Abstract
Recurrent hypoglycemia is a common problem among infants and children that is associated with several metabolic disorders and insulin-dependent diabetes mellitus. Although studies have reported a relationship between a history of juvenile hypoglycemia and psychological health problems, the direct effects of recurrent moderate hypoglycemia have not been fully determined. Thus, in this study, we used an animal model to examine the effects of recurrent hypoglycemia during the juvenile period on affective, social, and motor function (assessed under euglycemic conditions) across development. To model recurrent hypoglycemia, rats were administered 5 U/kg of insulin or saline twice per day from postnatal day (P)10 to P19. Body weight gain was retarded in insulin-treated rats during the treatment period, but recovered by the end of treatment. However, insulin-treated rats displayed increases in affective reactivity that emerged early during treatment and persisted after treatment into early adulthood. Specifically, insulin-treated pups showed increased maternal separation-induced vocalizations as infants, and an exaggerated acoustic startle reflex as juveniles and young adults. Moreover, young adult rats with a history of recurrent juvenile hypoglycemia exhibited increased fear-potentiated startle and increases in behavioral and hormonal responses to restraint stress. Some of these effects were sex-dependent. The changes in affective behavior in insulin-exposed pups were accompanied by decreases in adolescent social play behavior. These results provide evidence that recurrent, transient hypoglycemia during juvenile development can lead to increases in fear-related behavior and stress reactivity. Importantly, these phenotypes are not reversed with normalization of blood glucose and may persist into adulthood.
Keywords: Hypoglycemia, Insulin-dependent diabetes mellitus, Infant vocalization, Acoustic startle reflex, Prepulse inhibition of startle, Fear-potentiated startle, Conditioned emotional response, Social behavior, Stress, Postnatal development, Fluoro-Jade, Rat
1. Introduction
Glucose is the predominant metabolic fuel for the mammalian brain under physiologic conditions and a continual supply of glucose is essential for normal brain development (Vannucci and Vannucci, 2000, 2001). Unfortunately, however, hypoglycemia is the most common metabolic dysfunction in infants and children. Recurrent hypoglycemia is often seen in children with conditions associated with hormone deficiencies or excess, such as hyperinsulinism, and hereditary defects in carbohydrate, amino acid, or lipid metabolism. It is also highly associated with insulin treatment in insulin-dependent diabetes mellitus (IDDM) (Cornblath and Ichord, 2000; Cryer, 2006; De Leon and Stanley, 2007; Vannucci and Vannucci, 2001). Young children with IDDM are particularly susceptible to frequent bouts of hypoglycemia as a result of caretakers' efforts to maintain tight glycemic control via exogenous insulin administration (Bhatia and Wolfsdorf, 1991; Flykanaka-Gantenbein, 2004; Gonder-Frederick et al., 1989, 2008; Hershey et al., 1999; Perantie et al., 2008). Moreover, children often remain “asymptomatic” while experiencing moderate hypoglycemia (Becker and Ryan, 2000; Gonder-Frederick et al., 2008). The recurrence of these often undetected hypoglycemic episodes during critical periods of brain development may lead to deficits in cerebral and cognitive function (Becker and Ryan, 2000; Flykanaka-Gantenbein, 2004; Hershey et al., 1999; Northam et al., 2006; Perantie et al., 2008).
Many of the clinical and preclinical studies on the effects of hypoglycemia have focused on cognitive function (Cox et al., 2005; McNay and Sherwin, 2004; McNay et al., 2006; Perantie et al., 2008). However, hypoglycemia may also have significant effects on mood and affective behavior (e.g., Cox et al., 2005). Low blood glucose levels are associated with negative mood states, primarily self-reported “nervousness” (Boyle and Zrebiec, 2007; Gonder-Frederick et al., 1989). Moreover, patients with a history of severe hypoglycemia show significantly increased levels of anxiety (Wredling et al., 1992) or “negative mood” (Gonder-Frederick et al., 2008) relative to other patients with IDDM.
Information on the emotional, cognitive, and behavioral effects of recurrent hypoglycemia in children is scant. Similarly, few animal studies have examined the effects of moderate hypoglycemia on the developing brain. Given the increased utilization of alternate fuel substrates such as lactate and ketone bodies in the immature brain, it has been commonly believed that the developing brain is resistant to hypoglycemic injury. However, it is now known that the reduced capacity for glucose transport to the immature brain limits cerebral glucose utilization, especially during conditions of increased cerebral glycolytic demand or reduced availability, such as in hypoglycemia (Vannucci et al., 1981, 1998). Preclinical studies on effects of hypoglycemia on juvenile brain have examined neuronal injury (Ennis et al., 2008; Kim et al., 2005), hormonal stress responses (Grino et al., 1994), seizure susceptibility (Lee et al., 1988), and later glucose regulation (Thompson et al., 1997). However, these studies have not examined associations between hypoglycemic episodes and other functions regulated by the hippocampus and limbic circuits, including affective behavior.
Further study of the effects of recurrent juvenile hypoglycemia on neural and behavior development may be particularly important for children with IDDM, a population in which hypoglycemia often goes undetected. To address this, we have developed a rodent model of recurrent insulin-induced moderate “asymptomatic” hypoglycemia (defined as a significant but transient decrease in blood glucose that fails to produce gross behavioral changes) during juvenile development. In this study, we focus on the effects of this exposure on affective and social behavior throughout development. Moderate “asymptomatic” hypoglycemia was induced with insulin twice per day from postnatal day (P)10 to P19. Affective, social and motor behaviors were assessed throughout this period, and in adolescence and early adulthood. We report that recurrent, transient insulin-induced hypoglycemia during infant and juvenile development in the rat produces reliable and persistent increases in fear-related behaviors and reactivity to stressors. Moreover, this increase in affective reactivity is accompanied by specific deficits in social behavior.
2. Research design and methods
2.1. Animals and insulin treatment
All experiments were approved by the Institutional Animal Care and Use Committee of Columbia University and performed on Wistar rat pups bred on site in a dedicated holding room with the light:dark cycle set to 6:00 AM (lights on):6:00 PM. Pups were cross-fostered and randomized to litters of 10 pups each (5 male/5 female) on P1 to avoid predetermined litter effects. All pups were housed with the foster dam, and food and water were available ad libitum throughout their lifespan. On or prior to P9, pups were randomly assigned to hypoglycemia or saline treatment. Treatments began on the morning of P10 and were continued until P19. The hypoglycemia group received 5 U/kg (s.c.) injections of Humulin Regular Insulin (Eli Lilly, Indianapolis, IN, USA) (diluted in sterile saline to a volume of 0.05 cc) twice daily at 8:00 AM and 4:00 PM. On the basis of pilot experiments, this dose was found to be optimal for producing a significant but transient decrease in blood glucose levels to which pups rapidly developed behavioral tolerance such that after the first 1–2 administrations the treatment produced no gross behavioral changes. Control animals received injections of sterile saline (s.c.) of equal volume. All animals were returned to the dam immediately after injection. Blood glucose was monitored in 1–2 pups per litter per groups, chosen randomly, from 0.1 μl tail blood samples using LifeScan One-Touch Ultra Blood Glucose Monitoring System (Milpitas, CA, USA) to ensure the induction of hypoglycemia within 2 h and the return to euglycemia by 4 h. Daily weights were monitored on all animals, and pups that did not gain at least 1 g per day for two consecutive days were eliminated from the study.
2.2. Body growth and maturation
Body weights were recorded just prior to and recorded daily during administration of insulin or saline. For a subset of subjects, body weight continued to be monitored until P22. Anecdotal observations of the day of eye opening were also made.
Statistics
A mixed ANOVA in which juvenile treatment (INS or SAL) and sex were the between-subjects factors, and postnatal day the repeated measure, was used to assess the effect of INS treatment on growth. Significant interactions with postnatal day were interpreted as effects on growth rate; post-hoc analyses for this interaction are described in Section 2.1. To focus statistical analyses on the effects of treatment, the only effect of sex considered in this study was the interaction of sex with treatment.
2.3. Behavioral analyses
All behavioral testing was conducted during the light phase of the light:dark cycle.
