Abstract
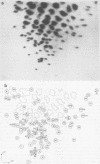
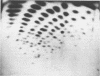
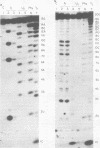
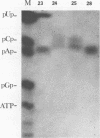
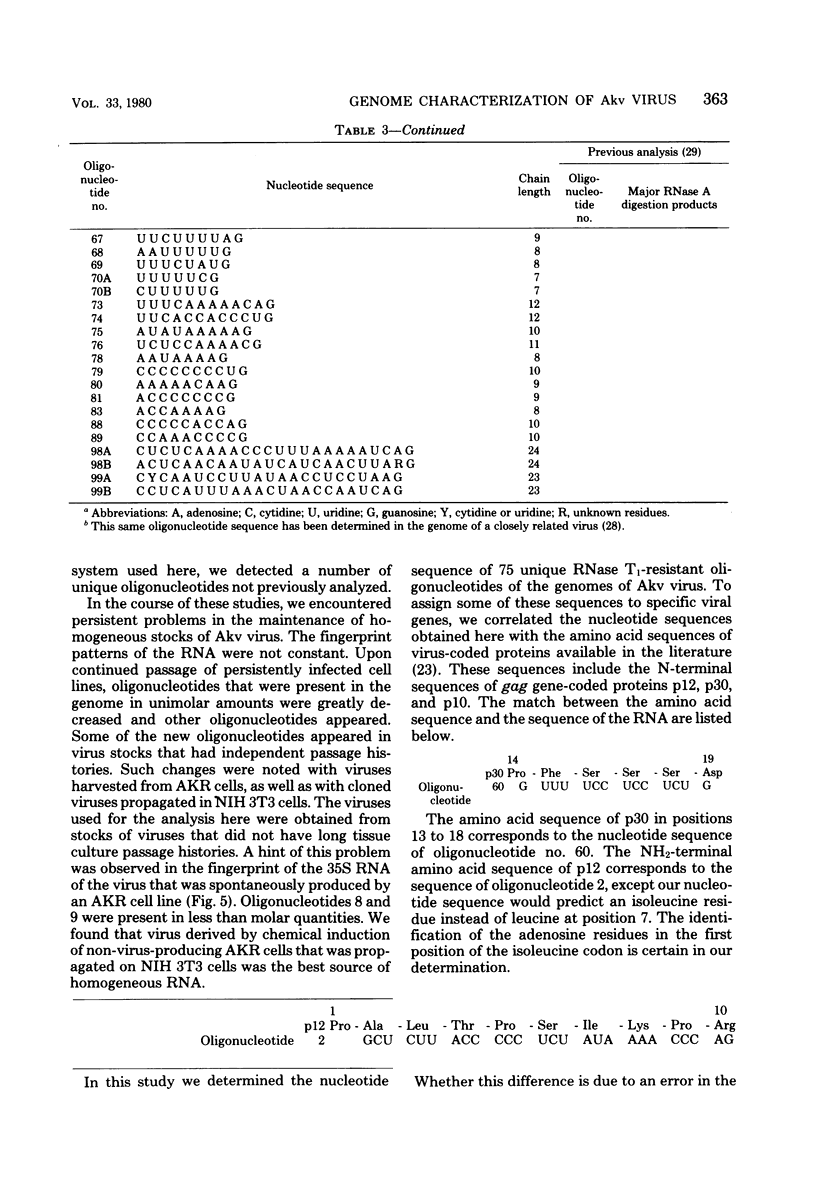
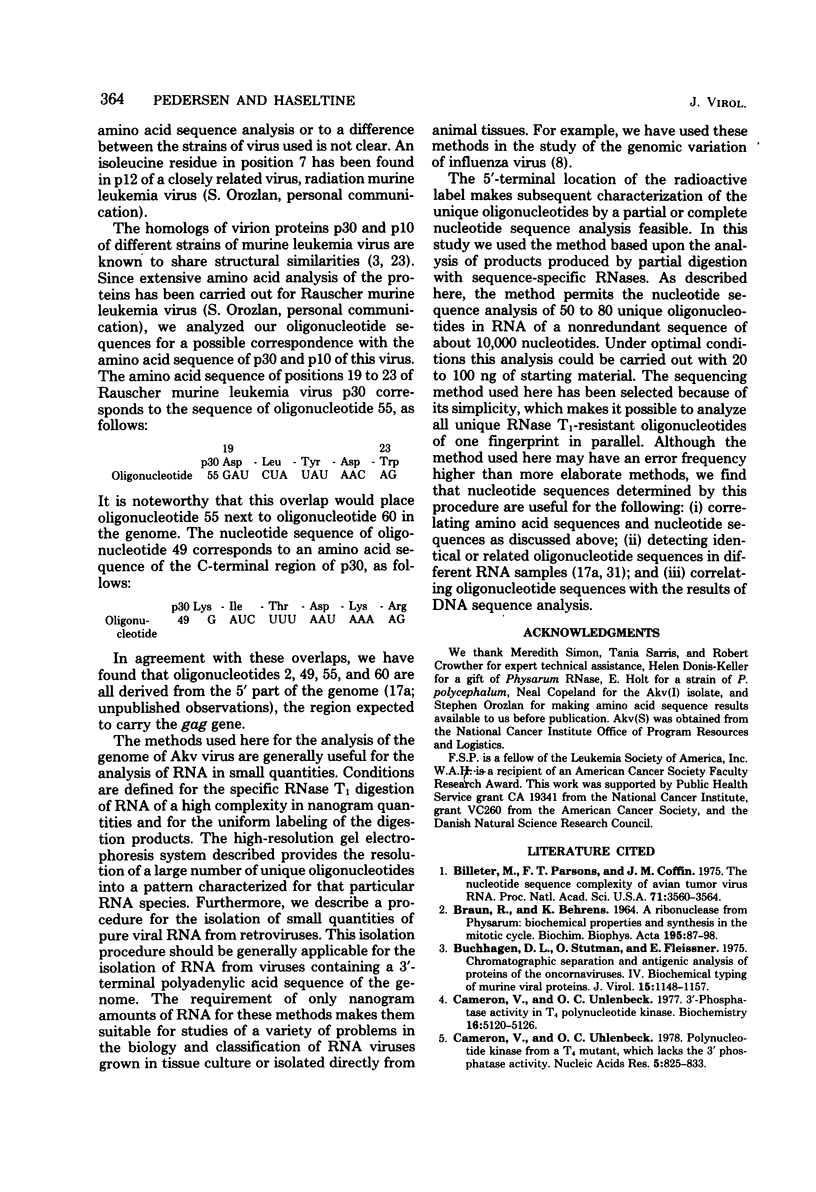
A detailed characterization of the genome of an endogenous, ecotropic type C virus, the Akv virus, is presented. Approximately 100 RNase T1-resistant oligonucleotides characteristic of the Akv genome were identified by two-dimensional gel electrophoresis, and the complete nucleotide sequence is presented for 75 of these oligonucleotides. A correspondence between the sequence of some of these oligonucleotides and the amino acid sequence of some virus-coded gag gene proteins is reported. For this study we developed methods suitable for the analysis of high-molecular-weight RNA species in nanogram quantities. The in vitro labeling procedures used here led to uniform labeling of the unique oligonucleotides.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R., Behrens K. A ribonuclease from Physarum. Biochemical properties and synthesis in the mitotic cycle. Biochim Biophys Acta. 1969 Nov 19;195(1):87–98. doi: 10.1016/0005-2787(69)90605-4. [DOI] [PubMed] [Google Scholar]
- Buchhagen D. L., Stutman O., Fleissner E. Chromatographic Separation and Antigenic Analysis of Proteins of the Oncornaviruses IV. Biochemical Typing of Murine Viral Proteins. J Virol. 1975 May;15(5):1148–1157. doi: 10.1128/jvi.15.5.1148-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron V., Soltis D., Uhlenbeck O. C. Polynucleotide kinase from a T4 mutant which lacks the 3' phosphatase activity. Nucleic Acids Res. 1978 Mar;5(3):825–833. doi: 10.1093/nar/5.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron V., Uhlenbeck O. C. 3'-Phosphatase activity in T4 polynucleotide kinase. Biochemistry. 1977 Nov 15;16(23):5120–5126. doi: 10.1021/bi00642a027. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Rowe W. P., Teich N. M., Lowy D. R. Definitive evidence that the murine C-type virus inducing locus Akv-1 is viral genetic material. Proc Natl Acad Sci U S A. 1975 Mar;72(3):906–910. doi: 10.1073/pnas.72.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Cooper G. M. Transfection by exogenous and endogenous murine retrovirus DNAs. Cell. 1979 Feb;16(2):347–356. doi: 10.1016/0092-8674(79)90011-4. [DOI] [PubMed] [Google Scholar]
