Abstract
The production of secretory proteins at the ER (endoplasmic reticulum) depends on a ready supply of energy and metabolites as well as the close monitoring of the chemical conditions that favor oxidative protein folding. ER oxidoreductases and chaperones fold nascent proteins into their export-competent three-dimensional structure. Interference with these protein folding enzymes leads to the accumulation of unfolded proteins within the ER lumen, causing an acute organellar stress that triggers the UPR (unfolded protein response). The UPR increases the transcription of ER chaperones commensurate with the load of newly synthesized proteins and can protect the cell from ER stress. Persistant stress, however, can force the UPR to commit cells to undergo apoptotic cell death, which requires the emptying of ER calcium stores.
Conversely, a continuous ebb and flow of calcium occurs between the ER and mitochondria during resting conditions on a domain of the ER that forms close contacts with mitochondria, the MAM (mitochondria-associated membrane). On the MAM, ER folding chaperones such as calnexin and calreticulin and oxidoreductases such as ERp44, ERp57 and Ero1α regulate calcium flux from the ER through reversible, calcium and redox-dependent interactions with IP3Rs (inositol 1,4,5-trisphophate receptors) and with SERCAs (sarcoplasmic/endoplasmic reticulum calcium ATPases). During apoptosis progression and depending on the identity of the ER chaperone and oxidoreductase, these interactions increase or decrease, suggesting that the extent of MAM targeting of ER chaperones and oxidoreductases could shift the readout of ER-mitochondria calcium exchange from housekeeping to apoptotic. However, little is known about the cytosolic factors that mediate the on/off interactions between ER chaperones and oxidoreductases with ER calcium channels and pumps. One candidate regulator is the multi-functional molecule PACS-2 (phosphofurin acidic cluster sorting protein-2). Recent studies suggest that PACS-2 mediates localization of a mobile pool of calnexin to the MAM in addition to regulating homeostatic ER calcium signaling as well as MAM integrity. Together, these findings suggest cytosolic, membrane and lumenal proteins combine to form a two-way switch that determines the rate of protein secretion by providing ions and metabolites and that appears to participate in the pro-apoptotic ER-mitochondria calcium transfer.
Keywords: Endoplasmic reticulum (ER), Mitochondria, Mitochondria-Associated Membrane (MAM), Oxidative Protein Folding, Chaperone, Apoptosis
1. Introduction
In many cell types, the ER is the most expansive cellular organelle, encompassing close to 50% of the total membrane in liver cells and close to 20% of the total volume of insulin secreting pancreatic β cells [1, 2]. The expansive size of the ER membrane reflects the manifold functions associated with this organelle, including secretory protein folding, calcium signaling and lipid synthesis and phagocytosis [3–8]. To physically accommodate these diverse functions, the continuous ER membrane system is composed of several domains that can be distinguished microscopically and isolated biochemically [9]. Among others, these domains include the rER (rough ER), the tER (transitional ER), the peripheral or cortical ER (also called PAM, plasma membrane-associated membrane) and the MAM [10]. The rER is characterized by the presence of ribosomes and translocons, which form a complex that mediates protein translocation, protein folding and protein degradation [10]. Following their folding, secretory proteins made within the rER translocate to the tER, which orchestrates COPII-mediated transport to the Golgi complex [11]. The tER can also contain ribosomes, but is distinguished from the rER by the presence of membrane buds and tubules, which contain cargo destined for protein secretion [12]. Other ER domains, however, are void of ribosomes. One such domain is the PAM, which provides ER-synthesized lipids to the plasma membrane and mediates capacitative calcium entry from the extracellular space [13]. The MAM forms close contacts with mitochondria, supports the transfer of essential lipids from the ER to mitochondria and regulates calcium exchange between these two organelles [14, 15].
Our current understading of MAM structure and function has evolved over 50 years, beginning with an electron microscopy-based report in 1959 that demonstrated the close proximity of the ER with mitochondria in fish gills [16]. Subsequent studies from 1969 to 1971 reported that liver microsomes composed of ER and associated with mitochondria contained all enzymes required for the synthesis of mitochondrial phospholipids [17–19]. During the 1970’s, protocols designed to isolate ER using detergent-free homogenization uncovered the tenacious association of mitochondria with heavy ER membranes [20, 21], leading to the hypothesis that biochemically distinct domains of the ER mediate interaction with mitochondria [21]. However, this provocative idea did not gain support until 1990, when the bona fide biochemical isolation of an ER domain associated with crude mitochondria from a Percoll gradient was reported. This critical breakthrough led to the designation of this domain as the MAM and subsequently enabled further characterization of the MAM [22–24]. Also during that decade, studies demonstrated the extent and structure of the MAM by confocal and electron microscopy [25, 26].
In parallel to these biochemical and microscopical analyses, seminal studies demonstrated that ER-mitochondria contacts at the MAM accommodate calcium transfer between the two organelles [27, 28]. This direct transfer occurs in a “quasi-synaptic” manner without major spills into the cytosol [26, 29, 30]. This model is supported by the enrichment of IP3Rs on the MAM, where they mediate the bulk of calcium release from the ER [31]. It is not yet known whether this enrichment extends to the SERCA transmembrane ATPase calcium pumps [32, 33], which transport two calcium ions per hydrolyzed ATP molecule into the ER [34]. Together, these data have led to the conclusion that the MAM is not only a site of lipid synthesis and transfer, but also a calcium signaling hub.
The importance of ER-mitochondria calcium signaling is most evident during the onset of apoptosis. Upon apoptosis onset, a robust transfer of calcium from the ER to the cytosol and mitochondria promotes cell death [35]. The MAM accommodates this transfer, since the disruption of the MAM, achieved by siRNA knockdown of PACS-2, results in the inhibition of ER calcium release and apoptosis onset [36]. Surprisingly however, PACS-2 knockdown also induces an unfolded protein response (UPR), typically elicited by disruption of ER oxidative protein folding [36]. Together with the observation that the MAM appears to be particularly enriched in ER folding chaperones and oxidoreductases [37–39], this domain of the ER may be functionally linked to the folding of newly synthesized proteins and might bridge the control of oxidative protein folding to ER calcium homeostasis. This review will summarize the functional ties between the MAM and ER oxidative protein folding, in addition to the more established roles of the MAM in apoptosis onset and progression.
2. Proteins regulating the composition and formation of the MAM
Recent research has focused on the description of connecting structures between the ER and mitochondria at the MAM. The in vitro incubation with proteinase not only detaches the ER from mitochondria, but also disrupts calcium transfer, thus suggesting these two organelles may be connected by proteinaceous tethers [40]. Since phospholipid import into mitochondria only decreases by 50% upon incubation of the mitochondria and the MAM with proteinase K, membrane-membrane association or membrane fusion between the two organelles may also occur [41]. Both lipid import into mitochondria and calcium transfer between the ER and mitochondria could depend on ubiquitination of yet to be identified substrates on the MAM: In yeast, phosphatidylserine transport to the mitochondria requires the expression of Met30p, a subunit of the SCF ubiquitin-ligase complex [42], whereas the amount of total ER calcium in mammalian cells depends on the ubiquitination of the ER stress protein Herp (homocysteine-inducible ER protein) by the expression levels of the E3 ubiquitin ligase POSH (plenty of SH3s) [43].
In yeast, the integral ER membrane protein Mmm1p, the peripheral ER membrane protein Mdm12p, and the two mitochondrial outer membrane proteins Mdm10p and Mdm34p have been demonstrated to tether the ER to mitochondria and to mediate mitochondria inheritance and movement [44, 45]. Mdm12p and Mmm1p are members of the SMP proteins family, whose members are widespread amongst eukaryotic species and play roles ranging from endocytosis to lipid metabolism and mitochondrial inheritance [46]. The role of mammalian protein homologues of this protein family in the formation of the MAM is currently unclear.