2.3.1. Maternal separation-induced vocalizations
Maternal separation-induced vocalization rates were assessed with a minor modification of the protocol repeatedly used in the laboratory of Brunelli et al. (Brunelli et al., 1996; Hofer et al., 2002; Muller et al., 2005). Briefly, one pup at a time was removed from the home cage and held for 3 min in a clean testing chamber kept at approximately 36 °C with a heating pad. During this period, ultrasonic vocalizations (USVs) were detected with an ultrasonic detector (Peterson, Uppsala, Sweden) set to convert sounds in the 30–50 kHz range to audible sounds heard through earphones. Vocalizations were counted by hand. Once the recording period was complete, the pup was placed in a clean holding cage, maintained at approximately 36 °C with a heating pad. After USVs were recorded for each pup, the entire litter was returned to the dam in the home cage.
Maternal separation-induced USVs were measured prior to treatment on P9, to allow for baseline vocalization to be used as a covariate in subsequent analyses. Pups were then re-tested on either P12 or P14. Testing took place between 8:30 and 9:30 AM, a point at least 16 h after the previous insulin treatment when blood glucose was normal in INS pups (see Section 2). The morning insulin treatment was then administered after a 30-min reunion with the dam.
Statistics
Effects on the number of USVs in the 3-min separation period were assessed with a two-way ANOVA including treatment, age-at-testing and sex as between-subjects factors and baseline USV rate as a covariate.
2.3.2. Play behavior
Play behavior was assessed in juvenile rats from P32 to P34. Two same-sex, same-treatment littermates were used for each testing session. Prior to testing, the pups were weaned and divided into same-sex housing groups of no more than 4 pups per cage. Two days after weaning, rats were single-housed. For testing, one poly(methyl methacrylate) chamber (15h × 17w × 17d) was assigned per litter and filled with clean bedding plus one half cup of bedding from the home cage of each littermate to be tested in that chamber. These chambers were located in a sound-attenuated room dedicate to this purpose. All behavioral procedures were carried out during the light cycle, but in red light. Prior to testing, each rat was individually habituated to the test chamber for 3 days. For this procedure, the rat was placed in the designated chamber for 10 min with the room conditions used for testing (sound attenuation; red light only from a lamp suspended two feet above the chamber). After 3 days of habituation to the chambers, rats were subjected to three daily social interaction sessions in which littermate pairs were placed in the test cage for 10 min and an observer blind to treatment counted frequencies of rearing, partner sniffing, walkovers (including side mounts), napes, and pins. These behaviors were defined according to criteria developed by Pellis et al. (1992).
Statistics
The effect of treatment and its interaction with sex on the frequencies of the behaviors were analyzed using a multivariate analysis of variance (MANOVA), followed by univariate tests on each outcome measure.
2.3.3. The acoustic startle reflex and prepulse inhibition of startle (PPI)
The acoustic startle reflex, and habituation and prepulse inhibition of this reflex, were determined using a standard startle chambers (SR-Lab, San Diego Instruments, Inc., San Diego, CA; or Kinder Scientific, Julian, CA) using minor modification of a widely-used paradigm (Koch, 1999; Swerdlow et al., 2001). Rats were tested on P22 or P23. Each rat was placed inside a holding tube, which was, in turn, placed on a force-transduction platform within a sound-attenuating chamber equipped with a speaker and houselight. Following a 5 min resting period to habituate to the background noise (60 dB), startle threshold was determined by presenting ascending then descending series of 40 ms noise bursts ranging from 79 to 96 dB separated by varying inter-trial intervals (ITIs) (15 ± 6s). A startle stimulus (40 ms duration, 110 dB) was then presented for 16 consecutive trials to assess the initial startle reflex and habituation to the startle stimulus. Following this block of stimuli, rats were allowed to rest in the chamber for 5 min, were briefly re-habituated to the standard startle stimulus (110 dB), then exposed to a pseudorandomized presentation of “startle-only” and “prepulse-startle” trials. In one quarter of the trials, the 110 dB startle stimulus was presented alone; on the other 75% of the trials, a “prepulse” (20 ms noise burst; salience ranging from 8 to 24 dB above background) preceded the startle tone with an inter-stimulus interval (ISI) of 100 ms.
A separate cohort INS- and SAL-treated rats (from different litters) were tested as young adults (P55–P60) with methods identical to those above except that an abbreviated startle threshold/habituation protocol and PPI were combined into one session and the range of prepulse salience levels was shifted from 8–24 dB to 2–16 dB above background in accordance with our and others' observations of an increase in PPI in adults relative to juveniles (Farid et al., 2000; Lipska et al., 1995; Martinez et al., 2000).
Statistics
Effects of treatment and its interaction with sex on habituation of the startle response across trials were assessed with a mixed ANOVA using trial as the repeated measure. We also applied an alternate analysis of habituation that could be applied to both the juvenile and adult cohorts. For this, a habituation index was calculated as the percent difference in startle amplitude between early and late startle trials presented during the respective habituation protocols. “Early” was defined as the average across the first 2 presentations of the startle stimulus at 110 (juvenile) or 96–100 (adult) dB. “Late” was defined as the average of last 2 of these startle-only trials. The distribution of the habituation index failed assumptions underlying an ANOVA but met those for the Wald logistic regression test and, thus, the effects of treatment and sex were assessed with the latter method.
For prepulse inhibition, data from at least 7 trials of each of the above-described prepulse-startle and startle-stimulus-only trial types were averaged. The habituated baseline acoustic response was calculated as the average displacement on startle stimulus-only trials. Prepulse inhibition of startle was quantified as a percentage decrease from the baseline acoustic startle response ((1 − [PPx/ST]) * 100), where PP = mean response on prepulse-startle trials at the prepulse loudness “x”, and ST = mean response on startle-only trials interspersed among prepulse-startle trials). Mixed ANOVAs in which prepulse salience was the repeated measure, and juvenile treatment and sex were between-subjects factors, were applied.
2.3.4. Fear-potentiated startle
Fear-potentiated startle (FPS) was assessed in young adults 1–2 days after baseline startle and PPI testing. Methods based on those of Davis et al. (Davis, 1990; Davis et al., 1993; Liang et al., 1992b; Rosen et al., 1992). The same enclosures described above were used. These chambers were cleaned between sessions. On Day 1 (Conditioning), shock grids were attached to the floor of the animal holders. Animals were placed in the enclosure such that all four feet rest on the shock grid. The animal was given room to shift its posture, but not to rear. The chamber was dark and a white-noise background (60 dB) was present throughout the session. A conditioning trial consisted of the pairing of the conditioned stimulus (CS+), a 2 s illumination of the houselight, with the unconditioned stimulus, footshock (0.5 mA, 200 ms). The footshock was delivered to the floor grid during the last 200 ms of the light. Twenty conditioning trials were presented with ITIs of 45, 60, or 75 s, pseudorandomized. Eighteen to 24 h following the light-shock conditioning (Test Day), animals were placed in the same startle chambers without the shock-grid flooring. On the background of constant white noise (60 dB), 13 bursts of white noise (startle stimulus; 105 dB, 40 ms) were delivered unpredictably (ITIs of 12, 24, 36 s, randomized). The first of these bursts was delivered with the houselight off (CS− condition). For the remaining 12 trials, CS+ (houselight on) trials were pseudorandomly interspersed with CS− trials with no more than 2 consecutive presentation of either trial type. On CS+ trials, the houselight was illuminated for 2 s and the startle stimulus was presented 1.8 s following the onset of the light (the same time relative to the onset of the light that the shock was presented on the Conditioning Day).
Statistics
The FPS ratio was calculated for each CS+ trial as the ratio between the startle amplitude on the CS+ trial divided by average startle amplitude across CS− trials. The average of the FPS ratios on the first 3 CS+ trials (during steady-state expression of the conditioned response) was used for comparisons across treatment and sex groups. Further explanation and justification for this approach are provided in the Supplementary materials (Supplementary Fig. 1). The effect of treatment and its interaction with sex were assessed with a Wald logistic regression test due to non-normality of the distributions of the FPS ratio statistic across groups, but conformity of the difference distributions with the assumptions the logistic regression.
