Abstract
Investigations over the last decade have established the essential role of growth factors and their receptors during angiogenesis and carcinogenesis. The vascular endothelial growth factor receptor (VEGFR) family in mammals contains three members, VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1) and VEGFR-3 (Flt-4), which are transmembrane tyrosine kinase receptors that regulate the formation of blood and lymphatic vessels. In the early 1990s, the above VEGFR were structurally characterized by cDNA cloning. Among these three receptors, VEGFR-2 is generally recognized to have a principal role in mediating VEGF-induced responses. VEGFR-2 is considered as the earliest marker for endothelial cell development. Importantly, VEGFR-2 directly regulates tumor angiogenesis. Therefore, several inhibitors of VEGFR-2 have been developed and many of them are now in clinical trials. In addition to targeting endothelial cells, the VEGF/VEGFR-2 system works as an essential autocrine/paracrine process for cancer cell proliferation and survival. Recent studies mark the continuous and increased interest in this related, but distinct, function of VEGF/VEGFR-2 in cancer cells: the autocrine/paracrine loop. Several mechanisms regulate VEGFR-2 levels and modulate its role in tumor angiogenesis and physiologic functions, i.e.: cellular localization/trafficking, regulation of cis-elements of promoter, epigenetic regulation and signaling from Notch, cytokines/growth factors and estrogen, etc. In this review, we will focus on updated information regarding VEGFR-2 research with respect to the molecular mechanisms of VEGFR-2 regulation in human breast cancer. Investigations in the activation, function, and regulation of VEGFR-2 in breast cancer will allow the development of new pharmacological strategies aimed at directly targeting cancer cell proliferation and survival.
Keywords: VEGF, VEGFR-2, Tumor angiogenesis, Breast cancer, autocrine/paracrine loop, leptin
1. Introduction
Formation of the primary vascular plexus in the embryo is driven by vasculogenesis; additional blood vessels are generated by angiogenesis, which are progressively pruned and remodeled into a functional adult circulatory system [1]. Vasculogenesis encompasses the transformation of mesodermic angioblasts into endothelial cells (EC) and further development of new blood vessels. In contrast, angiogenesis is the sprouting of new capillaries from pre-existing blood vessels that involves endothelial cell differentiation, proliferation, migration, cord formation and tubulogenesis [1]. Many endothelium-specific molecules can influence these processes, including members of the VEGF, angiopoietin and ephrin families. In addition, non-vascular endothelium-specific factors contribute to blood vessel formation, i.e., platelet-derived growth factor, PDGF and transforming growth factor-β, TGF-β families [2].
Crucial factors for blood vessel formation are the VEGF members showing multiple interactions with receptor tyrosine kinases (RTK), namely VEGFR-1 (fms-like tyrosine kinase, Flt-1) [3], VEGFR-2 (fetal liver kinase, Flk-1 in mice or KDR in humans) [4–6], VEGFR-3 (Flt-4)[7]. A fourth receptor, Flt-3/Flk-2, belonging to the RTK family, was identified, but it does not bind to VEGF [8]. Receptor Neuropilins 1 and 2 (NRP-1/-2) are another class of high-affinity non-tyrosine kinase receptors for VEGF on endothelial and neuronal cell surfaces [9]. VEGF-A is the major form that binds and signals through VEGFR-2 to develop blood vessels and maintain the vascular network [10]. In this respect VEGFR-2 is essential for life [11]. This is supported by the fact that homozygous-deficient Flk-1 mice die in the second week of gestation as a consequence of insufficient development of hematopoietic and EC [4]. In contrast, VEGF signals through VEGFR-1 (Flt-1) are also required for the organization of embryonic vasculature, but they are not essential for endothelial cell differentiation. Indeed, homozygous mice for targeted mutation of VEGFR-1 gene produce EC from angioblasts, but develop non-functional blood vessels and die at around 10 days of gestation [12]. VEGFR-2 mediates the major growth and permeability actions of VEGF [13], whereas VEGFR-1 may have a negative role, either by acting as a decoy receptor or by suppressing signaling through VEGFR-2 [2]. VEGFR-3 binds VEGF-C and VEGF-D to mainly regulate lymphatic EC [9]. However, VEGFR-3 is also essential for early blood vessel development and plays a role in tumor angiogenesis [14, 15]. The Flt-3/Flk-2 receptor (CD135) binds the Flt-3 ligand to promote the growth and differentiation of primitive hematopoietic cells. Flt-3/Flk-2 is expressed on CD34+ hematopoietic stem cells, myelomonocytic progenitors, primitive B cell progenitors, and thymocytes and control differentiation of hematopoietic and non-hematopoietic cells [8, 16].
2. VEGFR-2: a committed pro-angiogenic receptor
VEGFR-2 is a transmembrane receptor that plays an important role in endothelial cell development [1, 17] and is thought to mediate the key effects of the endothelial-specific mitogen VEGF on cell proliferation and permeability. Therefore, the majority of VEGFR-2 actions are related to angiogenesis [18, 19].
3. Structure of VEGFR-2
VEGFR-2 was discovered before the identification of its ligand, VEGF [1, 2]. The receptor-tyrosine kinase named KDR in human [2, 20], Flk-1 [4] or NYW/FLK-1 in mice [21] and TKr-11 in rat [22] was earlier identified as a transducer of VEGF in EC [23]. VEGFR-2 consists of 1356 and 1345 amino acids in humans and mice, respectively, and can be separated into four regions: the extracellular ligand-binding domain, transmembrane domain, tyrosine kinase domain, and downstream carboxy terminal region [3–6]. The extracellular ligand-binding domain consists of seven immunoglobulin (Ig)-like domains with the ligand-binding region being localized within the second and third Ig domains [24–27]. Shinkai et al, further demonstrated that the third Ig-like domain is critical for ligand binding, the second and fourth domains are important for ligand association, and the fifth and sixth domains are required for retention of the ligand bound to the receptor molecule [26]. Ligand-binding specificities of VEGFR-2 differ from VEGFR-1; VEGFR-2 binds VEGF-A, VEGF-C, VEGF-D and VEGF-E [28–32], whereas VEGFR-1 binds VEGF-A, VEGF-B and PIGF [33–35].
The classic interpretation of ligand-binding specificities is currently used for all aspects of vascular effects driven by VEGF-VEGFRs, which have helped in elucidating their function. However, the potential formation of VEGFR heterodimers, i.e., VEGFR-2-VEGFR-1 [36] and VEGFR-2-VEGFR-3 [37] has generally been understated. It was noticed that substantial differences in signal transduction occur upon distinct VEGFR heterodimers complex formation because kinase domains and substrate specificities differ [33, 36–39]. These complexes can also differentially interact with VEGF and PIGF [33, 36] resulting in unique signaling pattern and likely different feedback mechanisms [38].
4. VEGFR-2 signaling pathways and targeted effects
VEGFR-2 actions heavily depend on the specific activation of tyrosine amino acid residues within the intracytoplasmatic tail of the receptor (for Review see [40, 41]). The majority of VEGFR-2 intracellular domains contain tyrosine residues that are involved in redundant actions on vasculogenesis or angiogenesis. VEGFR-2 signals affect vascular permeability and proliferation, survival and migration of EC [42, 43]. The upstream phosphorylation of a tyrosine residue (Y801) within the juxtamembrane domain of VEGFR-2 seems to be required for the subsequent activation of the catalytic domain. However, the biological implications of these findings need to be further investigated [44]. Specific serine residues in the VEGFR-2 intracytoplasmatic region are targeted by protein kinase C (PKC) and involved in regulatory mechanisms of receptor levels (ubiquitinylation and degradation) [45].
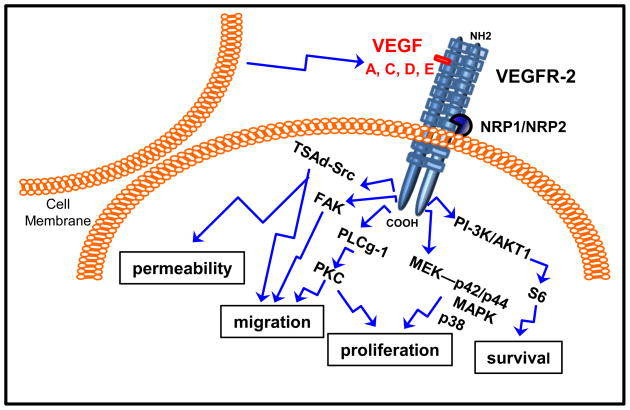
VEGF binding to VEGFR-2 triggers the specific activation of tyrosine amino acid residues within intracytoplasmatic tail of the receptor inducing multiple signaling networks that result in endothelial cell survival, proliferation, migration, focal adhesion turnover, actin remodeling and vascular permeability [40]. Signaling through mitogen-activated protein-kinase (MAP; including MEK-p42/p44 MAPK and p38 MAPK; linked to proliferation) [40, 46] and phosphatidylinositol 3′ kinase (PI-3K)/V-akt murine thymoma viral oncogene homolog 1 (Akt1; linked to survival) [47] are common receptor tyrosine kinase activation patterns. Additional signaling pathways are also triggered upon VEGFR-2 activation, i.e., phospholipase C gamma (PLCg)-protein kinase C (PKC; linked to proliferation) [48], focal adhesion kinase (FAK; linked to migration) [47] and T Cell–Specific Adapter (TSAd)-Src kinase (linked to migration and vascular permeability) [49] (See Fig 1). Recent data suggest that overexpression of caveolin-1 in prostate cancer cells specifically induce phosphorylation of tyrosine 951 in the VEGFR-2 intracytoplasmatic tail and increases angiogenesis [50]. On the other hand, tyrosine 1175 mediates both PLCγ-1 and protein kinase A (PKA)-dependent signaling pathways required for VEGF-induced release of von Willebrand factor from EC [51]. However, the involvement of specific VEGFR-2 intracytoplasmatic domains and associated activation of specific signaling pathways and driven functions is a point of controversial discussion. Dayanir et al, found that activation of PI-3K/S6 but not Ras/MAPK kinase pathway is responsible for VEGFR-2-mediated cell growth [52]. Inhibition of p38 MAPK activity enhances VEGF-induced angiogenesis in vitro and in vivo and prolonged ERK1/2 activation and increased endothelial survival, but abrogated VEGF-induced vascular permeability [53]. Intriguingly, VEGF-mediated proliferation of VEGFR-2 transfected fibroblasts was slower and weaker than in EC, suggesting the cell type-specific signaling mechanism(s) [46]. These results open the possibilities for differential signaling mechanisms/responses to VEGF via VEGFR-2 in cancer compared to EC. Inconsistent reports on VEGFR-2 signaling capabilities could be due to the complex interplay of signaling and inhibiting actions of other VEGF receptors. In addition, the activation and signaling of VEGFR-2 could also be modified by the formation of VEGFR-2 heterodimers exhibiting differential signaling potential as described above.
Fig. 1.
Schematic representation of how VEGFR-2 signaling pathways are linked to its main biological functions. Different VEGF isoforms can bind VEGFR-2 dimer. NRP-1/-2 are co-receptors that stabilize the VEGFR-2 dimer. Upon ligand binding to VEGFR-2 dimer several signaling pathways can be activated affecting diverse biological processes in endothelial and cancer cells.
5. The autocrine/paracrine VEGF/VEGFR-2 loop: a cancer cell survival process
Intensive research has been done on VEGF/VEGFR-2 roles in vascular functions [40]. However, only a small number of reports highlight a lesser known function of VEGF signaling that can directly impact cancer cell survival: the autocrine loop in cancer cells. Some reports suggest that a strict molecular requirement for these autocrine actions of VEGF is the expression of VEGFR-1 as it was found in colon carcinoma [54]. In line with these data, Wu et al, further reported that selective signaling through VEGFR-1 on breast cancer cells supports tumor growth through downstream activation of the p44/42 MAPK or Akt pathways [55]. However, in breast cancer cells, VEGFR-2 isoform was not initially linked to cell survival [54, 56]. The co-expression of NRP-1 [57] and α6β4 integrin [56] but not VEGFR-2, was found essential for the binding of VEGF and activation of the PI-3K survival signaling pathway in breast cancer cells. Moreover, it was suggested that breast cancer cells do not express VEGFR-2 [56, 57]. In contrast, VEGF/VEGFR-2 was found to be essential to cell survival in either estrogen receptor positive (MCF-7) [58, 59] or negative cells (MDA-MB-468) [59] after tamoxifen treatment. A signaling cascade from VEGFR-2 via ERK1/2 to Ets-2 phosphorylation was correlated to better survival of untreated patients [59]. Moreover, a VEGF/VEGFR-2/p38 kinase link was involved in poor outcome for tamoxifen-treated patients [58]. VEGF stimulation of Akt phosphorylation and activation of ERK1/2 correlated to VEGFR-2 expression and activation in various breast carcinoma cell lines and primary culture of breast carcinoma cells [60].
Findings from our laboratory suggest that mouse (4T1, ER +) [61] and human breast cancer cells (MCF-7, ER+ and MDA-MB-231, ER−) express VEGFR-2 in vitro and in vivo [62]. Interestingly in these cells the expression of VEGF and VEGFR-2 was linked to leptin signaling. Leptin is a small nonglycosilated protein (16 kD) product of the ob gene. White adipose tissue is the primary source of leptin in benign tissue but it is also expressed and secreted by cancer cells. Leptin is a known mitogenic, inflammatory and angiogenic factor for many tissues [63] and increases the levels of VEGF/VEGFR-2 in cancer cells [61, 62, 64]. Therefore, leptin from corporal, mammary adipose tissue or cancer cells could affect cancer cells through autocrine and paracrine actions. Leptin increased the proliferation of 4T1 cells, but treatment with anti-VEGFR-2 antibody resulted in a further increase in leptin-induced VEGF concentrations in the medium. This suggests a feedback loop between VEGF expression and VEGFR-2 that could be linked to cell survival/proliferation [58]. Remarkably, inhibition of leptin signaling mediated an impressive reduction of tumor growth in mice that paralleled a significant reduction of VEGF and VEGFR-2 levels [61, 62].
