Abstract
Platelet-derived growth factor-D (PDGF-D) can regulate many cellular processes, including cell proliferation, apoptosis, transformation, migration, invasion, angiogenesis and metastasis. Therefore PDGF-D signaling has been considered to be important in human malignancies, and thus PDGF-D signaling may represent a novel therapeutic target, and as such suggests that the development of agents that will target PDGF-D signaling is likely to have a significant therapeutic impact on human cancers. This mini-review describes the mechanisms of signal transduction associated with PDGF-D signaling to support the role of PDGF-D in the carcinogenesis. Moreover, we summarize data on several PDGF-D inhibitors especially naturally occurring “chemopreventive agent” such an indole compound, which we believe could serve as a novel agent for the prevention of tumor progression and/or treatment of human malignancies by targeted inactivation of PDGF-D signaling.
Keywords: PDGF-D, cancer, signaling, therapy, nutrition
1. Introduction
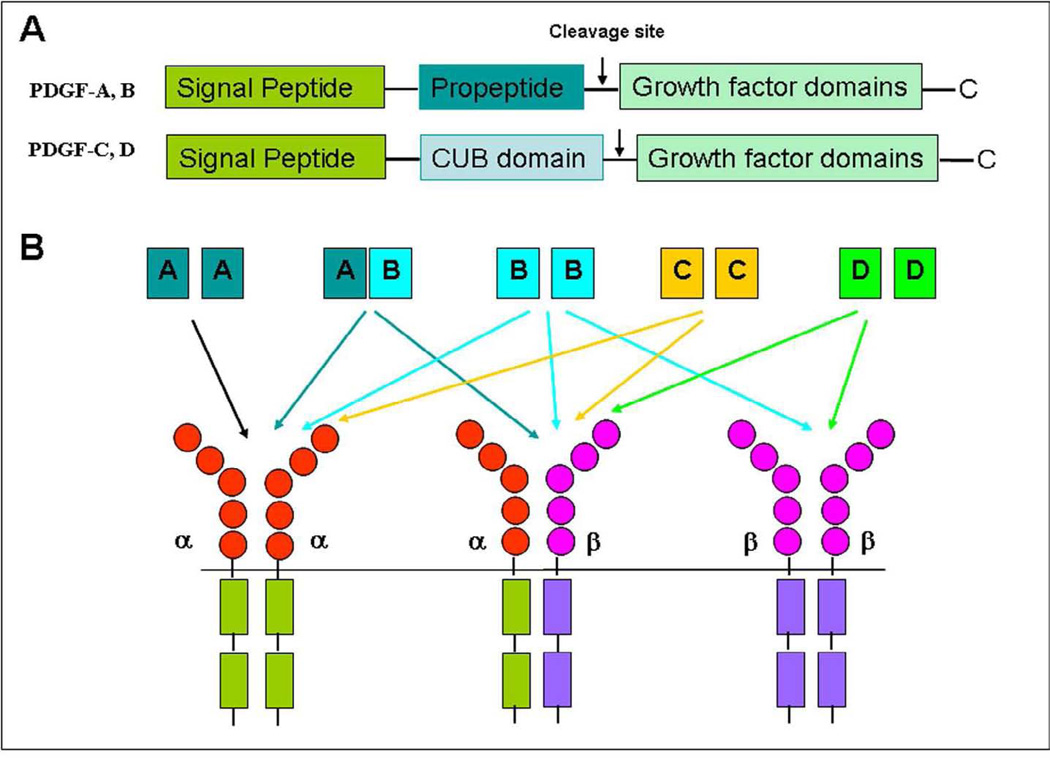
Platelet Derived Growth Factor (PDGF) signaling pathway has been extensively studied and well characterized since PDGF was first described in the 1970s as a serum factor that promoted the smooth muscle cell proliferation [1]. The PDGF family is comprised of four different polypeptide chains encoded by different genes, which have been identified: PDGF-A, PDGF-B, and recently discovered PDGF-C and PDGF-D [2–4]. PDGF need to be assembled into disulphide-bonded dimmers via homodimerization or heterodimerization in order to play their functional role. So far, four homodimers PDGF-AA, PDGF-BB, PDGF-CC and PDGF-DD, and one heterodimer, PDGF-AB have been described [5]. It is interesting to note that no heterodimers involving PDGF-C and PDGF-D chains have been described. In addition, it is notable that PDGF-A and PDGF-B are secreted in their active forms, while PDGF-C and PDGF-D are secreted as inactive forms requiring activation for their function. These differences could be due to their subtle structural differences although the PDGF have a common structure with typical growth factor domain involved in the dimerization of the two subunits, and in terms of their receptor binding and activation. Interestingly, the PDGF show high sequence identity with the vascular endothelial growth factor (VEGF), which may suggest their similar but distinct biological functions. Therefore, PDGF family is sometimes referred to as PDGF/VEGF family [1]. PDGF-A and PDGF-B mainly encode the growth factor domain and have short N-terminal extensions that undergo intracellular proteolytic processing for activation, while both PDGF-C and PDGF-D chains encode a unique two-domain structure with an N-terminal ‘Clr / Cls, urchin endothelial growth factor-like (UEGF) protein and bone morphogenic protein 1’ (CUB) domain, as part of their N-terminal extensions, in addition to the C-terminal growth factor domain. The basic domain structure of PDGF family members is provided in Figure 1.
Figure-1.
A; Schematic drawing of the four PDGF proteins (PDGF-A, B, C, D). B; Demonstrates the PDGF-PDGFR interactions.
Several reports have shown that the CUB domains of PDGF-D have to be cleaved extracellularly to make the COOH-terminal growth factor domain active for PDGF-D binding to its receptor. PDGFs exert their cellular effects by activating two structurally related receptor tyrosine kinases of the PDGF (PDGFR), PDGFR-α and PDGFR-β. The PDGF-AA activates PDGFRα, whereas PDGF-BB activates PDGFR-α, PDGFR-α/β and PDGFR-β. PDGF-AB and PDGF-CC activate PDGFR-α and PDGFR-α/β, while PDGF-DD specifically binds to and activates its cognate receptor PDGFR-β (Fig-1). The physiological relevance of the ability of PDGFs to activate PDGFR heterodimers is unclear at present [1]. The phosphorylation of PDGF receptor triggers a number of downstream signaling pathways including activation of phosphatidylinositol 3 kinase (PI3K), Akt, nuclear factor-κB (NF-κB), Notch, extracellular signal-regulated kinase (ERK), etc [5–8]. Although all four PDGF ligands play their oncogenic roles through two PDGFRs, they could promote carcinogenesis through different targets. Without doubt further investigations are needed to elucidate how PDGF ligands active different PDGFR downstream genes.
2. PDGF-D, the latest member of PDGF family, has unique functions
As mentioned above, all the PDGF isoforms elicits their biological functions through a total of two receptors. While this leads to overlapping cellular effects in some cases, it is increasingly being realized that different PDGFs exhibit mutually exclusive physiological effects as well [9]. Thus, PDGF signaling is a complex pathway, more so because of the addition of latest members, PDGF-C and PDGF-D [10]. Recognition of these new factors has, inspite of adding complexity to overall understanding of PDGF signaling, helped explain some key developmental processes, which shed more light on the overall inter-play and regulation of these growth factors. For example, in transdifferentiating hepatic stellate cells, different PDGF isoforms have been shown to be expressed at different stages [11]. PDGF-B is expressed predominantly at initial stages, whereas PDGF-D takes over at transitional stage while the levels of PDGF-C are high at later stages. This indicates that while different PDGFs might contribute to the same physiological process, each PDGF has a different and distinct role.
