Abstract
Objective:
To assess the potential role for Neuregulin-1 (NRG1) as a systemic endogenous protector in the setting of perinatal inflammatory brain damage.
Methods:
We measured NRG1- protein and mRNA levels in human umbilical venous endothelial cells (HUVECs) of different gestational ages at various durations of exposure to lipopolysaccharide (LPS). In parallel, we genotyped the donor individuals for SNP8NRG221533, a disease-related single nucleotide polymorphism in the 5′ region upstream of the NRG1 sequence. Intracellular NRG1 localization was visualized by confocal microscopy. Furthermore we analyzed the relationship between SNP8NRG221533 genotype and neurodevelopmental outcome in children born preterm.
Results:
We observed a positive dose-response-relationship between NRG1-mRNA and intracellular protein levels with both advancing gestational age and duration of LPS exposure in HUVECs. The presence of allele C at the SNP8NRG221533 locus was associated with an increased cellular production of NRG1 in HUVECs, and with a significantly reduced risk for cerebral palsy and developmental delay in children born preterm.
Interpretation:
In conclusion, our data indicate that gestational age, duration of LPS exposure, and the SNP8NRG221533 genotype affect NRG1 levels. Our results support the hypothesis that NRG1 may qualify as an endogenous protector during fetal development.
Keywords: Neuregulin-1, HUVECs, inflammation, SNP8NRG221533, neuroprotector, brain damage
Introduction
Preterm birth is associated with an increased risk of neonatal brain damage (Ferriero 2004) and long-term developmental delay (Stephens and Vohr 2009). Intrauterine infection leads to inflammatory responses (Dudley 1997; Dammann, Allred et al. 2004; Romero, Espinoza et al. 2006; Romero, Espinoza et al. 2007) that contribute to prematurity and neonatal morbidity. The umbilical vessel endothelium is one of the first fetal interfaces exposed to inflammatory challenges, e.g. lipopolysaccharides (LPS). Indeed, endothelial cells have a remarkable capacity to respond to stressors (Dauphinee and Karsan 2006; Magder, Neculcea et al. 2006).
Neuregulins (NRGs) play important roles during fetal brain (Bernstein, Lendeckel et al. 2006), heart (Gassmann, Casagranda et al. 1995) and lung development (Dammann, Nielsen et al. 2003), and are involved in inflammatory processes (Xu, Jiang et al. 2004; Xu, Ford et al. 2005). Several NRG isoforms (Meyer, Yamaai et al. 1997) are produced by alternative splicing (Wen, Suggs et al. 1994; Falls 2003). One of these, NRG1, appears to help signal the onset of surfactant synthesis in the fetal lung (Dammann, Nielsen et al. 2003) and might qualify as a brain protector in experimental ischemia (Xu, Jiang et al. 2004; Xu, Ford et al. 2005). We have recently suggested that NRG1 might play a role not only in adult (Deadwyler, Pouly et al. 2000; Gerecke, Wyss et al. 2004), but also in neonatal brain disorders (Dammann, Bueter et al. 2007).
The role of single nucleotide polymorphisms (SNPs) in inflammation-associated genes, e. g. interleukin (IL)-10, as risk-modulators for neonatal disorders has previously been investigated in a pilot study (Dordelmann, Kerk et al. 2006; Dammann, Brinkhaus et al. 2009). Now we wanted to expand this scenario to include NRG1 and one of its SNPs (SNP8NRG221533) that has been associated with schizophrenia (Stefansson, Sigurdsson et al. 2002; Stefansson, Sarginson et al. 2003).
We hypothesized, that
NRG1 is released by HUVECs in response to LPS-exposure
NRG1 gene expression and NRG1 protein production differ with both gestational age and LPS treatment duration
SNP8NRG221533 alters NRG1 gene expression; and
There is a reduced risk for neonatal brain damage and developmental delay among children with a high producer allele of SNP8NRG221533.
Methods and Patients
Cell culture
Umbilical cords were collected from babies born either before 30 weeks gestation (immature group) or after completion of 37th week of gestation (mature group). To reduce the likelihood of obtaining biased results all umbilical cords showing histological evidence for inflammation (funisitis) were excluded from our study. Endothelial cells were harvested from human umbilical cord vessels within 1 – 5 days of delivery (Bueter, Dammann et al. 2006). Briefly, umbilical veins and arteries were cannulated with blunt needles, rinsed with sterile PBS buffer, and treated with type I collagenase (0.04 %, GIBCO, Invitrogen, Karlsruhe) for 25 minutes at 37°C. Cell-collagenase suspension was collected and centrifuged at 250 × g for 5 minutes at 4°C. Cells were resuspended, and plated in endothelial cell growth medium containing 10 % fetal calf serum (FCS). Cells were washed after 2-3 hours of adherence and grown until confluence. We used cells up to the 5th passage for the experimental assays. Before treatment, cells were starved for two hours in Dulbecco's Modified Eagle's Medium (DMEM) containing 0.1 % FCS. Treatment was performed with 100 ng/ml LPS for 1.5, 3, 6, 12, and 24 hours. Untreated controls were kept in culture in DMEM for the same time.