However, three MAM-regulating proteins are nevertheless known in mammalian cells: mitofusin-2, GRP75 and PACS-2 (Figure 1) [36, 47, 48]. Mitofusin-2 is an outer membrane GTPase that stabilizes interaction between adjacent mitochondria, thus promoting their fusion [49–51]. Surprisingly, mitofusin-2 also regulates ER morphology and calcium homeostasis, and mediates tethering of the ER to mitochondria [47]. A similar bridging and calcium homeostasis function is performed by GRP75, an hsp70 homologous cytosolic chaperone, that bridges the mitochondrial voltage dependent anion channel (VDAC) to the large N-terminal IP3 binding regulatory domain of the IP3R [48].
Figure 1.
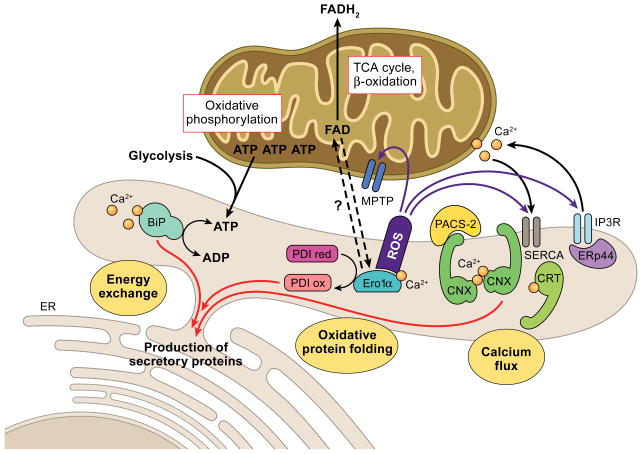
Three major metabolic exchanges between the ER and mitochondria impact on ER chaperone systems that catalyze the production of secretory proteins. 1. Energy exchange. GRP78/BiP requires ATP that is predominantly supplied by mitochondria, and to a lesser extent from glycolysis. GRP78/BiP also depends on ER calcium. 2. Oxidative protein folding. PDI forms disulfide bonds with the help of its recharger Ero1α, which can bind calcium and FAD derived from mitochondrial metabolism. Import proteins of FAD into the ER are currently unknown in mammalian systems. ROS produced by Ero1α could directly impact on the mitochondrial permeability transition pore (MPTP), SERCA and the IP3R. 3. Calcium flux. The calnexin/calreticulin folding cycle depends on ATP and ER calcium. Calnexin and calreticulin buffer free calcium within the ER, but also associate with SERCA to modulate calcium import. The localization of calnexin to the MAM and hence potentially its interaction with SERCA is under the control of PACS-2. The MAM-associated moiety of the oxidoreductase ERp44 interacts with the IP3R and regulates IP3R calcium release.
The role of PACS-2 in MAM tethering is complex. PACS-2 affects the composition of the MAM by mediating ER localization of the calcium release channel TRPP2 (the transient receptor potential protein 2, also known as polycystin-2) and of the chaperone calnexin, in particular on the MAM [38, 52]. Since the interaction of calnexin with SERCA in the ER inhibits calcium uptake [53] and calcium can exit the ER through TRPP2, siRNA knockdown of PACS-2, associated with reduced amounts of calnexin and TRPP2 at the MAM, increases the amount of IP3R-releasable calcium in the presence of extracellular calcium (see also section 5) [36]. PACS-2 siRNA knockdown also uncouples the ER from mitochondria, suggesting PACS-2 is required for MAM stabilization or formation [36]. Interestingly, PACS-2 knockdown increases ER calcium levels, but also induces the UPR, indicative of ER stress and suggesting that the MAM could impact on the control of ER oxidative protein folding as well [36].
3. ER folding mechanisms and stress
The ER maintains an optimized environment to support the production of secretory proteins. This includes maintaining millimolar concentrations of free calcium, which is more than 1,000-fold higher than the concentration of free calcium in cytosol [54, 55]. Depletion of ER calcium stalls oxidative protein folding and prevents export of secretory proteins from the ER [56, 57]. The orchestration of polypeptide folding and formation of disulfide bonds requires chaperones and oxidoreductases [58]. One of the best-characterized chaperones is the luminal protein BiP/GRP78, which is an ATPase that promotes the folding of hydrophobic regions in polypeptides to the interior in a calcium-dependent manner [59, 60]. Following the enzymatic action of BiP/GRP78, newly synthesized proteins interact with a pair of closely related lectins, the transmembrane protein calnexin and its luminal counterpart calreticulin, both of which require calcium for their folding activities as well [61–65]. These two proteins recognize monoglucosylated newly synthesized proteins and mediate their folding, assisted by disulfide bond formation catalyzed by the oxidoreductases protein disulfide isomerase (PDI) and ERp57 [66, 67]. To ensure the exclusive export of fully folded proteins, the ER uses sophisticated and stringent quality control mechanisms that retain incompletely folded intermediates by chaperones [68]. One example is the calnexin/calreticulin cycle, where nascent secretory proteins interact in their monoglucosylated forms with the two chaperones that promote their folding. This interaction is followed by a removal of the terminal glucose sugar by glucosidase II, leading either to export from the ER for proteins that have attained their mature conformation, or to re-glucosylation of incompletely folded proteins by UDP-glucose:glycoportein glucosyltransferase (UGGT) to allow another round of enzymatic action by calnexin and calreticulin [69, 70]. Interestingly, chaperones and oxidoreductases such as calnexin, BiP/GRP78 and, in particular, calreticulin not only depend on calcium for their functioning, but also buffer calcium within the ER and thus determine the amount of free calcium within the ER, essential for the functioning of ER chaperones and normally about one fifth of the total ER calcium content of 5 mM [61, 71]. For calreticulin, the calcium buffering function may even be more crucial than its role in ER protein folding, since this function can not be compensated by calnexin, the related lectin chaperone of the ER [72].
Interference with chaperone-assisted protein folding, either by depletion of ATP or ER calcium, or by a block of glycosylation or generation of unfavorable redox conditions that prevent disulfide bond formation can all result in ER stress and activation of the UPR [73, 74]. Thus, the block in cargo protein folding under conditions of low calcium appears to result from its manifold roles as a co-factor for chaperone-substrate interaction by calnexin and calreticulin [75, 76] and BiP/GRP78 [77], and also by its role in the formation of complexes between chaperones and oxidoreductases such as between calreticulin and ERp57 [78].
The UPR is controlled by the action of several ER-localized transmembrane stress sensors, Ire1, PERK and ATF6 [73, 74]. The monitoring of ER oxidative protein folding by the stress sensors is highly fine-tuned. The main mechanism that controls the signaling of the stress sensors depends on their ability to dimerize upon the accumulation of unfolded proteins. The chaperone BiP/GRP78 is a central regulator of this effect, since its increasing association with unfolded proteins under conditions of ER stress results in its decreased ability to interact with the stress sensors, but eventually also results in the accumulation of “naked” unfolded proteins that are not bound to this chaperone. Accumulation of unfolded proteins within the ER induces the transmembrane stress sensors to activate UPR signaling by different mechanisms. For instance monomeric ATF6 is transported to the Golgi complex [79], where it is cleaved by S1P/SKI-1 (site-1 protease/subtilisin kexin isozyme 1) and S2P [80, 81]. Cleaved ATF6 then translocates to the nucleus, where it activates transcription of genes encoding BiP/GRP78, and XBP1 [82, 83]. Conversely, Ire1 and PERK require oligomerization at the ER, which exposes and triggers their kinase activity [84–86]. Initially, the UPR utilizes all three sensor proteins to boost pro-survival signaling. Activated Ire1 becomes an endonuclease, which splices the XBP1 mRNA in mammalian cells, resulting in the expression of the active XBP1 transcription factor that promotes the production of ER chaperones and oxidoreductases [87, 88]. Actived PERK counteracts ER stress by phosphorylating eIF2α, leading to translational arrest and thus reducing the ER protein load [89, 90]. These responses to acute ER stress are intended to reset ER homeostasis by reducing the ER protein load, while increasing factors that combat ER stress (e.g., chaperones).