The report by Lokker et al. is a good example of how perception of PDGF signaling has changed with the discovery of PDGF-D [12]. This study was focused on glioblastoma mutiforme, a particularly aggressive brain tumor, where the median survival time is just 9–12 months. Before this study, PDGF-B was believed to be a crucial factor involved in growth of such brain tumors primarily because it was the only PDGF family member known to function through PDGFR-β [13]. This notion, however, changed with the detailed characterization of PDGF-D, along with other PDGFs, and the two receptors, studied in 11 glioma cell lines [12]. PDGF-D was found to be expressed in 10 of the 11 cell lines and PDGFR-β in 9 of 11 cell lines whereas PDGF-B was expressed in only 5 of the 11 cell lines. It was thus realized that PDGF-D and PDGFR-β might be the player of a novel autocrine loop that does not involve PDGF-B. These results suggest that PDGF-D can activate PDGFR-β and influence the aggressiveness of tumors without the involvement of classical PDGFR-β ligand PDGF-B.
In an earlier study [14], PDGF-D mRNA transcript was detected in many ovarian, lung, renal and brain cancer-derived cell lines. Further, a direct comparison between PDGF-B, PDGF-D and PDGFR-β in central nervous system-derived cancer cell lines in this study revealed that PDGF-D is expressed in 10 of 17 cell lines, PDGF-B in only 7 and PDGFR-β can be detected in 9 of those 17 cell lines. A total of five cell lines expressed only PDGF-D while there were only two cell lines that expressed only PDGF-B. Interestingly, PDGFR-β was detected in all of these 7 cell lines. These observations seem to suggest that a) PDGF-D might be a better molecular marker for aggressiveness than PDGF-B, and b) PDGF-D signaling can function independent of PDGF-B even though both activate PDGFR-β. Clearly, more investigations are needed to spell out the differences between PDGF-B and PDGF-D signaling pathways.
Based on these few preliminary reports, a consensus seem to emerge which suggest that PDGF-D might play a definitive role in human cancers through independent regulation of cellular signaling pathways. The other PDGF family members, particularly PDGF-A and PDGF-B, have been studied and reviewed in considerable detail [15–24] and because of that reason, we specifically focused our discussion on PDGF-D, the latest member of this family, for thus review article. Therefore, in the subsequent paragraphs we are presenting the biological functions of PDGF-D in human malignancies.
3. The role of PDGF-D in cancer
Since PDGF-A and PDGF-B has been well documented and characterized; however their roles in human cancers are questionable, thus we will not discuss their roles in cancer progression in this article. In spite of the discovery of PDGF-D over 10 years ago, the role of PDGF-D is just beginning to be understood. The growing body of literature strongly suggests that PDGF-D may function as a key player in the development and progression of human cancers by regulating the processes of cell proliferation, apoptosis, migration, invasion, angiogenesis, and metastasis. It has been reported that PDGF-D signaling is frequently deregulated in human malignancies with up-regulated expression of PDGF-D was found in prostate, lung, renal, ovarian, brain, and pancreatic cancer [7;8;12;25–27]. Ustach et al found that human prostate carcinoma LNCaP cells are capable of processing full-length PDGF-D into a biologically active PDGF form which binds and activate its cognate PDGFR-β. Moreover, PDGF-D expression greatly accelerates the tumor growth and enhances prostate carcinoma cell interaction with the surrounding stromal layers in a severe combined immunodeficient (SCID) mouse model, suggesting the potential oncogenic activity of PDGF-D in human prostate cancer progression [25]. Pancreatic Cancer like many other tumors has been shown to over-express the PDGF-D. We found that PDGF-D is highly expressed in human pancreatic adenocarcinoma specimens, in chronic pancreatitis associated with pancreatic adenocarcinoma, and in different human pancreatic cancer cell lines, suggesting that PDGF-D could be important in human pancreatic cancer progression [8]. Moreover, Xu et al reported that PDGF-D over-production in renal cancer SN12-C cells increased the proliferation and migration of cells in vitro and improved perivascular cell coverage in vivo [27]. Furthermore, blocking PDGF-D/PDGFR signaling inhibited survival and mitogenic pathways in the glioblastoma cell lines and prevented glioma formation in a nude mouse xenograft model [12].
The molecular mechanism(s) by which PDGF-D signaling induces tumor growth has not been fully elucidated. However, multiple oncogenic pathways, such as urokinase-type plasminogen activator (uPA), reactive oxygen species (ROS), proinflammatory cytokine interleukin-1β (IL-1β), phosphatidylinositol 3-kinase (PI3K)/Akt, NF-κB, Notch, ERK, mammalian target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK), vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), Cyclin D1, and Bcl-2 signaling have been reported to cross-talk with PDGF-D pathway, and thus it is believed that the cross-talk between PDGF-D and other signaling pathways plays important roles in tumor aggressiveness. Here, we will discuss the recent advances in our understanding on the role of PDGF-D in tumor progression. In this review, we will summarize the results of emerging studies on the PDGF-D signaling pathway, including the upstream regulators and the downstream effectors of the PDGF-D pathway, as well as its implication in human cancers. In addition, we sincerely apologize to those authors whose work could not be cited in this article because of space limitation.
4. Upstream regulators of PDGF-D
In recent years, studies on PDGF-D and cancer have burst onto the scene; however, the upstream regulators of PDGF-D in human cancer progression are largely unknown. The promoters for PDGF-A, PDGF-B as well as PDGF-C have been studied and our understanding of the mechanism of gene regulation of these three PDGFs is much more robust than that of PDGF-D [9]. Khachigian and colleagues have reported gene promoter studies for all the PDGFs. Their studies with PDGF-A and PDGF-C revealed the involvement of transcription factors Egr1 and Sp1 [28;29]. Egr1 was also found to interact with a novel element in PDGF-B promoter [30]. The investigations of this research group on the latest PDGF member, PDGF-D have identified Ets-1 and Sp1 as the transcription factors that regulates the expression of PDGF-D [31–33]. Clearly there has been a renewed interest to fully understand the gene regulation of PDGF-D. Also, available data points to differential regulation of PDGF-D gene compared to the other PDGF isoforms, thus pointing to the unique identity of PDGF-D among this family of growth factors.
In addition to transcription factors Ets-1 and Sp1, other factors such as uPA, H2O2, and IL-1β have also been reported to regulate the expression of PDGF-D signaling through different mechanisms [26;31;33]. H2O2 activates PDGF-D transcription and translation, whereas uPA is capable of processing recombinant latent PDGF-D into the active form through removal of the CUB domain. IL-1β suppresses PDGF-D promoter activity and mRNA and protein expression. The mechanisms by which these three upstream genes regulate PDGF-D are discussed in the following paragraphs.
4.1 Urokinase plasminogen activator (uPA) and its role in PDGF-D signaling
The urokinase plasminogen activator (uPA) system is a serine protease family comprising of urokinase-type plasminogen activator (uPA), plasminogen activator inhibitors (PAI’s), tissue-type plasminogen activator (tPA) and the uPA receptor (uPAR) [34]. It is well known that urokinase plasminogen system plays important roles in cell migration, angiogenesis, invasion and metastasis, and thus the spread of primary tumors to distant organs is in part associated uPA system, which correlate with poor prognosis, resulting in high mortality [35]. It has been reported that PDGF-D is activated by uPA [26]. We have indicated earlier that PDGF- DD is secreted as full-length, latent dimers, and the proteolytic cleavage of the CUB domain is required for the COOH-terminal growth factor domain to activate the PDGF receptor. Ustach et al found that uPA is capable of processing recombinant latent PDGF-D into the active form through removal of the CUB domain. The uPA cleavage site resides at the R247/R249 within the hinge region between the CUB and the growth factor domains. Interestingly, closely related protease tPA did not activate the PDGF-D [26]. Prostate cancer cells PC-3 and LNCaP can auto-activate latent full-length PDGF-D into its biologically active form under serum-independent conditions. However, this auto-activation is inhibited by PAI-1, an uPA/tPA inhibitor, and the serine protease inhibitor aprotinin. Very interestingly, uPA activates PDGF-D, which in turn regulates uPA expression and activity [26]. This evidence suggests that there is a direct link between uPA and PDGF-D-mediated cell signaling; however, the molecular mechanism of this feedback signaling loop is still unclear.
