Abstract
Aryl hydrocarbon receptor (AhR) activation by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces immune suppression. Dendritic cells (DCs) are key antigen presenting cells governing T cell activation and differentiation. However, the consequences of AhR activation in DCs are not fully defined. We hypothesized that AhR activation alters DC differentiation and generates dysfunctional DCs. To test this hypothesis, inflammatory bone marrow-derived DCs (BMDCs) from C57Bl/6 mice were generated in the presence of vehicle or TCDD. TCDD decreased CD11c expression but increased MHC class II, CD86 and CD25 expression on the BMDCs. The effects of TCDD were strictly AhR-dependent but not exclusively DRE-mediated. Similar effects were observed with two natural AhR ligands, 6-formylindolo[3,2-b]carbazole (FICZ) and 2-(1H-Indol-3-ylcarbonyl)-4-thiazolecarboxylic acid (ITE). TCDD increased LPS- and CpG-induced IL-6 and TNF-α production by BMDCs but decreased their NO production. TCDD decreased CpG-induced IL-12p70 production by BMDCs but did not affect their secretion of IL-10. TCDD downregulated LPS- and CpG-induced NF-kB p65 levels and induced a trend towards upregulation of RelB levels in the BMDCs. AhR activation by TCDD modulated BMDC uptake of both soluble and particulate antigens. Induction of indoleamine-2,3–dioxygenase (IDO) and TGF-β3 has been implicated in the generation of regulatory T cells following AhR activation. TCDD increased IDO1, IDO2 and TGF-β3 mRNA levels in BMDCs as compared to vehicle. Despite the induction of regulatory mediators, TCDD-treated BMDCs failed to suppress antigen-specific T cell activation. Thus, AhR activation can directly alter the differentiation and innate functions of inflammatory DCs without affecting their ability to successfully interact with T cells.
Keywords: Aryl hydrocarbon receptor (AhR); dendritic cells (DCs); 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD); NF-kB signaling; indoleamine-2,3-dioxygenase (IDO); immunotoxicity
Introduction
The Aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor mediating the toxic effects of environmental pollutants such as TCDD. Following ligand binding, the AhR translocates into the nucleus, where in conjunction with its dimeric partner the Aryl hydrocarbon receptor nuclear translocater (ARNT) it binds to dioxin response elements (DREs) in target genes. AhR activation has been recognized as the prime mechanism by which environmental pollutants such as dioxins exert their adverse effects. TCDD is a high affinity, exogenous AhR ligand. Exposure to very low doses of dioxin produces immune suppression via activation of the AhR in many animal species, making them highly susceptible to infectious diseases and cancer (Kerkvliet, 2002). More recently, various tryptophan metabolites such as 6-formylindolo[3,2-b]carbazole (FICZ) and 2-(1H-Indol-3-ylcarbonyl)-4-thiazolecarboxylic acid (ITE) have been described as high affinity, natural AhR ligands (Oberg et al., 2005; Henry et al., 2006). Few studies have examined the effects of these natural AhR ligands on the immune system. Initial studies in mice demonstrated that FICZ induces Th17 differentiation and inhibits Treg development (Quintana et al., 2008). In contrast, Kimura and co-workers subsequently demonstrated Treg induction by TGF-β that was enhanced following activation of the AhR by both FICZ and TCDD (Kimura et al., 2008). FICZ also affects the differentiation of immune cells other than T cells including human U937 monocyte-derived DCs (Veldhoen et al., 2008; Vogel et al., 2008) Studies conducted by Henry et al., showed that similar to TCDD, ITE alters thymocyte differentiation in vitro (Henry et al., 2006). However, the effects of these natural AhR ligands on dendritic cells have not been previously described. Therefore, studies aimed at defining the effects of TCDD and other AhR ligands on dendritic cells are expected to identify new biomarkers that should allow for better assessment of the potential immunotoxicity that may occur in humans following exposure to these environmental chemicals.
Dendritic cells (DCs) are key immune cells that bridge the gap between innate and adaptive immunity and defects in their development and/or function result in significant immunodeficiencies. Progressive differentiation of a DC is characterized by upregulation of MHC II and costimulatory molecules such as CD86 (B7-2) (Sato and Fujita, 2007). In addition to activating T cells, DCs constitute part of the first line of defense against invading pathogens and play a critical role in the generation of effective innate immunity to pathogenic challenge. The prime innate functions executed by DCs include recognition of unique pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs) such as toll like receptors (TLRs), and their ability to take up antigens and secrete cytokines. Additionally, studies have identified several distinct DC subsets in vivo (Shortman and Naik, 2007). These include the steady-state DCs and inflammatory DCs. Steady-state DCs are primarily located in lymphoid organs. The inflammatory DCs, on the other hand, constitute a novel DC population that are absent in the steady-state, and as their name implies, appear at sites of ongoing inflammation. These DCs, characterized by the production of TNF-α and the expression of inducible nitric oxide synthase (iNOS), are derived in vitro using the growth factor, granulocyte-macrophage colony stimulating factor (GM-CSF) (Xu et al., 2007).
Studies by Vorderstrasse and colleagues established DCs as a sensitive target of TCDD, demonstrating a loss of splenic DCs following AhR activation (Vorderstrasse and Kerkvliet, 2001). Additional studies have linked functional alterations of DCs to defective T cell responses and immune suppression following AhR activation. For instance, studies conducted by Ruby et al., showed that TCDD-treatment of BMDCs enhanced TNF-α-induced maturation, and augmented CD95-mediated BMDC apoptosis (Ruby et al., 2002; Ruby et al., 2005). Recently, AhR activation by TCDD was also demonstrated to induce down regulation of RelB mRNA levels in GM-CSF-derived BMDCs (Lee et al., 2007). DCs play a central role in both innate and adaptive immunity; however, the effects of AhR activation on DCs remain poorly understood. Thus, the aim of this study was to characterize the effects of AhR activation on the fate and function of inflammatory DCs, a subpopulation of DCs that are critical in the response to microbial infection. We hypothesized that AhR activation alters the differentiation of murine inflammatory DCs, an effect that may underlie the generation of TCDD-induced immune suppression. In this study, we explored the effects of AhR activation on DC differentiation, antigen uptake, TLR responsiveness and production of Treg-inducing mediators. Mechanistically, we examined the role of DREs in the generation of AhR-induced effects on DCs. The data presented in this paper provide new information on the consequences associated with AhR activation in DCs.
Materials and methods
Animals
6-8 week old C57Bl/6 (AhR+/+) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Breeding pairs of AhR knockout mice (AhR−/−) were kindly provided by Dr. Paige Lawrence (URMC, Rochester, NY). AhRdbd/dbd and AhRnls/nls mutant mice were generously provided by Dr. Chris Bradfield and Dr. Ed Glover (University of Wisconsin, Madison, WI) and subsequently maintained at UM. Animal experiments were approved by the UM IACUC and adhered to the current NIH guidelines for animal usage. Animals were provided rodent chow and tap water ad libitum.
