed. Since effects on protein phosphorylation were observed in hippocampus and cortex were comparable, we have chosen to present only data from hippocampus and striatum here.
4.5. Determination of Binding of PCH to Muscarinic and Nicotinic Receptor Subclasses
The ability of PCH to antagonize binding to subclasses of muscarinic (M1–M5) and nicotinic cholinergic receptors was determined in radioligand binding assays conducted in recombinant cells by Caliper Life Sciences, Inc (Hopkinton, MA). Inhibition of binding by PCH was calculated by comparison of receptor binding for each receptor subclass in the presence versus the absence of PCH (100 nM). The M1–M5 assays were conducted in human recombinant/CHO cells expressing a single muscarinic receptor subclass using N-Methyl [3H] Scopolamine as radioligand at concentration of 0.5 nM. Each assay was validated using (−)-Scopolamine MeBr as reference agent. The α7 assay was conducted as a radioligand binding assay in rat PC12 cells using [125I] α-Bungarotxin as radioligand at a concentration of 0.1 nM. This assay was validated using Methllycaconitine as reference agent (Ki = 2.65 nM). The α4β2 assay was performed as a radioligand binding assay in human SK-N-F1 cells using [3H]-Epibatidine as radioligand at concentration of 0.1 nM. This assay was validated using (+/−)-Epibatidine as reference agent (Ki = 0.061 nM).
4.6. Statistical Analysis
Phosphorylation at each site detected by phospho-specific antibodies was quantified, normalized to total levels of the protein (non-phosphorylated), and expressed as percent±SEM of level of phosphorylation in vehicle-treated control mice (n=6–10). Statistical analysis was performed using Student’s paired t-test or ANOVA with Newman-Keuls post hoc test as indicated using GraphPad Prism 4.2 (GraphPad Software Inc., San Diego, CA), with p<0.05 considered significant. For presentation purposes, results from representative experiments are displayed in a heat map format generated by plotting changes in phosphorylation state for each phospho-site (drug treatment-control) using dChip 1.3 software (W. Cheng and C. Li, Harvard School of Public Health and Dana Farber Cancer Research Institute).
Acknowledgments
The excellent technical assistance of Christopher Felton and Brenda Billig is gratefully acknowledged. We thank Dr. Angus Nairn of Yale University and The Rockefeller University for providing some of the antibodies for these studies. This work was supported, in part, by funding from the NIH (R43 MH067488-01 to Intra-Cellular Therapies Inc) and the United States Army Medical Research and Materiel Command NETRP Program (DAMD 17-03-2-0019, W81XWH-05-1-0400and W81XWH-06-C-0013 to Intra-Cellular Therapies Inc).
Abbreviations
- OP
organophosphorus
- PCH
phencynonate hydrochloride
- AChE
acetylcholinesterase
- DFP
diisopropyl fluorophosphate
- Cdk5
cyclin-dependent kinase-5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA. 2000;97:2910–5. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffeth LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai L-H, Kwon YT, Girault J-A, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Protein kinase and phosphatase control by distinct phosphorylation sites within a single regulatory protein. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17:1403–10. doi: 10.1046/j.1460-9568.2003.02588.x. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelina MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FW, III, O’Neill BT. Varenicline: An a4B2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. TiPS. 2009;30:148–55. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCamilli P, Cameron R, Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein I: Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983;96:1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of a-amino-3hydroxy-5-methyl-4-isoxazoleproprionate type glutamate receptors. Proc Natl Acad Sci USA. 1999;96:3269–74. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, Kameyama K, Huganir RL, Konradi C. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem. 2003;87:922–34. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J. 1995;9:619–625. [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein P, Song W-J, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller D, Cole D, Corbett R, Haile C, Cooper D, Onn S, Grace AA, Ouimet C, White FJ, Hyman SE, Surmeier DJ, Girault JA, Nestler E, Greengard P. DARPP-32, regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Golomb B. Acetylcholinesterase inhibitors and Gulf War Illnesses. Proc Natl Acad Sci USA. 2008;105:4295–4300. doi: 10.1073/pnas.0711986105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HYL, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin 1-actin interactions. Proc Natl Acad Sci USA. 1996;93:3679–83. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC, Jr, Greengard P, Czernik AJ. Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci. 2001;21:7944–53. doi: 10.1523/JNEUROSCI.21-20-07944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallement G, Carpentier P, Collet A, Pernot-Marino I, Baubichon D, Blanchet G. Effects of soman-induced seizures on different extracellular amino acid levels and on glutamate uptake in rat hippocampus. Brain Res. 1991;563:234–40. doi: 10.1016/0006-8993(91)91539-d. [DOI] [PubMed] [Google Scholar]