2.3.5. Behavioral and hormonal response to restraint stress
Rats were restrained in the crouched position in tubular chambers that allowed no movement except minor head and paw movements. The frequency of facial tremors during restraint was quantified. Stress-induced facial tremors were defined as a tremor starting rostrally as an tremulous jaw movements, progressing caudally to recruit peri-ocular muscles causing the appearance of high-frequency eye-bulging. Tremors of this structure are exhibited spontaneously by rats, but we have observed them to be increased by stress (unpublished observations). They are most similar to tremors described as being induced by stimulation of striatal DA receptors (Collins et al., 1993). Following 30 min of restraint stress, blood samples were collected from the tail vein. Samples were kept on ice following collection until (within 60 min) centrifuged at 2000g, for 25 min. Plasma supernatant was collected into new tubes in 20 μl aliquots then stored frozen until sent for quantification of corticosterone. Corticosterone was quantified by standard radioimmunoassay methods by the laboratory of Thomas Cooper, Nathan Kline Institute, Orangeburg, New York.
Statistics
The effects of treatment its interaction with sex on stress-induced orofacial tremor and plasma CORT levels were analyzed with either an ANOVA or the Wald method of logistic regression, depending on the distribution of the data.
2.4. Fluoro-Jade histochemistry
Animals were subjected to perfusion-fixation for histological analysis of brain tissue between P23 and P51, depending upon the behavioral testing performed per group. The rats were deeply anesthetized with pentobarbital 100 mg/kg i.p. and transcardially perfused with cold heparinized saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were post-fixed in formalin at 4 °C for 48 h then cryoprotected by sinking in increasing concentrations of sucrose (10%, 20%, 30%) in PBS for 24 h per concentration. Brains were then rapidly frozen in isopentanes. Fifty micron serial coronal cryosections were obtained through the hippocampus, hypothalamus and cerebral cortex (50–60 sections per brain) and floated in PBS. Sections were stained with Fluoro-Jade B (Histo-Chem, Jefferson, AR, USA) to identify degenerating neurons, and DAPI (Pierce, Rockford, IL, USA) to counterstain viable neurons, as previously described by Schmued and Hopkins (2000). The original protocol was modified to extend the incubation in potassium permanganate to 20 min. Fluorescent signals were detected using an Olympus Vanox-T microscope (Olympus, Japan) with a FITC filter for Fluoro-Jade B and UV-2 filter for DAPI. A qualitative assessment of cell death was made by surveying each section through the hippocampus, hypothalamus and cerebral cortex.
3. Results
3.1. Blood glucose changes and mortality
Total mortality during the treatment period was 1% and all deaths occurred in the insulin treated group. In randomly selected litters, blood glucose was tested in 1–2 subjects per condition for the first 3–5 days of treatment. This testing showed that all insulin-treated animals had blood glucose values of ∼30–40 mg/dL by 0.5 h after insulin which normalized to 125–140 mg/dL by 3.5 h. This time course is similar to that reported by Thompson et al. (1997), for once-daily insulin treatments (2 or 8 U/kg) of rats between P9 and P20. Saline treated animals had normal blood glucose levels at all times.
3.2. Body growth and maturation
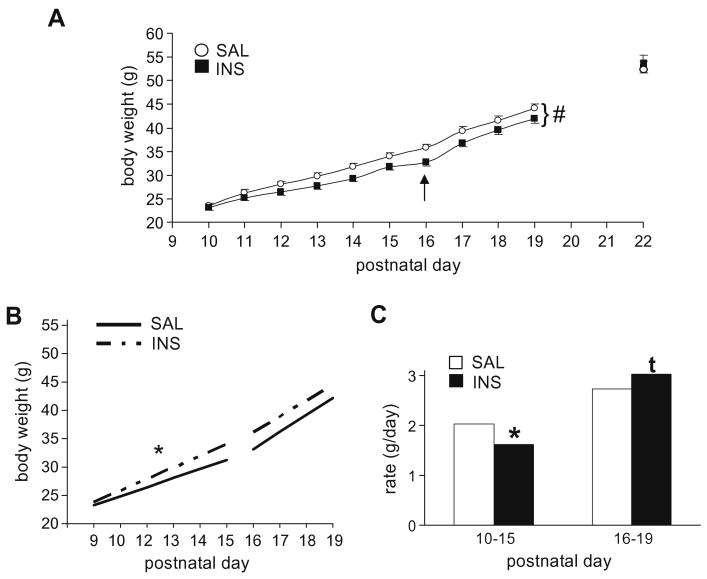
Body weights at P10–P19 were analyzed for 41 INS and 36 SAL pups from 12 litters. The sex of the pups did not interact with treatment (p > 0.9); thus sex was not included as a factor in the final analysis. The mixed ANOVA revealed a significant effect of insulin treatment (F[1, 75] = 3.7, p = 0.05) and a highly significant interaction with postnatal day indicating differences in growth rates (F[9, 675] = 6.0, p < 0.001) (Fig. 1A). Post-hoc analyses fitting polynomial functions to the growth rate functions (body weight × postnatal day) showed that the interaction of insulin treatment was best fit with a quadratic function (contrast F[1, 75] = 14.0, p < 0.001). The growth functions were then further analyzed based on qualitative observation of the growth curves and other aspects of maturation. We noted that the rate of growth appeared to increase around P16 for all animals (Fig. 1A). This increase occurred around the time of eye opening which, consistent with previous reports (Hoath, 1986; Philipps et al., 1988), was completed between P15 and P16 in both groups (no differences between groups; data not shown), and was associated with a rapid increase in consumption of chow. Thus, we divided the growth functions into two periods: P10–P15 (prior to full eye opening) and P16–P19 (after eye opening and initiation of consumption of chow). Between P10 and P15, the growth functions for both groups were linear (linear F[1, 75] = 1070, p < 0.001; no other polynomial significant; Fig. 1B) and the growth rate was significantly decreased in INS pups (Interaction: linear F[1, 75] = 13.4, p < 0.001; Fig. 1B and C). After eye opening (>P16), the difference in growth rates between treatment groups showed the opposite trend (linear F[1, 75] = 2.7, p = 0.1) with INS pups gaining weight at a slightly faster rate than SAL pups (Fig. 1B and C). For a subset of the pups (20 SAL, 25 INS) in the above cohort, weights continued to be monitored until P22. At P20, P21, and P22, there were no differences in body weights between SAL and INS pups (t's[43] < 0.74, p's > 0.45). Body weights for both groups at P22 are shown in Fig. 1A Rats were also weighed prior to each of the behavioral tests reported below; no differences were observed between SAL and INS pups after P20 (data not shown).
Fig. 1.
Body growth for saline- (SAL) or insulin-treated (INS) pups prior to, during, and after the treatment period for cohorts of rats receiving twice daily treatments from P10 to P19. (A) Daily morning body weights beginning on P10 (prior to the first treatment), continuing through P19, then at 3 days post-treatment on P22. INS pups showed a significantly different growth rate as indicated by a significant “treatment × postnatal day” interaction (indicated by “#”; see text). By P22, however, body weights of the two groups were nearly identical. The arrow marks the age of eye opening, after which solid food consumption and rate of weight gain appeared to increase in both groups. (B and C) Linear growth functions (weight × day) regressed across the period from P10 to P15, prior to full eye opening in all animals, and P16 to P19, from eye opening until the end of treatment. (B) The linear functions; (C) shows a bar graph of the slopes (growth rates). INS pups showed a significantly slower rate of growth between P10 and P15, but a trend for a faster rate of growth from P16 to P19. #p < 0.001, treatment × postnatal day interaction (A); *p < 0.05, main effect of treatment (B and C); tp < 0.1, main effect of treatment (C).