Chemicals
TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) in DMSO was obtained from Cambridge Isotope laboratories Inc., (Woburn, MA). 6-formylindolo[3,2-b]carbazole (FICZ) and 2-(1H-Indol-3-ylcarbonyl)-4-thiazolecarboxylic acid (ITE) were obtained from BIOMOL (Plymouth Meeting, PA) and Tocris (Ellisville, MO), respectively. DMSO (Sigma-Aldrich, St. Louis, MO) was used as a vehicle for all AhR ligands. Unless specified, TCDD was used at a concentration of 10nM. Final concentration of the solvent in culture was below 0.1% DMSO and did not induce cytotoxicity. Whole chicken ovalbumin (ova; Grade V) was obtained from Sigma-Aldrich.
Generation of inflammatory BMDCs
Bone marrow-derived dendritic cells were prepared using methods as described by Inaba and coworkers (Inaba et al., 1992). Briefly, bone marrow cells were flushed from the femurs of C57Bl/6 or AhR mutant mice using complete RPMI (cRPMI) media. Bone marrow cells were centrifuged at low speed and red blood cells were removed by gradient centrifugation using lympholyte-M (Cedarlane, Hornby, Canada). Bone marrow cells were washed twice and plated at a density of 1×106 cells/ml in tissue culture flasks or 6-well plates. Cells were cultured in cRPMI media with 30ng/ml GM-CSF and vehicle or TCDD at 37° C and 5 % CO2. On days 3 and 5, non-adherent cells were collected washed once and reseeded. Fresh media, GM-CSF (30ng/ml), vehicle or TCDD was added to the culture flask at these time points. Non-adherent cells were harvested on day 7 and BMDCs were purified (≥85-90%) with anti-CD11c-APC and anti-APC beads (Miltenyi Biotec, Auburn, CA) using MACS separation columns as per the manufacturer’s instructions.
Immunophenotypic analysis of BMDCs
BMDCs were stained for FACS analysis as previously described (Shepherd et al., 2001). Briefly, rat, hamster or mouse IgG block was added to prepared leukocytes for 10 minutes on ice to prevent non-specific binding. Cells were stained for 10 minutes on ice cells with monoclonal antibodies (mAbs) from BD Pharmingen (San Diego, CA): CD11c (HL3), MHC class II (2G9), CD86 (GL1), CD45.2 (104), CD62L (MEL14), CD44 (IM7) and BioLegend (San Diego, CA): CD25 (PC61). All mAbs used were optimally titrated and isotype-matched immunoglobulins were used as controls. Acquisition of 40,000-300,000 events was performed using a FACS Aria flow cytometer (BD Biosciences, San Jose, CA) and analyzed by BD FACS Diva software (Version 4.0).
Activation of BMDCs with TLR ligands and cytokine assays
Purified vehicle- or TCDD-treated BMDCs were cultured in 6-well plates at a density of 1×106/ml per well and stimulated with LPS at 1 μg/ml (Sigma-Aldrich) or CpG at 0.5μM (InvivoGen, San Diego, CA) for 24 hrs. IL-6, TNF-α, IL-12p70 and IL-10 levels in the supernatants from BMDC cultures were measured by enzyme linked immunosorbent assay (ELISA). Samples were analyzed as per the manufacturer’s instructions using cytokine-specific BD ELISA kits (BD Biosciences).
Nitric oxide (NO) measurement
NO levels in supernatants from TLR-activated BMDCs were assessed using the Griess assay. The assay was conducted as per the manufacturer’s instructions (Promega, Madison, WI).
Antigen uptake
To measure pinocytic and/or phagocytic potential, BMDCs were incubated with acetylated LDL-FITC (Molecular Probes, Eugene, OR) for 1.5 hours, FITC–ovalbumin (Molecular Probes) for 12 hours or latex beads-FITC (Polysciences, Warrington, PA) for 6 hours. Antigen uptake was subsequently assessed by flow cytometry.
T cell activation
To assess the ability of BMDCs to activate CD4+ T cells in an Ag-specific fashion, the OT II adoptive transfer model was employed. Briefly, CD45.2+ BMDCs were generated in the presence of GM-CSF and TCDD or vehicle and loaded with whole ova. Antigen-laden BMDCs were injected (1×106/hind footpad) into CD45.1+ host mice that had received ova-specific OT II T cells intravenously (2×106/mouse) one day prior. Lymph nodes were harvested on day 4 post-immunization and donor DCs and OT II T cells analyzed by flow cytometry. Co-expression of CD45.2/CD11c or CD4/Thy1.1 was used to track donor DCs or OT II T cells, respectively. The activation status of the OT II T cells was assessed phenotypically using the markers, CD44 and CD62L.
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated using Trizol reagent and quantified by absorbance at 260nm. Single strand cDNA synthesis was performed using a RT-PCR kit (SABiosciences, Frederick, MD) and prepared as per the manufacturer’s instructions. Specific primers for IDO1, IDO2, TGFβ-1, TGF-β2, TGF-β3, Latent transforming growth factor beta binding protein 3 (Ltbp3), Aldehyde dehydrogenase family 1, subfamily A2 (Aldh1a2), Thrombospondin 1 (Thbs1), Plasminogen activator (Plat) and β-actin were obtained from SABiosciences. Quantitative detection of mRNA expression was performed using a Light cycler Instrument (Biorad IQ™ 5, Multicolor Real Time PCR Detection Systems) using SYBR green (SABiosciences) according to the manufacturer’s instructions.
NF-kB activity
Purified BMDCs (1.5×106/well) were stimulated with LPS (1μg/ml) or CpG (0.5μM) for 30 minutes in 6-well plates. Cells were harvested and nuclear protein extracts prepared using the Active Motif nuclear lysis kit (Active Motif, Carlsbad, CA). The protein content of the nuclear extract was measured using a BCA protein assay kit (Pierce, Rockford, IL). Nuclear protein (2.5 μg/ml per well) was then added to the Active Motif TransAM NF (Motif, Carlsbad, CA) and assayed according to the manufacturer’s specifications.
Statistical analysis
Student’s t test was used to compare means of vehicle-treated groups to TCDD–exposed groups. Data sets with multiple comparisions were evaluated by one-way analysis of variance (ANOVA) with Bonferroni’s post test. For analysis, values of p≤0.05 were considered significant.