The endothelial-independent VEGF/VEGFR-2 autocrine loop was found essential for leukemia cell survival and migration in vivo [65]. Results from these studies suggest that effective antiangiogenic therapies to treat VEGF-producing and VEGFR-expressing leukemias may require blocking both paracrine and autocrine VEGF/VEGFR-2 angiogenic loops to achieve long term remission and cure [65]. On the other hand, stimulation of the proliferation of prostate cancer cells by VEGF depends on the presence of VEGFR-2 through autocrine loops generated by IL-6 [66]. Overall these data suggest that targeting the VEGF/VEGFR-2 autocrine loop in cancer may be an innovative way to treat the disease. However, the relationships between activation of specific intracellular domains of VEGFR-2 and autocrine actions of VEGF in cancer cells remains to be studied in detail.
6. Expression of VEGFR-2 in human Breast Cancer and other cancer types
Because of the potential role of VEGFR-2 in tumor angiogenesis, its expression was investigated in breast carcinoma soon after it was identified as a tyrosine kinase-receptor for VEGF [23]. Upregulation of VEGFR-2 mRNA was found earlier in invasive primary and metastatic breast cancers [67]. Western blot and immunohistochemical analyses of endothelium and epithelium of mammary ducts in carcinomas, fibroadenomas and fibrocystic breast disease showed positive expression of VEGFR-2, which was also found in tumor stroma [68]. VEGFR-2 expression correlated with VEGF expression that suggested these molecules were co-expressed in breast cancer; however, this was not significantly associated with patient survival [69]. In addition, tumor-specific expression of VEGFR-2 correlated strongly with expression of VEGF-A and progesterone receptor (PR) negativity, whereas VEGF-A was not associated with hormone receptor status [70].
In vitro studies demonstrated that VEGFR-2 receptors are largely expressed in breast cancer [58–62, 64] and colon cancer cells as well as intra-tumoral vasculature [71] and VEGFR-2 mRNA is expressed in breast cancer [64] as well as several pancreatic cancer cell lines [72]. In situ hybridization analyses revealed that 85% of human ovarian cancer specimens showed moderate to high VEGFR-2 expression [73] and some western blotting in small cell lung cancer (SCLC) cells analyses detected high VEGFR-2 [74].
Invasive and in situ breast cancers express many angiogenic factors and this process was found throughout all tumor stages [75]. Nakopoulou et al, detected VEGFR-2 in 64.5% (91/141) of invasive breast carcinomas showing a widespread cytoplasmic expression in most of the neoplastic cells [76]. VEGFR-2 expression was well correlated with the nuclear grade of the invasive breast carcinoma (P = 0.003), but demonstrated no correlation with histologic grade, stage, and patient survival [76] as was previously noticed [69]. In cervical adenosquamous carcinoma, VEGFR-2 positivity was observed in 22 of 30 cases (73.3%) but was significantly associated with lack of metastasis (P = 0.038) [77].
In contrast, other studies suggest that not only the overexpression of VEGFR-2 occurs in cancer, but also the expression of VEGFR-2 relates to the disease stage, recurrence and worse outcome. VEGFR-2 was found weakly expressed in normal tissues or cells, but VEGFR-2 overexpression was reported in various cancers including lung, colon, uterus, ovarian cancer, as well as breast cancers [71]. In colon cancer, VEGFR-2 positive rate was found 46.7% [78]. In esophageal adenocarcinoma and squamous cell cancer, VEGFR-2 positive rate was 100% [79]. The VEGFR-2 protein-positive phenotype of kidney clear cell carcinoma was relatively frequent (7/20, 35%), but was lost in bone metastases (2/20, 10%) [80]. Increased VEGFR-2 expression correlates with several features that predict progression of urothelial cancer, including disease stage and invasive phenotype. VEGFR-2 expression correlated with disease stage (coefficient 0.23, P = 0.05). In addition, VEGFR-2 expression increased with tumor invasion into the muscle (P <0.01) [81]. Moreover, in primary glioblastomas amplification of VEGFR-2 occurs frequently but also occurs in lower-grade gliomas and in their recurrent tumors [82]. Elevated expression of VEGFR-2 in HCC (hepatocellular carcinoma) was correlated with worse outcome following liver transplantation. Vascular invasion was consistently associated with HCC recurrence (P<0.01) and overall mortality (P<0.05). Subjects with VEGFR-2 overexpression in tumor arterioles (P<0.01), venules (P<0.05) had worse overall survival [83]. Table I shows relative levels of expression of VEGFR-2 in different cancer types.
Table I.
VEGFR-2 expression in human tumors and tumor cell lines
| Tissue | Subtype | Positive rate | Reference |
|---|---|---|---|
| Bladder | Carcinoma | 50% | [81] |
| Brain | Glioma | 6~17% | [82] |
| Breast | Adenocarcinoma | 64.5% | [76] |
| Cervix | Adenosquamous carcinoma | 73.3% | [77] |
| Colon | Carcinoma | 46.7% | [78] |
| Esophagus | Carcinoma | 100% | [79] |
| Kidney | Clear cell cancer | 35% | [80] |
| Lung | Non-small cell carcinoma | 54.2% | [198] |
| Oral | Carcinoma | ↑↑ | [199] |
| Ovary | Carcinoma | 100% | [200] |
| Pancreas | Carcinoma | 80% | [201] |
| Prostate | Cancer* | 100% | [202] |
| Skin | Melanoma | ↑↑ | [203] |
Cell lines; ↑↑, overexpression of VEGFR-2 in cancer cells
Interestingly, in breast cancer VEGFR-2 expression was significantly correlated with two well-established proliferation indices, Ki-67 (P = 0.037) and topo-IIα (P = 0.009). This suggests VEGF may exert growth factor activities on mammary cancer cells through its receptor VEGFR-2 [76]. Taken together, these data suggest that high-levels of VEGFR-2 may potentially serve as a biomarker in cancer.
7. Regulation of VEGFR-2 levels
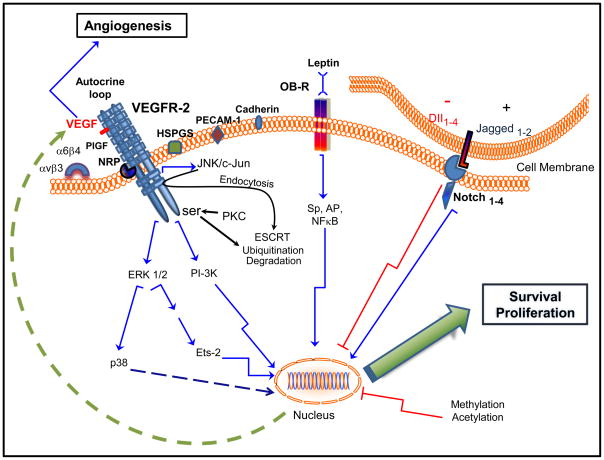
Despite the essential role of VEGFR-2 in angiogenesis and carcinogenesis the molecular mechanisms controlling its expression are only partially known. Regulation of VEGFR-2 expression involves a series of complex mechanisms, which include epigenetic changes, transcriptional regulation, cellular localization/trafficking, ligand binding, co-activator activity, adhesion molecule expression, constitutive-embryonic derived signaling pathways and cytokine-growth factor regulation. In addition, VEGFR-2 can assemble functional complexes composed of homodimers and heterodimers with other VEGF receptors and co-receptors that can bind VEGF or other angiogenic ligands thereby affecting VEGFR-2 signaling capabilities (Fig 2).
Fig. 2.
Possible mechanisms involved in the regulation of VEGFR-2 levels in cancer cells. VEGF promotes tumor angiogenesis and upregulates VEGFR-2 in cancer cells increasing survival and proliferation through an autocrine/paracrine loop (MAPK/ERK1/2/p38 and PI-3K signaling pathways). VEGFR-2 can form homodimers or heterodimers (VEGFR-2/VEGFR-1 and VEGFR-2/VEGFR-3). PIGF binds with high affinity to VEGFR-1 but also to VEGFR-2/VEGFR-1 heterodimers. VEGFR-1, when activated by PIGF, switches on intermolecular cross talk, which transactivates VEGFR-2 and thereby enhances the response to VEGF [33]. NRP stabilizes the VEGF/VEGFR-2 complex but this could be hampered by β3 integrin. The α6 integrin can decrease VEGFR-2 expression. HSPGS, PECAM-1 and VE-cadherin can regulate ligand binding and phosphorylation of VEGFR-2. Leptin and Jagged1-Notch signaling upregulates VEGFR-2 expression. In contrast, DII4-Notch signaling downregulates VEGFR-2 expression. Endocytosis and ubiquitin-mediated downregulation of VEGFR-2 linked to ESCRT (endosomal sorting complex required for transport) is mediated by JNK/c-Jun and PKC signaling pathways activated by VEGF. Deacetylation/methylation of VEGFR-2 gene could also play a role in its epigenetic control.
7.1. VEGFR-2 co-activators, cellular localization and trafficking
Heparin and heparan sulfates (components of proteoglycans, HSPGS) have affinity for VEGF165 (VEGF-A), the major isoform of VEGF, promoting enhanced phosphorylation of VEGFR-2. This probably occurs by enhancing ligand binding capabilities to VEGFR-2. Therefore, HSPGs affect the localization, extent and intensity of VEGFR signaling [84]. NRP-1 can stabilize the VEGF/VEGFR-2 complex and particularly increase tumor angiogenesis [85]. Blood flow could likely activate VEGFR-2 through the formation of mechanosensory complexes [86]. Adhesion molecules (PECAM-1 and VE-cadherin) [86, 87] and αvβ3 integrin [88] interplay with VEGFR-2 under diverse biological scenarios controlling VEGFR-2 expression and signaling. β3-integrin can limit the interaction of NRP-1 with VEGFR-2, thus negatively affecting VEGF-mediated angiogenesis [89]. Invasive ductal carcinoma has decreased expression of α6-integrin associated with higher tumor angiogenesis presumably linked to VEGFR-2 expression. Indeed, loss of α6-integrin correlates to overexpression of activated VEGFR-2 in murine melanoma and lung carcinoma in endothelial-specific α6-knockout mice [90]. However, the specific mechanisms involved in VEGFR-2 overexpression in tumors from these mice remains to be investigated. A negative regulator of angiogenesis is thrombospondin-1 (TSP-1 or CD36). This molecule negatively modulates VEGF actions through a complex with β1-integrin and VEGFR-2 [91].
Inverse regulation of VEGFR-1 and VEGFR-2 could play an important role in controlling the growth and differentiation of tumor-associated EC. VEGF signaling through JNK/c-Jun pathway induces endocytosis, nuclear translocation and ubiquitin-mediated downregulation of VEGFR-2 in human squamous-cell carcinomas [92]. A model recently proposed suggests that a negative feed-back loop regulates VEGFR-2 activities through the differential segregation/localization of VEGFR-1 and VEGFR-2 [89, 90]. The higher affinity of VEGFR-1 for VEGF blocks VEGFR-2 activation. In the model, Ca (2+) induces the translocation of VEGFR-1 from the trans-Golgi network to the plasma membrane allowing preferential binding of VEGF. VEGFR-2 is degraded after activation by ubiquitin-mediated proteolysis that is linked to ESCRT (endosomal sorting complex required for transport) and to Rab GTPases [93]. This could also be stimulated by PKC pathway that requires the removal of VEGFR-2 carboxyl terminus [94]. Differential trafficking of VEGFR-2 is potentially due to the formation of complexes with diverse angiogenic regulators. These processes occur through the endosomal pathway controlling angiogenesis [95].
7.2. Regulation of VEGFR-2 expression: Cis-elements of VEGFR-2 promoter
The VEGFR-2 gene promoter is highly complex with multiple cis-elements [96]. Regulation of VEGFR-2 expression is dependent on a number of factors, including cell context. VEGFR-2 gene promoter in humans (KDR) and in mice (Flk-1) does not have a TATA box region, but does have several DNA binding sites for general and tissue-specific transcription factors. Therefore, VEGFR-2 belongs to class II promoter structure. This TATA-less gene contains four upstream Sp1 sites and a single transcription start site [97] that binds multi-functional transcription factor TFII-I for gene expression [98]. Transfection of VEGFR-2 promoter constructs into bovine aortic EC (BAECs) showed that basal activity of VEGFR gene was primarily associated with the GC-rich −95 to −60 region of the promoter, which contains Sp, AP-2, and nuclear factor-κB (NFκB motifs) [96]. cAMP response element binding protein (CREB) and NFκB–related antigens bind specific sequences in the VEGFR-2 promoter of BAECs [98].