3.3. Behavioral analyses
3.3.1. Maternal separation-induced vocalizations
Maternal separation-induced USVs prior to the first treatment did not differ between SAL (Mean ± SEM: 125 ± 14) and INS (Mean ± SEM: 132 ± 14) pups (treatment × age-at-baseline AVOVA: F[1, 48] = 0.13, p > 0.7). Similarly, there were no effects of sex, or interaction of sex with other factors, on baseline USVs. By contrast, at P12 and P14, following 2 and 4 treatments, respectively, pups in the INS group showed significantly higher rates of USVs. The 2-way ANOVA which included pre-treatment (baseline) USVs as a covariate revealed significant main effects of treatment (INS > SAL; F[1, 1,47] = 5.6, p < 0.05) and age-at-testing (P12 > P14; F[1, 1,47] = 11.0, p < 0.01). Consistent with previous longitudinal studies of this behavior (cf. Brunelli et al., 1996), the rate of USVs during the period of separation from the dam showed an age-related decline between P12 and P14 for both groups (Fig. 2). However, at both ages, INS pups vocalized more than SAL pups (Fig. 2). There was no significant interaction between treatment and age-at-testing (p > 0.9). Moreover, there was no significant effect of sex or interaction of sex with treatment or age-at-testing (all p values > 0.25).
Fig. 2.
Ultrasonic vocalizations emitted by P12 and P14 pups during a 3 min period of separation from the dam. Baseline maternal separation-induced vocalizations assessed prior to the beginning of treatment on P10 were not different between groups (see text). The response to separation was assessed when all pups were euglycemic (see text). INS pups showed significant increases in separation-induced vocalizations. *p < 0.05 main effect of treatment; #p < 0.05 main effect of age.
3.3.2. Play behavior
The social behaviors exhibited reliably by all juvenile pairs were the play-related behaviors side mounts/walkovers, napes, and pins, as well as the non-play social behavior, partner sniffing. Rats also exhibited frequent rearing; thus, this behavior served as a non-social behavioral positive control for general motor development and activity. These behaviors were averaged across testing days. A MANOVA assessing the effect of treatment and sex on these behaviors showed a significant effect of treatment (Pillai's Trace F[5, 18] = 2.9, p < 0.05). As shown in Table 1, social play behaviors were selectively decreased in INS pups. Univariate analyses of the effect of treatment on each outcome measure revealed a significant decrease in pins in INS pups. Other social play behaviors were similarly decreased but not to a statistically significant level. In contrast to the decrease in play behaviors, sniffing was increased non-significantly in INS pups and rearing between the two groups was nearly identical (see Table 1).
Table 1.
Frequencies of social play, social non-play and non-social behaviors expressed during social interaction testing.
| Category | Behavior | SAL (Mean± SEM) | INS (Mean ± SEM) | F[1, 24] | p |
|---|---|---|---|---|---|
| Social play | Pins | 17.0 ± 1.5 | 10.5 ± 1.4 | 15.4 | 0.001 |
| Social play | Napes | 6.1 ± 1.3 | 3.9 ± 1.2 | 1.5 | 0.23 |
| Social play | Side mounts/walkovers | 12.2 ± 1.0 | 10.3 ± 0.95 | 1.7 | 0.21 |
| Non-play | Sniffing | 3.0 ± 0.85 | 4.3 ± 0.79 | 1.4 | 0.28 |
| Non-social | Rearing | 46.3 ± 2.7 | 45.9 ± 2.5 | 0.002 | 0.97 |
3.3.3. Habituation of the acoustic startle response
The acoustic startle response was assessed at age P22, 3 days following the termination of insulin treatment and a point at which average body weight between treatment groups was equal (see Fig. 1A). Startle habituation was defined as the decline in the startle response across 16 consecutive presentations of a startle stimulus-only trial. The initial startle response appeared to be increased in INS pups and the mixed ANOVA revealed a significant trial × treatment interaction (F[15, 375] = 1.7, p < 0.05; Fig. 3A). This was further explored by comparing the habituation index, the percent decrease in startle response between the early and late startle stimulus trials (see Section 1), across treatment groups. INS-treated pups exhibited lower habituation index values (caused by a relative increase in startle during late trials) (Wald Chi-Square = 4.7, p < 0.05; Fig. 3A, inset). In a separate cohort tested as young adults, the logistic regression revealed a significant interaction between treatment and sex (Wald Chi-Square = 12.2, p < 0.05). Underlying this interaction was a trend for decreased startle habituation in INS males relative to SAL males (Fig. 3B, top), but a trend for the opposite effect of INS within females (Fig. 3B, bottom).
Fig. 3.
Habituation of the acoustic startle reflex in INS- and SAL-treated rats assessed at P22, 3 days following the final treatment. (A) A polynomial function for the acoustic startle reflex regressed across the 16 consecutive startle stimulus presentation for SAL (open symbols; dotted line) and INS (solid symbols and line) rats, showing persistent increase in the startle reflex in INS rats. The inset shows the habituation index, defined as the percent decrease in startle amplitude between the first two and last two trials of the series. (B) Habituation index values for a separate cohort tested as young adults (see text for minor differences in testing conditions). There was a significant interaction between treatment and sex on this measure in adults (see text). Contributing to this was a trend for decreased startle habituation in INS males (top panel) but the opposite trend in females (bottom panel). *p < 0.05, effect of treatment; tp < 0.1, effect of treatment.
3.3.4. Prepulse inhibition of startle
Prepulse inhibition of startle was assessed in separate cohorts of juvenile (P22) and young adult (P55–P60) rats. In juvenile rats, a Student's t-test showed no significant difference in baseline startle amplitude between INS and SAL pups (p > 0.5) (Fig. 4A, inset) following habituation. The mixed ANOVA showed a that PPI increased with increased prepulse salience (F[4, 104] = 69, p < 0.001)(Fig. 4A), but there were no main or interactive effects of INS treatment. In early adulthood, baseline startle remained similar between rats with a history of juvenile INS or SAL treatment (p > 0.6; Fig. 4B, inset). The mixed ANOVA showed that PPI increased significantly with increased prepulse salience (F[4, 72] = 90.7, p < 0.001) and there was a “prepulse salience × condition” interaction (F[4, 72] = 4.4, p < 0.05) (Fig. 4B). Modified Bonferroni corrected comparisons of INS and SAL groups at each prepulse salience level revealed a selective enhancement of PPI at the lowest prepulse salience.
Fig. 4.
Baseline acoustic startle and prepulse inhibition of startle in SAL- or INS-treated pups tested as juveniles or young adults. Baseline startle was determined following full habituation of the response (see Section 1). (A) Baseline acoustic startle (inset) and prepulse inhibition of startle (as a function of the salience of a prepulse delivered 100 ms prior to the startle stimulus) in rats exposed to SAL or INS from P10 to P19, and tested on P22. (B) Baseline acoustic startle (inset) and prepulse inhibition of startle from a different cohort of rats tested as young adults. Consistent with previous reports (see Section 1) prepulse inhibition is more sensitive to prepulse salience in adults than in juveniles. An interaction between juvenile treatment and prepulse salience was found in the adult cohort, produced by a selective difference between groups at with the quietest prepulse. *p < 0.05, effect of treatment.
3.3.5. Fear-potentiated startle
Because each presentation of the CS+ on the test day represents an extinction trial, we first explored FPS on the Test Day as a function of trial. Startle amplitude was plotted across the 6 CS+ and 6 CS− trials and a polynomial function was fitted to allow exploratory analysis. This revealed that while startle amplitude did not change across CS− trials, it was significantly modulated across CS+ trials (Supplementary Fig. 1A). Thus, the FPS ratio for each
CS+ trial was calculated as the ratio between startle amplitude on that trial and the average amplitude across CS− trials. The FPS ratio was greater than a value of l by at least 1 standard error of the mean for the first 3 trial blocks, then extinguished across subsequent trials (Supplementary Fig. 1B). The effect of INS treatment was thus compared on the average FPS across the first three trials. The logistic regression model revealed that rats with a history of juvenile INS treatment showed a significantly higher level of conditioned fear (Wald Chi-Square = 4.5, p < 0.05) (Fig. 5). There was no interaction with sex.
Fig. 5.
Fear-potentiated startle (FPS) in young adult rats with a history of juvenile treatment with INS or SAL. FPS ratio calculated as the startle response on CS+ trials divided by the average startle response on CS− trials. Shown is the average FPS across the first 3 test trials, prior to evidence for extinction. See Supplementary material for further explanation of the calculation of average FPS. *p < 0.05, effect of treatment.
