Abstract
The goal of this study was to examine whether the A3 adenosine receptor (A3AR) agonist Cl-IB-MECA protects against myocardial ischemia/reperfusion injury when administered at the time of reperfusion in an in vivo mouse model of infarction induced by 30 min of coronary occlusion and 24 h of reperfusion. Treating B6 wild-type with Cl-IB-MECA during the reperfusion phase (100 μg/kg i.v. bolus + 0.3 μg/kg/min subcutaneously via implantation of Alzet mini-osmotic pumps) reduced myocardial infarct size ~37% from 50.1 ± 2.5% in vehicle-treated mice to 31.6 ± 2.8% in Cl-IB-MECA-treated mice, and significantly reduced the number of leukocytes that infiltrated into the ischemic-reperfused myocardium. Cl-IB-MECA did not reduce infarct size or limit leukocyte accumulation in studies using B6 congenic A3AR gene “knock-out” mice or in chimeric mice lacking the expression of A3ARs in bone marrow (BM)-derived cells. Subsequent mechanistic studies demonstrated that Cl-IB-MECA inhibited migration of mouse neutrophils isolated from BM towards the chemotactic substance c5a in trans-well migration assays, and inhibited leukocyte migration into the peritoneal cavity in a mouse model of thioglycollate-induced peritonitis. We conclude that treating with the A3AR agonist Cl-IB-MECA at the time of reperfusion provides effective protection from ischemia/reperfusion injury in the heart through activation of the A3AR expressed in BM-derived cells, potentially by suppressing the robust inflammatory reaction that occurs during reperfusion and neutrophil-mediated tissue injury.
Introduction
Agonists for the A3 adenosine receptor (A3AR) subtype are effective at limiting infarct size and reducing contractile dysfunction during myocardial ischemia/reperfusion injury [1–8]. A3AR agonists are attractive as cardioprotective agents, because they provide remarkable protection at doses that exert no adverse hemodynamic effects including changes in blood pressure and heart rate [1, 2, 8]. Currently available A3AR agonists demonstrated to be effective at reducing ischemia/reperfusion injury include the N6-benzyladenosine-5′-N-methylcarboxamide derivative IB-MECA and its 2-chloro-derivative Cl-IB-MECA [1, 2, 6–8]. The related 3′-amino-substituted derivatives CP-608,039 and CP-532,903 have also been shown to be highly effective cardioprotective agents [8, 9].
Most previous studies examining the cardioprotective profile of A3AR agonists have administered the agents prior to the onset of ischemia. In these studies, protection is blocked by glibenclamide and is absent in Kir6.2 gene ablated mice, suggesting that the mechanism of protection involves activation of ATP-sensitive potassium (KATP) channels [2, 5–7, 9]. It has yet to be examined in great detail, however, whether or not A3AR agonists are effective when they are administered at the time of reperfusion. Theoretically, A3AR activation could limit reperfusion-mediated injury by activating pro-survival signaling pathways in cardiac muscle [10, 11] and/or by suppressing the robust inflammatory reaction associated with reperfusion injury, characterized by pro-inflammatory cytokine production, edema, and leukocyte recruitment [12]. In support of this latter theory, recent studies have demonstrated that A3AR agonists provide benefit in various animal models of inflammation (rheumatoid arthritis, sepsis, inflammatory bowel disease), and that the A3AR is highly expressed in various leukocyte populations [13]. Most notably, the A3AR has been found to be abundantly expressed in the neutrophil, where it signals to inhibit stimulated superoxide production and to regulate chemotaxis [14–16].
In this study, we examined the effectiveness of administering the potent and selective A3AR agonist Cl-IB-MECA at the time of reperfusion in an in vivo mouse model of infarction. We found that treating with Cl-IB-MECA effectively reduced infarct size, and that this protection was associated with a reduction in leukocyte infiltration into the reperfused myocardium. The results of mechanistic studies with A3AR gene knock-out mice and bone marrow (BM) chimeric mice lacking the expression of A3ARs in BM-derived cells support the concept that A3AR activation reduces reperfusion-mediated injury through an anti-inflammatory mechanism.
Materials and Methods
Animals
Male C57BL/6 mice (B6) and congenic B6 mice expressing the CD45.1 gene (B6.SJL-PtprcaPep3b/BoyJ) weighing 25–30 g were purchased from The Jackson Laboratory (Bar Harbor, Maine). A3AR gene knock-out (A3KO) mice back-crossed > 12 generations onto the C57BL/6 genetic background were generously provided by Dr. Marlene Jacobson from Merck Research Laboratories. All experiments were performed in accordance with the guidelines of the Animal Care Committee of the Medical College of Wisconsin, which is accredited by the American Association of Laboratory Animal Care.