DNA footprinting studies also showed that protected sequences between −110 and −25 in human umbilical vein EC (HUVECs) were critical for VEGFR-2 promoter activity. However, comparable interactions were not observed in fibroblasts or HeLa cells [99]. Other studies also showed that the GC-rich −79 to −68 region of the promoter was essential for activity in EC [100]. This region bound both Sp1 and Sp3; however, their results suggested that Sp1 expression enhanced VEGFR-2 expression but that Sp3 attenuated this response. In contrast, another report showed that basal and shear stress-induced activation of VEGFR-2 promoter constructs in HUVECs was primarily dependent on two more proximal GC-rich sites at −58 and −44 bp [101]. Sp1-dependent DNA binding within −77 and −60 region of VEGFR-2 promoter seems to be essential for the regulation of both mRNA and protein in human EC by Rac-1 (a small Rho-GTPase) [102]. Consensus or nonconsensus estrogen receptor element (ERE motifs) has not been found in 5′ VEGFR-2 promoter region [96]. Results from chromatin immunoprecipitation assays (ChiPs) demonstrated that ER is constitutively bound to the VEGFR-2 promoter and that these interactions are not enhanced after treatment with estradiol (E2), whereas ER binding to the region of the pS2 promoter (a human breast cancer marker) containing an ERE element is enhanced by E2 [103]. Hormone-induced activation of the VEGFR-2 promoter constructs requires Sp3 and Sp4 but not Sp1, demonstrating that hormonal activation of VEGFR-2 involves a nonclassical mechanism in which ERα/Sp3 and ERα/Sp4 complexes activate GC-rich sites where Sp proteins, but not ERα, bind DNA [103]. Analysis of the mouse VEGFR-2 promoter sequence revealed the presence of binding sites of additional transcriptional factors, which related to angiogenesis previously reported. Putative transcription factor binding sites are systematically listed in Table II. Investigations using assays for transcription factor binding (EMSA, electrophoretic mobility shift assay, or others), ChiPs and luciferase reporter gene (containing binding site mutations) will be essential to further elucidate the molecular mechanisms involved in the regulation of VEGFR-2.
Table II.
Putative regulatory elements in the 5′-flanking region of the mouse VEGFR-2 gene
| Factor | Sequence | Position * | Reference |
|---|---|---|---|
| IRF-1 | TTTCCTCTT | −932/−924 | [204] |
| ROR α1 | TGAAAGGTCA | −859/−850 | [205] |
| RXR-α | GAAAGGTCA | −858/−850 | [206] |
| RAR-α1 | GAAAGGTCAG | −858/−849 | [207] |
| Sp-3 | CCAGCTCCCTG | −712/−702 | [100] |
| FOXO3a | TGGATAAG | −565/−558 | [208] |
| Elf-1 | AAAACAAAAC | −497/−488 | [209] |
| NF-κB | TTGGGACTTTCA | −385/−374 | [210] |
| AhR | GGGGCGTGG | −118/−108 | [211] |
| Pbx1b | CGGACGCAGGG | −108/−98 | [212] |
| PPAR-α | TGACCCC | −76/−70 | [213] |
| JunD | TGACCCC | −76/−69 | [214] |
| Sp1 | ACCCCGCCCC | −74/−65 | [102] |
| TFII-I | CTGCCCTGAGTCC | −34/−22 | [98] |
| P53 | Not shown | 21 sites | [215] |
| STAT5 | Not shown | 10 sites | [216] |
| STAT3 | Not shown | 6 sites | [217] |
| ER α | Not shown | 5 sites | [135, 136] |
List of the putative regulatory elements corresponding to the consensus sequence and their positions relatively to the transcriptional start site.
IRF-1: interferon regulatory factor I; RORα-1: the RAR-related orphan receptor or NR1F1 (nuclear receptor subfamily 1, group F, member 1); RXR-α: Retinoid X receptor alpha or NR2B1 (nuclear receptor subfamily 2, group B, member 1); FOXO3a: Forkhead box receptor transcription factor 3a; Elf-1: Ets domain transcription factor; AhR: Aryl hydrocarbon receptor; Pbx1b: Pre-B-cell leukemia transcription factor 1; PPAR-α: Peroxisome proliferator-activated receptor; JunD: Transcription factor.
7.3. Epigenetic regulation of VEGFR-2
Growth of malignant cells including breast cancer and metastasis involves coordinating changes in expression programs of multiple genes. Since genomic expression programs are regulated by the epigenome, it stands to reason that epigenomic changes plays a critical role in oncogenesis. Histone modifications could be promoted by several processes including methylation, acetylation, phosphorylation, and ubiquitination that modulate binding interactions with specific proteins and chromatin structure. Transcriptionally active regions are generally hypoacetylated or hypermethylated. Main mechanisms involved in epigenetic regulation of gene expression are methylation of chromatin proteins and deacetylation of DNA nitrogen bases related mainly to methyltransferase and deacetylase activities, respectively [104].
The promoter DNA-methylation of specific genes is a well-known mechanism of epigenetic gene silencing in oncogenesis [105]. DNA methylation, especially in CpG-rich 5Prime; regions, inhibits transcription by interfering with the initiation or by reducing the binding affinity of sequence-specific transcription factors. Yamada et al, reported that aberrant promoter methylation of VEGFR-1 (Flt-1) was found in 24 (38.1%) of 63 primary local prostate cancer samples, while in all 13 benign prostate samples it was not [106]. These findings suggest that methylation of VEGFR-1 is related to prostate carcinogenesis. Nevertheless, it is still unclear what role the VEGFR-1 plays in the development and progression of prostate cancer [106]. A putative oncogenic enzyme, Smyd3, dimethylates VEGFR-1 at lysine 831 (K831me2). However, the biological consequences on tumor growth of these epigenetic changes are unknown [107].
Scarce information on VEGFR-2 methylation is currently available. In a recent report, VEGFR-2 methylation was detected at a significantly higher level in cancer tissues of stomach, colon and hepatocellular cancer [108]. It is not know whether VEGFR-2 methylation also occurs in human breast cancer. As VEGFR-2 was not detected in some cases of invasive breast carcinomas, this may implicate hypermethylation of VEGFR-2 as a cause for this subset of breast cancer patients [76]. Results from our laboratories show that the inhibition of VEGFR-2 gene methylation using aza-2′-deoxycytidine (5-AZA) significantly increases the expression of VEGFR-2 mRNA in mouse mammary cancer cells. These data support the hypothesis that VEGFR-2 methylation could play an important role in regulating VEGFR-2 expression in breast cancer (unpublished results).
Another epigenomic regulatory mechanism of VEGFR-2 is chromatin modification. Chromatin packages the genetic information in either accessible or inaccessible configurations [109, 110]. Some extensively studied modifications are methylation and acetylation of lysines at the tails of H3 and H4 histones [111, 112]. The state of histone acetylation is dynamic and is determined by equilibrium of acetylation catalyzed by histone acetyltransferases (HAT) and deacetylation catalyzed by histone deacetylases (HDAC) [113]. HATs and HDACs are recruited to target genes by either activator or repressor complexes [113]. Deacetylation of VEGFR-2 gene could also play a role in its epigenetic control. Trichostatin A (TSA, an inhibitor of HDAC) upregulates the expression of VEGFR-1 and VEGFR-2, but downregulated NRP-1 and NRP-2 in non-small cell lung cancer [114]. Functional studies in BAECs demonstrate that cAMP represses whereas tumor necrosis factor-α (TNF-α) an activator of NFκB, stimulates promoter activity. Histone acetyltransferases (HATs) P/CAF and CBP/p300 together with p65/RelA, the catalytic subunit of NFκB, significantly increase VEGFR-2 promoter activity [98]. We have used TSA to study VEGFR-2 gene transcription in mouse mammary cancer cell lines. TSA induced dramatic elevations of VEGFR-2 mRNA and protein levels in some cell lines. These results suggest that, in breast cancer cells, histone acetylation does modulate VEGFR-2 gene transcription (unpublished data).
7.4 The Notch signaling-VEGFR-2 link
Notch signaling pathway functions in an enormous diversity of developmental processes and its dysfunction is implicated in many cancers. This evolutionarily conserved pathway in multicellular organisms regulates embryonic and stem cell fate [115]. It is generally believed that tumor angiogenesis will not occur in absence of Notch signaling. When a Notch decoy is introduced in place of a functional Notch receptor at a tumor site in the skin during angiogenesis, cell proliferation stops and the development of the new blood vessels ceases. This suggests that the Notch protein has some significant role in angiogenesis [116, 117]. Indeed, active Notch1 [118] or Notch4 [119] signaling are involved in breast cancer angiogenesis. Soares et al, first demonstrated that a cross talk between Notch and E2 signaling occurs in breast cancer and EC. Notch gene expression was required for tubule-like structure formation in EC. Notch gene expression clustered with hypoxia inducible factor-1 alpha (HIF-1α) and was upregulated by E2. Thus, Notch has significant role in human breast carcinogenesis and angiogenesis [120].
Notch is a family of four mammalian transmembrane proteins that function as a receptor for membrane bound ligands. There are four mammalian Notch genes, Notch1–Notch4, and five ligands, Jagged1 and Jagged2 (homologs of Drosophila Serrate-like proteins) and Delta-like 1 (DLL1), DLL3 and DLL4. Notch receptors have an extracellular domain made up of multiple epidermal growth factor (EGF) domain; yet, its intracellular domain is made up of many domain types [121]. The Notch proteins have been proven to affect diverse cell programs (proliferation, differentiation, and apoptosis) and as result Notch influences organogenesis and morphogenesis [122]. The activation of a Notch receptor is triggered by ligands expressed on adjacent Jagged and Delta cells. However, it has been found that Dll4 and Jagged1-Notch signaling pathways have opposing effects on angiogenesis [123]. While Jagged1-Notch signaling serves as a proangiogenic regulator [123], Dll4-Notch signaling has been shown to significantly decrease the expression of VEGFR-2 thus inhibiting the proliferation of angiogenic cells [124]. These two signals operate in equilibrium with one another, so that as the concentration of one signal increases the other will decrease proportionally. As a result of the ligand signaling competitive nature towards one another, it is plausible to speculate that the two are used as antagonistic mechanisms to help regulate the processes of angiogenesis [123, 124]. In vitro, the activation of Notch1 or Notch4 in EC induces the expression of the HESR-1 transcription factor (expressed in mature vasculature, but reduced in proliferating EC) that in turns downregulates VEGFR-2. Notch-mediated reduction in VEGFR-2 levels results in decreased EC proliferation. This Notch mechanism may be involved in the phenotypic changes during EC proliferation and migration to network formation [125]. Overall, one could speculate that the two types of Notch ligands operate to signal the VEGFR-2 to either continue to promote cell proliferation or move onward in the angiogenic process to EC differentiation. Activation of Notch signaling in ER-negative breast cancer cells results in direct transcriptional up-regulation of the apoptosis inhibitor and cell cycle regulator survivin (baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5). Survivin is highly expressed by cancer cells and binds and inhibits caspase-3, controlling the checkpoint in the G2/M-phase of the cell cycle. Therefore, the Notch-survivin functional gene signature is a hallmark of basal breast cancer, and may contribute to disease pathogenesis [126, 127]. In addition, Notch signaling modulates other pathways, such as PI-3K–Akt and NF-κB, also activated by VEGFR-2 signaling as discussed above. Therefore, crosstalk between Notch and VEGFR-2 signaling may be crucial for angiogenic processes.
In human MCF-7 BC cells over-expression of the γ-secretase (the enzyme that catalyzes intramembrane cleavage of the Notch receptor upon ligand binding required for Notch activation) liberated Notch intercellular domain and increased HIF-1α protein levels by an unknown mechanism [126, 127]. Notch1 signaling can promote NFκB translocation to the nucleus and DNA binding by increasing both phosphorylation of the IκB kinase α/β complex (a repressor of NFκB activation) and the expression of some NFκB family members [128]. We have found that leptin (an adipocytokine) activated NFκB, Sp1 and HIF-1α and increased the expression of Notch mRNA and protein in breast cancer cells under normoxic conditions. Remarkably, leptin induced the expression of Notch1–4, Jagged1 and VEGFR-2 in these cells. In particular, leptin-mediated activation of NFκB increased VEGFR-2 and Notch. Furthermore, leptin increased through several signaling pathways promoter activities of VEGFR-2-Luc transfected-cells. Interestingly, leptin effects on VEGFR-2 were abrogated by a γ–secretase inhibitor. Moreover, VEGFR-2 transcription and expression was heavily dependent on VEGFR-2 gene methylation and histone acetylation that could be linked to leptin and Notch effects (unpublished data). However, the role of Notch-mediated regulation of VEGFR-2 and signaling crosstalk in cancer cells is so far unknown.
7.5 Estrogen-mediated regulation of VEGFR-2
Estrogens exert important regulatory functions on vessel wall components, which may contribute to the increased prevalence and severity of certain chronic inflammatory, autoimmune diseases, as well as tumor initiation, progression, particularly in tumors of the breast, endometrium, ovary and prostate [129–131]. EC have also been identified as targets for estrogens. ERs have been found in EC from various vascular beds. The regulatory functions of estrogen in EC responses are relevant to vessel inflammation, injury, and repair. In these cells, estrogen affects nitric oxide production and release, modulates the expression of EC-adhesion molecules and regulates angiogenesis [132–134].
The mechanisms through which estrogen regulates VEGFR-2 in angiogenesis are complex and may involve both genomic and non-genomic effects. It was earlier reported that estrogen stimulates EC growth as well as VEGF-dependent angiogenesis by the receptor-mediated pathway, especially ERα [135, 136]. However, non-classical mechanisms through ERα/Sp3 and ERα/Sp4 complexes were found in some cancer cell lines, such as ZR-75 breast cancer cells. In these cells E2 activates GC-rich sites where Sp proteins but not ER-α bind to VEGFR-2 promoter to stimulate mRNA and protein expression [103]. In contrast, in MCF-7 cells, the ERα/Sp protein-VEGFR-2 promoter interactions involve the recruitment of the co-repressors SMRT (silencing mediator of retinoid and thyroid hormone receptor) and NCoR (nuclear receptor corepressor) resulting in decreased VEGFR-2 mRNA levels [137].