NRG1 localization by confocal microscopy
HUVECs were grown on glass slides for 24 hours and starved in serum-free DMEM for 2 hours. Cells were either treated with LPS (100ng/ml) for 30 minutes, 24 hours or left in DMEM as a control for the same time at 37°C. Immunofluorescence staining was performed as previously described (Padmakumar, Abraham et al. 2004). Briefly, cells were fixed with 3% paraformaldehyde for 20 minutes followed by permeabilisation with 0.2% Triton X-100 for 2 minutes. After 1 hour blocking in 10% normal goat serum, fixed cells were incubated with the specific primary NRG1 antibody (rabbit polyclonal IgG Neuregulin-Ab 2; Neo Markers, Fremont, CA) at room temperature for 1-2 hours. Cells were washed with PBS and incubated with the appropriate secondary antibody conjugated with Alexa568. Subsequently cells were incubated in 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma, Heidelberg, Germany) for 10 minutes. Cells were mounted in Gelvatol/DABCO and analyzed using a Leica TCS-SP2 confocal laser scanning microscope.
Genotyping for SNP8NRG221533
We genotyped both HUVECs obtained from umbilical cords and lymphocyte DNA obtained from whole blood samples (Dordelmann, Kerk et al. 2006). Genomic DNA was isolated using standard phenol-chloroform extraction. Polymerase chain reactions (PCR) were performed on 100 ng of genomic DNA using primers 5′-ACCTAAGATGTCCAAGAGACAG-3′ (forward) and 5′-GACTGGAAGCCATGTATCTTTATTGT -3′ (reverse) and HotStart Taq DNA polymerase (5 U/μl, Qiagen, Hilden, Germany). Thirty-six cycles were performed with 15 minutes denaturation at 95°C, 1 minute annealing at 62° and 1 minute extension at 72°C. The artificially introduced mismatch in the reverse primer allowed for a subsequent allelic discrimination by the restriction enzyme RsaI. PCR products were incubated with RsaI (New England BioLabs, Frankfurt, Germany) at 37°C overnight and restriction fragment length analysis (RFLP) was performed by 3 % agarose gel electrophoresis.
ELISA
All sample-measurements were carried out in quadruplicates. Cells were scraped, lysed by sonication (3 × 30 seconds), lyophilized, and resuspended in 200μl PBS. The amount of total protein was measured by DC Protein Assay (Bio-Rad, Munich, Germany). ELISA was performed using the Human NRG1-beta 1/HRG1-beta 1 DuoSet (DY 377 R&D Systems, Wiesbaden, Germany). 96-well-plates were coated overnight with 100 μl capture antibody (4 μg/ml Heregulin Ab-1 Clone 7D5, Neo Markers, Fremont, CA) at 4°C. After overnight blocking with 1 % protease-free BSA and 5 % sucrose, 100 μl of sample or standard were incubated overnight at 4°C. 100 μl detection antibody (200 ng/ml Heregulin/Neuregulin Ab-2, biotin-labeled, Neomarkers), solved in PBS with 1 % protease-free BSA and 2 % normal goat serum was added for two hours. Cells were incubated in the dark in 50 μl Streptavidin HRP for 20 minutes followed by 100 μl substrate solution for 20 minutes. Substrate reaction was stopped by adding 50 μl 2N H2SO4. Photometric extinction was measured at 450 nm vs 570 nm. NRG1 concentration was calculated based on 1mg of total protein and presented as % of controls.
RT-Real-time-PCR
Total RNA was isolated using a guanidinium-phenol based extraction procedure (Abgene, Hamburg, Germany). 1 μg of total RNA was subjected to reverse transcription using a First-Strand cDNA Synthesis Kit (GE Healthcare). Real-time PCR was performed using a commercially available primer assay for NRG1 and a SYBR Green Master Mix (Qiagen, Hilden, Germany) following the manufacturer's protocol with 40 cycles and an annealing at 60°C on a Sequence Detection System 7000 (Applied Biosystems). β-actin served as the housekeeping gene and was amplified on the same plate using primers 5′-AGATGACCCAGATCATGTTTGAG-3′ and 5′-GAGTCCATCACGATGCCAGTG -3′. We calculated means and DCt values using the ABI PRISM 7000 SDS software (Applied Biosystems, Darmstadt, Germany) for relative quantification. Corresponding control values were subtracted from treated samples to calculate DDCt values. A negative result indicates a higher NRG1 gene expression in the treated sample compared to the respective untreated control.
Statistics
P-values were calculated using the two-tailed t-test for equal variances. Equality of variances was tested by Levene Test. Potential associations between genotypes, antenatal characteristics, and clinical outcome were calculated using logistic regression and the one-sided Cochran-Armitage test for trend (publicly available syntax was obtained from http://listserv.uga.edu/). All analyses were performed with SPSS 15.0 (SPSS Inc., 2006).