4. ER stress and calcium signaling at the MAM
Prolonged ER stress, lasting typically more than 4h, can induce apoptosis. Under these conditions, the kinase domain of oligomerized Ire1 bind TRAF2 (tumor necrosis factor receptor-associated factor 2), an adaptor protein that bridges Ire1 to the protein kinase ASK1, leading to the activation of the pro-apoptotic kinase JNK [91]. Additionally, ATF6, the PERK target ATF4, and XBP1 induce CHOP, the CCAAT/enhancer-binding protein (C/EBP) homologous protein [92, 93]. CHOP is a transcription factor that promotes apoptosis by repressing Bcl2 transcription and promoting Bim transcription [94, 95].
Paralleling the modulation of UPR signaling, the readout of ER-mitochondria calcium exchange also undergoes dramatic changes when ER stress persists. Under homeostatic conditions, there is a continuous ebb and flow of calcium between the ER and mitochondria [3], mediated on the ER face by MAM-localized IP3Rs that passively release puffs of calcium into calcium hot spots in the cytosol [30, 96–99]. Mitochondria take up these local high amounts of calcium in a quasi-synaptic manner through a gated and highly selective calcium uniporter [100–102]. Within the mitochondria, increased calcium levels promote the activity of ATP synthase [103, 104]. Calcium efflux from mitochondria is likely mediated via an as yet unidentified Na/Ca exchanger [102]. The released calcium is retrieved to the ER by SERCA calcium pumps, completing the cycle.
A sustained ER stress of more than 24h or the induction of apoptosis result in the release of ER calcium at the MAM that triggers loss of mitochondrial membrane potential to promote apoptosis [105, 106]. Under these conditions, cytochrome c gradually translocates from mitochondria to the ER, where it binds the IP3R [107, 108]. This binding abolishes the calcium-mediated inhibition of IP3-associated calcium release and results in a feed-forward amplification of the ER calcium release in early apoptosis. Blocking this interaction inhibits the progression of apoptosis [109, 110], whereas allowing it leads to increased release of cytochrome c and a robust transfer of calcium from the ER to the cytosol and mitochondria to promote cell death [35]. Whereas the IP3R type 3 (IP3R3) apparently is the preferential calcium channel for apoptotic signal transmission from the ER to mitochondria, modulating the IP3R type 1 (IP3R1) activity also interferes with apoptotic signaling [111–114]. Moreover, calcium chelation or disruption of IP3R by pharmacological inhibiton can efficiently block apoptosis onset [109, 115]. Given the localization of IP3Rs to MAMs [32, 99, 114], the quantity and quality of this ER microdomain appears to regulate the strength and speed of cell death. Consistent with this hypothesis, proteins that mediate MAM composition and integrity have key roles in the onset of apoptosis.
Overexpression of mitofusin-2 or GRP75 inhibits apoptosis [116–119]. However, it is currently unclear whether this function stems from an influence on calcium exchange between the ER and mitochondria during either resting conditions or apoptosis onset. PACS-2 plays multiple roles in apoptosis: First, its expression is required for the ER localization of TRPP2 and for maintaining contacts between the ER and mitochondria that accommodate pro-apoptotic calcium release [36]. Second, PACS-2 is required for extrinsic and intrinsic apoptosis induction in virus-infected or transformed cells at the level of Bid translocation to the mitochondria [36, 120, 121]. Phosphorylation of PACS-2 Ser437 by Akt1 regulates the ability of PACS-2 to switch between its roles in homeostasis and apoptosis. Akt1 phosphorylation of PACS-2 promotes its binding to 14-3-3 proteins and its role in the ER homeostasis, whereas dephosphorylation of PACS-2 Ser437 triggered by death ligands promotes apoptotic Bid activation at mitochondria [120, 122, 123].
Proteins of the Bcl2 family also regulate the death-promoting calcium transfer from the ER to mitochondria. Overexpression of anti-apoptotic Bcl2 reduces the ER calcium content by increasing the calcium permeability of the ER [124], a characteristic of this organelle that may depend on Bcl2 itself or the translocon [125]. Similarly, Bcl-XL binds to the IP3R and promotes its ability to release calcium [126]. Furthermore, ER-targeting of the pro-apoptotic BH3-only protein Nix increases ER calcium content and promotes the opening of the mitochondrial permeability transition pore [127]. Recently, an interesting link between ER-localized Bax and Bak and ER stress signaling has been discovered, since Ire1 signaling requires expression of these two Bcl2 family proteins [128]. The ER-localized Bax inhibitor 1 (BI-1) opposes this role of Bax and Bak by inhibiting Ire1 and blocking ER oxidative protein folding, while at the same time reducing the overall calcium content of the ER [129–131].
5. MAM-localized chaperones and oxidoreductases modulate calcium exchange with mitochondria
ER oxidative protein folding itself may be another important regulator of calcium homeostasis and signaling, since numerous ER chaperones and oxidoreductases localize to the MAM and associate with the IP3R and SERCA. For instance, ERK1-phosphorylation of calnexin on Ser563 blocks calcium uptake by SERCA2b [53, 132, 133]. However, prolonged ER stress or ER calcium depletion lead to calnexin dephosphorylation and a removal of calnexin from the MAM, thus removing the calnexin-dependent clamp on SERCA activity [38, 53, 134]. PACS-2 binds to calnexin, when this SERCA regulator is dephosphorylated on Ser534, 544, two protein kinase CK2 target sites, which can boost the role of Ser563-phosphorylated calnexin in its folding and retention activities close to the translocon [132, 133, 135]. Thus, PACS-2 may modulate the extent of calnexin/SERCA interaction and its associated regulatory mechanism [38, 132]. The thioredoxin-related oxidoreductases ERp57 and ERp44 appear to modulate ER calcium signaling in a similar way. ERp57 interacts with ER-facing cysteines of SERCA2b under oxidizing ER conditions, thus inhibiting SERCA2b calcium pumping, whereas ERp44 interacts with lumenal cysteines of the IP3R1 to inhibit calcium transfer to mitochondria when ER conditions are reducing [113, 136].
The structure of the MAM directly determines the extent of this chaperone and oxidoreductase-regulated calcium flux, as observed following PACS-2 knockdown, which leads to an increase in ER calcium or in the absence of mitofusin-2, which results in lower ER calcium, but increased calcium levels within mitochondria [36, 47]. Additionally, the amount of calcium on the ER cytosolic face and within the ER lumen dictates the activity of both the IP3R and SERCA; high cytosolic calcium levels block the IP3R opening, whereas high concentrations of calcium within the ER lumen block SERCA [96, 137].
Besides the MAM structure itself, this calcium transfer could additionally require the formation of a glycosphingolipid-enriched microdomain fraction of MAMs [138], possibly to confine the chaperoning activity of sigma-1 receptors on IP3R3 to the MAM [139]. This chaperoning activity of sigma-1 receptors inhibits proteasomal degradation of the channel [112]. By doing so, the sigma-1 receptor maintains ER-mitochondria calcium signaling that is active during periods of extended ER stress. A DRM-like lipid composition of the MAM might also be required for the formation of a complex of erlin-1 and erlin-2 (ER lipid raft-associated proteins, also known as SPFH1/2, proteins with similarities to stomatin, prohibitin, flotillin, and HflC/K) that degrades IP3Rs by ERAD [140–142].
Together, these findings demonstrate that MAM formation, and MAM quality, together with the regulated enrichment of select chaperones and oxidoreductases control ER calcium homeostasis and signaling with mitochondria.
6. Role of ATP for the ER-mitochondria interaction and the MAM
The exchange of calcium between the ER and mitochondria has functions that go beyond the maintenance of homeostasis in the two organelles. High cytosolic calcium impedes mitochondria movement along microtubules, at sites of IP3R-mediated calcium release [143]. This suggests that mitochondria may coalesce near IP3R-enriched MAMs, allowing mitochondria to supply fuel to the ER, one of the major sites of ATP consumption in the cell [144, 145]. Numerous activities associated with the ER consume ATP, in particular the import of calcium by SERCA [146, 147], mRNA translation [148], protein translocation [149], the folding of newly synthesized polypeptides (see below), ERAD [150, 151] and the transfer of lipids into mitochondria [152]. However, because many of these mechanisms are interdependent, it has been challenging to determine how much ATP is used for each process. In particular, it appears difficult to assess the individual consumption specifically used for ER oxidative protein folding, although it is clear that the correct functioning of many ER chaperones such as BiP/GRP78, GRP94 and calnexin depends on a constant supply of ATP [153–155].