7.6 Cytokine and growth factor regulation of VEGFR-2
VEGF is hypoxia-inducible showing a temporal expression pattern that generally parallels VEGFR-2 expression. Contradictory data on the direct role of hypoxia in the regulation of VEGFR-2 were reported. The differential and synergistic regulation of VEGF and VEGFR-2 by hypoxia in an organotypic cerebral slice culture system for EC was linked to a direct induction of VEGF that subsequently up-regulates VEGFR-2 in EC. VEGF-induced VEGFR-2 up-regulation was abrogated by a neutralizing anti-VEGF antibody [138]. In contrast, it was later reported that hypoxia up-regulates VEGFR-2 in cultured cells by a posttranscriptional mechanism [139].
Inflammation and angiogenesis are frequently coupled in pathological situations like breast cancer. One of the hallmarks of inflammation is an increase in vascular permeability frequently driven by an excess of VEGF and other mediators. Inflammation induces EC activation and capillary sprouting [140]. Pathological angiogenesis is associated with the secretion of cytokines. However, the molecular and cellular mechanisms linking chronic inflammation to tumorigenesis remain largely unresolved. Many cytokines and growth factors are able to increase VEGF expression [141]. However, a reduced number of these factors have been confirmed to regulate VEGFR-2 expression. The majority of inflammatory cytokines exert inflammatory effects through the induction of NFκB. a hallmark of inflammatory responses. Activation of NFκB is essential for promoting inflammation-associated cancer [142]. VEGF [142] and VEGFR-2 [98] promoters have NFκB responsive cis-elements. Therefore, cytokine-activated NFκB increases angiogenesis by direct upregulation of pro-angiogenic genes. VEGFR-2 is associated with inflammatory breast cancer and is, therefore, a target for cancer prevention.
Cytokines have diverse effects on VEGFR-2. PIGF, erythropoietin or PDGF were unable to up-regulate VEGFR-2 [138]. Transforming growth factor-beta (TGF- β) can down-regulate VEGFR-2 [143] but discordant results on TNF-α mediated down-regulation [144] and up-regulation [145] of VEGFR-2 have been reported. Moreover, TNF showed contradictory effects on VEGFR-2 activity. TNF induced VEGFR-2, but blocked it signals, thus delaying the VEGF-driven angiogenic response [146]. Members of the chemokine family can also regulate VEGFR-2. CCL23 (also known as MPIF-1, MIP-3, or Ckb8) is a CC chemokine initially characterized as a chemoattractant for monocytes and dendritic cells. In HUVEC, CCL23 mainly induced KDR/Flk-1 expression at the transcriptional level. These effects were linked to CCL23-mediated phosphorylation of SAPK/JNK [147].
7.6.1 Leptin regulation of VEGFR-2
Recently, leptin was added to the list of factors that upregulate VEGF and VEGFR-2 [61, 62, 64]. Leptin actions are more often than not related to energy balance. However, leptin is also recognized for its contributions to reproduction, angiogenesis, proliferation and inflammation. Leptin’s actions are now being linked to the development and pathogenesis of cancer [148, 149]. Higher levels of leptin are found in female, postmenopausal women and obese individuals. The leptin levels have been related to the incidence of various types of cancer, most notably breast cancer [150, 151]. Leptin is a pleitropic adipocytokine, with mitogenic and angiogenic effects, that promotes anchorage, proliferation of breast cancer cells, microvessel and hematopoiesis and increase the levels of several factors including cell cycle regulators [61, 62, 149]. Breast carcinoma cells express higher levels of leptin and leptin receptor, OB-R, than normal mammary cells and a significant correlation between leptin/OB-R levels with metastasis and lower survival of breast cancer patients has been found [148, 152, 153]. A role for leptin in rodent mammary tumors has been documented. Restriction of caloric intake (25%) reduces mammary tumor incidence and growth in rats treated with 7,12-dimethylbenz[α]anthracene (DMBA) either fed with low or high-fat diets [154]. Moreover, studies on leptin (ob/ob) and OB-R (db/db) deficient mice have provided compelling data supporting a role for leptin in breast cancer development. Obese mice with deficiency in leptin signaling show a significantly lower incidence of mammary tumors than their lean littermates. MMTV (mouse mammary tumor virus)-TGF-α mice have a proclivity to develop mammary tumors but when crossed with leptin/OB-R deficient mice, there is a reduced incidence of mammary tumors in their progeny [155, 156].
Leptin acts through binding to specific membrane receptors of which six isoforms (OB-R a–f) have been identified at the present time [157–159]. Binding of leptin to its receptor induces activation of several canonical (JAK2/STAT; MAPK/ERK 1/2 and PI-3K/AKT1) and non-canonical signaling pathways (PKC; stress-activated protein kinase c-Jun N-terminal kinase, JNK and p38 MAP kinase) to exert its biological effects in food intake, energy balance, and adiposity as well as in the immune and endocrine systems [160, 161]. We have previously found that leptin signaling plays an important role in the growth of breast cancer that is associated with the regulation of pro-angiogenic, pro-inflammatory and pro-proliferative molecules [62]. Leptin increases VEGFR-2 expression in endometrial cancer cells in vitro [64] and in breast cancer cells in vitro and in vivo [61, 62]. In 4T1 cells leptin-mediated upregulation of VEGFR-2 was unaffected by treatment with siRNA targeted against STAT3 whereas inhibition of JAK2, PI-3K and MAPK cascades attenuated the leptin induced increase in VEGFR-2 [62]. In human breast cancer cells ER-positive (MCF-7) or ER-negative (MDA-MB-231), leptin in a dose-response manner significantly increased the levels of VEGFR-2 protein and mRNA [59]. However, the molecular mechanisms of how leptin signaling regulates VEGFR-2 are largely unknown [162–164].
Leptin is able to induce the growth of breast cancer cells through activation of the JAK2/STAT3, ERK1/2, and/or PI-3K pathways, and can mediate angiogenesis by inducing the expression of VEGF [163, 165]. Leptin-mediated activation of mTOR (mammalian target of rapamycin), mainly linked to MAPK, played a central role in leptin regulation of VEGF and VEGFR-2 in endometrial cancer cells [64]. In addition, leptin induces transactivation of the HER2/neu proto-oncogene (c-erbB-2), and interacts in triple negative breast cancer cells with insulin like growth factor-1 (IGF-1) to transactivate the EGF-receptor (EGFR), thus promoting invasion and migration [166, 167]. Leptin can also affect the growth of ER-positive breast cancer cells by stimulating aromatase expression and thereby increasing estrogen levels through the aromatization of androgens, and by inducing MAPK-dependent activation of ER [163, 168]. On the other hand, as it was mentioned in section 7.4, leptin modulates Notch expression in mouse mammary cancer cells. Therefore, leptin-mediated activation of PI-3K/Akt1/NFκB could be part of the crosstalk linking leptin and Notch signaling to regulate VEGFR-2.
In recent reports, the disruption of leptin signaling using pegylated leptin peptide receptor antagonist (PEG-LPrA2) markedly reduced the growth of tumors and the expression of VEGF/VEGFR-2 in mouse models of syngeneic and human breast cancer xenografts [61, 62]. The mice treated with PEG-LPrA2 had diminished expression of VEGF/VEGFR-2, OB-R, leptin, IL-1R tI, PCNA and cyclin D1 [59]. PEG-LPrA treatment did not affect mouse leptin levels in plasma suggesting that this compound did not interfere with the systemic leptin metabolism. Pharmacokinetic and toxicological studies suggested that the high molecular weight PEG-LPrA2 derivative does not travel through the blood-brain barrier and therefore it is not bound to hypothalamic OB-R or accumulated in the central nervous system of mice. This suggests PEG-LPrA2 may not interfere with leptin biological actions at hypothalamic level. Indeed, no significant effects on body or carcass weight were found between mice treated with PEG-LPrA2 or control [59]. These data suggest that inhibition of leptin signaling may serve as a novel adjuvant for prevention and treatment of breast cancer. The dramatic reduction of tumor growth could be directly related to the reduction of VEGF/VEGFR-2 levels and thereby to the abrogation of the VEGF/VEGFR-2 autocrine/paracrine cancer cell survival loop. This could be especially important for populations under higher risk and exhibiting higher levels of leptin such as obese and post-menopausal women. The alarming increase of incidence of obesity in the Western countries emphasizes the importance of these findings [126, 127].
7.7 Clinical Significance of Targeting VEGFR-2
Solid tumor malignancies, including breast, lung and prostate carcinomas, are considered to be angiogenesis dependent. However, antiangiogenic therapies have shown varying results partly because each tumor type secretes a distinct panel of angiogenic factors to sustain its own microvascular network. Additionally, recent evidence has demonstrated that tumors develop resistance to antiangiogenic therapy by turning on alternate angiogenic pathways when one pathway is therapeutically inhibited. The redundancy of these angiogenic pathways provides a plethora of targets for intervention. It is likely that successful complete inhibition of angiogenesis will rely on the use of combination and/or sequential therapies [169, 170]. In this section we will briefly discuss experimental therapies for angiogenesis inhibition in breast cancer.
As already discussed, the role of angiogenesis in carcinogenesis is complex and mediated by many different factors. The central role of the Notch receptor, and its attendant Jagged and Delta cell ligands, and their role in angiogenesis has led to the development of Notch signaling inhibition as a therapeutic intervention. The GSIs or small molecule inhibitors of γ-secretase have shown inhibition of tumorigenesis. The main disadvantage with the use of GSIs is their non-specificity. Many physiological processes require Notch signaling, therefore, toxicity profiles may be profound. Additionally, subsequent development of antibodies directed specifically against the Notch receptor or its ligands would offer another therapeutic alternative [171].
Tumor hypoxia occurs as tumors grow subsequently leading to an increase in HIF-1α. Acetylation and deacetylation post-translational modifications are critical to HIF-1α signaling. As such, HDAC inhibitors can be considered as a possible therapeutic intervention in breast cancer treatment [172]. The use of HDAC inhibitors in mouse models in combination with VEGF receptor tyrosine kinase inhibitor decreases the expression of angiogenesis-related genes such as angiopoietin-2 and its receptor Tie-2, and survivin in EC. HDAC inhibitors are already in clinical use in treating hematologic malignanices such as myelodisplastic syndrome (MDS) and acute myeloid leukemia (AML) [173].
Regulation of VEGFR-2 expression by Sp proteins has been previously discussed in this paper (see section 7.2). VEGFR-2 can be targeted by drugs that down regulate Sp proteins or block Sp-dependent trans-activation [72]. Activation of VEGFR-2 via binding of Sp3 and Sp4 with ERα to promoter region of VEGFR-2 is enhanced by E2. It could be hypothesized that blocking secretion of E2 would downregulate this effect and hence inhibit angiogenesis [103, 174].
Some anti-angiogenic treatment strategies have entered the clinic to date. These agents include a humanized monoclonal antibody directed to VEGF-A (Bevacizumab; Avastin; Genentech Inc, South San Francisco, CA), a chimeric monoclonal antibody directed to the VEGFR-2 (IMC-1C11), several small molecule inhibitors of the VEGFR-2 tyrosine kinase, and a nuclease-stabilized ribozyme (Angiozyme), that specific cleaves both Flt-1 and KDR mRNA [175]. A compilation of antiangiogenetic agents targeting VEGF or VEGFRs now being tested in clinical trials can be found in Table III. Preclinical models have shown regression of solid tumor growth and angiogenesis with anti-VEGF monoclonal antibodies alone or in combination with chemotherapy [176–178]. Clinical benefit with bevacizumab has been reported from clinically trials in metastatic colon, renal cell, and breast cancer [179–182]. Bevacizumab was able to significant decrease (66.7%) phosphorylated VEGFR-2 (Y951) in tumor cells and increase in tumor apoptosis after one cycle of bevacizumab alone [181]. Applications of bevacizumab therapy are not confined to cancer. Macular degeneration, a disorder of the retina that is enhanced by age, is the most common cause of irreversible vision loss in older people. The disease is characterized by abnormal blood vessels grow beneath the retina. Therefore, several anti-angiogenic treatments are being tested in the clinic. Among these, anti-VEGF therapies have potential successful applications. Investigations from intravitreal bevacizumab (Avastin) therapy showed promising 6-month results in patients with neovascular macular degeneration [183].
Table III.