Study population
We evaluated 54 children < 32 weeks gestation from the Developmental Follow Up Program at Hannover Medical School (Hannover, Germany). Children were at least two years of age. A comprehensive physical examination and the Denver developmental screening (DDST) II test were administered by one paediatrician. Clinical data were abstracted from medical charts. Peripheral lymphocytes were obtained from whole blood samples for SNP8NRG221533 genotyping as described above.
The study was approved by the institutional review board at Hannover Medical School. The parents of all study participants gave their written informed consent. Clinical data were extracted from mothers' and infants' medical records.
Outcome measures and potential confounders (Dordelmann, Kerk et al. 2006)
Cystic periventricular leukomalacia (cPVL) was defined by echolucent areas in the white matter at any time, location, and of any extent. Cerebral palsy (CP) was defined as abnormal control of movement or posture and not as a result of progressive disease (Kuban and Leviton 1994). Developmental delay (DD) was defined at ≥2 years of age corrected for prematurity as a delay ≥12 months on at least one of the 4 Denver Developmental Screening Test (DDST) II subscales (Frankenburg, Dodds et al. 1992). Examinations were performed by the same pediatrician.
Gestational age was recorded as completed weeks based on the last menstrual period and on early ultrasound examinations during pregnancy. Children whose birth weight had been below the 10th percentile compared to gestation-, sex-, singleton-/multiple-specific German reference data (Voigt, Schneider et al. 1996) were defined as being small for gestational age (SGA). Prenatal glucocorticoid exposure was defined as complete when two doses of betamethasone were administered at a 24-hour interval, with the first dose >48 h prior to delivery. We reported whether the mother had ruptures of membranes (ROM) >12 hours, preterm labour (PTL) unresponsive to tocolytic therapy, or histological or clinical chorioamnionitis (CAM: white blood cells ≥15 × 103/μl, C-reactive protein ≥20 mg/dL, pyrexia ≥38°C, uterine pain or tenderness). One infant was randomly chosen out of each multiple pregnancy.
Data Analysis
We report univariable and multivariable relationships between clinical variables, SNPs and outcomes in terms of odds ratios (ORs) and their 95% confidence intervals (CIs). In cases where we found an association with an OR whose CI excluded the null (i.e., 1.0) we still considered it worthwhile reporting this result. However, in constellations where the CI included the null, we do not believe that our study bears the potential of proving the absence of an association.
As the multifactorial pathogenesis of our clinical outcomes is widely known, we first performed univariable analyses to identify potential associations between antenatal characteristics and both the SNP and clinical outcomes. In a second step we utilized logistic regression analyzes adjusting for NRG1 genotype and one potential confounding factor at a time.
Results
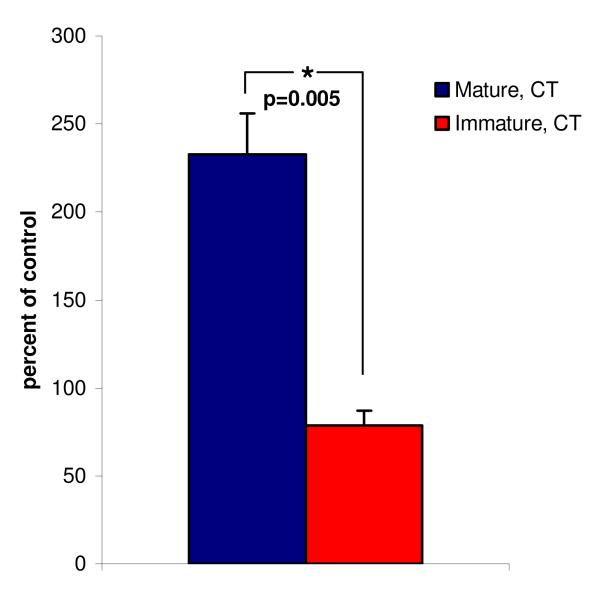
NRG1 protein levels after LPS exposure (Figure 1)
Figure 1. NRG1 protein concentration in human umbilical vein endothelial cells (HUVECs) from mature compared with premature newborns.
Mean intracellular concentration of NRG1 protein in HUVECs from term (mature) (N=2) vs. preterm (immature) newborns (N=3). All HUVECs carried the same genotype (CT) at the SNP8NRG221533 locus. NRG1 concentration was measured by ELISA after 24 hours of LPS-treatment (100ng/ml) and is presented as percent of the experiment specific non-treated control cells. Error bars indicate standard errors of the mean (SEM).
