Abstract
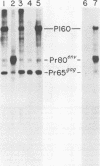
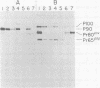
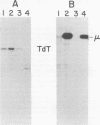
Abelson murine leukemia virus (A-MuLV) is a replication-defective virus that transforms both fibroblasts and hematopoietic cells in vitro. The virus encodes a 120,000-molecular-weight protein (P120) that is composed of Moloney murine leukemia virus-derived gag gene sequences and A-MuLV--specific sequences. This protein is the only A-MuLV--encoded protein that has been detected, and thus P120 is a candidate for the transforming protein of A-MuLV. We now report isolation and characterization of three new A-MuLV isolates that do not synthesize P120 but do produce analogous proteins of larger (160,000 molecular weight) and smaller (100,000 and 90,000 molecular weight) size. All of these A-MuLV isolates transform fibroblasts and lymphoid cells in vitro. Because the different A-MuLV proteins vary in the A-MuLV--specific region of the molecule, these variants may set a maximum limit on the size of the A-MuLV transforming protein.
Full text
PDF

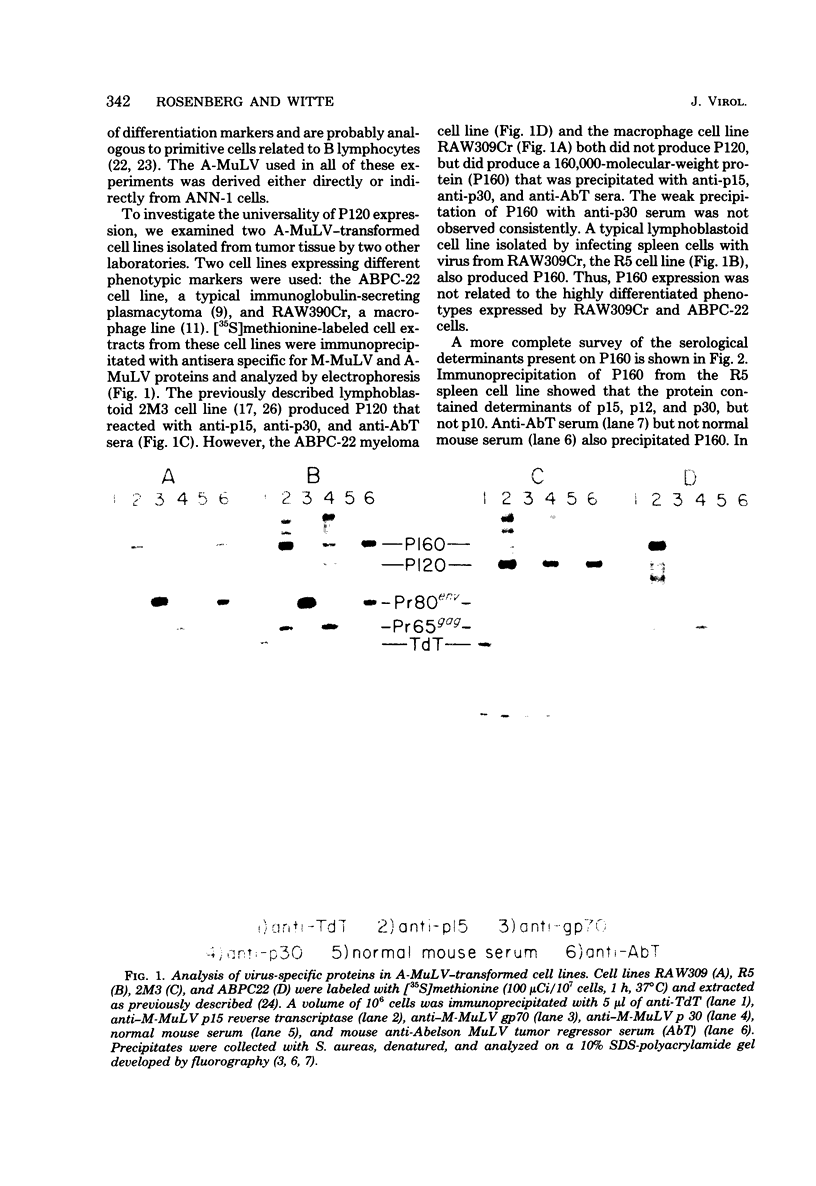





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Influence of prednisolone on Moloney leukemogenic virus in BALB-c mice. Cancer Res. 1970 Aug;30(8):2208–2212. [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boss M., Greaves M., Teich N. Abelson virus-transformed haematopoietic cell lines with pre-B-cell characteristics. Nature. 1979 Apr 5;278(5704):551–553. doi: 10.1038/278551a0. [DOI] [PubMed] [Google Scholar]
- Fan H., Paskind M. Measurement of the sequence complexity of cloned Moloney murine leukemia virus 60 to 70S RNA: evidence for a haploid genome. J Virol. 1974 Sep;14(3):421–429. doi: 10.1128/jvi.14.3.421-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Anisowicz A., Scolnick E. M. Deletion mapping of moloney type C virus: polypeptide and nucleic acid expression in different transforming virus isolates. J Virol. 1976 May;18(2):491–503. doi: 10.1128/jvi.18.2.491-503.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Sklar M. D., Rowe W. P. Rapid viral induction of plasmacytomas in pristane-primed BALB-c mice. Science. 1973 Nov 9;182(4112):592–594. doi: 10.1126/science.182.4112.592. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Megson M., Owen J. J., Cooper M. D. Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature. 1976 Jan 22;259(5540):224–226. doi: 10.1038/259224a0. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Sacks T. L., Deobagkar D. N., Stephenson J. R. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. doi: 10.1073/pnas.75.8.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. K., van de Ven W. J., Stephenson J. R. Translation of type C viral RNAs in Xenopus laevis oocytes: evidence that the 120,000-molecular-weight polyprotein expressed in Abelson leukemia virus-transformed cells is virus coded. J Virol. 1978 Nov;28(2):665–670. doi: 10.1128/jvi.28.2.665-670.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Potter M., Rowe W. P. Abelson virus-induced lymphomagenesis in mice. J Exp Med. 1978 Sep 1;148(3):714–726. doi: 10.1084/jem.148.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N. E., Baltimore D. A normal cell protein cross-reactive to the major Abelson murine leukaemia virus gene product. Nature. 1979 Oct 4;281(5730):396–398. doi: 10.1038/281396a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Baltimore D. Preparation of syngeneic tumor regressor serum reactive with the unique determinants of the Abelson murine leukemia virus-encoded P120 protein at the cell surface. J Virol. 1979 Sep;31(3):776–784. doi: 10.1128/jvi.31.3.776-784.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Wirth D. F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979 Feb;29(2):735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]