Generation of BM chimeric mice
Chimeras were produced using standard techniques [17, 18]. Briefly, donor mice (9–12 weeks of age) were sacrificed by CO2 inhalation. The BM from tibia and femur bones was isolated under sterile conditions in RPMI-1640 media (Invitrogen; Carlsbad, California) containing 10% fetal calf serum and repeatedly passed through a 27-gauge needle to obtain a single-cell suspension. The cells were washed by centrifugation, re-suspended in isolation media, and viable cells were counted. The yield was ~5–8 × 107 nucleated cells/mouse. Recipient mice (6–8 weeks of age) were subjected to lethal whole body irradiation (~1,100 cGy) using a Shepherd Mark I cesium irradiator. Twenty four hours later, irradiated mice were injected with 5 × 106 BM cells via the tail vein. During each irradiation procedure, three control mice were not reconstituted with BM cells. Irradiated/transplanted mice were housed in microisolator cages for a minimum of eight weeks before being used in experiments. Real-time quantitative RT-PCR was used to evaluate the level of mRNA expression of the A3AR in mouse spleen tissue randomly obtained from BM chimeric mice at the conclusion of the studies to monitor the transplantation procedure, according to procedures described previously in detail [16, 19]. A3AR transcript levels were expressed as copies per 100 ng of total RNA [16, 19].
Additional control experiments were conducted using CD45-congenic B6 mouse strains as donor/recipient pairs to evaluate the efficacy of the reconstitution procedure. C56BL/6 control mice (CD45.2+) were lethally irradiated and received donor marrow (5 × 106 cells) from B6.SJL-PtpcraPep3b/BoyJ (CD45.1+) mice. After 8 weeks, single cell suspensions (1 × 106 cells in RPMI containing 5% fetal calf serum) from spleens of recipient mice were incubated with FITC-conjugated rat anti-mouse CD45.1 monoclonal antibody, and either phycoerythrin (PE)-conjugated rat anti-mouse CD3 monoclonal antibody, allophycocyanin (APC)-conjugated rat anti-mouse CD4 monoclonal antibody, PE-conjugated rat anti-mouse CD8 monoclonal antibody, APC-conjugated rat anti-mouse B220 monoclonal antibody, PE-conjugated rat anti-mouse CD11b antibody, or APC-conjugated rat anti-mouse GR-1 monoclonal antibody on ice for 20 min. All antibodies were from BD Biosciences Pharmingen (San Jose, California). CD3-, CD4-, CD8-, B220-, CD11b-, and GR-1-positive cells were assessed by flow cytometry, and the percentage of cells expressing CD45.1 antigen was determined in each population of cells (FACSCaliber, Becton Dickinson, San Jose, California).
Myocardial ischemia/reperfusion and drug administration
Mice were subjected to 30 min of coronary occlusion followed by up to 24 h of reperfusion, after which the experiments were terminated and the hearts were evaluated for infarct size or leukocyte accumulation. Five minutes before initiating reperfusion, the mice were injected intravenously via the tail vein with a bolus dose of the A3AR agonist Cl-IB-MECA (100 μg/kg; Sigma-Aldrich, St. Louis, Missouri) followed by a continuous subcutaneous infusion (0.3 μg/kg/min) maintained throughout the reperfusion period by implanting Alzet mini-osmotic pumps (Cupertino, California). Control mice received an equivalent volume of vehicle (50% solution of DMSO in phosphate-buffered saline [PBS]). In all experiments, the surgeon/infarct size quantitator was blinded to the experimental protocol (i.e., vehicle or drug treatment) and the genotype of the mice (i.e., wild-type, A3KO, or BM chimeric mice).
A standard ischemia/reperfusion procedure was used, as described previously in detail [3, 9, 20]. Briefly, mice were anesthetized with sodium pentobarbital (100 mg/kg i.p.) and ventilated (model 845; Hugo Sachs Elektronic, Hugsteten, Germany) via an orally placed tracheal tube at a rate of 125 strokes/min and a stroke volume of 200 μl to maintain blood gasses within normal physiological limits. The heart was exposed via a lateral incision at the level of the 4th intercostal space and a ligature (8-0 nylon) was placed under the left coronary artery. The ligature was used to induce coronary occlusion and reperfusion by gently tightening and loosening the snare around a piece of wetted gauze. Approximately 15 min after completing the ischemia/reperfusion protocol, the chest cavity was closed using 6-0 polypropylene sutures, and the mice were allowed to recover.
Myocardial infarct size measurement
After 24 h of reperfusion, infarct size was measured by dual staining with phthalo blue dye and triphenyltetrazolium [3, 9, 20]. To delineate the risk region, the left coronary artery was re-occluded in situ under pentobarbital anesthesia while a 5% solution of phthalo blue dye was injected into the aortic root. To delineate infarcted tissue, the excised left ventricle was sliced into 5–6 transverse sections and stained for 10 min in a solution of 1% triphenyltetrazolium chloride at 37° C. Triphenyltetrazolium stains the viable tissue red leaving infarcted tissue unstained. The slices were weighed and digitally photographed to determine infarct size as a percent of the risk region, as described previously [3, 9, 20].
Histological examination of leukocyte infiltration
At the times indicated, the risk region was delineated in situ following coronary occlusion, as described above. The hearts were rapidly removed, rinsed briefly in cold PBS, and then flash frozen in O.C.T. compound (Sakura Finetek; Torrance, California). Cryosections (5–8 μm) prepared perpendicularly to the long-axis of the heart were prepared and stained with hematoxylin & eosin (H&E). The total number of clearly identifiable leukocytes was counted within viable tissue directly adjacent to infarcted areas (i.e., the border zone) by a blinded observer in 50 high-power fields from 10 different sections spanning the entire risk region.