3.4. Restraint stress-induced behavior and corticosterone secretion
The logistic regression analysis of stress-related facial tremor frequency revealed significant increases in the INS group (Wald Chi-Square = 3.8, p = 0.05) (Fig. 6A). However, the effect of treatment on post-stress plasma CORT levels interacted with sex (Wald Chi-Squareinteraction = 38.1, p < 0.01). Although our data set lacked the power to fully analyze this interaction, individual comparisons using logistic regression methods showed a trend for CORT to be increased in males with a history of juvenile INS treatment, relative to SAL males (Wald Chi-Square = 2.8, p = 0.09), with no effect of treatment in females (p > 0.25) (Fig. 6B).
Fig. 6.
Behavioral and hormonal responses to restraint stress in young adult rats with a history of juvenile treatment with INS or SAL (A) Frequency of bouts of facial tremor during 30 min of restraint. (B) Plasma corticosterone measured following at the end of the restraint period. *p < 0.05, main effect of treatment; tp < 0.1, main effect of treatment; #p < 0.05, treatment × sex interaction.
3.5. Neuronal degeneration
A total of 21 rats were analyzed for neuronal degeneration using Fluoro-Jade B staining (10 INS-treated and 11 controls). Fluoro-Jade B positive neurons were found only in INS-treated rats and were observed in singlets or clusters mainly in the parasagittal cortex (Supplementary Fig. 2), and rarely in the temporal cortical regions, such as the perirhinal cortex. Sampling these regions starting anteriorly at the level of the anterior cingulate cortex (adjacent to the anterior border of the genu of the corpus callosum) and progressing to the level of the splenium, we observed a very low incidence of Fluoro-Jade-B-positive neurons in INS-treated offspring overall, with no more than 10 occurrences (singlets or clusters) observed throughout the parasaggital and temporal cortex.
4. Discussion
In this study, we used a regimen of INS administration that models recurrent, transient hypoglycemia (Thompson et al., 1997). Rats that experienced recurrent, transient hypoglycemia, induced with INS twice daily from P10 to P19, showed a decrease in body growth during the treatment then a “catch-up” in growth after P16, a time when the pups began to feed independently. We note that Thompson et al. (1997) did not observe retarded body growth in pups once daily with INS (2–8 U/kg), although there appeared to be an increase in growth rate after P16 similar to what we observed in this study. Following the termination of INS exposure at P19, rats with a history of recurrent hypoglycemia showed largely normal sensorimotor gating. In contrast to the subtle effects on growth and motor development, recurrent moderate hypoglycemia had marked and/or persistent effects on affective regulation, social behavior, and stress responses. INS-exposed pups showed increased maternal-separation-induced vocalizations at P12 and P14 (tested under normoglycemic conditions), and an exaggerated (resistant to habituation) acoustic startle reflex at P22, 3 days after the termination of treatment. These rats also showed selective decreases in social play behavior during adolescence. As young adults, rats with a history of hypoglycemia showed, again, an exaggerated acoustic startle response, an exaggerated conditional emotional response (fear-potentiated startle), and altered behavioral and hormonal responses to restraint stress. Although not all of the experiments were sufficiently powered to assess sex-dependent effects, it appears from the current data that some of these effects become more prominent in males in adulthood. Moreover, while the effects of recurrent hypoglycemia in neonates and juveniles did not differ by sex, there was preliminary evidence for sexual dimorphism in some of the behavioral abnormalities expressed in adolescence or adulthood in rats with a history of juvenile hypoglycemia. These sex-related results will not be further discussed except to note that further examination of sex differences in the long-term effects of juvenile hypoglycemia is warranted. Together, the current results provide evidence that recurrent, transient hypoglycemia during juvenile development can lead to increases in fear-related behavior and stress reactivity that are (1) not reversed with normalization of blood glucose, and (2) may persist into adulthood. Below, we discuss possible neural substrates for these effects and their implication for functional outcomes in patients who experience recurrent hypoglycemia associated with early-onset IDDM and other metabolic disorders.
Many of the studies on the effects of recurrent hypoglycemia have focused on cognitive functions mediated by the medial temporal lobe or the hippocampus, an archi-cortical structure that resides within this region (Lavenex and Amaral, 2000). Several clinical studies have suggested that in children with IDDM the severity and frequency of hypoglycemia is predictive of deficits in cognitive functions, primarily forms of memory presumed to depend on the medial temporal lobe or hippocampus (Hershey et al., 1999, 2005). Similar data have been reported in rodents, with short-term (3 day) and long-term (11 months) induction of hypoglycemia resulting in impaired performance in cognitive tasks (McNay and Sherwin, 2004; McNay et al., 2006). However, the hippocampal and paralimbic (including parasagittal) cortical regions also mediate stress responses and affective behavior, including emotional learning and memory, via their extensive connections with limbic structures including the amygdala and hypothalamus (Bannerman et al., 2004; Herman et al., 2003; Izquierdo and Medina, 1997). Consistent with this, Wredling et al. (1992) reported that a history of severe hypoglycemia was significantly and selectively associated with anxiety in patients with IDDM. Similarly, more recent studies have reported relationships between frequency of hypoglycemia (or history of difficulty with detection) and self-reported nervousness or depression (Boyle and Zrebiec, 2007; Gonder-Frederick et al., 2008). This study supports the idea that increased anxiety and/or responsiveness to stress may be directly related to recurrent hypoglycemia, particularly if the hypoglycemic episodes occurred during juvenile development.
In this study, within 3 days (6 episodes) of recurrent hypoglycemia, rats exhibited significant increases in maternal separation-induced USVs. This phenotype has been proposed to be an early developmental indicator of anxiety. In mammals, acute maternal separation produces a stress response in the infant that includes activation of the HPA axis and autonomic changes; ultrasonic vocalizations are considered to be a behavioral component of this response (Brunelli, 2005; Hennessy et al., 1999). These USVs are modulated by stress hormones (Harvey et al., 1994; Hennessy et al., 1999; Ise et al., 2008) and depend on brain regions known in adults to mediate other distress responses (Wiedenmayer et al., 2000). Moreover, they are increased and decreased by neuroactive compounds known in adults to be anxiogenic and anxiolytic, respectively (Hofer, 1996; Ise et al., 2008; Podhorna and Brown, 2000; Trezza et al., 2008). The phenotype may also be predictive of a persistent anxiety-like phenotype, as rats selectively bred for high distress vocalizations show a number of anxiety-related phenotypes as adults (Brunelli, 2005).
Persistent increases in emotional reactivity following juvenile recurrent hypoglycemia were also evidenced as alterations in the acoustic startle response. Enhancement of the acoustic startle response can be produced by electrical or chemical stimulation of the amygdala or the periaquaductal gray – two nodes in a circuit mediating fear-related behavior. Moreover, elevations in corticotrophin releasing factor produce elevations in the startle reflex (Koch, 1999), an effect previously shown to be mediated by the central amygdala (Liang et al., 1992a), but more recently also shown to involve the bed nucleus of stria terminalis and hippocampus (Koch, 1999). The amygdala, on the other hand, is necessary for cue-conditioned fear and potentiation of startle by aversively-conditioned stimuli (Davis, 1990; Davis et al., 1993; Koch, 1999). This study did not examine functional neuroanatomical or physiological indices within the brain. However, the enhancement of stress-related hormones (in males), and increases in the spontaneous startle response and fear-potentiated startle may indicate that multiple nodes in the distinct but overlapping circuits mediating stress responses, anxiety, and conditioned fear are affected by recurrent hypoglycemia during juvenile development. Moreover, given the overlap in circuitry between these responses and maternal separation-induced USVs, we postulate that recurrent juvenile hypoglycemia may produce persistent increases in responsiveness of limbic circuits regulating responses to threat or stress.