4.2 The role of reactive oxygen species (ROS) in PDGF-D signaling
Reactive oxygen species (ROS), continuously generated from mitochondrial respiratory chain, includes hydrogen peroxide (H2O2), superoxide radical (O2−), hydroxyl radical (.OH) and singlet oxygen.[36] In eukaryotic cells, the most significant intracellular sources of ROS are the mitochondrial respiratory chain, microsomal cytochrome P450 enzymes, flavoprotein oxidases, and peroxisomal fatty acid metabolism. Mammalian cells possesses an efficient antioxidant defense system, such as glutathione peroxidase (GPx), superoxide dismutase (SOD), glutathione S-transferase (GST), catalase and peroxidases, and glutathione (GSH), which can scavenge the excessive ROS produced through cellular metabolism, and thus these antioxidant mechanisms when functioning correctly will make ROS level relatively stable under physiological conditions in order to maintain cellular homeostasis [37]. However, inefficient antioxidant mechanisms and/or over-production of ROS will lead to chronic diseases. It has been documented that when the intracellular homeostatic mechanism fails then the overproduced ROS could cause cellular oxidative stress, where DNA, lipids, proteins and other cellular components are oxidatively damaged. DNA damage induced by ROS is sufficient to convert normal cells to malignant cells [36]; therefore, aberrant ROS signaling has profound consequence in inducing human malignancies.
It has been reported that ROS is a signaling stimuli that is critical in mediating several cellular functions including tissue homeostasis and adaptation. A specific role of ROS in PDGF signaling has been suggested documenting that increased levels of ROS could lead to tyrosine phosphorylation of many proteins including the PDGF receptors [38;39]. PDGFR-β has been suggested to influence proliferation and migration [40] and chemotaxis [41] through an ROS-dependent pathway. Among the PDGF family, the involvement of ROS has been shown to influence PDGF-A, PDGF-B, and PDGF-D signaling pathways [31]. Liu et al. found that basal and inducible PDGF-D transcription is regulated by the winged helix-turn-helix factor and prototypic Ets family member, Ets-1 present in the PDGF-D promoter. Moreover, they found that H2O2 activates PDGF-D transcription at nanomolar concentration, and H2O2 also stimulates Ets-1 expression thereby linking ROS, regulation of Ets-1 and the regulation of PDGF-D transcription. Moreover, these authors have also demonstrated that angiotensin II, a well known factor that stimulate the production of ROS from NAPDH oxidase, could induce Ets-1 and PDGF-D expression via the endogenous generation of H2O2 [31], suggesting the importance of ROS in the regulation of Ets-1 and PDGF-D. Emerging evidence suggests that activation of PDGF receptors can be accomplished by factors other than PDGFs as well [1] and ROS seem to be crucial for this phenomenon [39]. Further research toward exploration of the molecular mechanisms by which ROS regulates PDGF-D requires in-depth investigations.
4.3 The role of interleukin-1β in the regulation of PDGF-D signaling
A growing body of evidence has shown that numerous cytokine polymorphisms are associated with increased risk of inflammatory diseases and cancer. Key pro-inflammatory cytokines include IL-1, −6, −8, −12 and −18, TNF-α and macrophage MIF (migration inhibitory factor). Anti-inflammatory cytokines include IL-4 and −10, IFN (interferon)-α and -β [42]. These cytokines have been linked to many immune reactions, including the recruitment of inflammatory cells to the site of infection. Moreover, the pro-inflammatory cytokines are known to activate critical transcription factors such as NF-κB, AP-1, STAT1 and STAT3, all of which have been implicated in inflammation mediated development of tumors depending on cell types [42]. In pancreatic cancer, autocrine production of IL-1β have been reported to promote growth and conferring chemo-resistance to conventional therapeutic agents. Recently, the cross-talk between IL-1β and PDGF-D has been demonstrated. Li et al reported that IL-1β could abolish the cell migration and proliferation induced by PDGF-D [43]. Moreover, Liu et al. reported that IL-1β suppresses PDGF-D promoter activity and mRNA and protein expression in a time- and dose-dependent manner [33]. IL-1β induced NF-κB p65 and interferon regulatory factor-1 (IRF-1) have been reported to bind to different elements in the PDGF-D promoter, leading to the inhibition of PDGF-D transcription. Furthermore, PDGF-D repression by IL-1β was reported to be mediated via histone deacetylation and interaction of histone deacetylase (HDAC)-1 with IRF-1 and p65 [33], suggesting that IL-1β inhibition of PDGF-D expression through IRF-1/p65/HDAC-1 may represent a negative regulatory mechanism, which could be a novel target for the inactivation of PDGF-D signaling.
5. Downstream effectors of PDGF-D signaling
PDGF-D regulates many cellular processes, including cell proliferation, transformation, invasion, and angiogenesis by specifically binding to and activating its cognate receptor PDGFR-β. Specifically, PDGF-D interacts with PDGFR-β and activates multiple downstream oncogenic pathways, resulting in tumor development and progression. For example, PDGF-D promotes cancer cell survival and growth through PI3K/Akt, mTOR, NF-κB, ERK, MAPK, and Notch pathway. PDGF-D increases cancer cell invasion, angiogenesis, and metastasis via upregulation of VEGF, MMP, miRNA, E-cadherin, and snail expression. Here, we discuss the recent advances in the understanding on the role of PDGF-D in tumor progression.
5.1 Regulation of cell survival and growth
5.1.1 Phosphatidylinositol 3-kinase (PI3K)/Akt signaling and its cross-talks with PDGF-D signaling
PDGF-D has been reported to cross-talk with PI3K/Akt, which is an evolutionarily conserved serine/threonine kinase [7;44]. It is well accepted that cross-talk means that one signal will affect another neighboring signal pathway. Akt (also known as protein kinase B) is one of the major cell growth and apoptosis regulatory pathways. There are three isoforms of Akt such as Akt 1, Akt 2 and Akt 3, which are encoded by the genes PKBα, PKBβ and PKBγ in mammals, respectively. Akt is activated by phosphorylation at Thr308 by 3-phosphoinositide-dependent protein kinase 1 (PDK1), and also by phosphorylation within the C-terminus at Ser473 by PDK2 [45]. PI3K activates Akt, which transmits signals from cytokines, growth factors, and oncoproteins to multiple targets. Activated Akt could promote cell survival by inhibiting apoptosis through inactivation of several pro-apoptotic factors including Bcl-xL/Bcl-2-Associated Death (BAD), Forkhead transcription factors and caspase-9 [45].
Recently, PDGF-D has been shown to cross-talk with the PI3K/Akt pathway. It has been reported that the PDGF signaling pathways could be evaluated by detection of Tyr residue autophosphorylation of PDGF-activated PDGFR, and phospho-Akt of PI3K after PDGF-D treatment. The results suggest that PDGF-D is important to induce PDGFR-β autophosphorylation, and phosphorylation of Akt [44]. Ammoun et al. also reported that PDGF-D activated PDGFR-β and pAkt in human Schwannoma cells [46]. Blocking PDGFR signaling using CT52923, a potent selective small molecule piperazinyl quinazoline kinase inhibitor of the PDGFR, also inhibited the phospho-Akt in glioblastoma cell lines [12]. Interestingly, we found that the total Akt expression was up-regulated in PDGF-D over-expressing PC3 (PC-3 PDGF-D) cells while p-Akt was reduced in PC-3 PDGF-D cells. Moreover, LY294002, a PI3K inhibitor, reversed the rapamycin (a mTOR inhibitor)-induced activation of Akt in PC3 PDGF-D cells, suggesting that prolonged exposure of cells to PDGF-D activates the mTOR pathway, which, in turn, represses Akt activity in PC3 PDGF-D cells through a PI3K-dependent manner [7]. These results suggest that prolonged exposure of cells to PDGF-D leads to hyperactivation of mTOR, which is responsible for inactivation of Akt [7]. Further research toward exploration of the molecular mechanisms by which PDGF-D regulates PI3K/Akt requires immediate attention.