Results
AhR activation alters the growth and differentiation of inflammatory BMDCs
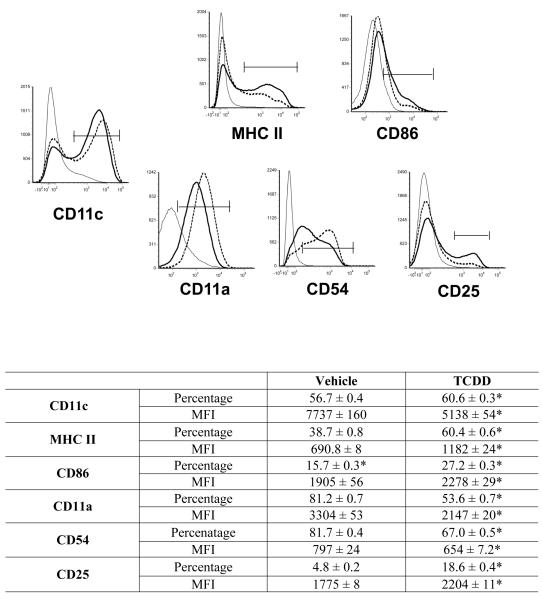
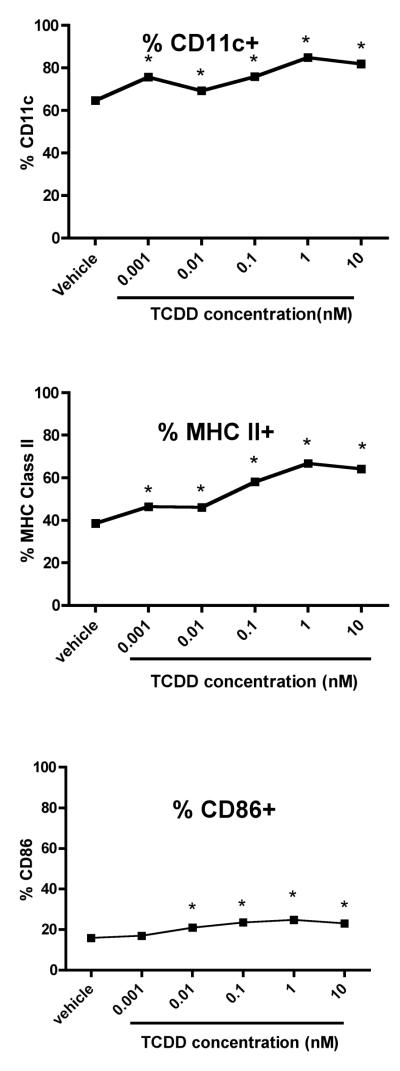
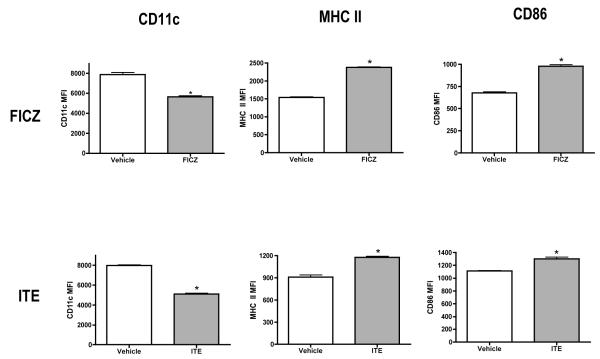
We first examined the effects of TCDD on the growth of BMDCs. Bone marrow cells were induced to differentiate into inflammatory DCs with GM-CSF in the presence of vehicle or TCDD for 7 days. On day 7, both adherent and non-adherent fractions were harvested. TCDD increased the number of non-adherent cells by 50 percent, but had no effect on the adherent cells when compared to vehicle-treated controls (Supplementary Table 1). Both vehicle- and TCDD-treated BMDCs were >95% viable (data not shown). Non-adherent cells were immunophenotyped using the murine DC marker, CD11c and the costimulatory/differentiation markers MHC class II, CD86, CD11a, CD54 and CD25. TCDD increased the frequency of CD11c+ BMDCs by 7 percent but decreased the relative expression of this lineage marker by 34 percent when compared to vehicle-treated controls. Additionally, TCDD increased MHC class II, CD86 and CD25 expression by 71, 20 and 24 percent, respectively, but decreased CD11a and CD54 expression by 35 and 18 percent, respectively, on the BMDCs (Fig. 1). These effects (as evaluated for CD11c, MHC class II and CD86) persisted on the BMDCs for up to four days in culture after removal of TCDD and growth factor (Supplementary Figure 1). TCDD altered BMDC differentiation at very low concentrations. A concentration of 0.01nM TCDD upregulated the frequency of BMDCs expressing CD11c, MHC class IIhigh and CD86high by 9, 31 and 30 percent, respectively, when compared to vehicle-treated controls (Fig. 2). In addition to TCDD, many natural (TCDD-like) ligands bind and activate the AhR. To examine the effects of non-TCDD chemicals on BMDC differentiation, cells were treated with two natural AhR ligands, FICZ and ITE. Similar to TCDD, FICZ and ITE decreased CD11c expression and increased levels of MHC class II and CD86 on the BMDCs (Fig. 3).
Figure 1. TCDD alters the expression of differentiation markers on BMDCs.
Bone marrow cells were induced to differentiate into immature DCs with GM-CSF in the presence of vehicle (represented by dotted line in the histograms) or 10 nM TCDD (thick line as indicated in the histograms). Isotype staining is shown in gray lines. Non-adherent cells were harvested on day 7 and stained with CD11c as a DC lineage marker. MHC class II, CD86, CD54, CD11a and CD25 expression was determined on CD11c+ BMDCs by FACS analysis. Data shown represents non-adherent cells obtained from 4 separate flasks per treatment group and is representative of 3 independent experiments. *p<0.05 indicates significant differences between vehicle- and TCDD-treated groups.
Figure 2. Low concentrations of TCDD alter BMDC differentiation.
Bone marrow cells were generated in the presence of GM-CSF and vehicle or varying TCDD concentrations (0.001nM-10nM). Non-adherent cells were harvested on day 7 and stained for CD11c, MHC II and CD86. Expression of MHC II and CD86 was determined on the CD11c+-gated population. Data represents mean ± SEM of 6 samples at each concentration. *p<0.05 indicates significant differences between the vehicle- and TCDD-treated groups.
Figure 3. Natural AhR agonists alter BMDC differentiation.
Bone marrow-derived dendritic cells were grown in presence of vehicle or the natural AhR agonists, FICZ and ITE. Non-adherent cells were harvested on day 7 and stained for CD11c, MHC II and CD86. Expression of MHC II and CD86 was determined on CD11c+ BMDCs. *indicates significant differences between treated and respective vehicle control groups. Data represents mean ± SEM of 4 samples per treatment group and is representative of 2 independent experiments.