Mature and immature HUVECs responded significantly different to 24 hours of LPS-treatment. Treated mature cells seemed to increase their intracellular NRG1 protein expression about two-fold, to a mean of 233± 23% (mean±SEM, N=2, p=0.11) of control cells, while NRG1 protein expression was slightly reduced in immature cells to 79±8% (mean±SEM, N= 3, p=0.13) of control cells. The difference between mature and immature cells was statistically significant (p=0.005). Despite different basal expression levels both immature (144±5% of control, mean±SEM, N= 3, p=0.01) and mature HUVECs (167±50%, mean±SEM, N= 2, p=0.41) seemed to increase NRG1 secretion into their supernatants in response to 24 hours of LPS exposure.
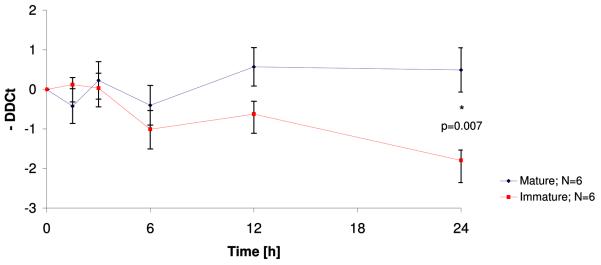
Both maturity and genotype affect NRG1-mRNA-levels (Figure 2)
Figure 2. Relative NRG1 mRNA concentrations in HUVECs dependent on gestational age (2.1) and stratified by NRG1 genotype (2.2).
NRG1 mRNA concentration was measured by real time RT-PCR. Data are presented as mean DDCts±SEM. Cells were treated with LPS (100ng/ml) for 1.5, 3, 6, 12 and 24 hours. DDCts were calculated as the difference between treated sample and their non-treated experiment-specific control cells with a negative result in case of a lead in crossing the threshold line thereby indicating more NRG1 mRNA within the raw material.
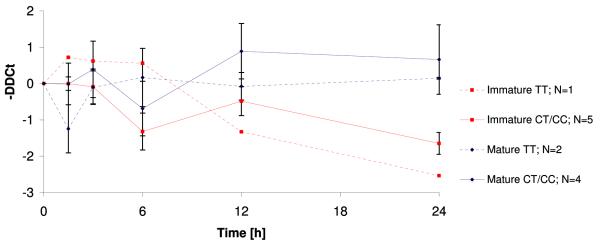
We confirmed that HUVECs do indeed produce NRG1 mRNA transcripts. Similar to protein levels, NRG1 expression was significantly higher in mature cells than in cells from immature infants after 24 hours of LPS exposure (Fig. 2.1). The difference in NRG1 gene expression between mature and immature cells became more prominent with increasing duration of LPS exposure and reached nominal statistical significance (N=12, p=0.007) after 24 hours. At that time point, LPS-treated mature cells surpassed their controls by 0.5±0.6 cycles (mean DDCt±SEM, N=6, p=0.36) whereas LPS-treated immature cells exceeded the threshold almost two PCR cycles later (mean DDCt±SEM =1.8±0.3, N=6, p=0.006) than untreated controls which is consistent with an about three-fold lower mRNA abundance. Stratification by SNP8NRG221533 genotype revealed that cells with at least one C-allele seemed to express more NRG1 mRNA than TT homozygous cells in both immature and mature cells (Fig.2.2).
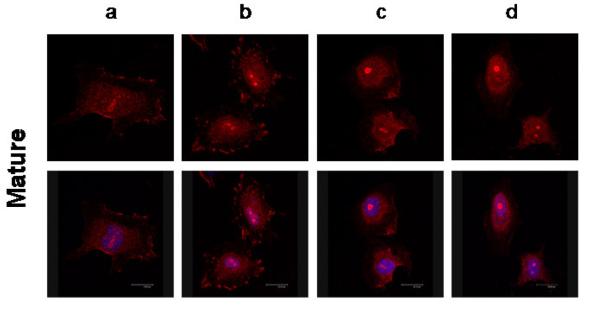
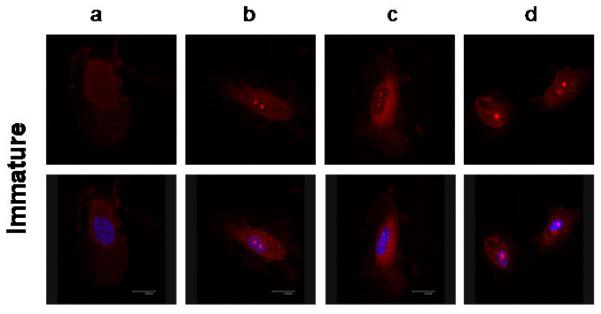
NRG1-localization in HUVECs in response to LPS-treatment (Figure 3)
Figure 3. Nuclear localization of NRG1 in human umbilical venous endothelial cells (HUVECs) in response to LPS-treatment.
Cellular localization of NRG1 (red) in mature (3.1) and immature (3.2) HUVECs after 30 minutes (a) and 24 hours (c) serum starvation and equivalent durations of LPS-treatment (b, d). The nucleus marked by DAPI is indicated by blue color in the bottom rows.