5.1.2 Mammalian target of rapamycin signaling and its role in the regulation of PDGF-D
The mTOR pathway has been reported to cross-talk with the PDGF-D pathway [7]. mTOR regulates translation rates and cell proliferation mainly by phosphorylating two major targets, the ribosomal protein S6 kinases (S6K1 and S6K2) and the eukaryotic translation initiation factor 4E (eIF4E)–binding protein 1 (4E-BP1). S6K1 directly phosphorylates the 40S ribosomal protein S6, and then promotes ribosome biogenesis. 4E-BP1 is released from eIF4E after phosphorylation, allowing eIF4E to assemble with other translation initiation factors to initiate cap-dependent translation [45]. mTOR exists in two distinct complexes (mTORC1 and mTORC2): mTORC1 consists of mTOR, G-protein β-subunit-like protein (GβL), raptor and proline-rich Akt substrate of 40 kilodaltons (PRAS40) protein, whereas mTORC2 contains mTOR, GβL, rictor and stress-activated protein kinase interacting protein 1 (SIN1). The raptor-containing complex is sensitive to rapamycin and regulates cell proliferation through phosphorylating S6K and 4E-BP1, while the rictor-containing complex is not sensitive to rapamycin [47].
Recently, the mTOR protein kinase has emerged as a critical player for controlling many cellular processes, such as cell growth and migration, by receiving stimulatory signals from PDGF-D. We have found that prostate cancer PC3 cells transfected with PDGF-D exhibit a rapid growth rate and increased invasion in vitro, which were associated with a high level of mTOR activity [7]. Specifically, PDGF-D markedly increased the levels of p-mTOR, p-4E-BP1, and p-S6K in prostate cancer cells. Moreover, we found that rapamycin increased phosphorylation of Akt in PC3 PDGF-D cells [7], suggesting that inactivation of Akt is attributed to a negative feedback regulation mediated by the mTOR pathway. However, the molecular mechanism(s) by which PDGF-D may be involved in the regulation of mTOR pathway or vice versa remains to be elucidated.
5.1.3 Nuclear factor-κB signaling and its role in PDGF-D signaling
Recently, several studies have shown that PDGF-D regulates the NF-κB pathway [8]. NF-κB plays important roles in the control of cell growth, differentiation, apoptosis and stress response. Without stimulation, NF-κB is sequestered in the cytoplasm through tight association with the specific inhibitory proteins IκB. Many stimuli can activate NF-κB, which leads to IKK (IκB kinase)-dependent phosphorylation and subsequent proteasome-mediated degradation of IκB proteins. Activated NF-κB, migrates into the nucleus and binds to the NF-κB-specific DNA-binding sites or interact with other transcription factors, and thus regulates gene transcription, including cytokines, chemokines, and anti-apoptotic factors [48]. A key regulatory step in the NF-κB pathway is the activation of IKK complex in which catalysis is thought to be via kinases, including IKKα and IKKβ, which directly phosphorylate IκB proteins. It has been reported that the interplay between the NF-κB and PDGF-D is biologically important. For example, we found that over-expression of PDGF-D in prostate cancer cells increased NF-κB DNA-binding activity [6]. Moreover, studies from our lab also showed that the down-regulation of PDGF-D in pancreatic cancer cells leads to the inactivation of NF-κB DNA-binding activity and, in turn, down-regulates the expression of its target genes, such as MMP-9 and VEGF [8]. Recently, we also found that down-regulation of PDGF-D in breast cancer cells inhibited the NF-κB DNA-binding activity (unpublished data). Further in-depth studies are needed to ascertain the precise molecular regulation of PDGF-D and NF-κB, and their cross-talks for elucidating the role of PDGF-D in cell growth, invasion and angiogenesis of cancer cells.
5.1.4 Notch signaling and its cross-talks with PDGF-D signaling
To date, in mammals, the Notch family of trans-membrane receptors consists of four members: Notch-1-4. Mammals also express Notch ligands and five such members have been found: Dll-1 (Delta-like 1), Dll-3 (Delta-like 3), Dll-4 (Delta-like 4), Jagged-1 and Jagged-2 [49]. Although these four Notch receptors show subtle differences in their extracellular and cytoplasmic domains, they are very similar. The extracellular domain of Notch possesses EGF-like repeats, which participate in ligand binding. The amino-terminal EGF-like repeats are followed by cysteine-rich Notch Lin12 repeats (N/Lin12) that prevent signaling in the absence of the ligand. The cytoplasmic region of Notch conveys the signal to the nucleus; it contains a RBP-J (Recombination Signal-Binding Protein 1 for J-kappa)–association molecule (RAM) domain, ankyrin repeats, TAD (trans-activation domain), NLS (nuclear localization signals) and a region rich in PEST (proline, glutamine, serine and threonine residues) sequence [50–52]. Notch signaling is initiated when Notch ligand binds to an adjacent Notch receptor between two neighboring cells. Upon activation, Notch is cleaved, releasing the intracellular domain of the Notch (ICN) which occurs through a cascade of proteolytic cleavages by the metalloprotease tumor necrosis factor-α-converting enzyme (TACE) and γ-secretase complex (comprised of presenilin-1/2, nicastrin, Pen-2, and Aph-1), resulting in the translocation of ICN into the nucleus for transcriptional activation of Notch target genes [53].
The growing body of literature strongly suggests the cross-talk between PDGF-D signaling and Notch pathway in cancer. We found that down-regulation of PDGF-D leads to the inactivation of Notch-1 in pancreatic cancer cells [8]. Therefore, the inactivation of PDGF-D– mediated cell invasion and angiogenesis could in part be due to inactivation of Notch-1 activity [8]. Moreover, we found that down-regulation of PDGF-D inhibited the Notch-1 expression in breast cancer cells (unpublished data). Recently, we reported that PDGF-D signaling contributes to epithelial to mesenchymal (EMT) phenotype which regulates cancer cell invasion and angiogenesis [6]. We also found that the expression of both mRNA and protein levels of Notch 1-4, Dll-1, Dll-3, Dll-4, Jagged-2 as well as Notch downstream targets, such as Hes and Hey, were significantly higher in PC3 PDGF-D cells (unpublished data). More importantly, we found that Notch-1 could be one of many miR-200b targets because over-expression of miR-200b significantly inhibited Notch-1 expression (unpublished data). However, how the miR-200b regulates Notch gene expression will certainly require further in-depth investigations.
5.1.5 Mitogen-activated protein kinase (MAPK) in relation to PDGF-D signaling
The MAPK has been known to play critical roles in controlling the balance between cell survival and apoptosis. A variety of cellular stimuli can activate the MAPK cascade, leading to the regulation of cell growth and apoptosis. It has been reported that MAPK pathway consists of three-tiered kinase core where a MAP3K activates MAP2K that in turn activates MAPK, resulting in the regulation of cell growth, and cell survival [54]. It has been well documented that activation of MAPK are also linked with cancer invasion, angiogenesis, and metastasis. Recent evidences suggest that PDGF-D is an effector of MAPK signaling. PDGF-D induced PDGFR-β autophosphorylation, and phosphorylation of JNK, p38 MAPK [44]. Blocking PDGFR signaling using PDGFR inhibitor CT52923 also inhibited the phospho-MAPK in glioblastoma cell lines [12]. However, more studies are required to understand how PDGF-D regulates MAPK signaling pathway in human malignancies.