Measurement of systemic blood pressure and plasma histamine levels
BM chimeric mice were anesthetized with pentobarbital and ventilated. A saline-filled cannula (stretched PE-10 tubing) was inserted into the right femoral artery with the aid of a dissecting microscope and connected to a pressure transducer (ADInstruments model MLT 0699; Colorado Springs, Colorado). Systemic blood pressure was continuously recorded using a PowerLab data acquisition system (ADInstrumnents) at baseline and then for up to 45 min after intravenous administration of Cl-IB-MECA. For measurement of plasma histamine levels, mice were anesthetized, ventilated, and subjected to thoracotomy to expose the heart. Blood samples (~150 μl) were collected by cardiac puncture into EDTA-coated syringes containing the histamine N-methyltransferase inhibitor SKF-9148 (20 μM final concentration; Sigma-Aldrich) at baseline and at 15, 30, and 45 min after administration of Cl-IB-MECA. The blood samples were centrifuged (1,000 × g for 10 min at 4° C) and the plasma was analyzed for histamine content by ELISA (Immunotech; Marseille, France).
Neutrophil transmigration assays
Neutrophils were purified from mouse BM by isotonic Percoll gradient centrifugation, as described previously [16]. In brief, mice were euthanized with carbon dioxide. Tibias and femurs were flushed with neutrophil isolation buffer (1 × Hank’s balanced salt solution [HBSS] without Ca2+ and Mg2+ containing 0.4% sodium citrate) and layered on a three-step Percoll density gradient (72, 64, and 52%; Amersham Biosciences, Pscataway, New Jersey). After centrifugation at 1,060 × g for 30 min, cells at the 72:64% interface were removed and washed once with isolation buffer before use in experiments. We have previously confirmed that >85% of the cells at the 72:64% interface consist of morphologically mature neutrophils [16].
Neutrophil migration towards complement component c5a was determined using 5 μm polycarbonate membrane Transwell inserts in 24-well tissue culture polystyrene plates (Costar; Corning, New York). Neutrophils were suspended in RPMI-1640 cell culture media (without phenol red) containing 0.5% BSA and 5 μg/ml Calcein-AM (Molecular Probes-Invitrogen; Eugene, Oregon) and incubated for 30 min at 37° C. The neutrophils were washed twice and re-suspended at a concentration of 1 × 107 cells/ml in RPMI-1640 media containing 0.5% BSA and 1 unit/ml adenosine deaminase. Neutrophils (1 × 106) in a total volume of 200 μl media were placed into the inserts. The inserts were then placed into the wells containing 500 μl media alone or media containing c5a (100 nM; Sigma-Aldrich). The plates were incubated in a humidified environment containing 5% CO2 at 37° C. After 90 min, the inserts were carefully removed and the fluorescence in each well was measured using a plate reader (excitation, 485 nm; emission, 530 nm). Additional wells were included in each assay in which an aliquot of cells (1 × 106 in 500 μl media) was added to wells lacking an insert to measure total fluorescence. Neutrophil migration into the lower chamber was calculated as follows: (fluorescence/total fluorescence) × 100.
In vivo neutrophil migration
Thioglycollate-induced peritonitis was used as an in vivo model of neutrophil chemotaxis [16]. Mice were injected with vehicle or Cl-IB-MECA (100 μg/kg i.v.) followed by intraperitoneal injection (1 ml) of a sterile solution of 3% thioglycollate (Sigma-Aldrich). At 3 h, the mice were euthanized by CO2 inhalation and injected intraperitoneally with 5 ml of PBS (without Ca2+ and Mg2+) containing 2 mM EDTA. The abdomens were briefly massaged and the total lavage fluid was collected through a small slit cut into the peritoneal cavity. Collected cells were pelleted by centrifugation, resuspended in neutrophil isolation buffer, diluted with Trypan blue, and counted using a standard hemocytometer. Most of the cells (>80%) found in the peritoneal exudate consisted of neutrophils (data not shown; [16, 21]).
Statistical analysis
All values are presented as means ± SEM. Hemodynamic variables and plasma histamine values, and in some cases leukocyte recruitment data, were analyzed by two-way ANOVA (time and drug treatment) to determine whether there was a main effect of time, a main effect of treatment, or a time-treatment interaction. If global tests showed a main effect or interaction, post hoc contrasts between time-points or treatments were performed with Student’s t-test for unpaired or paired data, as appropriate, with the Bonferroni correction. For all other comparisons, one-way ANOVA or a standard Student’s t-test was used, depending on the number of groups involved in the analysis.