- Desselberger U., Nakajima K., Alfino P., Pedersen F. S., Haseltine W. A., Hannoun C., Palese P. Biochemical evidence that "new" influenza virus strains in nature may arise by recombination (reassortment). Proc Natl Acad Sci U S A. 1978 Jul;75(7):3341–3345. doi: 10.1073/pnas.75.7.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Frisby D. P., Newton C., Carey N. H., Fellner P., Newman J. F., Harris T. J., Brown F. Oligonucleotide mapping of picornavirus RNAs by two-dimensional electrophoresis. Virology. 1976 Jun;71(2):379–388. doi: 10.1016/0042-6822(76)90365-2. [DOI] [PubMed] [Google Scholar]
- Frisby D. Oligonucleotide mapping of non-radioactive virus and messenger RNAs. Nucleic Acids Res. 1977 Sep;4(9):2975–2996. doi: 10.1093/nar/4.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Use of specific endonuclease cleavage in RNA sequencing-an enzymic method for distinguishing between cytidine and uridine residues. Nucleic Acids Res. 1977 Oct;4(10):3441–3454. doi: 10.1093/nar/4.10.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Pedersen F. S., Sahagan B. G., Rosenberg Z. F., Kozlov J. Comparative analysis of RNA tumor virus genomes. Haematol Blood Transfus. 1979;23:529–552. doi: 10.1007/978-3-642-67057-2_70. [DOI] [PubMed] [Google Scholar]
- Hays E. F., Vredevoe D. L. A discrepancy in XC and oncogenicity assays for murine leukemia virus in AKR mice. Cancer Res. 1977 Mar;37(3):726–730. [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Duran-Reynals M. L., Lilly F. Fv-1 regulation of lymphoma development and of thymic ecotropic and xenotropic MuLV expression in mice of the AKR/J x RF/J cross. Cell. 1978 Oct;15(2):429–435. doi: 10.1016/0092-8674(78)90012-0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Hays E. F., Doyle T., Linkhart S., Medeiros E., Pickering R. Oncornaviruses produced by murine leukemia cells in culture. Virology. 