5.1.6 Extracellular signal-regulated kinase (ERK) and its role in PDGF-D signaling
ERK activities were found to be up-regulated in many human tumors, and higher activity in tumors was associated with a poor prognosis, suggesting the crucial role of ERK in tumor progression [55]. ERK family has ERK1 and ERK2, which are members of the MAPK super family that can mediate cell proliferation and could regulate apoptosis. It has been well documented that multiple phosphatases (such as MAPK phosphatases) inactivate ERKs, suggesting that the duration and extent of ERK activation is tightly controlled by maintaining the balanced activities of (MAPK/ERK) kinase MEKs and respective phosphatases [56]. Recently, it has been found that PDGF-D can activate the ERK, suggesting that ERK is a major PDGF-D effector [44;46]. Ammoun et al. reported that PDGF-D activates PDGFR-β and ERK1/2 in human Schwannoma cells. PDGF-D promoted schwannoma cell proliferation, while inhibition of MAPK/ERK kinase 1/2 (MEK1/2) decreased PDGF-D-mediated proliferation, suggesting that ERK1/2 pathway is involved in this process [46]. Very interestingly, PDGF-D has different pathways to activate ERK1/2 that is localized to different intracellular compartments. The p-ERK1/2 pathway at 42/44 kDa (cytosol) uses PI3K-PKC-Src-c-Raf–dependent pathway and cross-talk with Akt at the level of PI3K, whereas PDGF-DD–mediated ERK1/2 activation at the 300-kDa level engages c-Raf, PKC, src, and PAK and localizes to the different cellular compartments than active ERK1/2 42/44 kDa [46]. These results suggest cooperation between PI3K, PKC, and Src in PDGF-D-mediated ERK1/2 activation. These results also suggest that a combined therapy targeting different pathways including PDGF-D pathway might be appropriate for treating schwannoma. The results from our laboratory also showed that condition medium form PC3-PDGF-D and LNCaP-PDGF-D, but not PC3 and LNCaP cells, induced an increase in ERK1/2 activation in NIH 3T3 cells, indicating that PDGF-D can regulate the ERK activation [26]. However, it remains to be determined how PDGF-D regulates the ERK activation in human cancers and whether inhibition of both PDGF-D and ERK signaling could be superior than targeting only PDGF-D or ERK in killing cancer cells.
5.2 Regulation of epithelial-mesenchymal transition
5.2.1 E-cadherin and Snail expression and their role in PDGF-D signaling
In recent years, PDGF-D has been found to play important roles in the acquisition of epithelial-mesenchymal transition (EMT) phenotype of cancer cell [6;57]. It is now widely accepted that epithelial cells can convert into mesenchymal cells by a fundamental process that is defined as EMT. Epithelial cells undergo remarkable morphologic changes from epithelial cobblestone phenotype to elongated fibroblastic phenotype (mesenchymal phenotype) and during the acquisition of EMT characteristics, cells lose epithelial cell-cell junction, actin cytoskeleton reorganization and the expression of proteins that promote cell-cell contact such as E-cadherin and γ-catenin [58]. Cells gain the expression of mesenchymal markers such as vimentin, α-smooth muscle actin (SMA), fibronectin, fibrillar collagen (type I and III), fibroblast-specific protein-1 and N-cadherin. EMT-type cells also showed increased activity of matrix metalloproteinases (MMPs) like MMP-2, MMP-3 and MMP-9, leading to increased migration and invasion [58]. A number of factors, including the zinc finger Snail homologues (Snail1, Snail2/Slug, and Snail3) and several basic helix-loop-helix factors such as Twist, zinc-finger E-box binding homeobox 1 (ZEB1), ZEB2/SIP1,and TCF3/E47/E12, have emerged as potent EMT drivers during normal development and cancer [58], suggesting that these molecules are important regulator of EMT.
During the acquisition of EMT phenotype, the E-cadherin down-regulation is the crucial step in reducing cell-cell adhesion, leading to destabilization of the epithelial architecture. Indeed, we found that over-expression of PDGF-D in prostate cancer cells resulted in a significant induction of EMT concomitant with the loss of E-cadherin [6]. We also found that the treatment of PC-3 prostate cancer cells with epithelial characteristics by purified PDGF-D protein resulted in a significantly decreased expression of E-cadherin at both the mRNA and protein levels [57]. It is known that a central role in E-cadherin gene repression is attributed to the Snail that is activated and triggers EMT. Snail binds to the two E-boxes of human E-cadherin promoter and then function as a repressor of E-cadherin gene. Snail is activated by most of the signaling pathways that are known to trigger EMT phenotype. As expected, PDGF-D was found to promote EMT through Snail because we found that the treatment of PC-3 prostate cancer cells (epithelial-like cells) by purified PDGF-D protein resulted in increased expression of Snail2 associated with induction of EMT characteristics. Moreover, Snail2 expression was dramatically up-regulated in PC3 PDGF-D cells, indicating that PDGF-D triggers EMT phenotype, which occurs in part through up-regulation of Snail2 [57]. It is important to note that PDGF-D also significantly increased the expression of ZEB1, ZEB2, N-cadherin, and vimentin [57], with concomitant loss of E-cadherin; however, further in-depth mechanistic studies are required for understanding how PDGF-D regulates E-cadherin and the processes of EMT phenotype.
5.2.2 The regulatory role of microRNAs (miRNAs) with respect to PDGF-D signaling
In recent years, PDGF-D has been found to crosstalk with miRNA [57]. It has been well documented that miRNAs work as integral players in cancer biology. The miRNAs elicit their regulatory effects in post-transcriptional regulation by binding to the 3’ untranslated region (3’UTR) of target messenger RNA (mRNA) [59]. Either perfect or near perfect complimentary base pairing results in the degradation of the mRNA, while partial base pairing leads to translational inhibition of functional proteins. It is known that miRNAs are key players in human cancer because miRNAs are involved in the biological processes of cell proliferation and apoptosis, which are critically involved in the development and progression of human malignancies [60;61]. Recent studies also suggest that miRNAs could have diagnostic, prognostic, and therapeutic value [62]. Although, studies elucidating the role of miRNAs in cancer have exploded in recent years, it is still not clear how specific miRNA is regulated and what specific genes are the real targets of specific miRNA in tumor progression. Once that becomes clear then strategies to eith inactivate of activate specific miRNA would become newer targeted approach for the prevention of tumor progression and/or treatment of most human malignancies.
Recently, PDGF-D has been reported to crosstalk with miRNA-200 [57]. The microRNA-200 family has five members: miR-200a, miR-200b, miR-200c, miR-141 and miR-429. Recently many studies have shown that the miR-200 family regulates EMT by targeting ZEB1 and ZEB2 [63;64]. We found that the treatment of cells with purified PDGF-D protein resulted in significant repression of the expression of miR-200a, miR-200b, and miR-200c. Moreover, we found that PDGF-D over-expression led to the acquisition of EMT phenotype in PC-3 prostate cells consistent with the loss of miR-200 expression, and that the re-expression of miR-200b in PC3 PDGF-D cells led to the reversal of the EMT phenotype, which was associated with the down-regulation of ZEB1, ZEB2, and Snail2 expression [57]. Moreover, transfection of PC3 PDGF-D cells with miR-200b inhibited cell migration and invasion with concomitant repression of cell adhesion to the culture surface and cell detachment, suggesting that PDGF-D is indeed responsible for the induction of the EMT phenotype in PC3 cells, which is in part mediated via down-regulation of miR-200 expression, and the regulation of its target genes [57]. Furthermore, we also found that PDGF-D triggers the EMT partly through down-regulation of let-7 in prostate cancer cells (unpublished data). Clearly, specific role of selected miRNA in PDGF-D signaling pathway has begun to be explored, and therefore further studies are needed in elucidating the role of specific miRNA in the regulation of PDGF-D mediated signaling pathway.