Glucose has long been viewed as the chief energy provider for ER oxidative protein folding, because lack of glucose can induce ER stress [156, 157] and ongoing glycolysis contributes to ER homeostasis [158]. Many of these studies, however, were performed using tumor cell lines cultured in media containing high glucose, exacerbating the abnormally high contribution of glycolysis of these transformed cells to the energy landscape [159]. However, most cell types, including the commonly used HeLa cells, still produce up to 50% of their total ATP supply within mitochondria when glucose is low [104, 160–162]. Consistent with this observation, even in laboratory culture settings, the inhibition of the mitochondrial electron transport chain with Antimycin A leads to the induction of a UPR in MDCK cells and inhibition of ER-mitochondria calcium flux with CGP 37157 blocks production of secretory proteins at the ER level in CHO cells [163, 164]. The disruption of the MAM and induction of the UPR by PACS-2 knockdown highlights the role of the MAM for oxidative protein folding in the ER [36]. Accordingly, the number of tight contacts between the ER and mitochondria double under conditions of ER stress, although future experiments will have to determine whether ER-stressed cells aim to increase metabolite exchange between the two organelles or whether this observation is explained by pro-apoptotic calcium exchange [40].
Overexpression of mitochondrial adenine nucleotide translocase (ANT) increases ATP export from mitochondria and reduces ER-mitochondria calcium flux, suggesting transfer of ATP from mitochondria influences ER lumenal calcium homeostasis [165]. This finding raises the question as to how ATP is imported into the ER, which is a particularly relevant question for ER oxidative protein folding. One candidate ATP importer associates with ER membranes [166]. Confirming this finding, partial purification showed that an ADP/ATP antiporter of 56 kDa mediates ATP import into the ER in rat liver [167, 168], but it is not known to which ER microdomain these candidate transport proteins are localized. Thus, like calcium, ATP production in mitochondria and its consumption in the ER may influence mitochondrial activity or proximity.
7. The MAM and the regulation of ER redox
The discovery that ER oxidative protein folding and mitochondrial oxidative phosphorylation compete for oxygen further supports a link between ER and mitochondria metabolism [169]. The ER enzyme that is responsible for oxygen consumption in yeast is Ero1p, a glycosylated membrane-associated flavoenzyme localized to the lumenal side of the ER [170]. Ero1p forms an electron transport chain within the ER lumen using the thioredoxin-related enzyme PDI and oxygen [171]. Ero1p uses molecular oxygen to oxidize PDI, which can then catalyze the formation of disulfide bonds within newly synthesized polypeptides [172]. The human Ero1 paralogs, Ero1-Lα and Ero1-Lβ catalyze the same reaction in human cells [173, 174]. Whereas Ero1-Lα shows a widespread distribution, Ero1-Lβ is mostly found in tissues that secrete large amounts of protein, suggesting that Ero1-Lβ specifically increases the efficiency of the ER secretion machinery [173–175]. The transcription of Ero1-Lα increases upon ER stress and hypoxia, whereas the transcription of Ero1-Lβ is exclusively under the control of ER stress [173, 176, 177], although the significance of this different regulation is currently unclear.
Similar to oxidative phosphorylation within mitochondria, the formation of disulfide bonds by Ero1-Lα and Ero1-Lβ leads to production of ER-localized hydrogen peroxide (H2O2), making the ER the cellular organelle with the highest content of H2O2 [178]. High levels of this ROS, which occurs under ER stress [129, 179–181], lead to the inactivation of SERCA by S-glutathionylation [182] and the activation of IP3R by oxidation, increasing the level of calcium on the cytosolic face of the ER [183]. In addition, H2O2 also evokes an increase of mitochondrial calcium [184]. Thus, ROS production by Ero1 proteins may provide an additional mechanism for the ER to attract mitochondria under conditions of ER stress. Consistent with this hypothesis, Ero1-Lα is highly enriched on the MAM [39], where it potentiates the release of calcium during ER stress [185]. It remains to be demonstrated, however, whether Ero1-Lα directly links the control of ER redox to the functioning and formation of the MAM as well.
All Ero1 proteins require flavin adenine dinucleotide (FAD) as a co-factor to remain in their active state and depend on free FAD levels within the ER [169, 186, 187]. FAD is made from riboflavin by FAD synthetase in the mitochondrial matrix and is converted to its reduced form (FADH2) by the tricarboxylic acid cycle and oxidative decarboxylation [188, 189]. In yeast, flavin carrier proteins import FAD into the ER, but they have not yet been detected in metazoans [190, 191]. FAD metabolism within the mitochondria is thought to control the redox homeostasis of the ER, since deficiency of riboflavin impairs oxidative folding and secretion [192, 193]. Together, these findings suggest multiple ties link the ER and the mitochondrial metabolism (Figure 1). The dependence of ER oxidative protein folding on the exchange of calcium, ATP and metabolites such as FAD with mitochondria may explain why ER chaperones and oxidoreductases regulate multiple aspects of the MAM. The coming years will lead to the identification of more MAM components and MAM regulating proteins that integrate the many interactions between the ER and the mitochondria. Of particular interest in this context appears the identification of ATP and ROS sensing and transporting proteins, which are currently unknown in most organisms.
8. Conclusions and Perspective
Critical progress has been made in the description of an apparently surprising link between ER oxidative protein folding and a domain of the ER called the MAM. Numerous ER chaperones are involved in the regulation of the function of this structure that controls cell metabolism and apoptosis. These findings suggest that proteins of the MAM and ER chaperones that regulate MAM signaling could play important roles in diseases where normal cellular life cycles are compromised. This could be relevant in tumor tissue. For instance, low levels of the ER calcium pump SERCA2b are associated with squamous cell carcinoma [194–196]. Similarly, increased levels of the sigma-1 receptor are found in breast cancer cell lines and are known to counteract ER stress response and apoptosis induction [197]. Furthermore, numerous members of ER oxidoreductases and chaperones (including calnexin and ERp57) show abnormal expression levels in tumors [198, 199]. Not surprisingly, mitofusin-2 markedly suppresses cell proliferation of tumor cells when overexpressed [200] and PACS-2, frequently absent from sporadic colorectal cancer cells [201], critically determines the outcome of TRAIL-mediated apoptosis [120]. Given that the MAM performs such important roles for cellular survival and apoptosis, interference with its natural function using small molecule inhibitors could tip the balance of life or death into a desired direction. These approaches have to await the discovery of more MAM sorting mechanisms and a more comprehensive description of the MAM composition and function.