1977 Sep;81(2):363–370. doi: 10.1016/0042-6822(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Henderson L. E., Stephenson J. R., Copeland T. D., Long C. W., Ihle J. N., Gilden R. V. Amino- and carboxyl-terminal amino acid sequences of proteins coded by gag gene of murine leukemia virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1404–1408. doi: 10.1073/pnas.75.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Haseltine W. A. A micromethod for detailed characterization of high molecular weight RNA. Methods Enzymol. 1980;65(1):680–687. doi: 10.1016/s0076-6879(80)65066-6. [DOI] [PubMed] [Google Scholar]
- Pilly D., Niemeyer A., Schmidt M., Bargetzi J. P. Enzymes for RNA sequence analysis. Preparation and specificity of exoplasmodial ribonucleases I and II from Physarum polycephalum. J Biol Chem. 1978 Jan 25;253(2):437–445. [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Donis-Keller H., Hopkins N. RNA sequencing provides evidence for allelism of determinants of the N-, B- or NB-tropism of murine leukemia viruses. Cell. 1979 Jan;16(1):43–50. doi: 10.1016/0092-8674(79)90186-7. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. RNase T1-resistant oligonucleotides of Akv-1 and Akv-2 type C viruses of AKR mice. J Virol. 1977 Nov;24(2):690–694. doi: 10.1128/jvi.24.2.690-694.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Genetic factors in the natural history of murine leukemia virus infection: G. H. A. Clowes Memorial Lecture. Cancer Res. 1973 Dec;33(12):3061–3068. [PubMed] [Google Scholar]
- Sahagan B. G., Haseltine W. A. Structural analysis of the genomes of gibbon ape and woolly monkey leukosis viruses. J Virol. 1979 Sep;31(3):657–667. doi: 10.1128/jvi.31.3.657-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Staal S. P., Hartley J. W., Rowe W. P. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Székely M., Sanger F. Use of polynucleotide kinase in fingerprinting non-radioactive nucleic acids. J Mol Biol. 1969 Aug 14;43(3):607–617. doi: 10.1016/0022-2836(69)90362-3. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]