5.3 Regulation of cell invasion, angiogenesis, and metastasis
5.3.1 The role of Matrix metalloproteinases (MMPs) in PDGF-D signaling
Tumor metastasis occurs through several steps requiring cell migration, invasion, degradation of basement membranes and the stromal extracellular matrix, ultimately leading to tumor cell metastasis, which is accomplished by many proteolytic enzymes including MMPs. The MMPs are a family of related enzymes which are capable of degrading extracellular matrix, and this process is crucial during tumor cell invasion, angiogenesis and metastasis. MMPs have also been implicated in the acquisition of EMT, a “hallmark” of tumor progression and metastasis. Evidence is emerging showing that members of the MMP family can serve not only as potential markers for diagnosis, prognosis, and early detection, but also as indicators of tumor recurrence, metastatic spread, and response to therapy for human cancers [65]. Among these MMPs, MMP-9 and MMP-2 have been found to be important factors in facilitating invasion and metastases in human cancers because they are directly linked with angiogenesis and degradation of the basement membrane collagen [65].
It has been reported that MMP-9 expression is regulated by PDGF-D in several kinds of human cancers. Xu et al. found that over-expression of PDGF-D in renal cancer SN12-C cells promoted tumor growth, angiogenesis and metastasis due to increased expression of MMP-9 and angiopoietin-1 in an orthotopic mouse model [27]. We also found that down-regulation of PDGF-D in pancreatic cancer cells leads to the inhibition of MMP-9 expression and MMP-9 activation [8]. Recently, we found that down-regulation of PDGF-D also decreased the expression of MMP-9 in breast cancer cells (unpublished data). More recently, Zhao et al. reported that inhibition of PDGF-D leads to decreased cell invasion and angiogenesis in gastric cancer through MMP-9 and MMP-2 [66]. In order to better understand the precise role of PDGF-D and its interrelationship with MMPs requires in-depth investigation.
5.3.2 Vascular endothelial cell growth factor (VEGF) and its relationship with PDGF-D signaling
Studies have shown that VEGF is very crucial for the induction of angiogenic processes because VEGF regulates most of the steps in the angiogenesis including migration, invasion, and tube formation of endothelial cells. In addition, studies have shown a trend towards an association between the expression of VEGF and migration, invasion and distant metastasis [67]. It has been reported that PDGF-D regulates VEGF signaling in various cancer cell types. Li et al. found that PDGF-D is a potent transforming growth factor for NIH/3T3 cells, and the transformed cells displayed increased proliferation rate, induced tumors in nude mice, and up-regulated VEGF [68]. We also found that down-regulation of PDGF-D in pancreatic cancer cells led to the inhibition of VEGF secretion [8]. Moreover, conditioned medium from PDGF-D siRNA–transfected cells showed reduced levels of VEGF, resulting in the inhibition of angiogenesis which was assessed by the tube formation of human umbilical vein endothelial cells (HUVECs), suggesting that down-regulation of PDGF-D is responsible for the inhibition of angiogenesis [8]. In contrast, condition medium from PDGF-D over-expressing PC3 cells induced tube formation of HUVECs [6]. Very recently, Zhao et al found that inhibition of PDGF-D leads to decreased cell invasion and angiogenesis in gastric cancer partly through the regulation of VEGF [66]. However, the molecular mechanism(s) by which VEGF is regulated by PDGF-D or vice versa is poorly understood, and thus in-depth investigation in this area is urgently needed.
5.3.3 The cross-talks of other effectors in the regulation of PDGF-D
Several recent studies have shown that PDGF-D regulates many other effectors, such as Bcl-2, Cyclin D1, and β-catenin, which have not been discussed above. For example, we found a dramatic increase in the levels of Bcl-2 expression in PDGF-D over-expressing prostate cancer cells, and the regulation of Bcl-2 expression by PDGF-D was shown to play an important role in PDGF-D-mediated acquisition of EMT [6;7]. Conversely, the down-regulation of PDGF-D was able to decrease the expression of Bcl-2 in pancreatic cancer cell [8]. In addition, we found that PDGF-D can up-regulate the expression of poly(ADPribose) polymerase-1 (PARP-1) in prostate cancer cells [6]. Further studies by Zhao et al have shown that down-regulation of PDGF-D inhibited Cyclin D1 and β-catenin in gastric cancer [66], and similar results were reported in pancreatic and prostate cancer [8;57]. Since PDGF-D signaling is an emerging area of research in multiple human malignancies, there is not doubt that we will be witnessing the role of additional effectors of PDGF-D signaling in years to come.
6. Conclusion and overall perspectives
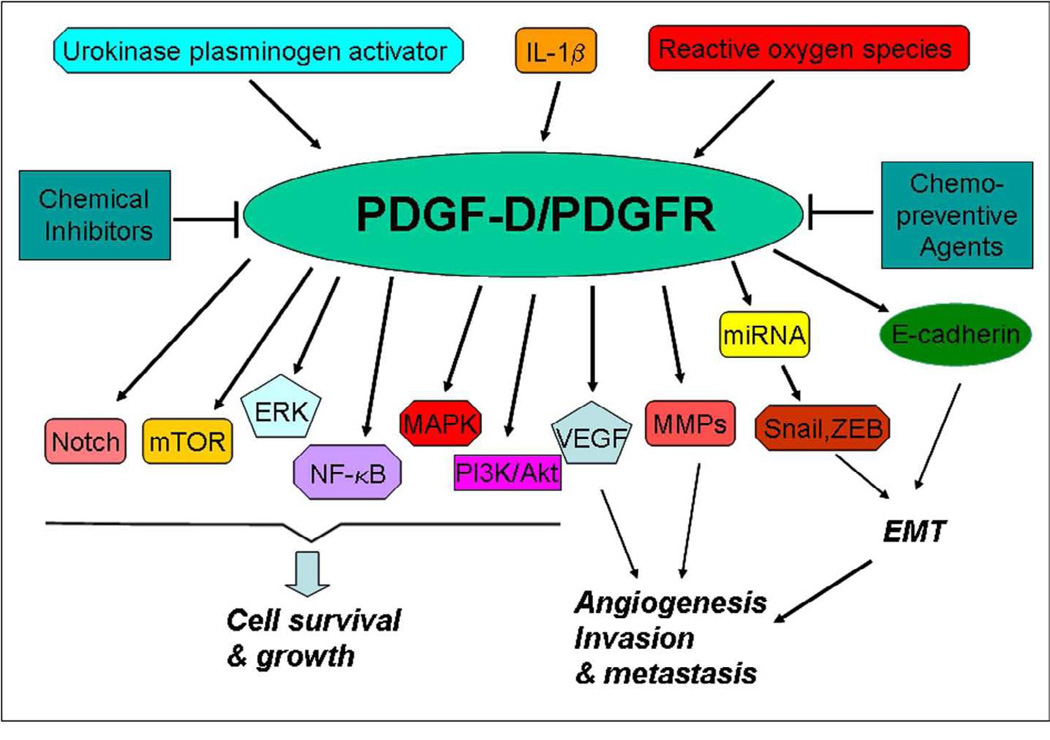
Given the importance of PDGF-D in tumor cell growth, migration, invasion, angiogenesis and metastasis and its cross-talks with many signaling pathways in human malignancies (Figure-2), significant attention has been paid in recent past toward the development of clinically useful antagonists of PDGF signaling. PDGF-D displays an oncogenic activity specifically through binding to and activating its cognate receptor PDGFR-β, suggesting that the inactivation of PDGF-D/PDGFR signaling by novel approaches is likely to have a significant impact in cancer therapy. Several small molecule tyrosine kinase inhibitors that block the PDGF receptor have been developed. For example, CT52923, one of PDGFR antagonists, inhibited survival and/or mitogenic pathways in the glioblastoma cell lines and prevented glioma formation in a nude mouse xenograft model [12]. Imatinib (STI571 or Gleveec), which is a selective tyrosine kinase inhibitor especially for the inhibition of PDGF-R, inhibited the cell growth and invasion in human breast cancer cell lines [69]. The combination of imatinib with chemotherapeutic agent paclitaxel or gemcitabine led to a further tumor growth inhibition in prostate cancer and malignant mesothelioma, suggesting that imatinib enhances the therapeutic response to chemotherapeutic agents [70;71]. CR002, a humanized monoclonal PDGF-D antibody, has been shown to be safe in a phase I study, and CR002 was able to reduce glomerular and secondary tubulointerstitial damage [72]; however, CR002 has not yet been tried for its effects in human cancer.