Recurrent hypoglycemic episodes between P10 and P19 led to decreases in play behavior in early adolescence. The decreases in napes and mounting during play did not reach statistical significance. However, pinning, which is generally dependent upon and initiated by napes in the normal stereotyped play sequence (Pellis et al., 1993, 1997), was significantly decreased. By contrast, non-play behaviors were increased or clearly not changed (p > 0.9). Social play behavior recruits and depends upon a network of structures known to support appetitive behavior (Panksepp et al., 1984; Pellis et al., 1993; Pellis and McKenna, 1995; Vanderschuren et al., 1997), including the ventral and dorsal striatum (Gordon et al., 2002). Play is generally inhibited by drugs that increase arousal or anxiety, and enhanced under most conditions by anxiolytics at doses that do not impair motor control (Vanderschuren et al., 1997). However, paradoxically, play behavior also activates the dorsal periaquaductal gray (Gordon et al., 2002); thus, perhaps this region is involved in the inverse “U” shaped relationship between arousal and play. Thus, we postulate that the decrease in social play behavior observed in rats with a history of juvenile hypoglycemia may be secondary to increased reactivity of anxiety- or fear-related neurobehavioral systems. Consistent with this, a recent study on the effects of perinatal exposure to delta-9-tetrahydrocannabinol (THC) found a similar relationship between affective reactivity and social play behavior. Specifically, early developmental exposure to THC ending on P9 was shown to produce increases in maternal separation-induced USV, as well as increased anxiety-related behavior later in development. Moreover, this increased anxiety was accompanied by a decrease in social play during adolescence (Trezza et al., 2008). It remains to be tested whether pharmacological or other manipulations known to decrease unconditioned fear can reverse the social play deficit produced by juvenile recurrent hypoglycemia.
Hypoglycemia is a potent physiological stressor that markedly alters activities of the mammalian adrenomedullary hormonal system, the HPA axis and the sympathetic nervous system (Goldstein and Kopin, 2008; Grino and Oliver, 1992; Havel and Taborsky, 1989). Although this response is blunted for part of the first two postnatal weeks in the rat (Arai and Widmaier, 1991), studies in the adult brain would predict that repeated exposure to hypoglycemia (Al-Noori et al., 2008b; Figlewicz et al., 2002; Sanders et al., 2006) and other stressors (Kim et al., 2006) would produce long-lasting adaptations in cortico-limbic circuits and the HPA axis. Although preliminary, the present data suggest that adult rats with a history of juvenile hypoglycemia showed increases in involuntary behaviors (facial tremors) induced by acute stress. Males in particular may also show an increased hormonal response. We note that our study did not have sufficient power to fully assess interactions between juvenile hypoglycemia and sex on the adrenal stress response. Moreover, data on resting corticosterone levels would be required to fully characterize the effects of juvenile recurrent hypoglycemia on regulation of the HPA axis. However, the selective increase in apparent responsiveness to stress in the males was consistent with their exaggerated acoustic startle response, a behavior that is also influenced by central action of stress-related hormones (see above).
Severe hypoglycemia has been shown in naïve adult rats and those with experimentally-induced diabetes to produce increases in markers of oxidative stress, DNA fragmentation and neuronal histopathology in multiple brain regions including the cerebral cortex, hippocampus, striatum, and diencephalon (including the hypothalamus) (Auer et al., 1984; Bhardwaj et al., 1998; Ferrand-Drake et al., 1999; Kim et al., 2005; Patockova et al., 2003; Singh et al., 2004). Consistent with evidence that the young juvenile (P14) rat brain may be less susceptible than the adult brain to these effects of hypoglycemia (Ennis et al., 2008), the INS-treated rats in this study showed little evidence for neuronal oxidative stress, with Fluoro-Jade-positive neurons being infrequently detected in parasagittal and paralimbic temporal cortex. However, no such markers were observed in SAL-treated rats. It may be the case that the transient decreases in blood glucose achieved by our insulin regimen induced adaptations in neuronal glucose metabolism (Jiang et al., 2009) that may have counteracted oxidative stress in the juvenile brain. However, these and other adaptations, including enhanced excitability in the amygdala (in juveniles) (Lee et al., 1988), increased activity in midline thalamic nuclei (Al-Noori et al., 2008a; Arbelaez et al., 2008), and alterations in multiple hypothalamic subnuclei (Chan et al., 2008; Evans et al., 2001), may also contribute to altered function of circuits that regulate fear-related behaviors and stress responses. Importantly, these changes in neuronal function may not be accompanied by standard histological markers of neuropathology.
The above data complement clinical and animal studies suggesting that recurrent hypoglycemia leads to persistent behavioral and cognitive impairments (Akyol et al., 2003; Gold et al., 1993; Wredling et al., 1990). However, the current study is the first to confirm an effect of recurrent hypoglycemia on affective regulation. The findings highlight the possibility that these effects may be particularly debilitating and persistent if hypoglycemic episodes begin earlier in development. Children with IDDM are at significant risk for recurrent hypoglycemia (Clarke et al., 1996); moreover, many of their hypoglycemic episodes go undetected (Gonder-Frederick et al., 2008). Indirect but compelling evidence to support this notion was provided by a recent study of paramedic calls for monitoring or treatment of acute hypoglycemia which showed that such calls are less frequent for children 0–9 years of age than for adolescents or young adults (Vilke et al., 2005). This is not likely due to better glycemic control in younger children, but rather to a lack of symptoms severe enough to warrant a call to paramedics. Importantly, whereas the relationship between a history of hypoglycemia and behavioral impairment is inconsistent in studies of adults with a relatively late (>18 years) age-of-onset of IDDM (Lincoln et al., 1996; The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research, 2007), multiple studies report a significant relationship between a history of severe or recurrent hypoglycemia and cognitive function in individuals with a juvenile age-of-onset (e.g., Akyol et al., 2003; Deary et al., 1993; Hershey et al., 1999, 2005; Wredling et al., 1990). Thus, as a whole, the literature suggests that the earlier in development that severe or recurrent hypoglycemia begins, the more it may contribute to persistent affective or cognitive impairments.
In summary, the current findings support the hypothesis that recurrent moderate hypoglycemia during postnatal/juvenile development can lead to persistent increases in fear-related behavior and alterations in stress responses. These effects may, in turn, negatively impact social play behavior. These findings may be particularly relevant to children with IDDM, who often have hypoglycemic episodes that go undetected and may experience frequent moderate hypoglycemia. Our findings highlight the need for more careful consideration of the impact of hypoglycemia on affective regulation. Specifically, the contribution of affective changes to differences in performance during cognitive assessments and, more importantly, to functional outcome should be further studied in IDDM patients. Moreover, determining the neural mechanisms underlying these effects will advance our understanding of links between energy homeostasis, neuronal function and mental health.
Supplementary Material
Acknowledgments
The authors thank Karin Krueger, Sara Steinfeld, and Alexei Shemyakin for excellent technical assistance and Thomas Cooper, Nathan Kline Institute, Orangeburg, New York, for analysis of plasma samples. This study was supported by PHS Grant NS045837 (S.J.V., PI).
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbi.2009.11.013.