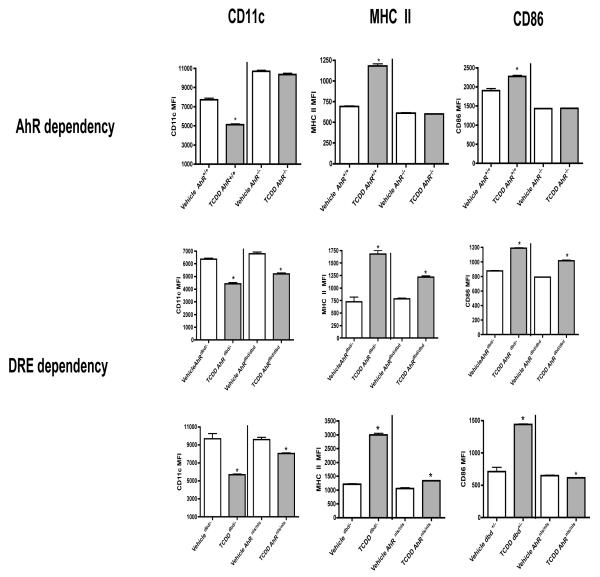
Effects of TCDD on BMDC differentiation are AhR-dependent but are not exclusively DRE-mediated
To define the role of the AhR in TCDD-induced alterations in the differentiation of BMDCs, we analyzed BMDCs from AhR−/−, AhRdbd/dbd and AhRnls/nls and wildtype mice. TCDD did not affect CD11c, MHC class II or CD86 expression on AhR−/− BMDCs when compared to vehicle-treated controls (Fig. 4). AhRnls/nls and AhRdbd/dbd mice express a mutant AhR that binds ligand but fails to translocate into the nucleus or bind DREs, respectively. BMDCs from AhRdbd/dbd mutant mice displayed decreased CD11c (by 24 percent) and increased MHC class II and CD86 expression (by 63 and 22 percent, respectively) following TCDD treatment (Fig. 4). On the other hand, BMDCs from AhRnls/nls mice displayed decreased levels of CD11c and CD86 (by 13 and 5 percent, respectively), and an increase (by 24 percent) in the expression of MHC class II following TCDD treatment.
Figure 4. The effects of TCDD on BMDCs are AhR-dependent and partially DRE-dependent.
BMDCs from AhR+/+, AhRdbd/-, AhRdbd/dbd or AhRnls/nls mice were treated with vehicle or TCDD. Non-adherent cells were harvested on day 7 and stained for CD11c, MHC II and CD86. Expression of MHCII and CD86 was determined on the CD11c+-gated population and analyzed by flow cytometry. Data represent mean ± SEM of non-adherent cells obtained from 3-4 samples per treatment group. *p<0.05 indicates significant differences between respective vehicle and TCDD-treated groups.
Effects of TCDD on TLR responsiveness and TLR ligand-induced production of inflammatory mediators
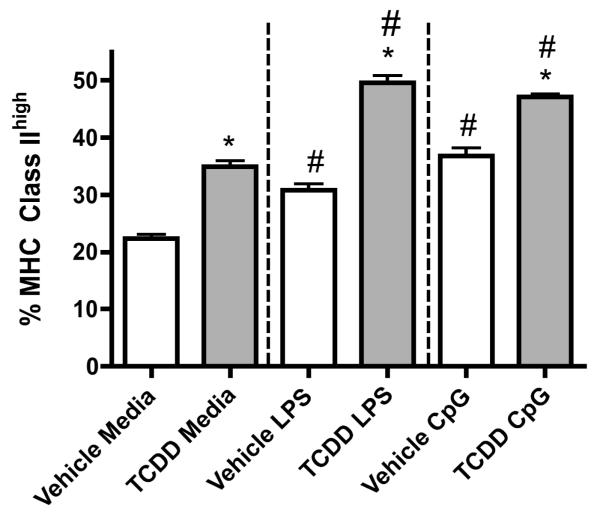
To evaluate the effects of TCDD on the innate responsiveness of BMDCs, cells were cultured with the TLR ligands, LPS (TLR4 agonist) or CpG (TLR9 agonist). Consistent with our previous results, TCDD increased MHC class IIhigh expression on unstimulated BMDCs by 35 percent when compared to the unstimulated vehicle controls (Fig. 5). As expected, LPS- and CpG-treatment increased the frequency of BMDCs expressing MHC class IIhigh when compared to the unstimulated controls. TCDD significantly increased the LPS- and CpG-induced frequency of BMDCs expressing MHC class IIhigh (by 60 and 25 percent, respectively) compared to respective vehicle-treated controls. TLR4 and TLR9 mRNA expression in the BMDCs was not affected by TCDD as measured by RT-PCR (data not shown). Additionally, AhR activation by TCDD increased both LPS- and CpG-induced IL-6 and TNF-α production but decreased NO production by BMDCs (Table 1). TCDD did not affect IL-10 production by the BMDCs when compared to the vehicle-treated controls. Although TCDD did not alter the LPS-induced IL-12p70 production, it decreased the CpG-induced IL-12p70 production by the BMDCs when compared to the vehicle-treated BMDCs.
Figure 5. Effects of TCDD on LPS- and CpG–induced MHC Class IIhigh expression.
BMDCs were grown in vehicle or TCDD and non-adherent cells were harvested on day 7. BMDCs were purified and treated with LPS or CpG for 24 hours. Cells were harvested after 24 hours and MHC class II expression was assessed. Data represents mean ± SEM of 9 samples per treatment group and is representative of 3 independent experiments. *p<0.05 indicates significant differences between the respective vehicle- and TCDD-treated groups. #p<0.05 indicates significant differences between TLR-stimulated and respective untreated groups.
Table 1.
Effects of TCDD on cytokine production by inflammatory BMDCsa
| IL-10 (pg/ml) | ||
|---|---|---|
| Vehicle | TCDD | |
| Media | BD | BD |
| LPS | 1310 ± 76 | 1538 ± 282 |
| CpG | 694± 64 | 719 ± 43 |
| IL-12p70 (pg/ml) | ||
|---|---|---|
| Vehicle | TCDD | |
| Media | BD | BD |
| LPS | 268 ± 36 | 271 ± 34 |
| CpG | 640 ± 28 | 581 ± 20* |
BMDCs were stimulated with LPS or CpG as described in Materials and Methods. Supernatants were collected and levels of TNF-α, IL-6, IL-10 and IL-12p70 determined by ELISA. Levels of NO were determined using the Griess reagent. Data represents mean±SEM of 4-9 samples for TNF-α, IL-6, IL-10 and NO and is representative of 2 independent experiments. Data represents mean ± SEM of 12-15 samples pooled from 2 separate experiments for IL-12p70.
p≤0.05 indicates significant differences between LPS- and CpG–stimulated BMDCs when compared to respective vehicle-treated controls. BD represents below detection.