- Lallement G, Carpentier A, Collet D, Baubichon D, Pernot-Marino I, Blanchet G. Extracellular acetylcholine changes in rat limbic structures during soman-induced seizures. NeuroToxicology. 1992;13:556–568. [PubMed] [Google Scholar]
- Marlo JE, Niswender CM, Days EL, Bridges TM, Xiang Y, Rodriguez AL, Shirey JK, Brady AE, Nalywajko T, Luo Q, Austin CA, Williams MB, Kim K, Williams R, Orton D, Brown HA, Lindsley CW, Weaver CW, Conn PJ. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol. 2009;75:577–588. doi: 10.1124/mol.108.052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara M, Kusubata M, Ishiguro K, Uchida T, Titani K, Taniguchi H. Site-specific phosphorylation of synapsin I by mitogen-activated protein kinase and Cdk5 and its effects on physiological functions. J Biol Chem. 1996;35:21108–21113. doi: 10.1074/jbc.271.35.21108. [DOI] [PubMed] [Google Scholar]
- Mesulam M. The cholinergic lesion of Alzheimer’s Disease: Pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Meyer DA, Richer E, Benkovic SA, Hayashi K, Kansy JW, Hale CF, Moy LY, Kim Y, O’Callaghan JP, Tsai LH, Greengard P, Nairn AC, Cowan CW, Miller DB, Antich P, Bibb JA. Striatal dysregulation of Cdk5 alters locomotor responses to cocaine, motor learning, and dendritic morphology. Proc Natl Acad Sci USA. 2008;105:18561–6. doi: 10.1073/pnas.0806078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki K, Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Yoshimura K, Etoh N, Matsumoto Y, Kikuchi Y, Kumagai N, Omae K. Effects of sarin on the nervous system of subway workers sever years after the Tokyo subway sarin attack. J Occup Health. 2005;47:299–304. doi: 10.1539/joh.47.299. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler JA, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neurosci. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki H, Hori S, Shinozawa S, Fujishima S, Takuma K, Sagoh M, Kimura H, Ohki T, Suzuki M, Aikawa N. Secondary exposure of medical staff to sarin vapor in the emergency room. Intensive Care Med. 1995;21:1032–1035. doi: 10.1007/BF01700667. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K. Focused microwave irradiation of the brain preserves in vivo protein phosphorylation: comparison with other methods of sacrifice and analysis of multiple phosphoproteins. J Neurosci Methods. 2004;135:159–68. doi: 10.1016/j.jneumeth.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillippens IH, Melchers BP, de Groot DM, Wolthuis OL. Behavioral performance, brain histology, and EEG sequela after immediate combined atropine/diazepam treatment of soman-intoxicated rats. Pharmacol Biochem Behav. 1992;42:711–9. doi: 10.1016/0091-3057(92)90019-c. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalog Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine levels in selective striatal regions promotes behavioral flexibility. Neurobiol Learn Mem. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–88. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Lasarev M, Anger WK, Scherer J, Stepfel J, McCauley L. Neurobehavioral performance of adult adolescent agricultural workers. NeuroToxicology. 2007;28:374–380. doi: 10.1016/j.neuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH., Jr Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicology Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH., Jr Neurochemical mechanisms in soman-induced seizures. J Appl Toxicol. 1997;17:255–264. doi: 10.1002/(sici)1099-1263(199707)17:4<255::aid-jat441>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/PKA/DARPP-32/protein phosphatase-1 pathway regulates dephosphorylation of the N-methyl-D-aspartate receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Kohno T, Tsukagosi M, Furuhata T, Yamazaki K. Eighteen cases exposed to sarin in Matsumoto, Japan. Intern Med. 1997;36:466–470. doi: 10.2169/internalmedicine.36.466. [DOI] [PubMed] [Google Scholar]
- Taylor P. Anticholinesterase agents. In: Gilman AG, Goodman LS, Rall TW, Murad F, editors. The Pharmacological Basis of Therapeutics. 6. Macmillan; New York: 1985. pp. 110–129. [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-Aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Veinbergs I, Davis RE. Antipsychotic-like behavioral effects and cognitive enhancement by a potent and selective muscarinic M1 receptor agonist, AC-260584. Behavioral Neuroscience. 2008;122:570–575. doi: 10.1037/0735-7044.122.3.570. [DOI] [PubMed] [Google Scholar]
- Wang LY, Wang Y, Zheng JQ, Zhong BH, Liu H, Dong SJ, Ruan JX, Liu KL. Pharmacological profiles of an anticholinergic agent, phencynonate hydrochloride and its optical isomers. Acta Pharmacol Sin. 2005a;26:527–32. doi: 10.1111/j.1745-7254.2005.00089.x. [DOI] [PubMed] [Google Scholar]
- Wang YA, Zhou WX, Li JX, Liu YQ, Yue YJ, Zheng JQ, Liu KL, Ruan JX. Anticonvulsant effects of phencynonate hydrochloride and other anticholinergic drugs in soman poisoning: neurochemical mechanisms. Life Sci. 2005b;78:210–23. doi: 10.1016/j.lfs.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Jovanovic JN, Czernik AJ, Greengard P, Obata K. Bidirectional changes in synapsin I phosphorylation at MAP kinase-dependent sites by acute neuronal excitation in vivo. J Neurochem. 2002;80:835–42. doi: 10.1046/j.0022-3042.2001.00753.x. [DOI] [PubMed] [Google Scholar]