Neuregulin-1 was diffusely expressed in the cytoplasm of untreated serum-starved mature and immature HUVECs (Fig. 3.1a and 3.2a). Mature cells also showed remarkable membrane NRG1 enrichment after serum starvation (Fig. 3.1a) and LPS-treatment (Fig. 3.1b). Over time, NRG1 seemed to accumulate in the nucleus (Fig. 3.1d) with a loss of membrane staining in LPS-treated cells. In immature HUVECs, NRG1 localization was more diffuse in and around the nuclear region with only very little membrane staining in the untreated cells (Fig. 3.2a and c). The localization in the cell membrane disappeared completely after LPS-treatment (Fig. 3.2d). With increasing duration of LPS-exposure, nuclear NRG1 localization increased in immature cells (Fig. 3.2c). LPS-treatment appeared to amplify this effect with special enhancement in the nucleoli in both gestational ages (Fig. 3.1d and 3.2d).
Human study population characteristics (Table 1)
Table 1.
Characteristics of the study population. Data are given for all children and for groups defined by NRG1 genotype CC/CT vs. TT, and the presence or absence of PVL, CP and DD >= 12 months. If not indicated otherwise, numbers are column percents. Variables tested as potential confounding factors are marked in grey.
|
All children |
SNP8NRG221533 genotype |
Cystic Periventricular Leukomalacia |
Cerebral Palsy |
Developmental Delay ≥ 12 months |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=54 |
CC, CT N=42 |
TT N=12 |
OR (CI 95%) |
Yes N=10 |
No N=44 |
OR (CI 95%) |
Yes N=5 |
No N=49 |
OR (CI 95%) |
Yes N=9 |
No N=45 |
OR (CI 95%) |
|
| % | % | % | % | % | % | % | % | % | |||||
| NRG1 CC/CT | - | - | - | 70 | 80 | 0.6 (0.1-2.8) | 40 | 82 | 0.2 (0.02-1.0) | 56 | 82 | 0.3 (0.1-1.2) | |
| Gestation < 30 wks | 63 | 62 | 67 | 0.8 (0.2-3.1) | 90 | 57 | 6.8 (0.8-59) | 80 | 61 | 2.5 (0.3-24) | 89 | 58 | 5.9 (0.7-51) |
| Birth weight < 1000g | 44 | 48 | 33 | 1.8 (0.5-7.0) | 70 | 39 | 3.7 (0.8-16) | 80 | 41 | 5.8 (0.6-56) | 67 | 40 | 3.0 (0.7-14) |
| Small for gestational age | 22 | 24 | 17 | 1.6 (0.3-8.4) | 20 | 23 | 0.9 (0.2-4.7) | 40 | 20 | 2.6 (0.4-18) | 33 | 20 | 2.0 (0.4-9.6) |
| Male | 50 | 45 | 67 | 0.4 (0.1-1.6) | 60 | 48 | 1.6 (0.4-6.6) | 60 | 49 | 1.6 (0.2-10) | 67 | 47 | 2.3 (0.5-10) |
| Mother German | 72 | 69 | 83 | 0.5 (0.1-2.3) | 60 | 75 | 0.5 (0.1-2.1) | 100 | 69 | - | 78 | 71 | 1.4 (0.3-7.8) |
| Multiple pregnancy | 17 | 14 | 25 | 0.5 (0.1-2.4) | 10 | 18 | 0.5 (0.1-4.5) | 20 | 16 | 1.3 (0.1-13) | 11 | 18 | 0.6 (0.1-5.3) |
| Prenatal glucocorticoid, complete |
81 | 76 | 100 | - | 70 | 84 | 0.4 (0.1-2.1) | 80 | 82 | 0.9 (0.1-9.1) | 78 | 82 | 0.8 (0.1-4.3) |
| Pregnancy induced hypertension |
19 | 19 | 17 | 1.2 (0.2-6.5) | 20 | 18 | 1.1 (0.2-6.3) | 20 | 18 | 1.1 (0.1-11) | 11 | 20 | 0.5 (0.1-4.5) |
| Preterm labor | 65 | 60 | 83 | 0.3 (0.1-1.5) | 80 | 61 | 2.5 (0.5-13) | 80 | 63 | 2.3 (0.2-22) | 89 | 60 | 5.3 (0.6-46) |
| Rupture of membranes >12hrs |
22 | 24 | 17 | 1.6 (0.3-8.4) | 30 | 20 | 1.7 (0.4-7.8) | 20 | 22 | 0.9 (0.1-8.5) | 22 | 22 | 1.0 (0.2-5.6) |
| Chorioamnionitis | 30 | 29 | 33 | 0.8 (0.2-3.2) | 60 | 23 | 5.1 (1.2-22) | 20 | 31 | 0.6 (0.1-5.5) | 44 | 27 | 2.2 (0.5-9.6) |
Among the 54 children born before 32 weeks of gestation, forty-two (77%) infants had at least one C-allele at the SNP8NRG221533 gene locus (CC: N=6; CT: N=36; TT: N=12) (Table 1). There were no significant associations between perinatal risk factors and SNP8NRG221533 genotype, although infants carrying at least one C-allele tended to be less likely to be born after preterm labor (60%) than those with two T-alleles (83%), and were also less likely to be male (45% vs. 67%).