Antiangiogenetic drugs targeting VEGF or VEGFRs in clinical trials
| Drug | Target | Clinical trial | Reference |
|---|---|---|---|
| Bevacizumab | VEGF/R | FDA approved | [179–182] |
| IMC-1C11 | VEGFR-2 | Phase I, II | [184–186] |
| SU5416 | VEGFR-2 | Phase I, II | [187, 188] |
| PTK 787 | VEGFR-1/2 | Phase I, II | [190] |
| ZD6474 | VEGFR-2, EGFR | Phase I, II | [189] |
| CP547632 | VEGFR-2, PDGFR, FGFR | Phase II | [191] |
| SU11248 | VEGFR-1/2, PDGFR, Kit | Phase I, II | [218] |
| SU6668 | VEGFR-2, PDGFR | Phase II | [219, 220] |
| YM-359445 | VEGFR-2 | Phase I, II | [221] |
| AZD2171 | VEGFR-2 | Phase I, III | [222–224] |
| CT-322 | VEGFR-2 | Phase I, II | [225] |
| CEP-7055 | VEGFR-2 | Phase I | [226, 227] |
IMC-1C11, a chimeric monoclonal antibody, binds specifically to the EC-surface extracellular domain of VEGFR-2, blocks VEGF-VEGFR-2 interaction and prevents VEGFR-2 activation of the intracellular tyrosine kinase pathway [184, 185]. The initial Phase I trial of IMC-1C11 was carried out in patients with metastatic colorectal carcinoma. This has provided evidence of the safety and low toxicity for antibody blockade of VEGFR-2, as well as insight into dose and schedule requirements [186]. A fully human anti-VEGFR-2 agent has been produced as a second-generation agent, which is anticipated to be nonimmunogenic for chronic administration as a single agent and in combination with chemotherapy or radiation. Semaxanib (SU5416) was the first specific synthesized potent and selective inhibitor of the VEGFR tyrosine kinase that is presently under evaluation in Phase I clinical studies for the treatment of human cancers [187]. SU5416 was showed to induce growth inhibition in mouse xenotransplants of human tumors. But in several phase II trials, results were disappointing, albeit providing a good security profile [188]. There other additional inhibitors of the VEGFR-2 tyrosine kinase that are currently being examined in clinical trials, such as PTK 787, ZD6474, and CP547632, which have been selected on the basis of relatively selective inhibition of the VEGFR-2 ATP binding site [189–191]. SCC-S2, a novel antiapoptotic molecule, has shown to decrease the proliferation and tumorigenicity of MDA-MB435 human breast cancer cells [192]. Treatment of these cells with a cationic liposomal formulation of SCC-S2 antisense oligo correlated with decreased expression of VEGFR-2 in tumor cells as well as human lung microvascular EC and loss of cell viability [193]. Targeted therapies with the introduction of adenoviral vector expressing inducible Caspase-9, (iCaspase-9) under transcriptional regulation with EC-specific VEGFR-2 promoter induced apoptosis of proliferating human dermal microvascular EC (HDMECs) [194].
New compounds/technologies are being developed to target VEGFR-2. Mice treated with VEGFR-2-based DNA vaccine showed significant reduction of renal carcinomas [195]. A series of dual c-Met/VEGFR-2 kinase inhibitors were found to significantly affect growth of human xenografts [196]. Medicinal plants could be new sources for anti-VEGFR-2 drugs. Acetyl-11-keto-beta-boswellic acid (AKBA) derived from Bowawellia serrata inhibits prostate tumor growth by blocking VEGFR-2 signaling [197]. Previous studies in our laboratory have demonstrated that leptin-signal inhibition resulted in decreased growth of mammary tumors derived from mouse and humans. The expression of VEGF and VEGFR-2 was increased under leptin signaling in cell culture and decreased by the actions of leptin peptide antagonists in vitro and in vivo [62]. Thus, our data strongly suggest that leptin signaling inhibition could serve as an additional preventative and/or therapeutic modality for breast cancer.
8. Conclusions
VEGFR-2 is an important factor for EC development and angiogenesis. Aberrant VEGFR-2 expression/signaling are found in cancer. Paracrine effects of VEGF and diverse cytokines/growth factors secreted by cancer cells up-regulated VEGFR-2 in EC. Cytokines/growth factors also orchestrate autocrine/paracrine upregulation of VEGFR-2 in cancer cells that is essential for the survival/proliferation actions of VEGF/VEGFR-2 loop. Targeting VEGFR-2 overexpression in endothelial/malignant cells could be an effective way to treat breast cancer. Additional studies are needed to further test and develop specific anti-VEGFR-2 therapies. Deeper understanding of VEGFR-2 regulation and signaling crosstalk mechanisms in cancer cells will likely lead to the development of new therapeutic modalities. Translational studies are needed to test these agents for efficacy and toxicity in the breast cancer patient population.
Acknowledgments
This work was supported in part by Grants from NIH/NCI 1SC1CA138658-01; NIH/UAB Breast SPORE Career Development Award, Susan Komen Foundation for the Cure, and the Georgia Cancer Coalition Distinguished Cancer Scholar Award to R.R.G-P., and facilities and support services at Morehouse School of Medicine (NIH RR03034 and 1C06 RR18386).
Glossary
- 4T1 cells
mouse mammary cancer cell line
- 5-AZA
aza-2′-deoxycytidine
- AhR
aryl hydrocarbon receptor
- Akt
protein kinase B
- AP-1/-2
the activator protein 1 and 2
- BAECs
bovine aortic endothelial cells
- Cdk-2
cyclin-dependent kinase 2
- ChiPs
chromatin immunoprecipitation assays
- CREB
cAMP response element binding protein
- Cyclin D1
kinase and regulator of cell cycle D1
- DLL1–4
Delta-like 1–4
- E2
estradiol
- EC
endothelial cells
- EGFR
epidermal growth factor receptor
- Elf-1
Ets domain transcription factor
- EMSA
electrophoretic mobility shift assay
- ER
estrogen receptor
- ERK 1/2
extracellular regulated kinase 1 and 2
- ESCRT
endosomal sorting complex required for transport
- Ets
E-twenty six family of transcription factor
- FAK
focal adhesion kinase
- FGFR
fibroblast growth factor receptor
- FOXO3a
forkhead box receptor transcription factor 3a
- HAT
histone acetyltransferases
- HDAC
histone deacetylases
- HIF-1α
hypoxia regulated factor-1 alpha
- HUVECs
human umbilical vein endothelial cells
- IGF-1
insulin like growth factor-1
- IRF- 1
interferon regulatory factor I
- JAK2
Janus kinase 2
- JNK
c-Jun N-terminal kinase or SAPK (stress activated protein kinase)
- JunD
Transcription factor jun-D
- MAPK
mitogen activated protein kinase
- MCF-7
ER positive human breast cancer cell line
- MDA-MB231
ER negative human breast cancer cell line
- MEK
mitogen-activated protein kinase/extracellular signal-regulated kinase
- mTOR
mammalian target of rapamycin
- NFκB
eukaryotic nuclear transcription factor kappa B
- NRP-1/-2
Neuropilins 1 and 2 receptors
- OB-R
leptin receptor
- P38 kinase
extracellular regulated kinase 38
- Pbx1b
pre-B-cell leukemia transcription factor 1
- PDGF
platelet-derived growth factor
- PIGF
placental growth factor or PGF
- PI-3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- PLCg
phospholipase C gamma
- PPAR-α
Peroxisome proliferator-activated receptor-alpha
- P53
tumor suppressor protein 53
- Rac-1
a small Rho-GTPase
- RORα-1
the RAR-related orphan receptor or NR1F1 (nuclear receptor subfamily 1, group F, member 1)
- RTK
receptor tyrosine kinase
- RXR-α
Retinoid X receptor alpha or NR2B1 (nuclear receptor subfamily 2, group B, member 1)
- Sp1–3
Specificity protein 1–3
- STAT3
signal transducer and activator of transcription 3
- TAM
tamoxifen
- TFII
COUP transcription factor 2 or NR2F2
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
- TSA
Trichostatin A
- TSAd
T Cell–Specific Adapter
- TSP-1
Thrombospondin-1
- VEGF
Vascular endothelial growth factor
- VEGFR-1
Vascular endothelial growth factor receptor 1 or Flt-1
- VEGFR-2
Vascular endothelial growth factor receptor 2 or KDR or Flk-1
- VEGFR-3
Vascular endothelial growth factor receptor 3 or Flt-4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 2.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 3.Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- 4.Matthews W, Jordan CT, Gavin M, Jenkins NA, Copeland NG, Lemischka IR. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci U S A. 1991;88:9026–9030. doi: 10.1073/pnas.88.20.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 6.Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL, Shows TB. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991;6:1677–1683. [PubMed] [Google Scholar]
- 7.Pajusola K, Aprelikova O, Korhonen J, Kaipainen A, Pertovaara L, Alitalo R, Alitalo K. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992;52:5738–5743. [PubMed] [Google Scholar]
- 8.Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan JF, Kastelein R, Hudak S, Wagner J, Mattson J, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368:643–648. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- 9.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82:673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 11.Kabrun N, Buhring HJ, Choi K, Ullrich A, Risau W, Keller G. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development. 1997;124:2039–2048. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- 12.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 13.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 14.Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, Alitalo K. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 15.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Rappold I, Ziegler BL, Kohler I, Marchetto S, Rosnet O, Birnbaum D, Simmons PJ, Zannettino AC, Hill B, Neu S, Knapp W, Alitalo R, Alitalo K, Ullrich A, Kanz L, Buhring HJ. Functional and phenotypic characterization of cord blood and bone marrow subsets expressing FLT3 (CD135) receptor tyrosine kinase. Blood. 1997;90:111–125. [PubMed] [Google Scholar]
- 17.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 19.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 21.Oelrichs RB, Reid HH, Bernard O, Ziemiecki A, Wilks AF. NYK/FLK-1: a putative receptor protein tyrosine kinase isolated from E10 embryonic neuroepithelium is expressed in endothelial cells of the developing embryo. Oncogene. 1993;8:11–18. [PubMed] [Google Scholar]
- 22.Sarzani R, Arnaldi G, De Pirro R, Moretti P, Schiaffino S, Rappelli A. A novel endothelial tyrosine kinase cDNA homologous to platelet-derived growth factor receptor cDNA. Biochem Biophys Res Commun. 1992;186:706–714. doi: 10.1016/0006-291x(92)90804-t. [DOI] [PubMed] [Google Scholar]
- 23.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 24.Barleon B, Totzke F, Herzog C, Blanke S, Kremmer E, Siemeister G, Marme D, Martiny-Baron G. Mapping of the sites for ligand binding and receptor dimerization at the extracellular domain of the vascular endothelial growth factor receptor FLT-1. J Biol Chem. 1997;272:10382–10388. doi: 10.1074/jbc.272.16.10382. [DOI] [PubMed] [Google Scholar]
- 25.Davis-Smyth T, Chen H, Park J, Presta LG, Ferrara N. The second immunoglobulin-like domain of the VEGF tyrosine kinase receptor Flt-1 determines ligand binding and may initiate a signal transduction cascade. EMBO J. 1996;15:4919–4927. [PMC free article] [PubMed] [Google Scholar]
- 26.Shinkai A, Ito M, Anazawa H, Yamaguchi S, Shitara K, Shibuya M. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J Biol Chem. 1998;273:31283–31288. doi: 10.1074/jbc.273.47.31283. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Yamaguchi S, Sawano A, Shibuya M. Characterization of the extracellular domain in vascular endothelial growth factor receptor-1 (Flt-1 tyrosine kinase) Jpn J Cancer Res. 1997;88:867–876. doi: 10.1111/j.1349-7006.1997.tb00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin HG, Ziche M, Lanz C, Buttner M, Rziha HJ, Dehio C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999;18:363–374. doi: 10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, Shibuya M. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem. 1998;273:31273–31282. doi: 10.1074/jbc.273.47.31273. [DOI] [PubMed] [Google Scholar]
- 32.Shibuya M. Vascular endothelial growth factor receptor-2: its unique signaling and specific ligand, VEGF-E. Cancer Sci. 2003;94:751–756. doi: 10.1111/j.1349-7006.2003.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Shibuya M, Collen D, Conway EM, Carmeliet P. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 34.Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 35.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 36.Huang K, Andersson C, Roomans GM, Ito N, Claesson-Welsh L. Signaling properties of VEGF receptor-1 and -2 homo- and heterodimers. Int J Biochem Cell Biol. 2001;33:315–324. doi: 10.1016/s1357-2725(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 37.Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, Claesson-Welsh L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem. 2003;278:40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- 38.Mac Gabhann F, Popel AS. Dimerization of VEGF receptors and implications for signal transduction: a computational study. Biophys Chem. 2007;128:125–139. doi: 10.1016/j.bpc.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neagoe PE, Lemieux C, Sirois MG. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J Biol Chem. 2005;280:9904–9912. doi: 10.1074/jbc.M412017200. [DOI] [PubMed] [Google Scholar]