Results
Effect of Cl-IB-MECA on ischemia/reperfusion injury in wild-type and A3KO mice
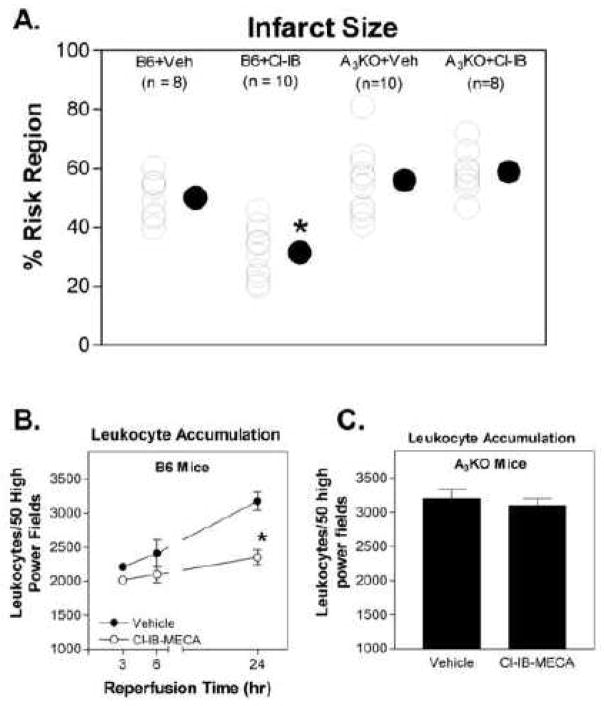
The effect of treating with the A3AR agonist Cl-IB-MECA during reperfusion on myocardial infarct size was evaluated in two groups of B6 wild-type mice and in two groups of A3KO mice subjected to 30 min of ischemia and 24 h of reperfusion. Cl-IB-MECA or equivalent vehicle were given throughout the reperfusion period starting 5 min before release of the occlusion (100 μg/kg i.v. bolus + 0.3 μg/kg/min subcutaneously via implantation of mini-osmotic pumps). There were no differences in the risk region sizes between the groups of mice (ranged between 39.7 ± 2.2% to 42.0 ± 2.4% of the left ventricle). Cl-IB-MECA treatment significantly reduced infarct size (percent of the risk region; n = 8–10) in B6 wild-type mice (vehicle-treated, 50.1 ± 2.5%; Cl-IB-MECA-treated, 31.6 ± 2.8%; Fig. 1A). However, infarct size was not significantly changed by treating with Cl-IB-MECA in A3KO mice (vehicle-treated, 55.4 ± 3.8%; Cl-IB-MECA-treated, 58.9 ± 2.7%; Fig 1A). No difference in infarct size was observed between B6 and A3KO mice (Fig. 1A). In parallel groups of mice, leukocyte infiltration measured 3, 6, or 24 hr after reperfusion was significantly reduced in the risk region in B6 mice treated with Cl-IB-MECA compared to vehicle-treated mice (Fig. 1B). At 24 h of reperfusion, treatment with Cl-IB-MECA did not reduce the infiltration of leukocytes into the risk region of A3KO mice (Fig. 1C).
Figure 1.
Infarct size (A) and leukocyte accumulation (B and C) induced by 30 min of coronary occlusion and 24 h of reperfusion in B6 wild-type mice and A3KO mice treated with either vehicle or Cl-IB-MECA throughout the 24 h reperfusion period (100 μg/kg i.v. bolus + 0.3 mg/kg/min by implanting Alzet mini-osmotic pumps). Infarct size is expressed as a percentage of the risk region and leukocyte accumulation determined from histological sections is expressed as the number of leukocytes found within 50 high power fields. n = 8–10 in the infarction studies and 6–8 in the leukocyte accumulation studies. For data shown in A, * indicates P < 0.05 versus the vehicle-treated B6 wild-type group by one-way ANOVA followed by post-hoc contrasts using Student’s t-test for unpaired data with the Bonferroni correction. For data shown in B, * indicates P < 0.05 versus the 24-h time-point in the vehicle-treated group by two-way ANOVA followed by Student’s t-test for unpaired data and the Bonferroni correction.
BM transplantation
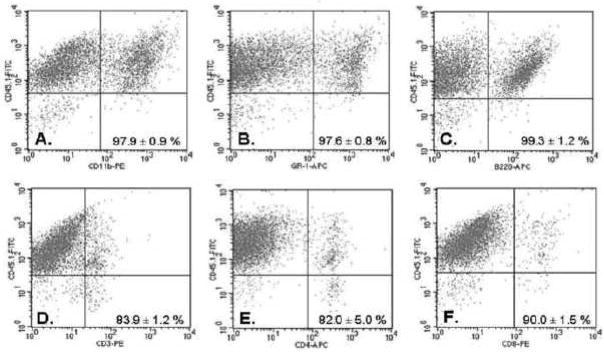
To investigate the site of action of Cl-IB-MECA to reduce infarct size, BM chimeric mice were created in which BM from B6 wild-type mice was destroyed and reconstituted with BM from A3KO mice (A3KO→B6) or with BM from B6 wild-type mice (B6→B6). In preliminary studies, chimera created with CD45-congenic B6 mouse strains were used to evaluate the extent of reconstitution of various BM-derived cell populations. Figure 2 illustrates the percentage of cells expressing the donor marker (CD45.1) in splenocytes that were positive for CD3 (T lymphocytes; 83.9 ± 1.2%), CD4 (T helper lymphocytes; 82.0 ± 5.0%), CD8 (cytotoxic T lymphocytes; 90.0 ± 1.5%), B220 (B lymphocytes; 99.3 ± 1.2%), CD11b (97.9 ± 0.9%; leukocytes), and GR-1 (97.6 ± 0.8%; granulocytes). These results demonstrate efficient repopulation of BM-derived cells in chimeric animals.