Ten infants (19%) had cystic periventricular leukomalacia (cPVL), 5 (9%) had cerebral palsy (CP), and 9 (17%) had developmental delay ≥12 months (DD). Low gestational age and birth weight were the strongest risk factors for all three outcomes. Only male gender, preterm labor and chorioamnionitis appeared to be potential confounders of the observed relationship between SNP8NRG221533 genotype and outcomes by virtue of being associated with both.
SNP8NRG221533 and reduced risk for neurodisability (Table 2)
Table 2.
Associations between SNP8NRG221533 genotypes and neurologic outcome.
| SNP8NRG221533 genotype | ||||||
|---|---|---|---|---|---|---|
| CC | CT | TT | Total | |||
| n | n | n | N | p | ||
| Cystic Periventricular Leukomalacia | yes | 0 | 7 | 3 | 10 | 0.121 |
| no | 6 | 29 | 9 | 44 | ||
| Cerebral Palsy | yes | 0 | 2 | 3 | 5 | 0.021 |
| no | 6 | 34 | 9 | 49 | ||
| Developmental Delay ≥ 12 months | yes | 0 | 5 | 4 | 9 | 0.027 |
| no | 6 | 31 | 8 | 45 | ||
The 42 children who were homo- or heterozygous for the C-allele had lower percentages of adverse clinical outcomes compared to those with a TT genotype. Among those homozygous for the C-allele, all outcomes were absent. A one-sided Cochran-Armitage test revealed a significant trend with p=0.02 for CP and p=0.03 for DD (Table 2) suggesting a linear relationship between the number of C-alleles and neurological outcome. Adjustment for single confounders did not result in a reduction of the effect of the SNP8NRG221533 genotype on outcome observed in univariable analyses (Table 3). We considered our dataset too small to adjust for more than one variable at a time.
Table 3.
Risk analyses for development of neurodevelopmental outcome using SNP8NRG221533 (CC/CT vs TT) and one confounder at a time
|
Cystic Periventricular Leukomalacia (n=10) |
Cerebral Palsy (n=5) |
Developmental Delay ≥ 12 months (n=9) |
||||
|---|---|---|---|---|---|---|
| OR | (CI 95 %) | OR | (CI 95 %) | OR | (CI 95 %) | |
| SNP8NRG221533 (CC/CT vs TT) | 0.6 | (0.1-3.1) | 0.2 | (0.02-1.1) | 0.3 | (0.1-1.3) |
| Gestational age < 30 wks | 6.8 | (0.8-58) | 2.5 | (0.2-26) | 6.0 | (0.7-54) |
| SNP8NRG221533 (CC/CT vs TT) | 0.5 | (0.1-2.4) | 0.1 | (0.01-0.8) | 0.2 | (0.04-1.0) |
| Birth weight < 1000g | 4.1 | (0.9-19) | 11 | (0.9-140) | 4.1 | (0.8-22) |
| SNP8NRG221533 (CC/CT vs TT) | 0.7 | (0.1-3.1) | 0.2 | (0.02-1.1) | 0.3 | (0.1-1.4) |
| Male | 1.5 | (0.4-6.4) | 1.1 | (0.2-8.1) | 1.9 | (0.4-9.0) |
| SNP8NRG221533 (CC/CT vs TT) | 0.7 | (0.2-3.5) | 0.2 | (0.02-1.2) | 0.4 | (0.1-1.6) |
| Preterm labor | 2.4 | (0.4-13) | 1.5 | (0.1-17) | 4.4 | (0.5-39) |
| SNP8NRG221533 (CC/CT vs TT) | 0.6 | (0.1-3.2) | 0.1 | (0.02-1.0) | 0.3 | (0.1-1.3) |
| Chorioamnionitis | 5.1 | (1.2-22) | 0.5 | (0.1-5.2) | 2.2 | (0.5-10) |
Discussion
The goal of our study was to explore a potentially protective role for NRG1 in neonatal brain damage (Dammann, Bueter et al. 2007) based on experimental evidence from other groups obtained in several damage models (Xu, Jiang et al. 2004; Xu, Ford et al. 2005; Li, Xu et al. 2007; Lok, Wang et al. 2007). Our results suggest that NRG1 is expressed and might even be systemically released by human umbilical venous endothelial cells in response to LPS challenge. We found support for a dose-response-relationship between the amount of NRG1 expression and (1) maturity level of cells and (2) the presence of the C-allele of SNP8NRG221533. In preterm infants, the presence of at least one C-allele was associated with a reduced risk for adverse neurologic outcome.