Acknowledgments
We thank Show-Ling Shyng and Ing-Swie Goping for critical reading of this manuscript. We also thank Lori Vaskalis for expert assistance with the artwork. Work in the Simmen laboratory was supported by Alberta Health Services (Bridge Grant #24170) and by the NCIC/CCSRI (Grant #17291). TS is supported by the Alberta Heritage Foundation for Medical Research (200500396). EML is supported by an ACRI studentship (#24136). GT is supported by NIH grant R01 DK37274.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Croze EM, Morre DJ. Isolation of plasma membrane, golgi apparatus, and endoplasmic reticulum fractions from single homogenates of mouse liver. J Cell Physiol. 1984;119:46–57. doi: 10.1002/jcp.1041190109. [DOI] [PubMed] [Google Scholar]
- 2.Dean PM. Ultrastructural morphometry of the pancreatic -cell. Diabetologia. 1973;9:115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 4.Brostrom MA, Brostrom CO. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium. 2003;34:345–363. doi: 10.1016/s0143-4160(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 5.Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem Cell Biol. 2004;82:113–128. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]
- 6.Tehlivets O, Scheuringer K, Kohlwein SD. Fatty acid synthesis and elongation in yeast. Biochim Biophys Acta. 2007;1771:255–270. doi: 10.1016/j.bbalip.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004;16:343–349. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, Paiement J, Bergeron JJ, Desjardins M. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 9.Lavoie C, Paiement J. Topology of molecular machines of the endoplasmic reticulum: a compilation of proteomics and cytological data. Histochem Cell Biol. 2008;129:117–128. doi: 10.1007/s00418-007-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. doi: 10.1038/nature06384. [DOI] [PubMed] [Google Scholar]
- 11.Tang BL, Wang Y, Ong YS, Hong W. COPII and exit from the endoplasmic reticulum. Biochim Biophys Acta. 2005;1744:293–303. doi: 10.1016/j.bbamcr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Fan JY, Roth J, Zuber C. Ultrastructural analysis of transitional endoplasmic reticulum and pre-Golgi intermediates: a highway for cars and trucks. Histochem Cell Biol. 2003;120:455–463. doi: 10.1007/s00418-003-0597-1. [DOI] [PubMed] [Google Scholar]
- 13.Lebiedzinska M, Szabadkai G, Jones AW, Duszynski J, Wieckowski MR. Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other subcellular organelles. Int J Biochem Cell Biol. 2009;41:1805–1816. doi: 10.1016/j.biocel.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz JG, Genty H, St-Pierre P, Dang T, Joshi B, Sauve R, Vogl W, Nabi IR. Reversible interactions between smooth domains of the endoplasmic reticulum and mitochondria are regulated by physiological cytosolic Ca2+ levels. J Cell Sci. 2007;120:3553–3564. doi: 10.1242/jcs.03486. [DOI] [PubMed] [Google Scholar]
- 16.Copeland DE, Dalton AJ. An association between mitochondria and the endoplasmic reticulum in cells of the pseudobranch gland of a teleost. J Biophys Biochem Cytol. 1959;5:393–396. doi: 10.1083/jcb.5.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungalwala FB, Dawson RM. The origin of mitochondrial phosphatidylcholine within the liver cell. Eur J Biochem. 1970;12:399–402. doi: 10.1111/j.1432-1033.1970.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 18.McMurray WC, Dawson RM. Phospholipid exchange reactions within the liver cell. Biochem J. 1969;112:91–108. doi: 10.1042/bj1120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauner MT, Levy M. Study of the transfer of phospholipids from the endoplasmic reticulum to the outer and inner mitochondrial membranes. J Lipid Res. 1971;12:71–75. [PubMed] [Google Scholar]
- 20.Lewis JA, Tata JR. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J Cell Sci. 1973;13:447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- 21.Shore GC, Tata JR. Two fractions of rough endoplasmic reticulum from rat liver. I. Recovery of rapidly sedimenting endoplasmic reticulum in association with mitochondria. J Cell Biol. 1977;72:714–725. doi: 10.1083/jcb.72.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem. 1994;269:27494–27502. [PubMed] [Google Scholar]
- 23.Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- 24.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 25.Mannella CA, Buttle K, Rath BK, Marko M. Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors. 1998;8:225–228. doi: 10.1002/biof.5520080309. [DOI] [PubMed] [Google Scholar]
- 26.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 27.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 28.Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 29.Filippin L, Magalhaes PJ, Di Benedetto G, Colella M, Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J Biol Chem. 2003;278:39224–39234. doi: 10.1074/jbc.M302301200. [DOI] [PubMed] [Google Scholar]
- 30.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. Embo J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikoshiba K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp. 2007:9–22. doi: 10.1042/BSS0740009. [DOI] [PubMed] [Google Scholar]
- 32.Csordas G, Hajnoczky G. Sorting of calcium signals at the junctions of endoplasmic reticulum and mitochondria. Cell Calcium. 2001;29:249–262. doi: 10.1054/ceca.2000.0191. [DOI] [PubMed] [Google Scholar]
- 33.Csordas G, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria in cardiac muscle. Trends Cardiovasc Med. 2001;11:269–275. doi: 10.1016/s1050-1738(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 34.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 35.Crompton M, Costi A, Hayat L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J. 1987;245:915–918. doi: 10.1042/bj2450915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. Embo J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T. The Subcellular Distribution of Calnexin Is Mediated by PACS-2. Mol Biol Cell. 2008;19:2777–2788. doi: 10.1091/mbc.E07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilady SY, Bui M, Lynes EM, Benson MD, Watts R, Vance JE, Simmen T. Ero1α requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM) Cell Stress Chaperones. MS ID #CSAC-253. 2010 doi: 10.1007/s12192-010-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein SD, Perktold A, Zellnig G, Daum G. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem. 1999;264:545–553. doi: 10.1046/j.1432-1327.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher MM, Choi JY, Voelker DR. Phosphatidylserine transport to the mitochondria is regulated by ubiquitination. J Biol Chem. 2002;277:51033–51042. doi: 10.1074/jbc.M205301200. [DOI] [PubMed] [Google Scholar]
- 43.Tuvia S, Taglicht D, Erez O, Alroy I, Alchanati I, Bicoviski V, Dori-Bachash M, Ben-Avraham D, Reiss Y. The ubiquitin E3 ligase POSH regulates calcium homeostasis through spatial control of Herp. J Cell Biol. 2007;177:51–61. doi: 10.1083/jcb.200611036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boldogh IR, Nowakowski DW, Yang HC, Chung H, Karmon S, Royes P, Pon LA. A protein complex containing Mdm10p, Mdm12p, and Mmm1p links mitochondrial membranes and DNA to the cytoskeleton-based segregation machinery. Mol Biol Cell. 2003;14:4618–4627. doi: 10.1091/mbc.E03-04-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee I, Hong W. Diverse membrane-associated proteins contain a novel SMP domain. Faseb J. 2006;20:202–206. doi: 10.1096/fj.05-4581hyp. [DOI] [PubMed] [Google Scholar]
- 47.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 48.Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 51.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 52.Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. Embo J. 2005;24:705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roderick HL, Lechleiter JD, Camacho P. Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b. J Cell Biol. 2000;149:1235–1248. doi: 10.1083/jcb.149.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 55.Robert V, De Giorgi F, Massimino ML, Cantini M, Pozzan T. Direct monitoring of the calcium concentration in the sarcoplasmic and endoplasmic reticulum of skeletal muscle myotubes. J Biol Chem. 1998;273:30372–30378. doi: 10.1074/jbc.273.46.30372. [DOI] [PubMed] [Google Scholar]
- 56.Lodish HF, Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990;265:10893–10899. [PubMed] [Google Scholar]