Figure 2.
Reconstitution efficiency of lymphocytes and granulocytes at 2 months after BM transplantation in control studies using CD45-congenic B6 mouse strains as donor (CD45.1+)/recipient (CD45.2+) pairs. Splenocytes from transplanted recipients were incubated with anti-CD45.1 as a marker for donor cells and either anti-CD11.b (A), anti-GR-1 (B), anti-B22 (C), anti-CD3 (D), anti-CD4 (E), or anti-CD8 (F) antibodies. Labeled cells were analyzed by flow cytometry. The number in each panel indicates the average percentage of cells that were positive for both CD45.1 and each of the other fluorescently marked antibodies from 3 independent experiments.
Effect of Cl-IB-MECA in infarct size in BM chimeric mice
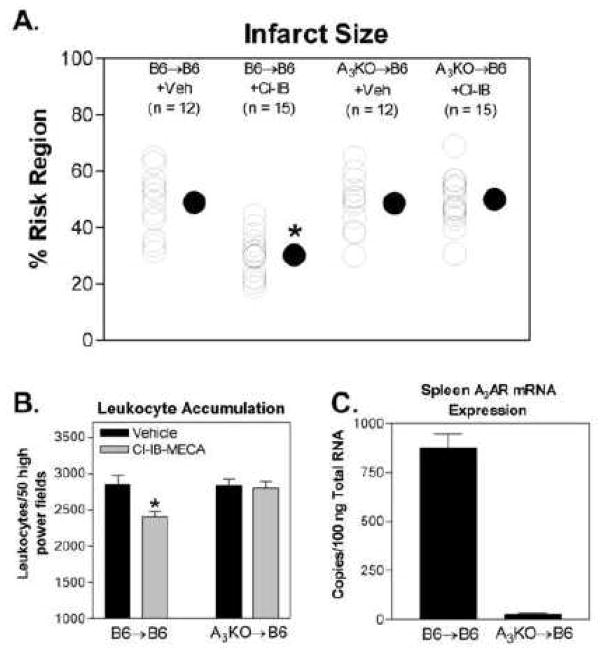
Infarct size in B6→B6 chimeras following 30 min of ischemia and 24 h of reperfusion was found to be similar to that of B6 wild-type mice, indicating that the BM transplantation procedure had no effect on the responsiveness of the heart to ischemia/reperfusion injury (Fig. 3A). Treating with Cl-IB-MECA during reperfusion produced a marked reduction in infarct size in B6→B6 chimeras (from 49.0 ± 3.0% in vehicle-treated chimeras to 30.3 ± 1.9% in Cl-IB-MECA-treated chimeras). In the A3KO→B6 chimeras the infarct-sparing effect of Cl-IB-MECA was not present, and infarct size (49.5 ± 2.3%) was similar to that observed with vehicle-treated B6→B6 chimeras and with vehicle-treated A3KO→B6 chimeras (48.8 ± 2.9%; Fig. 3A). Treating with Cl-IB-MECA also significantly reduced the accumulation of leukocytes in the ischemic-reperfused myocardium after 24 h of reperfusion in B6→B6 chimeras, but not in A3KO→B6 chimeras (Fig. 3B.). To confirm efficient reconstitution, mRNA expression of the A3AR in spleen tissue was assessed in 16 randomly chosen chimeras (8 per group) by quantitative RT-PCR. As shown (Figure 3C), A3AR mRNA levels were reduced from 878 ± 49 copies/100 ng total RNA in B6→B6 chimeras to 29 ± 1.5 copies/100ng of total RNA in A3KO→B6 chimeras, indicating a reconstitution efficiency of ~97%.
Figure 3.
Effect of treating with Cl-IB-MECA during the reperfusion period on infarct size (A) and leukocyte accumulation (B) in BM-chimeric mice. The infarct size-sparing actions of Cl-IB-MECA were observed in B6→B6 chimeras, but not in A3KO→B6 chimeras. n = 12–15 in the infarction studies and 8–10 in the leukocyte accumulation studies. Panel C shows the results of quantitative RT-PCR assays measuring A3AR mRNA expression in spleen tissue randomly chosen from 8 B6→B6 chimeras and 8 A3KO→B6 chimeras used in the infarction studies. For data shown in Panel A, * indicates P < 0.05 versus the B6→B6 vehicle-treated group by one-way ANOVA followed by Student’s t-test with the Bonferroni correction. For data shown in Panel B, * indicates P < 0.05 versus the vehicle-treated group by Student’s t-test.