- 40.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 41.Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front Biosci. 2006;11:818–829. doi: 10.2741/1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kliche S, Waltenberger J. VEGF receptor signaling and endothelial function. IUBMB Life. 2001;52:61–66. doi: 10.1080/15216540252774784. [DOI] [PubMed] [Google Scholar]
- 43.Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 44.Solowiej J, Bergqvist S, McTigue MA, Marrone T, Quenzer T, Cobbs M, Ryan K, Kania RS, Diehl W, Murray BW. Characterizing the effects of the juxtamembrane domain on vascular endothelial growth factor receptor-2 enzymatic activity, autophosphorylation, and inhibition by axitinib. Biochemistry. 2009;48:7019–7031. doi: 10.1021/bi900522y. [DOI] [PubMed] [Google Scholar]
- 45.Singh AJ, Meyer RD, Band H, Rahimi N. The carboxyl terminus of VEGFR-2 is required for PKC-mediated down-regulation. Mol Biol Cell. 2005;16:2106–2118. doi: 10.1091/mbc.E04-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T, Shibuya M. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 1997;14:2079–2089. doi: 10.1038/sj.onc.1201047. [DOI] [PubMed] [Google Scholar]
- 47.Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- 48.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- 49.Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, Wang L, Wikner C, Qi JH, Wernstedt C, Wu J, Bruheim S, Mugishima H, Mukhopadhyay D, Spurkland A, Claesson-Welsh L. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005;24:2342–2353. doi: 10.1038/sj.emboj.7600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tahir SA, Park S, Thompson TC. Caveolin-1 regulates VEGF-stimulated angiogenic activities in prostate cancer and endothelial cells. Cancer Biol Ther. 2009:8. doi: 10.4161/cbt.8.23.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong Y, Huo Y, Chen C, Zeng H, Lu X, Wei C, Ruan C, Zhang X, Hu Z, Shibuya M, Luo J. Vascular endothelial growth factor (VEGF) receptor-2 tyrosine 1175 signaling controls VEGF-induced von Willebrand factor release from endothelial cells via phospholipase C-gamma 1- and protein kinase A-dependent pathways. J Biol Chem. 2009;284:23217–23224. doi: 10.1074/jbc.M109.019679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dayanir V, Meyer RD, Lashkari K, Rahimi N. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem. 2001;276:17686–17692. doi: 10.1074/jbc.M009128200. [DOI] [PubMed] [Google Scholar]
- 53.Issbrucker K, Marti HH, Hippenstiel S, Springmann G, Voswinckel R, Gaumann A, Breier G, Drexler HC, Suttorp N, Clauss M. p38 MAP kinase--a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J. 2003;17:262–264. doi: 10.1096/fj.02-0329fje. [DOI] [PubMed] [Google Scholar]
- 54.Andre T, Kotelevets L, Vaillant JC, Coudray AM, Weber L, Prevot S, Parc R, Gespach C, Chastre E. Vegf, Vegf-B, Vegf-C and their receptors KDR, FLT-1 and FLT-4 during the neoplastic progression of human colonic mucosa. Int J Cancer. 2000;86:174–181. doi: 10.1002/(sici)1097-0215(20000415)86:2<174::aid-ijc5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, Hicklin DJ. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 56.Mercurio AM, Bachelder RE, Bates RC, Chung J. Autocrine signaling in carcinoma: VEGF and the alpha6beta4 integrin. Semin Cancer Biol. 2004;14:115–122. doi: 10.1016/j.semcancer.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 57.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- 58.Aesoy R, Sanchez BC, Norum JH, Lewensohn R, Viktorsson K, Linderholm B. An autocrine VEGF/VEGFR2 and p38 signaling loop confers resistance to 4-hydroxytamoxifen in MCF-7 breast cancer cells. Mol Cancer Res. 2008;6:1630–1638. doi: 10.1158/1541-7786.MCR-07-2172. [DOI] [PubMed] [Google Scholar]
- 59.Svensson S, Jirstrom K, Ryden L, Roos G, Emdin S, Ostrowski MC, Landberg G. ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene. 2005;24:4370–4379. doi: 10.1038/sj.onc.1208626. [DOI] [PubMed] [Google Scholar]
- 60.Weigand M, Hantel P, Kreienberg R, Waltenberger J. Autocrine vascular endothelial growth factor signalling in breast cancer. Evidence from cell lines and primary breast cancer cultures in vitro. Angiogenesis. 2005;8:197–204. doi: 10.1007/s10456-005-9010-0. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, Lynch MP, Rueda BR. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281:26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 62.Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, Penichet ML. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 64.Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, Gonzalez RR. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer. 2008;123:2782–2790. doi: 10.1002/ijc.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dias S, Hattori K, Heissig B, Zhu Z, Wu Y, Witte L, Hicklin DJ, Tateno M, Bohlen P, Moore MA, Rafii S. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci U S A. 2001;98:10857–10862. doi: 10.1073/pnas.191117498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steiner H, Berger AP, Godoy-Tundidor S, Bjartell A, Lilja H, Bartsch G, Hobisch A, Culig Z. An autocrine loop for vascular endothelial growth factor is established in prostate cancer cells generated after prolonged treatment with interleukin 6. Eur J Cancer. 2004;40:1066–1072. doi: 10.1016/j.ejca.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 67.Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF, Senger DR, Connolly JL, Schnitt SJ. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995;26:86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 68.Kranz A, Mattfeldt T, Waltenberger J. Molecular mediators of tumor angiogenesis: enhanced expression and activation of vascular endothelial growth factor receptor KDR in primary breast cancer. Int J Cancer. 1999;84:293–298. doi: 10.1002/(sici)1097-0215(19990621)84:3<293::aid-ijc16>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 69.Ryden L, Linderholm B, Nielsen NH, Emdin S, Jonsson PE, Landberg G. Tumor specific VEGF-A and VEGFR2/KDR protein are co-expressed in breast cancer. Breast Cancer Res Treat. 2003;82:147–154. doi: 10.1023/B:BREA.0000004357.92232.cb. [DOI] [PubMed] [Google Scholar]
- 70.Ryden L, Stendahl M, Jonsson H, Emdin S, Bengtsson NO, Landberg G. Tumor-specific VEGF-A and VEGFR2 in postmenopausal breast cancer patients with long-term follow-up. Implication of a link between VEGF pathway and tamoxifen response. Breast Cancer Res Treat. 2005;89:135–143. doi: 10.1007/s10549-004-1655-7. [DOI] [PubMed] [Google Scholar]
- 71.Giatromanolaki A, Koukourakis MI, Sivridis E, Chlouverakis G, Vourvouhaki E, Turley H, Harris AL, Gatter KC. Activated VEGFR2/KDR pathway in tumour cells and tumour associated vessels of colorectal cancer. Eur J Clin Invest. 2007;37:878–886. doi: 10.1111/j.1365-2362.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 72.Higgins KJ, Abdelrahim M, Liu S, Yoon K, Safe S. Regulation of vascular endothelial growth factor receptor-2 expression in pancreatic cancer cells by Sp proteins. Biochem Biophys Res Commun. 2006;345:292–301. doi: 10.1016/j.bbrc.2006.04.111. [DOI] [PubMed] [Google Scholar]
- 73.Spannuth WA, Nick AM, Jennings NB, Armaiz-Pena GN, Mangala LS, Danes CG, Lin YG, Merritt WM, Thaker PH, Kamat AA, Han LY, Tonra JR, Coleman RL, Ellis LM, Sood AK. Functional significance of VEGFR-2 on ovarian cancer cells. Int J Cancer. 2009;124:1045–1053. doi: 10.1002/ijc.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanno S, Ohsaki Y, Nakanishi K, Toyoshima E, Kikuchi K. Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer. 2004;46:11–19. doi: 10.1016/j.lungcan.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 75.Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res. 2007;9:216. doi: 10.1186/bcr1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakopoulou L, Stefanaki K, Panayotopoulou E, Giannopoulou I, Athanassiadou P, Gakiopoulou-Givalou H, Louvrou A. Expression of the vascular endothelial growth factor receptor-2/Flk-1 in breast carcinomas: correlation with proliferation. Hum Pathol. 2002;33:863–870. doi: 10.1053/hupa.2002.126879. [DOI] [PubMed] [Google Scholar]
- 77.Longatto-Filho A, Pinheiro C, Martinho O, Moreira MA, Ribeiro LF, Queiroz GS, Schmitt FC, Baltazar F, Reis RM. Molecular characterization of EGFR, PDGFRA and VEGFR2 in cervical adenosquamous carcinoma. BMC Cancer. 2009;9:212. doi: 10.1186/1471-2407-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 79.Gockel I, Moehler M, Frerichs K, Drescher D, Trinh TT, Duenschede F, Borschitz T, Schimanski K, Biesterfeld S, Herzer K, Galle PR, Lang H, Junginger T, Schimanski CC. Co-expression of receptor tyrosine kinases in esophageal adenocarcinoma and squamous cell cancer. Oncol Rep. 2008;20:845–850. [PubMed] [Google Scholar]
- 80.Badalian G, Derecskei K, Szendroi A, Szendroi M, Timar J. EGFR and VEGFR2 protein expressions in bone metastases of clear cell renal cancer. Anticancer Res. 2007;27:889–894. [PubMed] [Google Scholar]
- 81.Xia G, Kumar SR, Hawes D, Cai J, Hassanieh L, Groshen S, Zhu S, Masood R, Quinn DI, Broek D, Stein JP, Gill PS. Expression and significance of vascular endothelial growth factor receptor 2 in bladder cancer. J Urol. 2006;175:1245–1252. doi: 10.1016/S0022-5347(05)00736-6. [DOI] [PubMed] [Google Scholar]
- 82.Puputti M, Tynninen O, Sihto H, Blom T, Maenpaa H, Isola J, Paetau A, Joensuu H, Nupponen NN. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res. 2006;4:927–934. doi: 10.1158/1541-7786.MCR-06-0085. [DOI] [PubMed] [Google Scholar]
- 83.Kim ALRD, Aucejo F, et al. Vascular endothelial growth factor receptor 2 (VEGFr2) expression and recurrence of hepatocellular carcinoma following liver transplantation: The Cleveland Clinic experience. J Clin Oncol. 2008 abstr 4594. [Google Scholar]
- 84.Ashikari-Hada S, Habuchi H, Kariya Y, Kimata K. Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins. J Biol Chem. 2005;280:31508–31515. doi: 10.1074/jbc.M414581200. [DOI] [PubMed] [Google Scholar]
- 85.Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 86.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 87.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 88.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12:177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, Baker M, Marshall JF, Hodivala-Dilke KM. Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. J Biol Chem. 2009;284:33966–33981. doi: 10.1074/jbc.M109.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Germain M, De Arcangelis A, Robinson SD, Baker M, Tavora B, D’Amico G, Silva R, Kostourou V, Reynolds LE, Watson A, Jones JL, Georges-Labouesse E, Hodivala-Dilke K. Genetic ablation of the alpha 6-integrin subunit in Tie1Cre mice enhances tumour angiogenesis. J Pathol. 2009 doi: 10.1002/path.2654. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X, Kazerounian S, Duquette M, Perruzzi C, Nagy JA, Dvorak HF, Parangi S, Lawler J. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 2009;23:3368–3376. doi: 10.1096/fj.09-131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nor JE. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bruns AF, Bao L, Walker JH, Ponnambalam S. VEGF-A-stimulated signalling in endothelial cells via a dual receptor tyrosine kinase system is dependent on co-ordinated trafficking and proteolysis. Biochem Soc Trans. 2009;37:1193–1197. doi: 10.1042/BST0371193. [DOI] [PubMed] [Google Scholar]
- 94.Bruns AF, Herbert SP, Odell AF, Jopling HM, Hooper NM, Zachary IC, Walker JH, Ponnambalam S. Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic. 11:161–174. doi: 10.1111/j.1600-0854.2009.01001.x. [DOI] [PubMed] [Google Scholar]
- 95.Scott A, Mellor H. VEGF receptor trafficking in angiogenesis. Biochem Soc Trans. 2009;37:1184–1188. doi: 10.1042/BST0371184. [DOI] [PubMed] [Google Scholar]
- 96.Patterson C, Perrella MA, Hsieh CM, Yoshizumi M, Lee ME, Haber E. Cloning and functional analysis of the promoter for KDR/flk-1, a receptor for vascular endothelial growth factor. J Biol Chem. 1995;270:23111–23118. doi: 10.1074/jbc.270.39.23111. [DOI] [PubMed] [Google Scholar]
- 97.Ronicke V, Risau W, Breier G. Characterization of the endothelium-specific murine vascular endothelial growth factor receptor-2 (Flk-1) promoter. Circ Res. 1996;79:277–285. doi: 10.1161/01.res.79.2.277. [DOI] [PubMed] [Google Scholar]
- 98.Wu Y, Patterson C. The human KDR/flk-1 gene contains a functional initiator element that is bound and transactivated by TFII-I. J Biol Chem. 1999;274:3207–3214. doi: 10.1074/jbc.274.5.3207. [DOI] [PubMed] [Google Scholar]
- 99.Patterson C, Wu Y, Lee ME, DeVault JD, Runge MS, Haber E. Nuclear protein interactions with the human KDR/flk-1 promoter in vivo. Regulation of Sp1 binding is associated with cell type-specific expression. J Biol Chem. 1997;272:8410–8416. doi: 10.1074/jbc.272.13.8410. [DOI] [PubMed] [Google Scholar]
- 100.Hata Y, Duh E, Zhang K, Robinson GS, Aiello LP. Transcription factors Sp1 and Sp3 alter vascular endothelial growth factor receptor expression through a novel recognition sequence. J Biol Chem. 1998;273:19294–19303. doi: 10.1074/jbc.273.30.19294. [DOI] [PubMed] [Google Scholar]
- 101.Urbich C, Stein M, Reisinger K, Kaufmann R, Dimmeler S, Gille J. Fluid shear stress-induced transcriptional activation of the vascular endothelial growth factor receptor-2 gene requires Sp1-dependent DNA binding. FEBS Lett. 2003;535:87–93. doi: 10.1016/s0014-5793(02)03879-6. [DOI] [PubMed] [Google Scholar]