- 57.Lodish HF, Kong N, Wikstrom L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J Biol Chem. 1992;267:12753–12760. [PubMed] [Google Scholar]
- 58.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braakman I, Hoover-Litty H, Wagner KR, Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991;114:401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gething MJ. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- 61.Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multiprocess calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- 62.Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 63.Ou WJ, Cameron PH, Thomas DY, Bergeron JJ. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- 64.Rajagopalan S, Xu Y, Brenner MB. Retention of unassembled components of integral membrane proteins by calnexin. Science. 1994;263:387–390. doi: 10.1126/science.8278814. [DOI] [PubMed] [Google Scholar]
- 65.Wada I, Rindress D, Cameron PH, Ou WJ, Doherty JJ, 2nd, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJ. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- 66.Oliver JD, van der Wal FJ, Bulleid NJ, High S. Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science. 1997;275:86–88. doi: 10.1126/science.275.5296.86. [DOI] [PubMed] [Google Scholar]
- 67.Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci. 2006;119:615–623. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
- 68.Anelli T, Sitia R. Protein quality control in the early secretory pathway. Embo J. 2008;27:315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellgaard L, Frickel EM. Calnexin, calreticulin, and ERp57: teammates in glycoprotein folding. Cell Biochem Biophys. 2003;39:223–247. doi: 10.1385/CBB:39:3:223. [DOI] [PubMed] [Google Scholar]
- 70.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 71.Mogami H, Gardner J, Gerasimenko OV, Camello P, Petersen OH, Tepikin AV. Calcium binding capacity of the cytosol and endoplasmic reticulum of mouse pancreatic acinar cells. J Physiol. 1999;518(Pt 2):463–467. doi: 10.1111/j.1469-7793.1999.0463p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molinari M, Eriksson KK, Calanca V, Galli C, Cresswell P, Michalak M, Helenius A. Contrasting functions of calreticulin and calnexin in glycoprotein folding and ER quality control. Mol Cell. 2004;13:125–135. doi: 10.1016/s1097-2765(03)00494-5. [DOI] [PubMed] [Google Scholar]
- 73.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 74.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Jeso B, Ulianich L, Pacifico F, Leonardi A, Vito P, Consiglio E, Formisano S, Arvan P. Folding of thyroglobulin in the calnexin/calreticulin pathway and its alteration by loss of Ca2+ from the endoplasmic reticulum. Biochem J. 2003;370:449–458. doi: 10.1042/BJ20021257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ou WJ, Bergeron JJ, Li Y, Kang CY, Thomas DY. Conformational changes induced in the endoplasmic reticulum luminal domain of calnexin by Mg-ATP and Ca2+ J Biol Chem. 1995;270:18051–18059. doi: 10.1074/jbc.270.30.18051. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki CK, Bonifacino JS, Lin AY, Davis MM, Klausner RD. Regulating the retention of T-cell receptor alpha chain variants within the endoplasmic reticulum: Ca(2+)-dependent association with BiP. J Cell Biol. 1991;114:189–205. doi: 10.1083/jcb.114.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corbett EF, Oikawa K, Francois P, Tessier DC, Kay C, Bergeron JJ, Thomas DY, Krause KH, Michalak M. Ca2+ regulation of interactions between endoplasmic reticulum chaperones. J Biol Chem. 1999;274:6203–6211. doi: 10.1074/jbc.274.10.6203. [DOI] [PubMed] [Google Scholar]
- 79.Nadanaka S, Okada T, Yoshida H, Mori K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol. 2007;27:1027–1043. doi: 10.1128/MCB.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pullikotil P, Benjannet S, Mayne J, Seidah NG. The proprotein convertase SKI-1/S1P: alternate translation and subcellular localization. J Biol Chem. 2007;282:27402–27413. doi: 10.1074/jbc.M703200200. [DOI] [PubMed] [Google Scholar]
- 81.Shen J, Prywes R. Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. J Biol Chem. 2004;279:43046–43051. doi: 10.1074/jbc.M408466200. [DOI] [PubMed] [Google Scholar]
- 82.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 83.Shen J, Snapp EL, Lippincott-Schwartz J, Prywes R. Stable binding of ATF6 to BiP in the endoplasmic reticulum stress response. Mol Cell Biol. 2005;25:921–932. doi: 10.1128/MCB.25.3.921-932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 85.Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu CY, Schroder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2000;275:24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- 87.Tirasophon W, Lee K, Callaghan B, Welihinda A, Kaufman RJ. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 2000;14:2725–2736. doi: 10.1101/gad.839400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 89.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 91.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 92.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 93.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 94.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 96.Mogami H, Tepikin AV, Petersen OH. Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. Embo J. 1998;17:435–442. doi: 10.1093/emboj/17.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529(Pt 1):37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rutter GA, Rizzuto R. Regulation of mitochondrial metabolism by ER Ca2+ release: an intimate connection. Trends Biochem Sci. 2000;25:215–221. doi: 10.1016/s0968-0004(00)01585-1. [DOI] [PubMed] [Google Scholar]
- 99.Hajnoczky G, Csordas G, Madesh M, Pacher P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J Physiol. 2000;529(Pt 1):69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 101.Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 102.Graier WF, Frieden M, Malli R. Mitochondria and Ca(2+) signaling: old guests, new functions. Pflugers Arch. 2007;455:375–396. doi: 10.1007/s00424-007-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Griffiths EJ, Rutter GA. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta. 2009;1787:1324–1333. doi: 10.1016/j.bbabio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 104.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 106.Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 107.Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 108.Boehning D, Patterson RL, Snyder SH. Apoptosis and calcium: new roles for cytochrome c and inositol 1,4,5-trisphosphate. Cell Cycle. 2004;3:252–254. [PubMed] [Google Scholar]
- 109.Steinmann C, Landsverk ML, Barral JM, Boehning D. Requirement of inositol 1,4,5-trisphosphate receptors for tumor-mediated lymphocyte apoptosis. J Biol Chem. 2008;283:13506–13509. doi: 10.1074/jbc.C800029200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wozniak AL, Wang X, Stieren ES, Scarbrough SG, Elferink CJ, Boehning D. Requirement of biphasic calcium release from the endoplasmic reticulum for Fas-mediated apoptosis. J Cell Biol. 2006;175:709–714. doi: 10.1083/jcb.200608035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blackshaw S, Sawa A, Sharp AH, Ross CA, Snyder SH, Khan AA. Type 3 inositol 1,4,5-trisphosphate receptor modulates cell death. Faseb J. 2000;14:1375–1379. doi: 10.1096/fj.14.10.1375. [DOI] [PubMed] [Google Scholar]
- 112.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 113.Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. Subtype-Specific and ER Lumenal Environment-Dependent Regulation of Inositol 1,4,5-Trisphosphate Receptor Type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 114.Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- 115.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Voccoli V, Mazzoni F, Garcia-Gil M, Colombaioni L. Serum-withdrawal-dependent apoptosis of hippocampal neuroblasts involves Ca++ release by endoplasmic reticulum and caspase-12 activation. Brain Res. 2007;1147:1–11. doi: 10.1016/j.brainres.2007.01.145. [DOI] [PubMed] [Google Scholar]
- 117.Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab. 2009;29:365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jahani-Asl A, Cheung EC, Neuspiel M, MacLaurin JG, Fortin A, Park DS, McBride HM, Slack RS. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem. 2007;282:23788–23798. doi: 10.1074/jbc.M703812200. [DOI] [PubMed] [Google Scholar]
- 119.Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem. 2005;280:25060–25070. doi: 10.1074/jbc.M501599200. [DOI] [PubMed] [Google Scholar]