Effect of Cl-IB-MECA on systemic blood pressure and plasma histamine concentrations in BM chimera
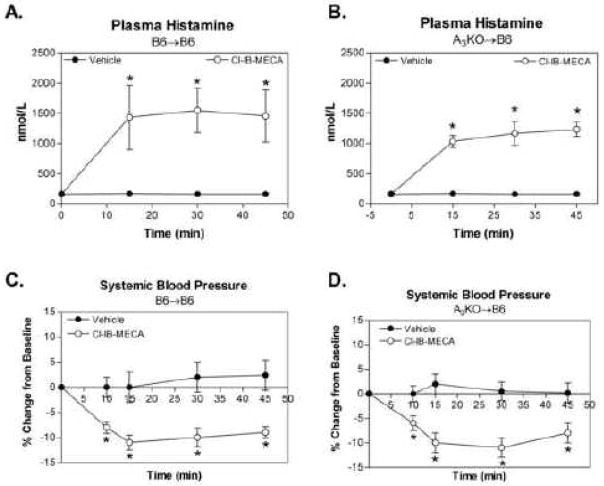
We have previously reported that Cl-IB-MECA increases plasma histamine levels in mice due to activation of A3ARs expressed in mast cells, which produces a modest decrease in systemic blood pressure insufficient to stimulate reflex changes in heart rate [3, 9]. In this investigation, we examined whether Cl-IB-MECA produces similar effects in A3AR BM chimeric mice. As shown in Figure 4, Cl-IB-MECA produced an equivalent increase in plasma histamine concentrations and an equivalent decrease (~10%) in systemic blood pressure in A3KO→B6 chimeras as compared to B6→B6 chimeras.
Figure 4.
Effect of Cl-IB-MECA (100 μg/kg i.v. bolus) on plasma histamine levels (A and B) and systemic blood pressure (C and D) in B6→B6 and A3KO→B6 chimeric mice. Baseline blood pressure was similar in B6→B6 (85 ± 5) and A3KO→B6 (89 ± 6) chimeras. *, P < 0.05 versus corresponding values in the vehicle-treated group by 2-way ANOVA followed by Student’s t-test with the Bonferroni correction, n = 6–8/group.
Cl-IB-MECA reduced neutrophil migration through A3AR activation
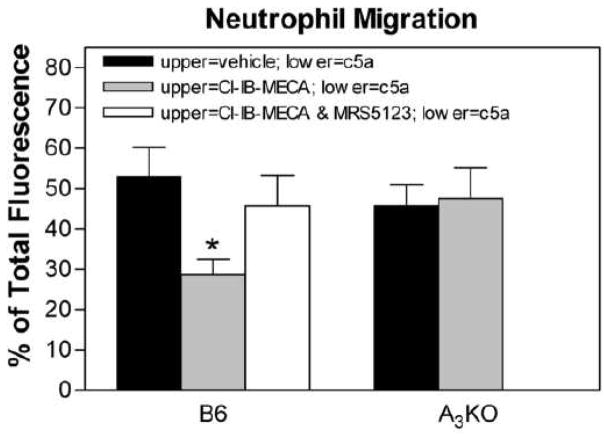
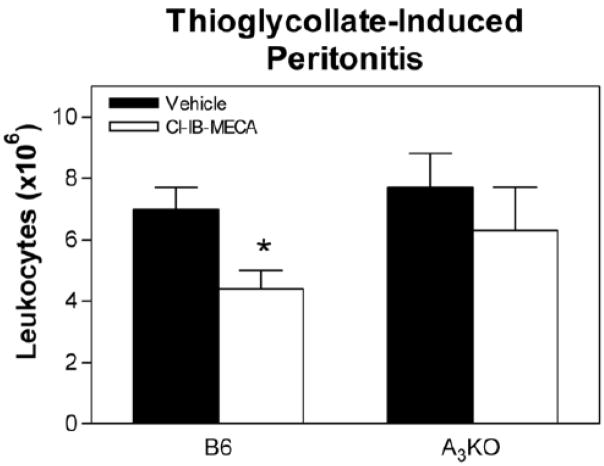
In transwell migration assays, treating isolated neutrophils with Cl-IB-MECA (100 nM) decreased migration towards the chemotactic compound complement component c5a (Fig. 5A). However, Cl-IB-MECA did not inhibit c5a-stimulated neutrophil chemotaxis in assays using neutrophils isolated from A3KO mice, indicating a specific inhibitory effect mediated through the A3AR. In the thioglycollate-induced peritonitis model, treating with Cl-IB-MECA (100 μg/kg i.v. bolus) reduced the accumulation of leukocytes in the peritoneal cavity of B6 wild-type mice but not A3KO mice (Fig 6).
Figure 5.
Effect of Cl-IB-MECA on neutrophil migration in trans-well assays using Percoll gradient-purified BM neutrophils. Fluorescently labeled (Calcein-AM) neutrophils were added into upper wells along with either vehicle, Cl-IB-MECA (100 nM), or Cl-IB-MECA (100 nM) in combination with the A3AR antagonist MRS 5123 (10 μM). The lower wells contained either vehicle or complement component c5a (100 nM). After incubating for 90 min at 37° C, the inserts were removed and fluorescence in each well was quantified. Neutrophil migration was calculated as the percentage of fluorescence found in the wells as a percentage of total fluorescence. Random migration was ~15% in experiments using either wild-type or A3KO neutrophils. *, P < 0.05 versus the vehicle-treated group by one-way ANOVA followed by Student’s t-test with the Bonferroni correction, n = 6–8/group.
Figure 6.