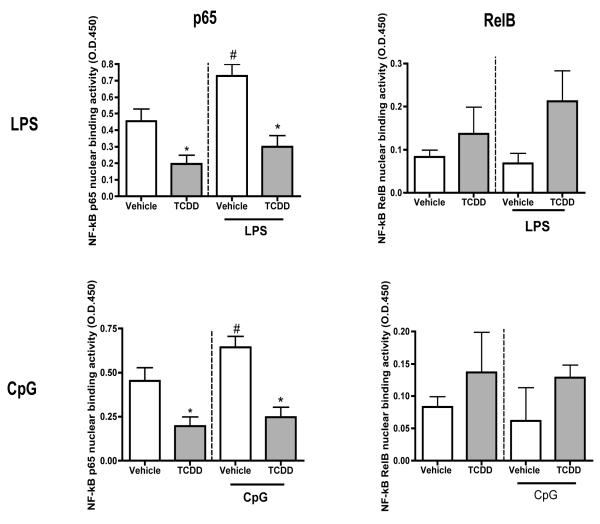
TCDD decreases LPS- and CpG-induced p65 but not RelB activity
NF-kB signaling in DCs is activated following TLR stimulation. Additionally, based on the potential of ligand-activated AhR to physically interact with NF-kB and alter immune function, we evaluated NF-kB signaling in BMDCs (Ruby et al., 2002; Vogel et al., 2007a). TCDD decreased p65, but not RelB activity in unstimulated BMDCs (by approximately 56 percent) when compared to unstimulated vehicle controls. LPS- and CpG-treatment increased p65 but not RelB activity in BMDCs when compared to the unstimulated controls. TCDD significantly decreased LPS- and CpG-induced p65 activity in BMDCs (by 57 and 65 percent, respectively) when compared to respective vehicle-treated controls. TCDD generated a trend towards upregulation of LPS- and CpG-induced RelB levels in BMDCs when compared to respective vehicle-treated controls (Fig. 6).
Figure 6. LPS- and CpG-induced p65 but not Rel B activity is reduced by TCDD.
Vehicle- or TCDD-treated BMDCs were stimulated with LPS (1 μg/ml) or CpG (0.5μM) for 0.5 hours. Cells were harvested and nuclear proteins isolated. NF-kB p65 and RelB activity was measured using the TransAM activity ELISA. * indicates significant differences between LPS- or CpG-stimulated groups compared to respective vehicle-treated control groups. # represents significant differences between LPS- or CpG-stimulated groups compared to respective untreated control group. Data represents mean ± SEM of 3 samples per treatment group.
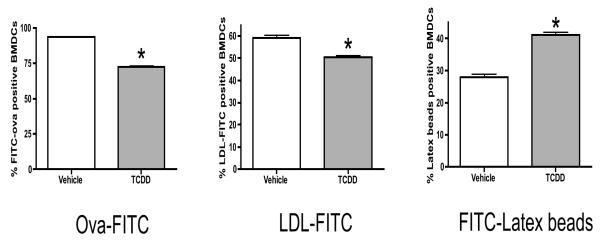
Antigen uptake by BMDCs is modulated by TCDD
Dendritic cells take up antigens by pinocytosis, phagocytosis and receptor-mediated endocytosis. To monitor the effects of TCDD on antigen uptake, BMDCs were evaluated for uptake of both soluble and particulate antigens. As determined by flow cytometry, TCDD decreased BMDC uptake of the soluble antigens, ova and acetylated-LDL, by 25 and 10 percent, respectively (Fig. 7). Conversely, TCDD increased the uptake of the particulate antigen, latex beads, by 40 percent when compared to the vehicle-treated controls.
Figure 7. TCDD alters antigen uptake by BMDCs.
Vehicle- or TCDD-treated BMDCs were purified and their ability to take up FITC-conjugated whole ovalbumin (FITC–Ova), low-density lipoprotein (LDL-FITC) and latex beads was assessed. Cells were exposed to FITC-conjugated ovalbumin overnight, LDL-FITC for 1.5 hours or FITC-latex beads for 6 hours prior to harvesting. Data represents mean ± SEM of 9-12 samples per treatment group for 2 independent experiments. * p≤ 0.05 indicates significant differences between vehicle- and TCDD-treated samples.
AhR activation increases mRNA levels of regulatory mediators
Potent Treg-inducing mediators such as IDO1, IDO2, Thbs1 and TGF-β3 have been implicated in the generation of immune suppression following AhR activation (Lawrence et al., 2008; Marshall et al., 2008; Vogel et al., 2008). In this context, we examined the effects of AhR activation on the expression of suppressive mediators in BMDCs. TCDD-treated BMDCs upregulated IDO1, IDO2 and TGF-β3 mRNA levels by 18-, 24- and 11-fold, respectively (Table 2). TCDD had no effect on the expression of LTBP, Aldh, Thbs1 or Plat in the BMDCs. Following LPS treatment, TCDD-treated BMDCs displayed increased levels of IDO1, IDO2, TGF-β1, TGF-β2 and Thbs1 when compared to vehicle-treated BMDCs.
Table 2.
AhR activation in inflammatory BMDCs induces expression of regulatory mediatorsa
| Unactivated | LPS-activated | |||
|---|---|---|---|---|
| Fold change | p value | Fold change | p value | |
| IDO1 | 18 * | 0.0001 | 22 * | ≤0.001 |
| IDO2 | 24 * | ≤0.001 | 25 * | ≤0.001 |
| TGFβ1 | 0.4 | 0.1 | 1.8 * | 0.02 |
| TGFβ2 | 0.9 | 0.9 | 2.3 * | 0.008 |
| TGFβ3 | 11 * | 0.001 | 0.01 | 0.4 |
| Ltbp3 | 0.2 | 0.3 | 1.3 * | 0.001 |
| Aldh1a2 | 0.8 | 0.6 | 0.6 | 0.09 |
| Thbs1 | 0.4 | 0.07 | 1.9 * | 0.01 |
| Plat | 1.2 | 0.4 | 1.0 | 0.8 |
Gene expression in resting and LPS-activated BMDCs was evaluated by realtime qRT-PCR. Results are expressed as TCDD-induced fold change after normalization with the housekeeping gene, β-actin. N = 3-4 per treatment group.
p≤0.05 indicates significant differences between vehicle-treated and TCDD-treated BMDCs.