- 120.Aslan JE, You H, Williamson DM, Endig J, Youker RT, Thomas L, Shu H, Du Y, Milewski RL, Brush MH, Possemato A, Sprott K, Fu H, Greis KD, Runckel DN, Vogel A, Thomas G. Akt and 14–3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis. Mol Cell. 2009;34:497–509. doi: 10.1016/j.molcel.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 122.Youker RT, Shinde U, Day R, Thomas G. At the crossroads of homoeostasis and disease: roles of the PACS proteins in membrane traffic and apoptosis. Biochem J. 2009;421:1–15. doi: 10.1042/BJ20081016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.You H, Thomas G. A homeostatic switch in PACS-2 links membrane traffic to TRAIL-induced apoptosis. Cell Cycle. 2009;8:2679–2680. doi: 10.4161/cc.8.17.9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Giunti R, Gamberucci A, Fulceri R, Banhegyi G, Benedetti A. Both translocon and a cation channel are involved in the passive Ca2+ leak from the endoplasmic reticulum: a mechanistic study on rat liver microsomes. Arch Biochem Biophys. 2007;462:115–121. doi: 10.1016/j.abb.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 126.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Diwan A, Matkovich SJ, Yuan Q, Zhao W, Yatani A, Brown JH, Molkentin JD, Kranias EG, Dorn GW., 2nd Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 129.Kim HR, Lee GH, Cho EY, Chae SW, Ahn T, Chae HJ. Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J Cell Sci. 2009;122:1126–1133. doi: 10.1242/jcs.038430. [DOI] [PubMed] [Google Scholar]
- 130.Kim HR, Lee GH, Ha KC, Ahn T, Moon JY, Lee BJ, Cho SG, Kim S, Seo YR, Shin YJ, Chae SW, Reed JC, Chae HJ. Bax Inhibitor-1 Is a pH-dependent regulator of Ca2+ channel activity in the endoplasmic reticulum. J Biol Chem. 2008;283:15946–15955. doi: 10.1074/jbc.M800075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P, Reed JC, Glimcher LH, Hetz C. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chevet E, Wong HN, Gerber D, Cochet C, Fazel A, Cameron PH, Gushue JN, Thomas DY, Bergeron JJ. Phosphorylation by CK2 and MAPK enhances calnexin association with ribosomes. Embo J. 1999;18:3655–3666. doi: 10.1093/emboj/18.13.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chevet E, Smirle J, Cameron PH, Thomas DY, Bergeron JJ. Calnexin phosphorylation: Linking cytoplasmic signalling to endoplasmic reticulum lumenal functions. Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 134.Delom F, Fessart D, Chevet E. Regulation of calnexin sub-cellular localization modulates endoplasmic reticulum stress-induced apoptosis in MCF-7 cells. Apoptosis. 2007;12:293–305. doi: 10.1007/s10495-006-0625-4. [DOI] [PubMed] [Google Scholar]
- 135.Cameron PH, Chevet E, Pluquet O, Thomas DY, Bergeron JJ. Calnexin phosphorylation attenuates the release of partially misfolded alpha1-antitrypsin to the secretory pathway. J Biol Chem. 2009;284:34570–34579. doi: 10.1074/jbc.M109.053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Y, Camacho P. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J Cell Biol. 2004;164:35–46. doi: 10.1083/jcb.200307010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 138.Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d’Azzo A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell. 2009;36:500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmacol. 2010 doi: 10.1124/mol.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Browman DT, Resek ME, Zajchowski LD, Robbins SM. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J Cell Sci. 2006;119:3149–3160. doi: 10.1242/jcs.03060. [DOI] [PubMed] [Google Scholar]
- 141.Pearce MM, Wormer DB, Wilkens S, Wojcikiewicz RJ. An endoplasmic reticulum (ER) membrane complex composed of SPFH1 and SPFH2 mediates the ER-associated degradation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2009;284:10433–10445. doi: 10.1074/jbc.M809801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hoegg MB, Browman DT, Resek ME, Robbins SM. Distinct regions within the erlins are required for oligomerization and association with high molecular weight complexes. J Biol Chem. 2009;284:7766–7776. doi: 10.1074/jbc.M809127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–354. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 145.Culic O, Gruwel ML, Schrader J. Energy turnover of vascular endothelial cells. Am J Physiol. 1997;273:C205–213. doi: 10.1152/ajpcell.1997.273.1.C205. [DOI] [PubMed] [Google Scholar]
- 146.Tsuura Y, Fujimoto S, Kajikawa M, Ishida H, Seino Y. Regulation of intracellular ATP concentration under conditions of reduced ATP consumption in pancreatic islets. Biochem Biophys Res Commun. 1999;261:439–444. doi: 10.1006/bbrc.1999.1052. [DOI] [PubMed] [Google Scholar]
- 147.de Meis L, Arruda AP, Carvalho DP. Role of sarco/endoplasmic reticulum Ca(2+)-ATPase in thermogenesis. Biosci Rep. 2005;25:181–190. doi: 10.1007/s10540-005-2884-7. [DOI] [PubMed] [Google Scholar]
- 148.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- 149.Awe K, Lambert C, Prange R. Mammalian BiP controls posttranslational ER translocation of the hepatitis B virus large envelope protein. FEBS Lett. 2008;582:3179–3184. doi: 10.1016/j.febslet.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 150.McCracken AA, Brodsky JL. Assembly of ER-associated protein degradation in vitro: dependence on cytosol, calnexin, and ATP. J Cell Biol. 1996;132:291–298. doi: 10.1083/jcb.132.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nakatsukasa K, Huyer G, Michaelis S, Brodsky JL. Dissecting the ER-associated degradation of a misfolded polytopic membrane protein. Cell. 2008;132:101–112. doi: 10.1016/j.cell.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shiao YJ, Lupo G, Vance JE. Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J Biol Chem. 1995;270:11190–11198. doi: 10.1074/jbc.270.19.11190. [DOI] [PubMed] [Google Scholar]
- 153.Braakman I, Helenius J, Helenius A. Role of ATP and disulphide bonds during protein folding in the endoplasmic reticulum. Nature. 1992;356:260–262. doi: 10.1038/356260a0. [DOI] [PubMed] [Google Scholar]
- 154.Ostrovsky O, Makarewich CA, Snapp EL, Argon Y. An essential role for ATP binding and hydrolysis in the chaperone activity of GRP94 in cells. Proc Natl Acad Sci U S A. 2009;106:11600–11605. doi: 10.1073/pnas.0902626106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wada I, Ou WJ, Liu MC, Scheele G. Chaperone function of calnexin for the folding intermediate of gp80, the major secretory protein in MDCK cells; regulation by redox state and ATP. JBC. 1994;269:7464–7472. [PubMed] [Google Scholar]
- 156.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 157.Shiu RP, Pouyssegur J, Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977;74:3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 159.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 160.Goh CH, Jung KH, Roberts SK, McAinsh MR, Hetherington AM, Park YI, Suh K, An G, Nam HG. Mitochondria provide the main source of cytosolic ATP for activation of outward-rectifying K+ channels in mesophyll protoplast of chlorophyll-deficient mutant rice (OsCHLH) seedlings. J Biol Chem. 2004;279:6874–6882. doi: 10.1074/jbc.M309071200. [DOI] [PubMed] [Google Scholar]
- 161.Knopp A, Thierfelder S, Doepner B, Benndorf K. Mitochondria are the main ATP source for a cytosolic pool controlling the activity of ATP-sensitive K(+) channels in mouse cardiac myocytes. Cardiovasc Res. 2001;52:236–245. doi: 10.1016/s0008-6363(01)00395-9. [DOI] [PubMed] [Google Scholar]
- 162.Piechota J, Szczesny R, Wolanin K, Chlebowski A, Bartnik E. Nuclear and mitochondrial genome responses in HeLa cells treated with inhibitors of mitochondrial DNA expression. Acta Biochim Pol. 2006;53:485–495. [PubMed] [Google Scholar]
- 163.Osibow K, Frank S, Malli R, Zechner R, Graier WF. Mitochondria maintain maturation and secretion of lipoprotein lipase in the endoplasmic reticulum. Biochem J. 2006;396:173–182. doi: 10.1042/BJ20060099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kuznetsov G, Bush KT, Zhang PL, Nigam SK. Perturbations in maturation of secretory proteins and their association with endoplasmic reticulum chaperones in a cell culture model for epithelial ischemia. Proc Natl Acad Sci U S A. 1996;93:8584–8589. doi: 10.1073/pnas.93.16.8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wieckowski MR, Szabadkai G, Wasilewski M, Pinton P, Duszynski J, Rizzuto R. Overexpression of adenine nucleotide translocase reduces Ca2+ signal transmission between the ER and mitochondria. Biochem Biophys Res Commun. 2006;348:393–399. doi: 10.1016/j.bbrc.2006.07.072. [DOI] [PubMed] [Google Scholar]