Effect of Cl-IB-MECA on leukocyte accumulation during thioglycollate-induced peritonitis in B6 wild-type mice and A3KO mice. Mice were injected i.v. with either vehicle or Cl-IB-MECA (100 μg/kg) immediately before intraperitoneal injection with thioglycollate (1 ml of a 3% solution). Three hours later, the number of leukocytes within the peritoneal exudates was quantified. *, P < 0.05 versus the vehicle-treated group by Student’s t-test, n = 8/group.
Discussion
This study demonstrates that treating with the potent A3AR agonist Cl-IB-MECA during reperfusion reduces myocardial infarct size in a mouse model of ischemia/reperfusion injury. The decrease in infarct size was associated with a reduction in the number of leukocytes that infiltrated into the ischemic/reperfused myocardium, was sustained for up to 24 h, and was mediated through specific activation of the A3AR. Using standard transplantation techniques, this study further shows that BM-derived cells are responsible for mediating the beneficial effects of A3AR activation. The identity of the specific BM-derived cell type(s) bearing the protective A3AR was not definitively determined in this study. However, supportive evidence suggests that it may be the neutrophil.
Several previous studies using in vivo models or isolated hearts have demonstrated that A3AR agonists reduce ischemia/reperfusion injury when administered prior to the ischemic insult [1–8]. It appears that benefit in these studies is explained by activation of A3ARs expressed in cardiac myocytes coupled to protective signaling pathways that may involve the KATP channel or pro-survival kinases [1, 2, 8, 10, 11]. This mechanism is akin to that of the similarly Gi protein-coupled A1AR. The present study demonstrates that A3AR activation also provides protection from ischemia/reperfusion injury when applied during the reperfusion phase. Although we have previously reported that the modestly selective A3AR agonist IB-MECA reduced infarct size when administered during reperfusion in an open-chest dog model [1], it was not clear in this study whether it was effective through an A3AR-mediated mechanism. The absence of cardioprotection in the present investigation in gene knock-out mice provides conclusive evidence that the beneficial effect of Cl-IB-MECA to reduce infarct size was through activation of the A3AR.
The findings that Cl-IB-MECA functioned by activating A3ARs expressed in BM-derived cells indicates that the mechanism of protection likely involves suppressing the robust inflammatory reaction that occurs during reperfusion [12]. An anti-inflammatory mechanism is consistent with our observation that leukocyte recruitment was reduced by Cl-IB-MECA treatment. We and others have recently discovered that the A3AR is abundantly expressed in murine neutrophils, where it regulates chemotaxis and other inflammatory activities such as stimulated superoxide production [14–16]. Thus, it is possible that benefit observed in the present investigation was due to a direct anti-neutrophil effect, supported by our findings that Cl-IB-MECA treatment reduced chemotaxis of isolated neutrophils and inhibited leukocyte infiltration in the thioglycollate-induced peritonitis model. Recent studies have suggested that inflammation associated with reperfusion injury in the heart may be triggered by rapid recruitment and activation of T lymphocytes [18, 22]. Using RAG1 immunodeficient mice that lack B and T cells and adoptive transfer approaches, Linden’s group [18, 22] has provided evidence that A2AAR agonists reduce reperfusion injury by inhibiting CD4+ T cell accumulation and activation. A2AAR activation has also been shown to suppress liver ischemia/reperfusion injury by inhibiting activation of resident CD4+ natural killer T cells expressing the invariant T cell receptor responsive to lipid molecules [23]. Thus, it remains possible that A3AR activation may function to reduce reperfusion injury through effects on lymphocytes. Other potential mechanisms include inhibition of pro-inflammatory cytokine production by resident leukocytes including macrophages or by newly recruited monocytes within the reperfused myocardium.
In rodents, administration of A3AR agonists stimulates degranulation of mast cells through an A3AR-mediated mechanism that consequently increases plasma histamine concentrations and produces a transient ~10% reduction in systemic blood pressure [3, 9]. Since tissue-resident mast cells are derived from BM, it was surprising that Cl-IB-MECA continued to increase plasma histamine levels in A3KO→B6 BM chimeras. This finding suggests that the 2-month recovery period following the transplantation procedure was not sufficiently long enough to allow for repopulation of mast cell subsets that express the A3AR with donor A3KO cells. It has previously been suggested that A3AR agonists may reduce infarct size and reduce ischemia/reperfusion-induced inflammation by depleting stored mediators within mast cells that may be released during reperfusion and contribute to the inflammatory reaction [24]. Our observation in the present study with A3KO→B6 BM chimeras whereby Cl-IB-MECA did not reduce infarct size while continuing to stimulate histamine release argues against this possibility.
We observed that infarct size was not significantly different between wild-type, A3KO, and A3KO→B6 chimeras, a finding that is consistent with previous reports by our group in ischemia/reperfusion studies using congenic B6 A3KO mice [3, 9]. Thus, it appears that the A3AR does not play a tonic role to regulate ischemia/reperfusion injury, but that activating the A3AR during reperfusion by administering an agonist provides significant tissue protection. In the present investigation, Cl-IB-MECA was administered immediately before and throughout the entire 24-h reperfusion phase. It will be important to determine in future studies whether the duration of treatment can be reduced and whether it needs to be provided at the time reperfusion is initiated.