Among candidates for endogenous protectors are growth factors that are “developmentally regulated”, i.e., they are more abundant in term than in preterm newborns (Dammann and Leviton 1999). This concept helps explain the prominently increased risk for brain damage among preterm infants by postulating a decreased availability of protectors.
To qualify as a perinatal endogenous protector, NRG1 first of all needs to be expressed at the human systemic level. Our present and previous (Bueter, Dammann et al. 2006) results suggest that NRG1 is expressed by human endothelial cells as early as mid-gestation.
Second, we would expect a developmentally-regulated endogenous protector to increase with (a) advanced gestational age and (b) after exposure to potential adverse stimuli. Indeed, we found support for both these hypotheses (Figures 1 and 2).
NRG1 plays major roles in lung (Dammann, Nielsen et al. 2003) and brain (Esper, Pankonin et al. 2006) development. We therefore wanted to begin exploring the possibility that NRG1 is involved in inflammation-related processes in preterm and term settings. Although studies on LPS-induced genes in HUVECs are available (Ahn, Choe et al. 2003; Dauphinee and Karsan 2006), our study is the first to show that LPS-exposure appears to influence NRG1 expression in HUVECs. Our failure to detect a prominent response to LPS of immature cells might be part of the reason why preterm infants are at increased risk for inflammation-associated organ damage (Dammann, Leviton et al. 2005). Certainly, we have to take into account that these results might be affected by independent events besides the maturity level of the cells. Indeed we did not have access to the medical history of umbilical cord donors and the etiology of preterm delivery. Nevertheless we eliminated the possibility of acute inflammation as the cause for preterm delivery, by excluding umbilical cords with histological signs of funisitis to prevent result biasing by activated endothelial cells. Taken together, our findings further support the contention that the concept of developmental regulation observed for a multitude of proteins (Dammann and Leviton 1999) might also apply to NRG1.
While no clear trends were present during the first hours of LPS-exposure (Fig. 2.1), the difference between immature and mature cells regarding mRNA production started increasing after 3 hours, reaching nominal statistical significance at 24 hours. Although immature cells might have a similar capacity of withstanding inflammatory challenge during the first hours, they seemed to “burn out” after 5-6 hours, while mature cells continued producing NRG1. Thus, although immature cells seem to be able to upregulate NRG1 mRNA production, they do not fully respond to demand by sustaining protein production.
Perinatal infection and inflammation appear to play a role in both, neonatal brain damage (Dammann and Leviton 2004) and in the etiology of adult neurological diseases such as schizophrenia (Brown 2006) or Parkinson's disease (Ling, Zhu et al. 2006; Snyder-Keller and Stark 2008). Others have identified potential interactions between pro-inflammatory and NRG1 SNPs in schizophrenia (Hanninen, Katila et al. 2007). We hypothesized that, if genetic polymorphisms of NRG1 were associated with differential gene expression and systemic availability of NRG1 protein, they might also be associated with an altered risk for neurodevelopmental outcome in preterm infants. Our results indicate that both immature and mature cells with at least one SNP8NRG221533 C-allele seem to be capable of producing higher quantities of NRG1 mRNA than cells with the homozygous TT-genotype (Fig. 2.2). Therefore, we explored in a small clinical population of children born before 32 weeks of gestation the relationship between SNP8NRG221533, one of the multiple SNPs in the NRG1 gene associated with schizophrenia (Li, Collier et al. 2006; Norton, Williams et al. 2006), and clinical outcome (Table 1). We found that, while the schizophrenia literature indicates a risk increase with the C-allele in the SNP8NRG221533 locus, our results suggest a decrease in neonatal brain damage and developmental long-term problems.
What might explain this “development-disease-paradox”? While we speculate that increased NRG1 levels (and the SNP8NRG221533 allele) protect the preterm newborn's brain by compensating for maturition deficits, increased NRG1 levels in the prefrontal cortex are associated with schizophrenia (Chong, Thompson et al. 2008). We propose that the function of growth factors such as NRG1 might change over time, just like cell proliferation is of critical importance during normal fetal development and also contributes to disease processes in aging cells (e.g., tumor growth). Two major parts in cell biology, namely chronic ER-stress induced changes in unfolding protein response (Naidoo, RevNeuro Sci 2009) and aging induced changes in autophagy (Salminen et Kaarmiranta 2008, Trends Mol Med 2009) might be major players in these events. Another explanation of this paradox its that it is a chance finding. However, the possibility that the finding is real deserves further investigation.
Interestingly, the C allele that appeared neuroprotective in our case-only study of preterm newborns, has recently been associated with an increased frontal brain activation in a working memory task of healthy individuals (Krug, Markov et al. 2008). If NRG1 was indeed neuroprotective, further studies are warranted to elucidate how exactly the protective mechanism might look like. For example, experiments are needed that incorporate the effects of inflammation on the developing NRG-system in concert with, e.g., the developing oligodendrocyte (Sussman, Vartanian et al. 2005), one of the key cellular components of the developing brain white matter that is likely to be a target for inflammatory challenge (Pang, Zheng et al. 2007).