References
- Akyol A, Kiylioglu N, Bolukbasi O, Guney E, Yurekli Y. Repeated hypoglycemia and cognitive decline. A case report Neuroendocrinol Lett. 2003;24:54–56. [PubMed] [Google Scholar]
- Al-Noori S, Sanders NM, Taborsky GJ, Jr, Wilkinson CW, Figlewicz DP. Acute THPVP inactivation decreases the glucagon and sympathoadrenal responses to recurrent hypoglycemia. Brain Res. 2008a;1194:65–72. doi: 10.1016/j.brainres.2007.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Noori S, Sanders NM, Taborsky GJ, Jr, Wilkinson CW, Zavosh A, West C, Sanders CM, Figlewicz DP. Recurrent hypoglycemia alters hypothalamic expression of the regulatory proteins FosB and synaptophysin. Am J Physiol Regul Integr Comp Physiol. 2008b;295:R1446–R1454. doi: 10.1152/ajpregu.90511.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M, Widmaier EP. Activation of the pituitary-adrenocortical axis in day-old rats by insulin-induced hypoglycemia. Endocrinology. 1991;129:1505–1512. doi: 10.1210/endo-129-3-1505. [DOI] [PubMed] [Google Scholar]
- Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes. 2008;57:470–475. doi: 10.2337/db07-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer RN, Wieloch T, Olsson Y, Siesjo BK. The distribution of hypoglycemic brain damage. Acta Neuropathol (Berl) 1984;64:177–191. doi: 10.1007/BF00688108. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang WN, Pothuizen HHJ, Feldon J. Regional dissociations within the hippocampus – memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Becker DJ, Ryan CM. Hypoglycemia: a complication of diabetes therapy in children. Trends Endocrinol Metab. 2000;11:198–202. doi: 10.1016/s1043-2760(00)00259-9. [DOI] [PubMed] [Google Scholar]
- Bhardwaj SK, Sharma ML, Gulati G, Chhabra A, Kaushik R, Sharma P, Kaur G. Effect of starvation and insulin-induced hypoglycemia on oxidative stress scavenger system and electron transport chain complexes from rat brain, liver, and kidney. Mol Chem Neuropathol. 1998;34:157–168. doi: 10.1007/BF02815077. [DOI] [PubMed] [Google Scholar]
- Bhatia V, Wolfsdorf JI. Severe hypoglycemia in youth With insulin-dependent diabetes mellitus: frequency and causative factors. Pediatrics. 1991;88:1187–1193. [PubMed] [Google Scholar]
- Boyle PJ, Zrebiec J. Physiological and behavioral aspects of glycemic control and hypoglycemia in diabetes. South Med J. 2007;100:175–182. doi: 10.1097/01.smj.0000242866.81791.70. summary for patients in. [DOI] [PubMed] [Google Scholar]; South Med J. 2007 Feb;100(2):231. [PubMed] [Google Scholar]
- Brunelli SA. Selective breeding for an infant phenotype: rat pup ultrasonic vocalization (USV) Behav Genet. 2005;35:53–65. doi: 10.1007/s10519-004-0855-6. [DOI] [PubMed] [Google Scholar]
- Brunelli SA, Keating CC, Hamilton NA, Hofer MA. Development of ultrasonic vocalization responses in genetically heterogeneous National Institute of Health (N:NIH) rats. I. Influence of age, testing experience, and associated factors. Dev Psychobiol. 1996;29:507–516. doi: 10.1002/(SICI)1098-2302(199609)29:6<507::AID-DEV3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes. 2008;57:1363–1370. doi: 10.2337/db07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke WL, Gonder-Frederick L, Cox DJ. The frequency of severe hypoglycaemia in children with insulin-dependent diabetes mellitus. Horm Res. 1996;45(Suppl. 1):48–52. doi: 10.1159/000184830. [DOI] [PubMed] [Google Scholar]
- Collins P, Broekkamp CL, Jenner P, Marsden CD. Electromyographical differentiation of the components of perioral movements induced by SKF 38393 and physostigmine in the rat. Psychopharmacology. 1993;112:428–436. doi: 10.1007/BF02244890. [DOI] [PubMed] [Google Scholar]
- Cornblath M, Ichord R. Hypoglycemia in the neonate. Semin Perinatol. 2000;24:136–149. doi: 10.1053/sp.2000.6364. [DOI] [PubMed] [Google Scholar]
- Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, Clarke WL. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care. 2005;28:71–77. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia in diabetes: pathophysiological mechanisms and diurnal variation. Prog Brain Res. 2006;153:361–365. doi: 10.1016/S0079-6123(06)53021-3. [DOI] [PubMed] [Google Scholar]
- Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmacol Ther. 1990;47:147–165. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- De Leon DD, Stanley CA. Mechanisms of disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab. 2007;3:57–68. doi: 10.1038/ncpendmet0368. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Crawford JR, Hepburn DA, Langan SJ, Blackmore LM, Frier BM. Severe hypoglycemia and intelligence in adult patients with insulin-treated diabetes. Diabetes. 1993;42:341–344. doi: 10.2337/diab.42.2.341. [DOI] [PubMed] [Google Scholar]
- Ennis K, Tran PV, Seaquist ER, Rao R. Postnatal age influences hypoglycemia-induced neuronal injury in the rat brain. Brain Res. 2008;1224:119–126. doi: 10.1016/j.brainres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SB, Wilkinson CW, Bentson K, Gronbeck P, Zavosh A, Figlewicz DP. PVN activation is suppressed by repeated hypoglycemia but not antecedent corticosterone in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1426–R1436. doi: 10.1152/ajpregu.2001.281.5.R1426. [DOI] [PubMed] [Google Scholar]
- Farid M, Martinez ZA, Geyer MA, Swerdlow NR. Regulation of sensorimotor gating of the startle reflex by serotonin 2A receptors. Ontogeny and strain differences Neuropsychopharmacology. 2000;23:623–632. doi: 10.1016/S0893-133X(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Ferrand-Drake M, Friberg H, Wieloch T. Mitochondrial permeability transition induced DNA-fragmentation in the rat hippocampus following hypoglycemia. Neuroscience. 1999;90:1325–1338. doi: 10.1016/s0306-4522(98)00559-4. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Van Dijk G, Wilkinson CW, Gronbeck P, Higgins M, Zavosh A. Effects of repetitive hypoglycemia on neuroendocrine response and brain tyrosine hydroxylase activity in the rat. Stress. 2002;5:217–226. doi: 10.1080/1025389021000010558. [DOI] [PubMed] [Google Scholar]
- Flykanaka-Gantenbein C. Hypoglycemia in childhood: long-term effects. Pediatr Endocrinol Rev. 2004;1(Suppl. 3):530–536. [PubMed] [Google Scholar]
- Gold AE, Deary IJ, Frier BM. Recurrent severe hypoglycaemia and cognitive function in type 1 diabetes. Diabet Med. 1993;10:503–508. doi: 10.1111/j.1464-5491.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul. 2008;42:111–119. [PMC free article] [PubMed] [Google Scholar]
- Gonder-Frederick L, Zrebiec J, Bauchowitz A, Lee J, Cox D, Ritterband L, Kovatchev B, Clarke W. Detection of hypoglycemia by children with type 1 diabetes 6 to 11 years of age and their parents: a field study. Pediatrics. 2008;121:e489–e495. doi: 10.1542/peds.2007-0808. [DOI] [PubMed] [Google Scholar]
- Gonder-Frederick LA, Cox DJ, Bobbitt SA, Pennebaker JW. Mood changes associated with blood glucose fluctuations in insulin-dependent diabetes mellitus. Health Psychol. 1989;8:45–59. doi: 10.1037//0278-6133.8.1.45. [DOI] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res Bull. 2002;57:651–659. doi: 10.1016/s0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- Grino M, Oliver C. Ontogeny of insulin-induced hypoglycemia stimulation of adrenocorticotropin secretion in the rat: role of catecholamines. Endocrinology. 1992;131:2763–2768. doi: 10.1210/endo.131.6.1359963. [DOI] [PubMed] [Google Scholar]
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. Blockade of alpha 2-adrenoceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology. 1994;135:2549–2557. doi: 10.1210/endo.135.6.7988443. [DOI] [PubMed] [Google Scholar]
- Harvey AT, Moore H, Lucot JB, Hennessy MB. Monoamine activity in anterior hypothalamus of guinea pig pups separated from their mothers. Behav Neurosci. 1994;108:171–176. doi: 10.1037//0735-7044.108.1.171. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Taborsky GJ., Jr The contribution of the autonomic nervous system to changes of glucagon and insulin secretion during hypoglycemic stress. Endocr Rev. 1989;10:332–350. doi: 10.1210/edrv-10-3-332. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Davis HN, McCrea AE, Harvey AT, Williams MT. Short- and long-term consequences of corticotropin-releasing factor in early development. Ann NY Acad Sci. 1999;897:76–91. doi: 10.1111/j.1749-6632.1999.tb07880.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hershey T, Bhargava N, Sadler M, White NH, Craft S. Conventional versus intensive diabetes therapy in children with type 1 diabetes: effects on memory and motor speed. Diabetes Care. 1999;22:1318–1324. doi: 10.2337/diacare.22.8.1318. see comment. [DOI] [PubMed] [Google Scholar]
- Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28:2372–2377. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- Hoath SB. Treatment of the neonatal rat with epidermal growth factor: differences in time and organ response. Pediatr Res. 1986;20:468–472. doi: 10.1203/00006450-198605000-00017. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology. 1996;21:203–217. doi: 10.1016/0306-4530(95)00042-9. erratum appears in. [DOI] [PubMed] [Google Scholar]; Psychoneuroendocrinology. 1996 Jul;21(5):501. [Google Scholar]
- Hofer MA, Shair HN, Brunelli SA. Ultrasonic vocalizations in rat and mouse pups. Current Protocols in Neuroscience. 2002 doi: 10.1002/0471142301.ns0814s17. (Chapter 8), Unit 8.14. [DOI] [PubMed] [Google Scholar]
- Ise S, Nagano N, Okuda S, Ohta H. Corticotropin-releasing factor modulates maternal separation-induced ultrasonic vocalization in rat pups via activation of CRF1 receptor. Brain Res. 2008;1234:59–65. doi: 10.1016/j.brainres.2008.07.079. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Jiang L, Herzog RI, Mason GF, de Graaf RA, Rothman DL, Sherwin RS, Behar KL. Recurrent antecedent hypoglycemia alters neuronal oxidative metabolism in vivo. Diabetes. 2009;58:1266–1274. doi: 10.2337/db08-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Song EY, Kosten TA. Stress effects in the hippocampus: synaptic plasticity and memory. Stress. 2006;9:1–11. doi: 10.1080/10253890600678004. [DOI] [PubMed] [Google Scholar]
- Kim M, Yu ZX, Fredholm BB, Rivkees SA. Susceptibility of the developing brain to acute hypoglycemia involving A1 adenosine receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E562–E569. doi: 10.1152/ajpendo.00112.2005. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal–neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lee SS, Murata R, Matsuura S. Effects of hypoglycemia on kindling seizures in suckling rats. Exp Neurol. 1988;99:142–153. doi: 10.1016/0014-4886(88)90134-3. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJ, Davis M. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. J Neurosci. 1992a;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992b;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln NB, Faleiro RM, Kelly C, Kirk BA, Jeffcoate WJ. Effect of long-term glycemic control on cognitive function. Diabetes Care. 1996;19:656–658. doi: 10.2337/diacare.19.6.656. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology. 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Martinez ZA, Halim ND, Oostwegel JL, Geyer MA, Swerdlow NR. Ontogeny of phencyclidine and apomorphine-induced startle gating deficits in rats. Pharmacol Biochem Behav. 2000;65:449–457. doi: 10.1016/s0091-3057(99)00217-8. [DOI] [PubMed] [Google Scholar]
- McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53:418–425. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes. 2006;55:1088–1095. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- Muller JM, Brunelli SA, Moore H, Myers MM, Shair HN. Maternally modulated infant separation responses are regulated by D2-family dopamine receptors. Behav Neurosci. 2005;119:1384–1388. doi: 10.1037/0735-7044.119.5.1384. [DOI] [PubMed] [Google Scholar]
- Northam EA, Rankins D, Cameron FJ. Therapy insight: the impact of type 1 diabetes on brain development and function. Nat Clin Pract Neurol. 2006;2:78–86. doi: 10.1038/ncpneuro0097. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Patockova J, Marhol P, Tumova E, Krsiak M, Rokyta R, Stipek S, Crkovska J, Andel M. Oxidative stress in the brain tissue of laboratory mice with acute post insulin hypoglycemia. Physiol Res. 2003;52:131–135. [PubMed] [Google Scholar]
- Pellis SM, Castaneda E, McKenna MM, Tran-Nguyen LT, Whishaw IQ. The role of the striatum in organizing sequences of play fighting in neonatally dopamine-depleted rats. Neurosci Lett. 1993;158:13–15. doi: 10.1016/0304-3940(93)90600-p. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev. 1997;21:105–120. doi: 10.1016/0149-7634(95)00060-7. [DOI] [PubMed] [Google Scholar]
- Pellis SM, McKenna M. What do rats find rewarding in play fighting? An analysis using drug-induced non-playful partners. Behav Brain Res. 1995;68:65–73. doi: 10.1016/0166-4328(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC, Whishaw IQ. The role of the cortex in play fighting by rats: developmental and evolutionary implications. Brain Behav Evol. 1992;39:270–284. doi: 10.1159/000114124. [DOI] [PubMed] [Google Scholar]
- Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey T. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Philipps AF, Persson B, Hall K, Lake M, Skottner A, Sanengen T, Sara VR. The effects of biosynthetic insulin-like growth factor-1 supplementation on somatic growth, maturation, and erythropoiesis on the neonatal rat. Pediatr Res. 1988;23:298–305. doi: 10.1203/00006450-198803000-00014. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Brown RE. Flibanserin has anxiolytic effects without locomotor side effects in the infant rat ultrasonic vocalization model of anxiety. Br J Pharmacol. 2000;130:739–746. doi: 10.1038/sj.bjp.0703364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Miserendino MJ, Falls WA, Campeau S, Davis M. Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. J Neurosci. 1992;12:4624–4633. doi: 10.1523/JNEUROSCI.12-12-04624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders NM, Figlewicz DP, Taborsky GJ, Jr, Wilkinson CW, Daumen W, Levin BE. Feeding and neuroendocrine responses after recurrent insulin-induced hypoglycemia. Physiol Behav. 2006;87:700–706. doi: 10.1016/j.physbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol Pathol. 2000;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- Singh P, Jain A, Kaur G. Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem. 2004;260:153–159. doi: 10.1023/b:mcbi.0000026067.08356.13. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-Term Effect of Diabetes and Its Treatment on Cognitive Function. 2007:1842–1852. [Google Scholar]
- Thompson CI, Munford JW, Ryker RM. Insulin during infancy attenuates insulin-induced hypoglycemia in adult male rats. Physiol Behav. 1997;62:841–848. doi: 10.1016/s0031-9384(97)00247-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Cassano T, Macheda T, Dipasquale P, Carratu MR, Gaetani S, Cuomo V. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology. 2008;198:529–537. doi: 10.1007/s00213-008-1162-3. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Nardis EE, Vannucci SJ, Campbell PA. Cerebral carbohydrate and energy metabolism during hypoglycemia in newborn dogs. Am J Physiol. 1981;240:R192–R199. doi: 10.1152/ajpregu.1981.240.3.R192. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. Glucose metabolism in the developing brain. Semin Perinatol. 2000;24:107–115. doi: 10.1053/sp.2000.6361. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. Hypoglycemic brain injury. Semin Neonatol. 2001;6:147–155. doi: 10.1053/siny.2001.0044. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Clark RR, Koehler-Stec E, Li K, Smith CB, Davies P, Maher F, Simpson IA. Glucose transporter expression in brain: relationship to cerebral glucose utilization. Dev Neurosci. 1998;20:369–379. doi: 10.1159/000017333. [DOI] [PubMed] [Google Scholar]
- Vilke GM, Castillo EM, Ray LU, Murrin PA, Chan TC. Evaluation of pediatric glucose monitoring and hypoglycemic therapy in the field. Pediatr Emerg Care. 2005;21:1–5. doi: 10.1097/01.pec.0000150980.94571.10. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Goodwin GA, Barr GA. The effect of periaqueductal gray lesions on responses to age-specific threats in infant rats. Brain Res Dev Brain Res. 2000;120:191–198. doi: 10.1016/s0165-3806(00)00009-2. [DOI] [PubMed] [Google Scholar]
- Wredling R, Levander S, Adamson U, Lins PE. Permanent neuropsychological impairment after recurrent episodes of severe hypoglycaemia in man. Diabetologia. 1990;33:152–157. doi: 10.1007/BF00404042. [DOI] [PubMed] [Google Scholar]
- Wredling RA, Theorell PG, Roll HM, Lins PE, Adamson UK. Psychosocial state of patients with IDDM prone to recurrent episodes of severe hypoglycemia. Diabetes Care. 1992;15:518–521. doi: 10.2337/diacare.15.4.518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.