Figure-2.
Diagram of PDGF-D cross-talks with other pathways. ERK: extracellular signal-regulated kinase; IL-1β: proinflammatory cytokine interleukin-1β; MAPK: mitogen-activated proteinkinase; MMPs: matrix metalloproteinases; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor-κB; PI3K: phosphatidylinositol 3-kinase; ROS: reactive oxygen species; uPA: urokinase-type plasminogen activator; VEGF: vascular endothelial growth factor. EMT: epithelial-mesenchymal transition.
To our knowledge, there is no report regarding the small chemical inhibitors of PDGF-D but we are confident that such search must be an active area of research. Interestingly, we found that 3,3'-Diindolylmethane (DIM, a well known chemopreventive agent) significantly inhibited the expression and activation of PDGF-D in prostate cancer cells [7]. Our results suggest that DIM could serve as a novel and efficient chemopreventive and/or therapeutic agent by inactivation of PDGF-D in prostate cancer cells especially because DIM was found to be non-toxic in most human studies. We and other investigators have demonstrated that increased expression of PDGF-D and its receptor is detected in many human cancer cells and tissues. More importantly, PDGF-D plays important roles in almost all aspects of cancer biology, such as proliferation, apoptosis, migration, invasion, angiogenesis and metastasis; however, further in-depth studies including mechanistic in vitro studies and in vivo animal experiments are needed to fully understand and appreciate the roles of PDGF-D in tumor progression. We believe that this article would be able to stimulate or promote further research in this field toward the development of novel approaches by which PDGF-D signaling could be targeted for the inhibition of tumor progression and/or therapy for most human malignancies.
Acknowledgements
The authors’ work cited in this review was funded by grants from the National Cancer Institute, NIH (5R01CA131151, 5R01CA083695, 1R01CA132794, 1R01CA101870) to F.H.S. and Department of Defense Postdoctoral Training Award W81XWH-08-1-0196 (Zhiwei Wang) and also partly supported by a subcontract award (F.H.S.) from the University of Texas MD Anderson Cancer Center through a SPORE grant (5P20-CA101936) on pancreatic cancer awarded to James Abbruzzese. We also sincerely thank both Puschelberg and Guido foundation for their generous contributions to our research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat.Cell Biol. 2001;3:512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- 3.LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M, Vernet C, Burgess CE, Fernandes E, Deegler LL, Rittman B, Shimkets J, Shimkets RA, Rothberg JM, Lichenstein HS. PDGF-D, a new protease-activated growth factor. Nat.Cell Biol. 2001;3:517–521. doi: 10.1038/35074593. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Eriksson U. Novel PDGF family members: PDGF-C and PDGF-D. Cytokine Growth Factor Rev. 2003;14:91–98. doi: 10.1016/s1359-6101(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Kong D, Li Y, Sarkar FH. PDGF-D signaling: a novel target in cancer therapy. Curr.Drug Targets. 2009;10:38–41. doi: 10.2174/138945009787122914. [DOI] [PubMed] [Google Scholar]
- 6.Kong D, Wang Z, Sarkar SH, Li Y, Banerjee S, Saliganan A, Kim HR, Cher ML, Sarkar FH. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26:1425–1435. doi: 10.1634/stemcells.2007-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong D, Banerjee S, Huang W, Li Y, Wang Z, Kim HR, Sarkar FH. Mammalian target of rapamycin repression by 3,3'-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68:1927–1934. doi: 10.1158/0008-5472.CAN-07-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Kong D, Banerjee S, Li Y, Adsay NV, Abbruzzese J, Sarkar FH. Down-regulation of platelet-derived growth factor-D inhibits cell growth and angiogenesis through inactivation of Notch-1 and nuclear factor-kappaB signaling. Cancer Res. 2007;67:11377–11385. doi: 10.1158/0008-5472.CAN-07-2803. [DOI] [PubMed] [Google Scholar]
- 9.Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J. 2005;272:5723–5741. doi: 10.1111/j.1742-4658.2005.04989.x. [DOI] [PubMed] [Google Scholar]
- 10.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Breitkopf K, Roeyen C, Sawitza I, Wickert L, Floege J, Gressner AM. Expression patterns of PDGF-A, -B, -C and -D and the PDGF-receptors alpha and beta in activated rat hepatic stellate cells (HSC) Cytokine. 2005;31:349–357. doi: 10.1016/j.cyto.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Research. 2002;62:3729–3735. [PubMed] [Google Scholar]
- 13.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 14.LaRochelle WJ, Jeffers M, Corvalan JR, Jia XC, Feng X, Vanegas S, Vickroy JD, Yang XD, Chen F, Gazit G, Mayotte J, Macaluso J, Rittman B, Wu F, Dhanabal M, Herrmann J, Lichenstein HS. Platelet-derived growth factor D: tumorigenicity in mice and dysregulated expression in human cancer. Cancer Research. 2002;62:2468–2473. [PubMed] [Google Scholar]
- 15.Ross R. Platelet-derived growth factor. Annu.Rev.Med. 1987;38:71–79. doi: 10.1146/annurev.me.38.020187.000443. [DOI] [PubMed] [Google Scholar]
- 16.Heldin CH, Westermark B. Platelet-derived growth factor: three isoforms and two receptor types. Trends Genet. 1989;5:108–111. doi: 10.1016/0168-9525(89)90040-1. [DOI] [PubMed] [Google Scholar]
- 17.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 18.Claesson-Welsh L. Mechanism of action of platelet-derived growth factor. Int.J.Biochem.Cell Biol. 1996;28:373–385. doi: 10.1016/1357-2725(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 19.Heldin CH, Hammacher A, Nister M, Westermark B. Structural and functional aspects of platelet-derived growth factor. Br.J.Cancer. 1988;57:591–593. doi: 10.1038/bjc.1988.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George D. Platelet-derived growth factor receptors: a therapeutic target in solid tumors. Semin.Oncol. 2001;28:27–33. [PubMed] [Google Scholar]
- 21.Yu J, Ustach C, Kim HR. Platelet-derived growth factor signaling and human cancer. J.Biochem.Mol.Biol. 2003;36:49–59. doi: 10.5483/bmbrep.2003.36.1.049. [DOI] [PubMed] [Google Scholar]
- 22.George D. Targeting PDGF receptors in cancer--rationales and proof of concept clinical trials. Adv.Exp.Med.Biol. 2003;532:141–151. doi: 10.1007/978-1-4615-0081-0_12. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Jendrossek V, Belka C. The role of PDGF in radiation oncology. Radiat.Oncol. 2007;2:5. doi: 10.1186/1748-717X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei H, Kazlauskas A. Focus on molecules: platelet-derived growth factor C, PDGF-C. Exp.Eye Res. 2008;86:711–712. doi: 10.1016/j.exer.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ustach CV, Taube ME, Hurst NJ, Jr, Bhagat S, Bonfil RD, Cher ML, Schuger L, Kim HR. A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer Research. 2004;64:1722–1729. doi: 10.1158/0008-5472.can-03-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ustach CV, Kim HR. Platelet-derived growth factor D is activated by urokinase plasminogen activator in prostate carcinoma cells. Mol.Cell Biol. 2005;25:6279–6288. doi: 10.1128/MCB.25.14.6279-6288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Tong R, Cochran DM, Jain RK. Blocking platelet-derived growth factor-D/platelet-derived growth factor receptor beta signaling inhibits human renal cell carcinoma progression in an orthotopic mouse model. Cancer Research. 2005;65:5711–5719. doi: 10.1158/0008-5472.CAN-04-4313. [DOI] [PubMed] [Google Scholar]
- 28.Khachigian LM, Williams AJ, Collins T. Interplay of Sp1 and Egr-1 in the proximal platelet-derived growth factor A-chain promoter in cultured vascular endothelial cells. J.Biol.Chem. 1995;270:27679–27686. doi: 10.1074/jbc.270.46.27679. [DOI] [PubMed] [Google Scholar]