- 166.Mayinger P, Meyer DI. An ATP transporter is required for protein translocation into the yeast endoplasmic reticulum. Embo J. 1993;12:659–666. doi: 10.1002/j.1460-2075.1993.tb05699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim SH, Shin SJ, Park JS. Identification of the ATP transporter of rat liver rough endoplasmic reticulum via photoaffinity labeling and partial purification. Biochemistry. 1996;35:5418–5425. doi: 10.1021/bi950485h. [DOI] [PubMed] [Google Scholar]
- 168.Shin SJ, Lee WK, Lim HW, Park J. Characterization of the ATP transporter in the reconstituted rough endoplasmic reticulum proteoliposomes. Biochim Biophys Acta. 2000;1468:55–62. doi: 10.1016/s0005-2736(00)00241-8. [DOI] [PubMed] [Google Scholar]
- 169.Tu BP, Weissman JS. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell. 2002;10:983–994. doi: 10.1016/s1097-2765(02)00696-2. [DOI] [PubMed] [Google Scholar]
- 170.Frand AR, Kaiser CA. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell. 1998;1:161–170. doi: 10.1016/s1097-2765(00)80017-9. [DOI] [PubMed] [Google Scholar]
- 171.Sevier CS, Kaiser CA. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim Biophys Acta. 2008;1783:549–556. doi: 10.1016/j.bbamcr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 172.Hatahet F, Ruddock LW, Ahn K, Benham A, Craik D, Ellgaard L, Ferrari D, Ventura S. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal. 2009;11:2807–2850. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- 173.Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R. Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J Biol Chem. 2000;275:23685–23692. doi: 10.1074/jbc.M003061200. [DOI] [PubMed] [Google Scholar]
- 174.Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J Biol Chem. 2000;275:4827–4833. doi: 10.1074/jbc.275.7.4827. [DOI] [PubMed] [Google Scholar]
- 175.Dias-Gunasekara S, Gubbens J, van Lith M, Dunne C, Williams JA, Kataky R, Scoones D, Lapthorn A, Bulleid NJ, Benham AM. Tissue-specific expression and dimerization of the endoplasmic reticulum oxidoreductase Ero1beta. J Biol Chem. 2005;280:33066–33075. doi: 10.1074/jbc.M505023200. [DOI] [PubMed] [Google Scholar]
- 176.Tanaka S, Uehara T, Nomura Y. Up-regulation of protein-disulfide isomerase in response to hypoxia/brain ischemia and its protective effect against apoptotic cell death. J Biol Chem. 2000;275:10388–10393. doi: 10.1074/jbc.275.14.10388. [DOI] [PubMed] [Google Scholar]
- 177.May D, Itin A, Gal O, Kalinski H, Feinstein E, Keshet E. Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene. 2005;24:1011–1020. doi: 10.1038/sj.onc.1208325. [DOI] [PubMed] [Google Scholar]
- 178.Enyedi B, Varnai P, Geiszt M. Redox state of the endoplasmic reticulum is controlled by ERO1L-ALPHA and intraluminal calcium. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2880. [DOI] [PubMed] [Google Scholar]
- 179.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 180.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 181.Gross E, Sevier CS, Heldman N, Vitu E, Bentzur M, Kaiser CA, Thorpe C, Fass D. Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc Natl Acad Sci U S A. 2006;103:299–304. doi: 10.1073/pnas.0506448103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 183.Hu Q, Zheng G, Zweier JL, Deshpande S, Irani K, Ziegelstein RC. NADPH oxidase activation increases the sensitivity of intracellular Ca2+ stores to inositol 1,4,5-trisphosphate in human endothelial cells. J Biol Chem. 2000;275:15749–15757. doi: 10.1074/jbc.M000381200. [DOI] [PubMed] [Google Scholar]
- 184.Gonzalez A, Granados MP, Salido GM, Pariente JA. H2O2-induced changes in mitochondrial activity in isolated mouse pancreatic acinar cells. Mol Cell Biochem. 2005;269:165–173. doi: 10.1007/s11010-005-3457-6. [DOI] [PubMed] [Google Scholar]
- 185.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science. 2000;290:1571–1574. doi: 10.1126/science.290.5496.1571. [DOI] [PubMed] [Google Scholar]
- 187.Papp E, Nardai G, Mandl J, Banhegyi G, Csermely P. FAD oxidizes the ERO1-PDI electron transfer chain: the role of membrane integrity. Biochem Biophys Res Commun. 2005;338:938–945. doi: 10.1016/j.bbrc.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 188.Barile M, Brizio C, Valenti D, De Virgilio C, Passarella S. The riboflavin/FAD cycle in rat liver mitochondria. Eur J Biochem. 2000;267:4888–4900. doi: 10.1046/j.1432-1327.2000.01552.x. [DOI] [PubMed] [Google Scholar]
- 189.Barile M, Brizio C, De Virgilio C, Delfine S, Quagliariello E, Passarella S. Flavin adenine dinucleotide and flavin mononucleotide metabolism in rat liver--the occurrence of FAD pyrophosphatase and FMN phosphohydrolase in isolated mitochondria. Eur J Biochem. 1997;249:777–785. doi: 10.1111/j.1432-1033.1997.00777.x. [DOI] [PubMed] [Google Scholar]
- 190.Protchenko O, Rodriguez-Suarez R, Androphy R, Bussey H, Philpott CC. A screen for genes of heme uptake identifies the FLC family required for import of FAD into the endoplasmic reticulum. J Biol Chem. 2006;281:21445–21457. doi: 10.1074/jbc.M512812200. [DOI] [PubMed] [Google Scholar]
- 191.Csala M, Marcolongo P, Lizak B, Senesi S, Margittai E, Fulceri R, Magyar JE, Benedetti A, Banhegyi G. Transport and transporters in the endoplasmic reticulum. Biochim Biophys Acta. 2007;1768:1325–1341. doi: 10.1016/j.bbamem.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 192.Depeint F, Bruce WR, Shangari N, Mehta R, O’Brien PJ. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006;163:94–112. doi: 10.1016/j.cbi.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 193.Manthey KC, Chew YC, Zempleni J. Riboflavin deficiency impairs oxidative folding and secretion of apolipoprotein B-100 in HepG2 cells, triggering stress response systems. J Nutr. 2005;135:978–982. doi: 10.1093/jn/135.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Endo Y, Uzawa K, Mochida Y, Shiiba M, Bukawa H, Yokoe H, Tanzawa H. Sarcoendoplasmic reticulum Ca(2+) ATPase type 2 downregulated in human oral squamous cell carcinoma. Int J Cancer. 2004;110:225–231. doi: 10.1002/ijc.20118. [DOI] [PubMed] [Google Scholar]
- 195.Prasad V, Boivin GP, Miller ML, Liu LH, Erwin CR, Warner BW, Shull GE. Haploinsufficiency of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump, predisposes mice to squamous cell tumors via a novel mode of cancer susceptibility. Cancer Res. 2005;65:8655–8661. doi: 10.1158/0008-5472.CAN-05-0026. [DOI] [PubMed] [Google Scholar]
- 196.Liu LH, Boivin GP, Prasad V, Periasamy M, Shull GE. Squamous cell tumors in mice heterozygous for a null allele of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump. J Biol Chem. 2001;276:26737–26740. doi: 10.1074/jbc.C100275200. [DOI] [PubMed] [Google Scholar]
- 197.Aydar E, Onganer P, Perrett R, Djamgoz MB, Palmer CP. The expression and functional characterization of sigma (sigma) 1 receptors in breast cancer cell lines. Cancer Lett. 2006;242:245–257. doi: 10.1016/j.canlet.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 198.Leys CM, Nomura S, LaFleur BJ, Ferrone S, Kaminishi M, Montgomery E, Goldenring JR. Expression and prognostic significance of prothymosin-alpha and ERp57 in human gastric cancer. Surgery. 2007;141:41–50. doi: 10.1016/j.surg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 199.Dissemond J, Busch M, Kothen T, Mors J, Weimann TK, Lindeke A, Goos M, Wagner SN. Differential downregulation of endoplasmic reticulum-residing chaperones calnexin and calreticulin in human metastatic melanoma. Cancer Lett. 2004;203:225–231. doi: 10.1016/j.canlet.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 200.Wu L, Li Z, Zhang Y, Zhang P, Zhu X, Huang J, Ma T, Lu T, Song Q, Li Q, Guo Y, Tang J, Ma D, Chen KH, Qiu X. Adenovirus-expressed human hyperplasia suppressor gene induces apoptosis in cancer cells. Mol Cancer Ther. 2008;7:222–232. doi: 10.1158/1535-7163.MCT-07-0382. [DOI] [PubMed] [Google Scholar]
- 201.Anderson GR, Brenner BM, Swede H, Chen N, Henry WM, Conroy JM, Karpenko MJ, Issa JP, Bartos JD, Brunelle JK, Jahreis GP, Kahlenberg MS, Basik M, Sait S, Rodriguez-Bigas MA, Nowak NJ, Petrelli NJ, Shows TB, Stoler DL. Intrachromosomal genomic instability in human sporadic colorectal cancer measured by genome-wide allelotyping and inter-(simple sequence repeat) PCR. Cancer Res. 2001;61:8274–8283. [PubMed] [Google Scholar]