In conclusion, the present study demonstrates that activation of the A3AR during reperfusion effectively reduces ischemia/reperfusion injury due to stimulation of A3ARs in BM-derived cells. We speculate that myocardial protection occurs because A3AR agonists inhibit migration and the pro-inflammatory actions of neutrophils. Taken together with previous studies assessing the effectiveness of pretreating with A3AR agonists, it appears that A3AR activation provides protection from ischemia/reperfusion by two contributing mechanisms: 1) by activating pro-survival signaling mechanisms in cardiac muscle, and 2) by reducing the inflammatory reaction during reperfusion.
Acknowledgments
We thank Dr. Jeffrey Woodliff (Flow Cytometry Core Facility, Department of Pediatrics, Medical College of Wisconsin) for assistance with the flow cytometry studies and Dr. Robert Truitt (Cesium Irradiator Core Facility, Department of Pediatrics, Medical College of Wisconsin) for assistance in generating BM-chimeric mice. The authors are grateful to the Medical College of Wisconsin Cardiovascular Research Center for providing the key facilities required to complete this project.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auchampach JA, Ge ZD, Wan TC, Moore J, Gross GJ. A3 adenosine receptor agonist IB-MECA reduces myocardial ischemia-reperfusion injury in dogs. Am J Physiol. 2003;285:H607–13. doi: 10.1152/ajpheart.01001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, et al. Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res. 1997;80:800–9. doi: 10.1161/01.res.80.6.800. [DOI] [PubMed] [Google Scholar]
- 3.Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, Gross GJ, et al. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;319:1200–10. doi: 10.1124/jpet.106.111351. [DOI] [PubMed] [Google Scholar]
- 4.Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, Vinten-Johansen J. A3 adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol. 1999;277:H1895–905. doi: 10.1152/ajpheart.1999.277.5.H1895. [DOI] [PubMed] [Google Scholar]
- 5.Thourani VH, Nakamura M, Ronson RS, Jordan JE, Zhao ZQ, Levy JH, et al. Adenosine A3-receptor stimulation attenuates postischemic dysfunction through KATP channels. Am J Physiol. 1999;277:H228–35. doi: 10.1152/ajpheart.1999.277.1.H228. [DOI] [PubMed] [Google Scholar]
- 6.Tracey WR, Magee W, Masamune H, Kennedy SP, Knight DR, Buchholz RA, et al. Selective adenosine A3 receptor stimulation reduces ischemic myocardial injury in the rabbit heart. Cardiovasc Res. 1997;33:410–5. doi: 10.1016/s0008-6363(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 7.Tracey WR, Magee W, Masamune H, Oleynek JJ, Hill RJ. Selective activation of adenosine A3 receptors with N6-(3-chlorobenzyl)-5′-N-methylcarboxamidoadenosine (CB-MECA) provides cardioprotection via KATP channel activation. Cardiovasc Res. 1998;40:138–45. doi: 10.1016/s0008-6363(98)00112-6. [DOI] [PubMed] [Google Scholar]
- 8.Tracey WR, Magee WP, Oleynek JJ, Hill RJ, Smith AH, Flynn DM, et al. Novel N6-substituted adenosine 5′-N-methyluronamides with high selectivity for human adenosine A3 receptors reduce ischemic myocardial injury. Am J Physiol. 2003;285:H2780–7. doi: 10.1152/ajpheart.00411.2003. [DOI] [PubMed] [Google Scholar]
- 9.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, et al. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther. 2008;324:234–43. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SS, Zhao H, Jang Y, Mueller RA, Xu Z. N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide confers cardioprotection at reperfusion by inhibiting mitochondrial permeability transition pore opening via glycogen synthase kinase 3 β. J Pharmacol Exp Ther. 2006;318:124–31. doi: 10.1124/jpet.106.101477. [DOI] [PubMed] [Google Scholar]
- 11.Gross GJ, Auchampach JA. Reperfusion injury: does it exist? J Mol Cell Cardiol. 2007;42:12–8. doi: 10.1016/j.yjmcc.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 13.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nature Rev. 2008;7:759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–5. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 15.Gessi S, Varani K, Merighi S, Cattabriga E, Iannotta V, Leung E, et al. A3 adenosine receptors in human neutrophils and promyelocytic HL60 cells: a pharmacological and biochemical study. Mol Pharmacol. 2002;61:415–24. doi: 10.1124/mol.61.2.415. [DOI] [PubMed] [Google Scholar]
- 16.van der Hoeven D, Wan TC, Auchampach JA. Activation of the A3 adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrophils. Mol Pharmacol. 2008;74:685–96. doi: 10.1124/mol.108.048066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–91. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–7. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 19.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-α release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–80. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 20.Black RG, Jr, Guo Y, Ge ZD, Murphree SS, Prabhu SD, Jones WK, et al. Gene dosage-dependent effects of cardiac-specific overexpression of the A3 adenosine receptor. Circ Res. 2002;91:165–72. doi: 10.1161/01.res.0000028007.91385.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunolog Meth. 1996;197:139–50. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–64. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 23.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–48. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]