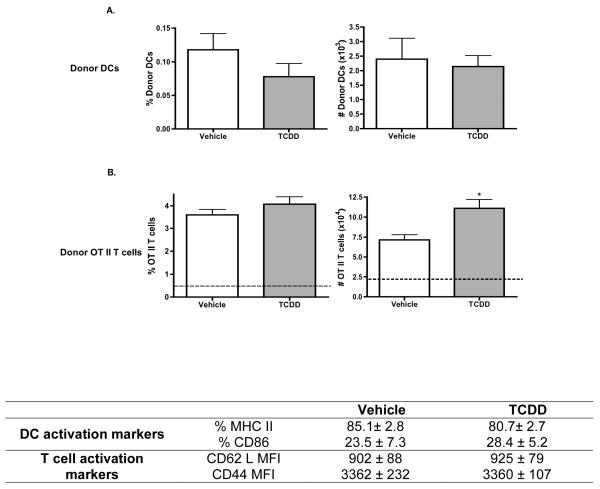
AhR-activated BMDCs do not inhibit antigen-specific CD4+ T cell activation in vivo or in vitro
We tested the ability of AhR-activated BMDCs to generate antigen-specific T cell activation using the modified OT II adoptive transfer model. Vehicle- or TCDD-treated, CD45.2+ BMDCs were pulsed in vitro with whole ovalbumin to load them with antigen. Ova-loaded DCs were then used to activate ova-specific OT II/Thy1.1 CD4+ T cells that had been adoptively transferred into CD45.1+ host mice. Peripheral lymph nodes were harvested on day 4 following BMDC immunizations. No differences were observed in the frequency or number of TCDD- or vehicle-treated donor DCs in the proximal, draining popliteal lymph nodes (PLNs) (Fig. 8A). In contrast to BMDCs that were harvested directly after in vitro culture (Fig. 1), no TCDD effects were identified on the frequency of BMDCs expressing either MHC class II or CD86 harvested from the PLNs on day 4 post-immunization. As anticipated, ova-specific OT II CD4+ T cells isolated from the PLNs exhibited significant clonal expansion when compared to OT-II CD4+ T cells isolated from the distal, non-draining brachial lymph nodes. Adoptively transferred mice immunized with TCDD-treated, ova-loaded BMDCs displayed an increase (by approximately 57 percent) in the number, but not the frequency, of OT II CD4+ T cells when compared to the respective control mice (Fig. 8B). Additionally, no differences were observed in the expression of the activation markers CD62L and CD44 on the OT II CD4+ T cells from mice receiving TCDD-treated BMDCs when compared to donor T cells from mice immunized with vehicle-treated BMDCs.
Figure 8. Ability of ova-laden BMDCs to activate ova-specific T cells in vivo.
CD4+ T cells from OT II/Thy1.1 mice were adoptively transferred into congenic, CD45.1+ host mice on day -1 relative to immunization. On day 0, vehicle- or TCDD-treated BMDCs that were loaded with whole ovalbumin were injected into adoptively transferred mice. On day 4 post-immunization, popliteal and brachial lymph nodes were harvested from host mice and analyzed by flow cytometry. Analysis was based on the differences in the phenotypic expression of cell surface molecule between donor DCs (CD45.2+/CD11c+) and donor OT II T cells (CD4+/V-alpha2+/Thy1.1+) from host mice (CD45.1+/Thy1.2+). Data are representative of 4-5 animals per treatment group. * p ≤ 0.05 indicates significant differences between vehicle- and TCDD–treated groups. Dashed line represents the donor OT II T cell control levels in the brachial lymph nodes.
Discussion
Dendritic cells are professional antigen presenting cells that play an important role in both the innate and adaptive arms of the immune system. In this study, we have characterized the consequences of AhR activation on the innate and adaptive functions of inflammatory BMDCs and examined the role of DRE-mediated events in the generation of these effects. We demonstrate that AhR activation alters BMDC differentiation and induces a regulatory phenotype, but surprisingly without altering the ability of the DCs to initiate antigen-specific activation of naïve, CD4+ T cells.
Dendritic cells exist in multiple, functionally distinct stages of differentiation. Several investigators have linked TCDD-induced modulation of DC differentiation to the generation of defective T cell responses (Vorderstrasse and Kerkvliet, 2001; Lee et al., 2007). In our study, TCDD decreased expression of CD11c but increased MHC II and CD86 levels on BMDCs. Engagement of B7 (CD86) molecules on a DC with CD28 or CTLA4 on the surface of the T cell can activate or impair T cell responses, respectively (Krummel and Allison, 1995; Lenschow et al., 1996). Successful T cell activation involves not only interactions between the TcR on the T cells and antigen-associated MHC class II on antigen presenting cells, but also costimulation via interactions between accessory molecules such as CD28 and CD86. In this context, studies have linked TCDD-induced increases in CD86 expression on DCs with increased T cell activation in an MLR response (Lee et al., 2007). However, AhR activation has also been shown to induce CTLA-4 expression on T cells which can bind to CD86 on a DC with high affinity and impair T cell responses (Funatake et al., 2005). Thus, in the context of AhR activation, ligation of CD86 on DCs with CTLA-4 on T cells could alternatively contribute towards the generation of immune suppression. Furthermore, consistent with previous studies documenting TCDD-induced modulation of the adhesion molecules CD11a and CD54, TCDD decreased the expression of both of these molecules on inflammatory BMDCs (Shepherd et al., 2001; Vorderstrasse and Kerkvliet, 2001). The decreased expression of adhesion molecules on DCs could contribute to defective T cell activation and subsequent immune suppression. TCDD-induced immune suppression has also been linked to increased CD25 expression on T cells (Funatake et al., 2005). More recently, CD25 was also identified as a phenotypic marker for regulatory DCs (DCregs) (von Bergwelt-Baildon et al., 2006; Driesen et al., 2008). TCDD increased CD25 expression on the BMDCs, suggesting the induction of a regulatory phenotype in these inflammatory BMDCs. Taken together, TCDD-induced alteration of molecules involved in antigen presentation, costimulation and adhesion could result in defective T cell activation contributing to immune suppression. Recently, ligand-specific activation of the AhR has been shown to generate Tregs or Th17 cells (Quintana et al., 2008). However, the effects of ligand-specific activation in inflammatory DCs had not been previously described. Interestingly, the AhR ligands, FICZ and ITE generated phenotypical alterations in BMDCs similar to TCDD, demonstrating a lack of differential responsiveness of DCs to an array of AhR ligands.
While the AhR is essential for TCDD-induced immunotoxicity, no information exists regarding the role of DREs in TCDD-induced alterations in DCs. We investigated the role of the AhR and DREs in TCDD-induced alteration of DC differentiation using BMDCs from AhR null, AhRnls/nls and AhRdbd/dbd mice. While the AhR null mice, lacking functional AhR receptor, enabled us to determine the role of the AhR in the generation of TCDD-induced alterations in DCs, involvement of DRE-mediated events was assessed using the AhRnls/nls and AhRdbd/dbd mice expressing a mutant AhR that binds ligand but fails to translocate into the nucleus or bind DREs, respectively. Our results show that the effects of AhR activation in BMDCs are strictly AhR-dependent but not exclusively DRE-mediated. TCDD-treated BMDCs from AhRdbd/dbd mice displayed increased MHC class II and CD86 expression when compared to vehicle-treated AhRdbd/dbd BMDCs. However, TCDD-induced increases in MHC class II and CD86 expression on AhR dbd/dbd BMDCs did not reach similar levels to those observed on TCDD-treated BMDCs from AhRdbd/- control mice. This suggested that a non-DRE-mediated mechanism was contributing to the TCDD-induced differentiation of the DCs. Of the several non-DRE-mediated mechanisms implicated in TCDD immunotoxicity, interactions of the activated AhR with NF-kB signaling components can induce defects in DCs following TCDD exposure (Ruby et al., 2002; Vogel and Matsumura, 2008). Thus, one possible explanation for the partial effects of TCDD on BMDC differentiation as observed in DCs from AhRdbd/dbd and AhRnls/nls mice could be due to altered NF-kB signaling.