- 102.Meissner M, Michailidou D, Stein M, Hrgovic I, Kaufmann R, Gille J. Inhibition of Rac1 GTPase downregulates vascular endothelial growth factor receptor-2 expression by suppressing Sp1-dependent DNA binding in human endothelial cells. Exp Dermatol. 2009;18:863–869. doi: 10.1111/j.1600-0625.2009.00867.x. [DOI] [PubMed] [Google Scholar]
- 103.Higgins KJ, Liu S, Abdelrahim M, Yoon K, Vanderlaag K, Porter W, Metz RP, Safe S. Vascular endothelial growth factor receptor-2 expression is induced by 17beta-estradiol in ZR-75 breast cancer cells by estrogen receptor alpha/Sp proteins. Endocrinology. 2006;147:3285–3295. doi: 10.1210/en.2006-0081. [DOI] [PubMed] [Google Scholar]
- 104.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 105.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 106.Yamada Y, Watanabe M, Yamanaka M, Hirokawa Y, Suzuki H, Takagi A, Matsuzaki T, Sugimura Y, Yatani R, Shiraishi T. Aberrant methylation of the vascular endothelial growth factor receptor-1 gene in prostate cancer. Cancer Sci. 2003;94:536–539. doi: 10.1111/j.1349-7006.2003.tb01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kunizaki M, Hamamoto R, Silva FP, Yamaguchi K, Nagayasu T, Shibuya M, Nakamura Y, Furukawa Y. The lysine 831 of vascular endothelial growth factor receptor 1 is a novel target of methylation by SMYD3. Cancer Res. 2007;67:10759–10765. doi: 10.1158/0008-5472.CAN-07-1132. [DOI] [PubMed] [Google Scholar]
- 108.Kim JY, Hwang JH, Zhou W, Shin J, Noh SM, Song IS, Lee SH, Kim J. The expression of VEGF receptor genes is concurrently influenced by epigenetic gene silencing of the genes and VEGF activation. Epigenetics. 2009;4:313–321. [PubMed] [Google Scholar]
- 109.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 110.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 111.Jenuwein T. Re-SET-ting heterochromatin by histone methyltransferases. Trends Cell Biol. 2001;11:266–273. doi: 10.1016/s0962-8924(01)02001-3. [DOI] [PubMed] [Google Scholar]
- 112.Wade PA, Wolffe AP. Histone acetyltransferases in control. Curr Biol. 1997;7:R82–84. doi: 10.1016/s0960-9822(06)00042-x. [DOI] [PubMed] [Google Scholar]
- 113.Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 114.Barr GM, Gately K, Al-Sarraf N, Pidgeon GP, O’Byrne KJ. VEGF and the epigenetic regulation of its receptors in non-small cell lung cancer. Lung Cancer. 2009;63(Supplement 1):S1, S1–S38. [Google Scholar]
- 115.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 116.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 117.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 118.Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- 119.Imatani A, Callahan R. Identification of a novel NOTCH-4/INT-3 RNA species encoding an activated gene product in certain human tumor cell lines. Oncogene. 2000;19:223–231. doi: 10.1038/sj.onc.1203295. [DOI] [PubMed] [Google Scholar]
- 120.Soares R, Balogh G, Guo S, Gartner F, Russo J, Schmitt F. Evidence for the notch signaling pathway on the role of estrogen in angiogenesis. Mol Endocrinol. 2004;18:2333–2343. doi: 10.1210/me.2003-0362. [DOI] [PubMed] [Google Scholar]
- 121.Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene. 2008;27:5099–5109. doi: 10.1038/onc.2008.223. [DOI] [PubMed] [Google Scholar]
- 122.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 123.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 124.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taylor KL, Henderson AM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc Res. 2002;64:372–383. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- 126.Lee CW, Raskett CM, Prudovsky I, Altieri DC. Molecular dependence of estrogen receptor-negative breast cancer on a notch-survivin signaling axis. Cancer Res. 2008;68:5273–5281. doi: 10.1158/0008-5472.CAN-07-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee CW, Simin K, Liu Q, Plescia J, Guha M, Khan A, Hsieh CC, Altieri DC. A functional Notch-survivin gene signature in basal breast cancer. Breast Cancer Res. 2008;10:R97. doi: 10.1186/bcr2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Monsalve E, Ruiz-Garcia A, Baladron V, Ruiz-Hidalgo MJ, Sanchez-Solana B, Rivero S, Garcia-Ramirez JJ, Rubio A, Laborda J, Diaz-Guerra MJ. Notch1 upregulates LPS-induced macrophage activation by increasing NF-kappaB activity. Eur J Immunol. 2009;39:2556–2570. doi: 10.1002/eji.200838722. [DOI] [PubMed] [Google Scholar]
- 129.Ferreira AM, Westers H, Albergaria A, Seruca R, Hofstra RM. Estrogens, MSI and Lynch syndrome-associated tumors. Biochim Biophys Acta. 2009;1796:194–200. doi: 10.1016/j.bbcan.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 130.Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102:89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Santen R, Cavalieri E, Rogan E, Russo J, Guttenplan J, Ingle J, Yue W. Estrogen mediation of breast tumor formation involves estrogen receptor-dependent, as well as independent, genotoxic effects. Ann N Y Acad Sci. 2009;1155:132–140. doi: 10.1111/j.1749-6632.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 132.Kim KH, Bender JR. Rapid, estrogen receptor-mediated signaling: why is the endothelium so special? Sci STKE. 2005;2005:pe28. doi: 10.1126/stke.2882005pe28. [DOI] [PubMed] [Google Scholar]
- 133.Rubanyi GM, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38:89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 134.Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR. Genomic and non-genomic effects of estrogens on endothelial cells. Steroids. 2004;69:537–542. doi: 10.1016/j.steroids.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 135.Suzuma I, Mandai M, Takagi H, Suzuma K, Otani A, Oh H, Kobayashi K, Honda Y. 17 Beta-estradiol increases VEGF receptor-2 and promotes DNA synthesis in retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci. 1999;40:2122–2129. [PubMed] [Google Scholar]
- 136.Tanemura M, Miyamoto N, Mandai M, Kamizuru H, Ooto S, Yasukawa T, Takahashi M, Honda Y. The role of estrogen and estrogen receptorbeta in choroidal neovascularization. Mol Vis. 2004;10:923–932. [PubMed] [Google Scholar]
- 137.Higgins KJ, Liu S, Abdelrahim M, Vanderlaag K, Liu X, Porter W, Metz R, Safe S. Vascular endothelial growth factor receptor-2 expression is down-regulated by 17beta-estradiol in MCF-7 breast cancer cells by estrogen receptor alpha/Sp proteins. Mol Endocrinol. 2008;22:388–402. doi: 10.1210/me.2007-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kremer C, Breier G, Risau W, Plate KH. Up-regulation of flk-1/vascular endothelial growth factor receptor 2 by its ligand in a cerebral slice culture system. Cancer Res. 1997;57:3852–3859. [PubMed] [Google Scholar]
- 139.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 140.Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010:10. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 142.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 143.Barleon B, Siemeister G, Martiny-Baron G, Weindel K, Herzog C, Marme D. Vascular endothelial growth factor up-regulates its receptor fms-like tyrosine kinase 1 (FLT-1) and a soluble variant of FLT-1 in human vascular endothelial cells. Cancer Res. 1997;57:5421–5425. [PubMed] [Google Scholar]
- 144.Patterson C, Perrella MA, Endege WO, Yoshizumi M, Lee ME, Haber E. Downregulation of vascular endothelial growth factor receptors by tumor necrosis factor-alpha in cultured human vascular endothelial cells. J Clin Invest. 1996;98:490–496. doi: 10.1172/JCI118816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Giraudo E, Primo L, Audero E, Gerber HP, Koolwijk P, Soker S, Klagsbrun M, Ferrara N, Bussolino F. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem. 1998;273:22128–22135. doi: 10.1074/jbc.273.34.22128. [DOI] [PubMed] [Google Scholar]
- 146.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Han KY, Kim CW, Lee TH, Son Y, Kim J. CCL23 up-regulates expression of KDR/Flk-1 and potentiates VEGF-induced proliferation and migration of human endothelial cells. Biochem Biophys Res Commun. 2009;382:124–128. doi: 10.1016/j.bbrc.2009.02.149. [DOI] [PubMed] [Google Scholar]
- 148.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 149.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 150.Cleary MP, Maihle NJ. The role of body mass index in the relative risk of developing premenopausal versus postmenopausal breast cancer. Proc Soc Exp Biol Med. 1997;216:28–43. doi: 10.3181/00379727-216-44153b. [DOI] [PubMed] [Google Scholar]
- 151.Ray A, Cleary MP. Leptin as a potential therapeutic target for breast cancer prevention and treatment. Expert Opin Ther Targets. 14:443–451. doi: 10.1517/14728221003716466. [DOI] [PubMed] [Google Scholar]
- 152.Laud K, Gourdou I, Pessemesse L, Peyrat JP, Djiane J. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol Cell Endocrinol. 2002;188:219–226. doi: 10.1016/s0303-7207(01)00678-5. [DOI] [PubMed] [Google Scholar]
- 153.Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, Benedetto C, Mussa A. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000;5:421–426. doi: 10.3892/ijmm.5.4.421. [DOI] [PubMed] [Google Scholar]
- 154.Klurfeld DM, Welch CB, Lloyd LM, Kritchevsky D. Inhibition of DMBA-induced mammary tumorigenesis by caloric restriction in rats fed high-fat diets. Int J Cancer. 1989;43:922–925. doi: 10.1002/ijc.2910430532. [DOI] [PubMed] [Google Scholar]
- 155.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 156.Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, Juneja SC, Grande JP, Maihle NJ. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–215. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- 157.Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail A, Platika D, Snodgrass HR. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med. 1996;2:585–589. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 158.Lollmann B, Gruninger S, Stricker-Krongrad A, Chiesi M. Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and, e in different mouse tissues. Biochem Biophys Res Commun. 1997;238:648–652. doi: 10.1006/bbrc.1997.7205. [DOI] [PubMed] [Google Scholar]
- 159.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 160.Akther A, Khan KH, Begum M, Parveen S, Kaiser MS, Chowdhury AZ. Leptin: a mysterious hormone; its physiology and pathophysiology. Mymensingh Med J. 2009;18:S140–144. [PubMed] [Google Scholar]
- 161.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 162.Beecken WD, Kramer W, Jonas D. New molecular mediators in tumor angiogenesis. J Cell Mol Med. 2000;4:262–269. doi: 10.1111/j.1582-4934.2000.tb00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J Cell Biochem. 2008;105:956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- 164.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 165.Binai NA, Damert A, Carra G, Steckelbroeck S, Lower J, Lower R, Wessler S. Expression of estrogen receptor alpha increases leptin-induced STAT3 activity in breast cancer cells. Int J Cancer. 2009 doi: 10.1002/ijc.25010. [DOI] [PubMed] [Google Scholar]
- 166.Fiorio E, Mercanti A, Terrasi M, Micciolo R, Remo A, Auriemma A, Molino A, Parolin V, Di Stefano B, Bonetti F, Giordano A, Cetto GL, Surmacz E. Leptin/HER2 crosstalk in breast cancer: in vitro study and preliminary in vivo analysis. BMC Cancer. 2008;8:305. doi: 10.1186/1471-2407-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Soma D, Kitayama J, Yamashita H, Miyato H, Ishikawa M, Nagawa H. Leptin augments proliferation of breast cancer cells via transactivation of HER2. J Surg Res. 2008;149:9–14. doi: 10.1016/j.jss.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 168.Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, Ando S. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- 169.Hayes DF, Miller K, Sledge G. Angiogenesis as targeted breast cancer therapy. Breast. 2007;16(Suppl 2):S17–19. doi: 10.1016/j.breast.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 170.Roy V, Perez EA. Biologic therapy of breast cancer: focus on co-inhibition of endocrine and angiogenesis pathways. Breast Cancer Res Treat. 2009;116:31–38. doi: 10.1007/s10549-008-0268-y. [DOI] [PubMed] [Google Scholar]
- 171.Shi W, Harris AL. Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;11:41–52. doi: 10.1007/s10911-006-9011-7. [DOI] [PubMed] [Google Scholar]
- 172.Ellis L, Hammers H, Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett. 2009;280:145–153. doi: 10.1016/j.canlet.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Qian DZ, Wang X, Kachhap SK, Kato Y, Wei Y, Zhang L, Atadja P, Pili R. The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2004;64:6626–6634. doi: 10.1158/0008-5472.CAN-04-0540. [DOI] [PubMed] [Google Scholar]
- 174.Liang Y, Brekken RA, Hyder SM. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr Relat Cancer. 2006;13:905–919. doi: 10.1677/erc.1.01221. [DOI] [PubMed] [Google Scholar]
- 175.Weng DE, Usman N. Angiozyme: a novel angiogenesis inhibitor. Curr Oncol Rep. 2001;3:141–146. doi: 10.1007/s11912-001-0014-7. [DOI] [PubMed] [Google Scholar]
- 176.Borgstrom P, Gold DP, Hillan KJ, Ferrara N. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res. 1999;19:4203–4214. [PubMed] [Google Scholar]