- 29.Midgley VC, Khachigian LM. Fibroblast growth factor-2 induction of platelet-derived growth factor-C chain transcription in vascular smooth muscle cells is ERK-dependent but not JNK-dependent and mediated by Egr-1 1. J.Biol.Chem. 2004;279:40289–40295. doi: 10.1074/jbc.M406063200. [DOI] [PubMed] [Google Scholar]
- 30.Khachigian LM, Lindner V, Williams AJ, Collins T. Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science. 1996;271:1427–1431. doi: 10.1126/science.271.5254.1427. [DOI] [PubMed] [Google Scholar]
- 31.Liu MY, Eyries M, Zhang C, Santiago FS, Khachigian LM. Inducible platelet-derived growth factor D-chain expression by angiotensin II and hydrogen peroxide involves transcriptional regulation by Ets-1 and Sp1. Blood. 2006;107:2322–2329. doi: 10.1182/blood-2005-06-2377. [DOI] [PubMed] [Google Scholar]
- 32.Tan NY, Midgley VC, Kavurma MM, Santiago FS, Luo X, Peden R, Fahmy RG, Berndt MC, Molloy MP, Khachigian LM. Angiotensin II-inducible platelet-derived growth factor-D transcription requires specific Ser/Thr residues in the second zinc finger region of Sp1. Circ.Res. 2008;102:e38–e51. doi: 10.1161/CIRCRESAHA.107.167395. [DOI] [PubMed] [Google Scholar]
- 33.Liu MY, Khachigian LM. Histone deacetylase-1 is enriched at the platelet-derived growth factor-D promoter in response to interleukin-1beta and forms a cytokine-inducible gene-silencing complex with NF-kappab p65 and interferon regulatory factor-1. J.Biol.Chem. 2009;284:35101–35112. doi: 10.1074/jbc.M109.061903. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat.Rev. 2008;34:122–136. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad A, Kong D, Wang Z, Sarkar SH, Banerjee S, Sarkar FH. Down-regulation of uPA and uPAR by 3,3'-diindolylmethane contributes to the inhibition of cell growth and migration of breast cancer cells. J.Cell Biochem. 2009;108:916–925. doi: 10.1002/jcb.22323. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat.Rev.Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Li Y, Sarkar FH. Signaling Mechanism(S) of Reactive Oxygen Species in Epithelial-Mesenchymal Transition Reminiscent of Cancer Stem Cells in Tumor Progression. Curr.Stem Cell Res.Ther. 2009 doi: 10.2174/157488810790442813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 39.Lei H, Kazlauskas A. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J.Biol.Chem. 2009;284:6329–6336. doi: 10.1074/jbc.M808426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu H, Hirose Y, Nishijima F, Tsubakihara Y, Miyazaki H. ROS and PDGF-beta [corrected] receptors are critically involved in indoxyl sulfate actions that promote vascular smooth muscle cell proliferation and migration. Am.J.Physiol Cell Physiol. 2009;297:C389–C396. doi: 10.1152/ajpcell.00206.2009. [DOI] [PubMed] [Google Scholar]
- 41.Ricono JM, Wagner B, Gorin Y, Arar M, Kazlauskas A, Choudhury GG, Abboud HE. PDGF receptor-{beta} modulates metanephric mesenchyme chemotaxis induced by PDGF AA. Am.J.Physiol Renal Physiol. 2009;296:F406–F417. doi: 10.1152/ajprenal.90368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad A, Banerjee S, Wang Z, Kong D, Majumdar AP, Sarkar FH. Aging and Inflammation: Etiological Culprits of Cancer. Curr.Aging Sci. 2009;2:174–186. doi: 10.2174/1874609810902030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li R, Maminishkis A, Wang FE, Miller SS. PDGF-C and -D induced proliferation/migration of human RPE is abolished by inflammatory cytokines. Invest Ophthalmol.Vis.Sci. 2007;48:5722–5732. doi: 10.1167/iovs.07-0327. [DOI] [PubMed] [Google Scholar]
- 44.Borkham-Kamphorst E, van Roeyen CR, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J.Hepatol. 2007;46:1064–1074. doi: 10.1016/j.jhep.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat.Rev.Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- 47.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat.Rev.Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 48.Sarkar FH, Li Y, Wang Z, Kong D. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int.Rev.Immunol. 2008;27:293–319. doi: 10.1080/08830180802276179. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Li Y, Banerjee S, Sarkar FH. Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res. 2008;28:3621–3630. [PubMed] [Google Scholar]
- 50.Wang Z, Li Y, Banerjee S, Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279:8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miele L, Miao H, Nickoloff BJ. Notch signaling as a novel cancer therapeutic target. Curr.Cancer Drug Targets. 2006;6:313–323. doi: 10.2174/156800906777441771. [DOI] [PubMed] [Google Scholar]
- 52.Miele L. Notch signaling. Clin.Cancer Res. 2006;12:1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol.Cancer Ther. 2006;5:483–493. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 54.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat.Rev.Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 55.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat.Rev.Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 56.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat.Rev.Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J.Clin.Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandenboom Ii TG, Li Y, Philip PA, Sarkar FH. MicroRNA and Cancer: Tiny Molecules with Major Implications 1262. Curr.Genomics. 2008;9:97–109. doi: 10.2174/138920208784139555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown BD, Naldini L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat.Rev.Genet. 2009;10:578–585. doi: 10.1038/nrg2628. [DOI] [PubMed] [Google Scholar]
- 63.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat.Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 64.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur HA, Brunton VG, Morton J, Sansom O, Schuler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat.Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 65.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J.Clin.Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao L, Zhang C, Liao G, Long J. RNAi-mediated inhibition of PDGF-D leads to decreased cell growth, invasion and angiogenesis in the SGC-7901 gastric cancer xenograft model. Cancer Biol.Ther. 2009;9:1–7. doi: 10.4161/cbt.9.1.10282. [DOI] [PubMed] [Google Scholar]
- 67.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat.Rev.Clin.Oncol. 2009;6:465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 68.Li H, Fredriksson L, Li X, Eriksson U. PDGF-D is a potent transforming and angiogenic growth factor. Oncogene. 2003;22:1501–1510. doi: 10.1038/sj.onc.1206223. [DOI] [PubMed] [Google Scholar]
- 69.Roussidis AE, Theocharis AD, Tzanakakis GN, Karamanos NK. The importance of c-Kit and PDGF receptors as potential targets for molecular therapy in breast cancer. Curr.Med.Chem. 2007;14:735–743. doi: 10.2174/092986707780090963. [DOI] [PubMed] [Google Scholar]
- 70.Bertino P, Piccardi F, Porta C, Favoni R, Cilli M, Mutti L, Gaudino G. Imatinib mesylate enhances therapeutic effects of gemcitabine in human malignant mesothelioma xenografts. Clin.Cancer Res. 2008;14:541–548. doi: 10.1158/1078-0432.CCR-07-1388. [DOI] [PubMed] [Google Scholar]
- 71.Kim SJ, Uehara H, Yazici S, Busby JE, Nakamura T, He J, Maya M, Logothetis C, Mathew P, Wang X, Do KA, Fan D, Fidler IJ. Targeting platelet-derived growth factor receptor on endothelial cells of multidrug-resistant prostate cancer. J.Natl.Cancer Inst. 2006;98:783–793. doi: 10.1093/jnci/djj211. [DOI] [PubMed] [Google Scholar]
- 72.Boor P, Konieczny A, Villa L, Kunter U, van Roeyen CR, LaRochelle WJ, Smithson G, Arrol S, Ostendorf T, Floege J. PDGF-D inhibition by CR002 ameliorates tubulointerstitial fibrosis following experimental glomerulonephritis. Nephrol. Dial. Transplant. 2007;22:1323–1331. doi: 10.1093/ndt/gfl691. [DOI] [PubMed] [Google Scholar]