A primary function of DCs is the recognition of pathogen associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs) such as TLRs. Based on the observed TCDD-induced modulation of DC differentiation, we assessed whether TCDD-exposed BMDCs were responsive to PAMPs via TLR activation. TCDD increased BMDC responsiveness to TLR stimulation via TLR4 and TLR9 without affecting the basal mRNA levels of these receptors. Following activation, DCs secrete cytokines that orchestrate developing immune responses. In our study, TCDD increased LPS- and CpG-induced IL-6 and TNF-α production by BMDCs but decreased their production of NO. Multiple possibilities could account for these observed effects. The NF-kB independent transcription factor, LPS-Induced TNF-α Factor (LITAF), is a transcription factor that has been implicated in the LPS-induced expression of TNF-α and other inflammatory cytokines (Sun et al., 2004; Tang et al., 2005). LITAF contains 7 DRE sequences and upregulation of LITAF in DCs could underlie our observed TCDD-induced increases in LPS-induced pro-inflammatory cytokine secretion (Sun et al., 2004). However, recent studies have documented the ability of activated AhR to negatively regulate inflammatory responses in human monocyte-derived DCs and murine macrophages (Lawrence et al., 2008; Kimura et al., 2009). Discrepancies in these results could be due to differences in the AhR agonists used in the respective studies (VAGF347 versus TCDD) or in the cells that were examined (human monocyte-derived DCs versus murine DCs or macrophages). In addition, activation of inflammatory DCs by LPS or CpG usually induces upregulation of inducible nitric oxide synthase (iNOS) and subsequent increases in NO production. AhR activation by TCDD has been shown to increase pulmonary iNOS levels contributing to an enhanced IFN-γ production in the lungs (Neff-LaFord et al., 2007). Contrary to these reports, in our study TCDD decreased NO production by both unstimulated and TLR-activated BMDCs. Additional investigations are thus warranted to further understand the effects of TCDD on NO production by BMDCs.
A previous study examining the effects of TCDD on BMDCs reported decreased mRNA levels of IL-10 but not IL-12 following LPS-stimulation (Lee et al., 2007). Contrary to this study, our data indicate that TCDD does not alter the production of IL-10 and decreases CpG-induced IL-12 production by BMDCs. Different DC preparations (unpurified versus purified BMDCs) used in these two studies could account for the observed differences. Under in vivo conditions, inflammatory DCs function to limit infection by their secretion of inflammatory cytokines. Induction of inflammation is a hallmark feature of TCDD-induced immunotoxicity and our results suggest a role for DCs in TCDD-induced inflammatory responses. However, how TCDD-induced alterations of inflammatory DCs impacts the development of acute inflammation remains to be determined.
NF-kB is a major signaling pathway involved in the differentiation of DCs and their responsiveness to TLR stimulation. Furthermore, several studies have linked physical interactions of activated AhR with NF-kB p65 and RelB in the generation of TCDD-induced cellular dysfunction and immune suppression (Ruby et al., 2002; Camacho et al., 2005; Vogel et al., 2007a; Vogel et al., 2007b; Vogel and Matsumura, 2008). Because TCDD alters the differentiation of DCs and their responsiveness to TLRs, we investigated the effects of AhR activation on NF-kB signaling in inflammatory BMDCs. TCDD suppressed basal p65 activity in unstimulated BMDCs as well as BMDCs activated by LPS and CpG. Furthermore, a trend towards increased RelB activity was also observed in TCDD-treated BMDCs. Binding of RelB/AhR to specific response elements (RelBAhRE) in certain target genes has been linked to the regulation of cytokines and chemokines such as IL-8 and BLC following TCDD exposure (Vogel et al., 2005; Vogel et al., 2007b; Vogel and Matsumura, 2008). It would be of interest to determine if the enhanced production of IL-6 and TNF-α by TCDD-treated BMDCs is also regulated by increased interactions between activated AhR and RelB. Overall, our data suggest that TCDD-induced modulation of DC differentiation and their responsiveness to TLR stimulation may be mediated via altered NF-kB signaling.
Dendritic cells capture antigens via distinct mechanisms. While soluble antigen uptake primarily involves macropinocytic or receptor-mediated endocytic events, particulate antigens are internalized primarily via phagocytosis (Banchereau et al., 2000). TCDD decreased the uptake of the soluble antigens ovalbumin and LDL but increased phagocytosis of latex beads by inflammatory DCs. These results suggest that TCDD differentially impacts distinct mechanisms of antigen uptake. Studies have shown macrophage mannose receptor and scavenger receptor A to mediate the endocytosis of ova and acetylated LDL, respectively (Pearson et al., 1993; Becker et al., 2006; Burgdorf et al., 2007). Therefore, based on our results, we hypothesize that decreased expression of these receptors in BMDCs following exposure to TCDD leads to decreased uptake of soluble antigens. Conversely, TCDD increased the uptake of latex beads by BMDCs. So far, only one study has described effects of TCDD on the uptake of latex beads. In this in vivo study, uptake of latex beads by splenic DCs was unaffected following TCDD exposure (Vorderstrasse et al., 2003). The observed discrepancy in our results could be attributed to distinct antigen uptake mechanisms in the two functionally distinct DC populations (steady-state versus inflammatory), which could be differentially affected by TCDD.
Because our data demonstrated that TCDD altered antigen uptake, we assessed the effects of TCDD on the T cell stimulatory capacity of inflammatory BMDCs. Surprisingly, TCDD-treated BMDCs generated normal antigen-specific T cell responses in vivo. These results were unexpected, as TCDD not only induced a regulatory phenotype in BMDCs, but also increased their expression of several regulatory mediators including IDO1, IDO2 and TGF-β3. Several possibilities could account for the lack of T cell suppression by TCDD-treated BMDCs. Though our results demonstrated that TCDD alters antigen uptake of ovalbumin by BMDCs, it is possible that the observed decreases in antigen uptake do not reach a physiological threshold below which T cell activation is affected. Additionally, TCDD may increase the expression, but not the function or activity of the suppressive mediators IDO1, IDO2 and TGF-β3. In this case, we would not expect TCDD-treated BMDCs to contribute to the suppression of T cell activation. Recent studies have linked AhR activation in DCs with suppression of T cell responses (Hauben et al., 2008). In these studies, AhR-activated BMDCs suppressed T cell responses only following LPS stimulation; immature BMDCs did not alter T cell responses. Thus, we also evaluated the expression of the suppressive mediators in BMDCs following LPS activation. TCDD-treated BMDCs stimulated with LPS displayed increased expression of IDO1, IDO2, TGF-β1, TGF-β2 and Thbs1. However, future experiments are needed to determine whether TCDD-treated BMDCs stimulated with LPS can affect antigen-specific T cell responses.