- 177.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 178.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 179.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 180.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 181.Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, Hewitt SM, Berman A, Steinberg SM, Liewehr DJ, Plehn J, Doshi A, Thomasson D, McCarthy N, Koeppen H, Sherman M, Zujewski J, Camphausen K, Chen H, Swain SM. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 182.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Weigert G, Michels S, Sacu S, Varga A, Prager F, Geitzenauer W, Schmidt-Erfurth U. Intravitreal bevacizumab (Avastin) therapy versus photodynamic therapy plus intravitreal triamcinolone for neovascular age-related macular degeneration: 6-month results of a prospective, randomised, controlled clinical study. Br J Ophthalmol. 2008;92:356–360. doi: 10.1136/bjo.2007.125823. [DOI] [PubMed] [Google Scholar]
- 184.Lu D, Kussie P, Pytowski B, Persaud K, Bohlen P, Witte L, Zhu Z. Identification of the residues in the extracellular region of KDR important for interaction with vascular endothelial growth factor and neutralizing anti-KDR antibodies. J Biol Chem. 2000;275:14321–14330. doi: 10.1074/jbc.275.19.14321. [DOI] [PubMed] [Google Scholar]
- 185.Zhu Z, Rockwell P, Lu D, Kotanides H, Pytowski B, Hicklin DJ, Bohlen P, Witte L. Inhibition of vascular endothelial growth factor-induced receptor activation with anti-kinase insert domain-containing receptor single-chain antibodies from a phage display library. Cancer Res. 1998;58:3209–3214. [PubMed] [Google Scholar]
- 186.Posey JA, Ng TC, Yang B, Khazaeli MB, Carpenter MD, Fox F, Needle M, Waksal H, LoBuglio AF. A phase I study of anti-kinase insert domain-containing receptor antibody, IMC-1C11, in patients with liver metastases from colorectal carcinoma. Clin Cancer Res. 2003;9:1323–1332. [PubMed] [Google Scholar]
- 187.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 188.Fury MG, Zahalsky A, Wong R, Venkatraman E, Lis E, Hann L, Aliff T, Gerald W, Fleisher M, Pfister DG. A Phase II study of SU5416 in patients with advanced or recurrent head and neck cancers. Invest New Drugs. 2007;25:165–172. doi: 10.1007/s10637-006-9011-x. [DOI] [PubMed] [Google Scholar]
- 189.Ansiaux R, Dewever J, Gregoire V, Feron O, Jordan BF, Gallez B. Decrease in tumor cell oxygen consumption after treatment with vandetanib (ZACTIMA; ZD6474) and its effect on response to radiotherapy. Radiat Res. 2009;172:584–591. doi: 10.1667/RR1744.1. [DOI] [PubMed] [Google Scholar]
- 190.Banerjee S, Zvelebil M, Furet P, Mueller-Vieira U, Evans DB, Dowsett M, Martin LA. The vascular endothelial growth factor receptor inhibitor PTK787/ZK222584 inhibits aromatase. Cancer Res. 2009;69:4716–4723. doi: 10.1158/0008-5472.CAN-08-4711. [DOI] [PubMed] [Google Scholar]
- 191.Beebe JS, Jani JP, Knauth E, Goodwin P, Higdon C, Rossi AM, Emerson E, Finkelstein M, Floyd E, Harriman S, Atherton J, Hillerman S, Soderstrom C, Kou K, Gant T, Noe MC, Foster B, Rastinejad F, Marx MA, Schaeffer T, Whalen PM, Roberts WG. Pharmacological characterization of CP-547,632, a novel vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for cancer therapy. Cancer Res. 2003;63:7301–7309. [PubMed] [Google Scholar]
- 192.Kumar D, Gokhale P, Broustas C, Chakravarty D, Ahmad I, Kasid U. Expression of SCC-S2, an antiapoptotic molecule, correlates with enhanced proliferation and tumorigenicity of MDA-MB 435 cells. Oncogene. 2004;23:612–616. doi: 10.1038/sj.onc.1207123. [DOI] [PubMed] [Google Scholar]
- 193.Zhang C, Chakravarty D, Sakabe I, Mewani RR, Boudreau HE, Kumar D, Ahmad I, Kasid UN. Role of SCC-S2 in experimental metastasis and modulation of VEGFR-2, MMP-1, and MMP-9 expression. Mol Ther. 2006;13:947–955. doi: 10.1016/j.ymthe.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 194.Song W, Dong Z, Jin T, Mantellini MG, Nunez G, Nor JE. Cancer gene therapy with iCaspase-9 transcriptionally targeted to tumor endothelial cells. Cancer Gene Ther. 2008;15:667–675. doi: 10.1038/cgt.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yan J, Jia R, Song H, Liu Y, Zhang L, Zhang W, Wang Y, Zhu Y, Yu J. A promising new approach of VEGFR2-based DNA vaccine for tumor immunotherapy. Immunol Lett. 2009;126:60–66. doi: 10.1016/j.imlet.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 196.Mannion M, Raeppel S, Claridge S, Zhou N, Saavedra O, Isakovic L, Zhan L, Gaudette F, Raeppel F, Deziel R, Beaulieu N, Nguyen H, Chute I, Beaulieu C, Dupont I, Robert MF, Lefebvre S, Dubay M, Rahil J, Wang J, Ste-Croix H, Robert Macleod A, Besterman JM, Vaisburg A. N-(4-(6,7-Disubstituted-quinolin-4-yloxy)-3-fluorophenyl)-2-oxo-3-phenylimidazoli dine-1-carboxamides: a novel series of dual c-Met/VEGFR2 receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2009;19:6552–6556. doi: 10.1016/j.bmcl.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 197.Pang X, Yi Z, Zhang X, Sung B, Qu W, Lian X, Aggarwal BB, Liu M. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009;69:5893–5900. doi: 10.1158/0008-5472.CAN-09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Carrillo de Santa Pau E, Arias FC, Caso Pelaez E, Munoz Molina GM, Sanchez Hernandez I, Muguruza Trueba I, Moreno Balsalobre R, Sacristan Lopez S, Gomez Pinillos A, del Val Toledo Lobo M. Prognostic significance of the expression of vascular endothelial growth factors A, B, C, and D and their receptors R1, R2, and R3 in patients with nonsmall cell lung cancer. Cancer. 2009;115:1701–1712. doi: 10.1002/cncr.24193. [DOI] [PubMed] [Google Scholar]
- 199.Sato H, Takeda Y. VEGFR2 expression and relationship between tumor neovascularization and histologic characteristics in oral squamous cell carcinoma. J Oral Sci. 2009;51:551–557. doi: 10.2334/josnusd.51.551. [DOI] [PubMed] [Google Scholar]
- 200.Chen H, Ye D, Xie X, Chen B, Lu W. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol Oncol. 2004;94:630–635. doi: 10.1016/j.ygyno.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 201.Itakura J, Ishiwata T, Shen B, Kornmann M, Korc M. Concomitant over-expression of vascular endothelial growth factor and its receptors in pancreatic cancer. Int J Cancer. 2000;85:27–34. doi: 10.1002/(sici)1097-0215(20000101)85:1<27::aid-ijc5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 202.Stadler WM, Cao D, Vogelzang NJ, Ryan CW, Hoving K, Wright R, Karrison T, Vokes EE. A randomized Phase II trial of the antiangiogenic agent SU5416 in hormone-refractory prostate cancer. Clin Cancer Res. 2004;10:3365–3370. doi: 10.1158/1078-0432.CCR-03-0404. [DOI] [PubMed] [Google Scholar]
- 203.Straume O, Akslen LA. Increased expression of VEGF-receptors (FLT-1, KDR, NRP-1) and thrombospondin-1 is associated with glomeruloid microvascular proliferation, an aggressive angiogenic phenotype, in malignant melanoma. Angiogenesis. 2003;6:295–301. doi: 10.1023/B:AGEN.0000029408.08638.aa. [DOI] [PubMed] [Google Scholar]
- 204.Lee JH, Chun T, Park SY, Rho SB. Interferon regulatory factor-1 (IRF-1) regulates VEGF-induced angiogenesis in HUVECs. Biochim Biophys Acta. 2008;1783:1654–1662. doi: 10.1016/j.bbamcr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 205.Chauvet C, Bois-Joyeux B, Berra E, Pouyssegur J, Danan JL. The gene encoding human retinoic acid-receptor-related orphan receptor alpha is a target for hypoxia-inducible factor 1. Biochem J. 2004;384:79–85. doi: 10.1042/BJ20040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Razny U, Wator L, Polus A, Wan YJ, Dyduch G, Tomaszewska R, Dembinska-Kiec A. Hepatocyte RXR alpha deletion in mice leads to inhibition of angiogenesis. Genes Nutr. 2009;4:69–72. doi: 10.1007/s12263-009-0111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gaetano C, Catalano A, Illi B, Felici A, Minucci S, Palumbo R, Facchiano F, Mangoni A, Mancarella S, Muhlhauser J, Capogrossi MC. Retinoids induce fibroblast growth factor-2 production in endothelial cells via retinoic acid receptor alpha activation and stimulate angiogenesis in vitro and in vivo. Circ Res. 2001;88:E38–47. doi: 10.1161/01.res.88.4.e38. [DOI] [PubMed] [Google Scholar]
- 208.Davis R, Singh KP, Kurzrock R, Shankar S. Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncol Rep. 2009;22:1473–1478. doi: 10.3892/or_00000589. [DOI] [PubMed] [Google Scholar]
- 209.Huang X, Brown C, Ni W, Maynard E, Rigby AC, Oettgen P. Critical role for the Ets transcription factor ELF-1 in the development of tumor angiogenesis. Blood. 2006;107:3153–3160. doi: 10.1182/blood-2005-08-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038–6042. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fritz WA, Lin TM, Peterson RE. The aryl hydrocarbon receptor (AhR) inhibits vanadate-induced vascular endothelial growth factor (VEGF) production in TRAMP prostates. Carcinogenesis. 2008;29:1077–1082. doi: 10.1093/carcin/bgn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Charboneau A, East L, Mulholland N, Rohde M, Boudreau N. Pbx1 is required for Hox D3-mediated angiogenesis. Angiogenesis. 2005;8:289–296. doi: 10.1007/s10456-005-9016-7. [DOI] [PubMed] [Google Scholar]
- 213.Biscetti F, Gaetani E, Flex A, Straface G, Pecorini G, Angelini F, Stigliano E, Aprahamian T, Smith RC, Castellot JJ, Pola R. Peroxisome proliferator-activated receptor alpha is crucial for iloprost-induced in vivo angiogenesis and vascular endothelial growth factor upregulation. J Vasc Res. 2009;46:103–108. doi: 10.1159/000143793. [DOI] [PubMed] [Google Scholar]
- 214.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 215.Teodoro JG, Evans SK, Green MR. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med. 2007;85:1175–1186. doi: 10.1007/s00109-007-0221-2. [DOI] [PubMed] [Google Scholar]
- 216.Joung YH, Lim EJ, Lee MY, Park JH, Ye SK, Park EU, Kim SY, Zhang Z, Lee KJ, Park DK, Park T, Moon WK, Yang YM. Hypoxia activates the cyclin D1 promoter via the Jak2/STAT5b pathway in breast cancer cells. Exp Mol Med. 2005;37:353–364. doi: 10.1038/emm.2005.45. [DOI] [PubMed] [Google Scholar]
- 217.Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev. 2008;28:185–200. doi: 10.1002/med.20101. [DOI] [PubMed] [Google Scholar]
- 218.Polyzos A. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma and various other solid tumors. J Steroid Biochem Mol Biol. 2008;108:261–266. doi: 10.1016/j.jsbmb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 219.Kuenen BC, Giaccone G, Ruijter R, Kok A, Schalkwijk C, Hoekman K, Pinedo HM. Dose-finding study of the multitargeted tyrosine kinase inhibitor SU6668 in patients with advanced malignancies. Clin Cancer Res. 2005;11:6240–6246. doi: 10.1158/1078-0432.CCR-04-2466. [DOI] [PubMed] [Google Scholar]
- 220.Xiong HQ, Herbst R, Faria SC, Scholz C, Davis D, Jackson EF, Madden T, McConkey D, Hicks M, Hess K, Charnsangavej CA, Abbruzzese JL. A phase I surrogate endpoint study of SU6668 in patients with solid tumors. Invest New Drugs. 2004;22:459–466. doi: 10.1023/B:DRUG.0000036688.96453.8d. [DOI] [PubMed] [Google Scholar]
- 221.Amino N, Ideyama Y, Yamano M, Kuromitsu S, Tajinda K, Samizu K, Hisamichi H, Matsuhisa A, Shirasuna K, Kudoh M, Shibasaki M. YM-359445, an orally bioavailable vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor, has highly potent antitumor activity against established tumors. Clin Cancer Res. 2006;12:1630–1638. doi: 10.1158/1078-0432.CCR-05-2028. [DOI] [PubMed] [Google Scholar]
- 222.Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, Harder J, Blum H, Robertson J, Jurgensmeier JM, Puchalski TA, Young H, Saunders O, Unger C. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 223.Laurie SA, Gauthier I, Arnold A, Shepherd FA, Ellis PM, Chen E, Goss G, Powers J, Walsh W, Tu D, Robertson J, Puchalski TA, Seymour L. Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 2008;26:1871–1878. doi: 10.1200/JCO.2007.14.4741. [DOI] [PubMed] [Google Scholar]
- 224.Robertson JD, Botwood NA, Rothenberg ML, Schmoll HJ. Phase III trial of FOLFOX plus bevacizumab or cediranib (AZD2171) as first-line treatment of patients with metastatic colorectal cancer: HORIZON III. Clin Colorectal Cancer. 2009;8:59–60. doi: 10.3816/CCC.2009.n.010. [DOI] [PubMed] [Google Scholar]
- 225.Dineen SP, Sullivan LA, Beck AW, Miller AF, Carbon JG, Mamluk R, Wong H, Brekken RA. The Adnectin CT-322 is a novel VEGF receptor 2 inhibitor that decreases tumor burden in an orthotopic mouse model of pancreatic cancer. BMC Cancer. 2008;8:352. doi: 10.1186/1471-2407-8-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jones-Bolin S, Zhao H, Hunter K, Klein-Szanto A, Ruggeri B. The effects of the oral, pan-VEGF-R kinase inhibitor CEP-7055 and chemotherapy in orthotopic models of glioblastoma and colon carcinoma in mice. Mol Cancer Ther. 2006;5:1744–1753. doi: 10.1158/1535-7163.MCT-05-0327. [DOI] [PubMed] [Google Scholar]
- 227.Ruggeri B, Singh J, Gingrich D, Angeles T, Albom M, Yang S, Chang H, Robinson C, Hunter K, Dobrzanski P, Jones-Bolin S, Pritchard S, Aimone L, Klein-Szanto A, Herbert JM, Bono F, Schaeffer P, Casellas P, Bourie B, Pili R, Isaacs J, Ator M, Hudkins R, Vaught J, Mallamo J, Dionne C. CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models. Cancer Res. 2003;63:5978–5991. [PubMed] [Google Scholar]