Collectively, we show that AhR activation alters inflammatory DC differentiation, generating DCs that are hyperresponsive to TLR stimulation and exhibit a regulatory phenotype in vitro. Surprisingly, these BMDCs fail to alter antigen-specific T cell activation. However, further studies are needed to examine the potential for AhR-activated DCs to contribute to the generation of regulatory T cells and the suppression of T cell-mediated immune responses. One of the novelties of our data is the identification of biomarkers of exposure to TCDD and TCDD-like chemicals on a highly sensitive and extremely important immune cell population, the inflammatory DCs. Moreover, these effects were detected at very low, environmentally relevant levels of exposure. Because our results indicate that specific modulation occurs in these cells, it would be of great value to evaluate human inflammatory DCs. Similarly, our data further define mechanisms of action of TCDD on the immune system. This information may be useful for the generation of focused therapeutic approaches to treat humans exposed to environmental chemicals that can activate the AhR. Furthermore, it highlights the potential to develop selective AhR modulators that may be beneficial in the treatment of allergies and autoimmune diseases.
Supplementary Material
Acknowledgements
This research was supported by the NIH grant, ES013784 (DMS). We would like to acknowledge the assistance of the Fluorescence Cytometry Core facility at the University of Montana (supported in part by NIH grant, RR017670) and the Molecular Biology Core facility at the University of Montana (supported in part by NIH grants, RR017670 and RR015583).
Abbreviations
- AhR
Aryl hydrocarbon receptor
- Ag
Antigen
- ova
Ovalbumin
- BMDCs
bone marrow-derived dendritic cells
- DCs
dendritic cells
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TLR
Toll-like receptor
- FICZ
6-formylindolo[3,2-b]carbazole
- IDO
Indoleamine-2,3-dioxygenase
- ITE
2-(1H-Indol-3-ylcarbonyl)-4-thiazolecarboxylic acid
- Treg
regulatory T cell.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Becker M, Cotena A, Gordon S, Platt N. Expression of the class A macrophage scavenger receptor on specific subpopulations of murine dendritic cells limits their endotoxin response. Eur J Immunol. 2006;36:950–960. doi: 10.1002/eji.200535660. [DOI] [PubMed] [Google Scholar]
- Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappaB and expression of Fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol. 2005;175:90–103. doi: 10.4049/jimmunol.175.1.90. [DOI] [PubMed] [Google Scholar]
- Driesen J, Popov A, Schultze JL. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology. 2008;213:849–858. doi: 10.1016/j.imbio.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175:4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- Hauben E, Gregori S, Draghici E, Migliavacca B, Olivieri S, Woisetschlager M, Roncarolo MG. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008;112:1214–1222. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- Henry EC, Bemis JC, Henry O, Kende AS, Gasiewicz TA. A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys. 2006;450:67–77. doi: 10.1016/j.abb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int Immunopharmacol. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009 doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BP, Denison MS, Novak H, Vorderstrasse BA, Harrer N, Neruda W, Reichel C, Woisetschlager M. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112:1158–1165. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Hwang JA, Sung HN, Jeon CH, Gill BC, Youn HJ, Park JH. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modulates functional differentiation of mouse bone marrow-derived dendritic cells Downregulation of RelB by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 2007;173:31–40. doi: 10.1016/j.toxlet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181:2382–2391. doi: 10.4049/jimmunol.181.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff-LaFord H, Teske S, Bushnell TP, Lawrence BP. Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-gamma production by phagocytic cells. J Immunol. 2007;179:247–255. doi: 10.4049/jimmunol.179.1.247. [DOI] [PubMed] [Google Scholar]
- Oberg M, Bergander L, Hakansson H, Rannug U, Rannug A. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci. 2005;85:935–943. doi: 10.1093/toxsci/kfi154. [DOI] [PubMed] [Google Scholar]
- Pearson AM, Rich A, Krieger M. Polynucleotide binding to macrophage scavenger receptors depends on the formation of base-quartet-stabilized four-stranded helices. J Biol Chem. 1993;268:3546–3554. [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Ruby CE, Funatake CJ, Kerkvliet NI. 2,3,7,8 Tetrachlorodibenzo-p-Dioxin (TCDD) Directly Enhances the Maturation and Apoptosis of Dendritic Cells In Vitro. J Immunotoxicol. 2005;1:159–166. doi: 10.1080/15476910490920968. [DOI] [PubMed] [Google Scholar]
- Ruby CE, Leid M, Kerkvliet NI. 2,3,7,8-Tetrachlorodibenzo-p-dioxin suppresses tumor necrosis factor-alpha and anti-CD40-induced activation of NF-kappaB/Rel in dendritic cells: p50 homodimer activation is not affected. Mol Pharmacol. 2002;62:722–728. doi: 10.1124/mol.62.3.722. [DOI] [PubMed] [Google Scholar]
- Sato K, Fujita S. Dendritic cells: nature and classification. Allergol Int. 2007;56:183–191. doi: 10.2332/allergolint.R-06-139. [DOI] [PubMed] [Google Scholar]
- Shepherd DM, Steppan LB, Hedstrom OR, Kerkvliet NI. Anti-CD40 Treatment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-exposed C57Bl/6 mice induces activation of antigen presenting cells yet fails to overcome TCDD-induced suppression of allograft immunity. Toxicol Appl Pharmacol. 2001;170:10–22. doi: 10.1006/taap.2000.9080. [DOI] [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- Sun YV, Boverhof DR, Burgoon LD, Fielden MR, Zacharewski TR. Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res. 2004;32:4512–4523. doi: 10.1093/nar/gkh782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Marciano DL, Leeman SE, Amar S. LPS induces the interaction of a transcription factor, LPS-induced TNF-alpha factor, and STAT6(B) with effects on multiple cytokines. Proc Natl Acad Sci U S A. 2005;102:5132–5137. doi: 10.1073/pnas.0501159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2008 doi: 10.1016/j.bcp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007a;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem Biophys Res Commun. 2007b;363:722–726. doi: 10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect. 2005;113:1536–1541. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, Fiore F, Roth U, Beyer M, Debey S, Wickenhauser C, Hanisch FG, Schultze JL. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- Vorderstrasse BA, Dearstyne EA, Kerkvliet NI. Influence of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the antigen-presenting activity of dendritic cells. Toxicol Sci. 2003;72:103–112. doi: 10.1093/toxsci/kfg012. [DOI] [PubMed] [Google Scholar]
- Vorderstrasse BA, Kerkvliet NI. 2,3,7,8-Tetrachlorodibenzo-p-dioxin affects the number and function of murine splenic dendritic cells and their expression of accessory molecules. Toxicol Appl Pharmacol. 2001;171:117–125. doi: 10.1006/taap.2000.9119. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–7584. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.