It is well-established that neuregulins act locally and in a paracrine manner (Meyer and Birchmeier 1995). The overall hypothesis of our study, however, was that NRG1 might play a role as a responder to inflammation on the systemic level. In earlier studies, NRG1 expression had been identified in cardiac endothelium (Carraway, Weber et al. 1997; Zhang, Sliwkowski et al. 1997; Zhao, Sawyer et al. 1998), brain white matter (Winterer, Konrad et al. 2008), and mesenchymal stem cells (Gui, Wang et al. 2007). More recently, Neuregulin-1 mRNA, protein expression, and activity towards the receptors erbB2 and erbB3 were detected in brain microvascular endothelial cells (BMECs), and evidence for a cytoprotective effect of NRG1 in this cell type has been presented (Lok, Sardi et al. 2009).
We previously found that expression of erbB receptors for NRG in HUVECs was altered by stimulation with NRG1 (Bueter, Dammann et al. 2006). We therefore hypothesized that HUVECs themselves should be capable of expressing the NRG1 protein. Indeed, we found support for this using both ELISA techniques (Fig. 1) and confocal microscopy (Fig. 3). Our previous (Bueter, Dammann et al. 2006) findings also indicate that nuclear shifting of erbB4, another one of the NRG1 receptors, after NRG1 stimulation is amplified by LPS-treatment. We speculate that NRG1 uses the erbB4 receptor for nuclear transport, since erbB4 contains a nuclear localization signal. During systemic inflammation the NRG1-erbB4 complex might play a central role in the cellular immune response, just as erbB2, an orphan receptor of the erbB receptor family, appears to play a role in interleukin-6 signalling (Qiu, Ravi et al. 1998).
In summary, our data suggest that carrying at least one C-allele of the SNP8NRG221533 might lead to an increase in NRG1 gene expression in human umbilical venous endothelial cells, that exposure to pro-inflammatory stimuli further increases this effect, and that NRG1 might be a systemic endogenous neuroprotector in preterm newborns. Future research should include studies that correlate systemic NRG1 level in fetuses/newborns with both, fetal and maternal genotypes and neurologic outcomes.
Ackowledgements
Our work was generously supported by the Deutsche Forschungsgemeinschaft (Da378-3/1), the European Union (LSHM -CT-2006-036534) and National Institutes of Health (NIH HL-04436).
Abbreviations
- BPD
Bronchopulmonary dysplasia
- CAM
Chorioamnionitis
- CI
Confidence interval
- CLD
Chronic lung disease
- CP
Cerebral palsy
- cPVL
Cystic periventricular leukomalacia
- DD
Developmental delay
- NRG
Neuregulin
- OR
Odds ratio
- PTL
Preterm labor
- PVE
Periventricular echodensity
- ROM
Rupture of membranes
- SGA
Small for gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn SK, Choe TB, et al. The gene expression profile of human umbilical vein endothelial cells stimulated with lipopolysaccharide using cDNA microarray analysis. Int J Mol Med. 2003;12(2):231–6. [PubMed] [Google Scholar]
- Bernstein HG, Lendeckel U, et al. Localization of neuregulin-1alpha (heregulin-alpha) and one of its receptors, ErbB-4 tyrosine kinase, in developing and adult human brain. Brain Res Bull. 2006;69(5):546–59. doi: 10.1016/j.brainresbull.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32(2):200–2. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueter W, Dammann O, et al. ErbB receptors in fetal endothelium-A potential linkage point for inflammation-associated neonatal disorders. Cytokine. 2006;36(5-6):267–75. doi: 10.1016/j.cyto.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway KL, 3rd, Weber JL, et al. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387(6632):512–6. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- Chong VZ, Thompson M, et al. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr Res. 2008;100(1-3):270–80. doi: 10.1016/j.schres.2007.12.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann CE, Nielsen HC, et al. Role of neuregulin-1 beta in the developing lung. Am J Respir Crit Care Med. 2003;167(12):1711–6. doi: 10.1164/rccm.200205-468OC. [DOI] [PubMed] [Google Scholar]
- Dammann O, Allred EN, et al. Fetal vasculitis in preterm newborns: interrelationships, modifiers, and antecedents. Placenta. 2004;25(10):788–96. doi: 10.1016/j.placenta.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Dammann O, Brinkhaus MJ, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: A multi-hit hypothesis. Early Hum Dev. 2009 doi: 10.1016/j.earlhumdev.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Dammann O, Bueter W, et al. Neuregulin-1: A Potential Endogenous Protector in Perinatal Brain White Matter Damage. Neonatology. 2007;93(3):182–187. doi: 10.1159/000111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Brain damage in preterm newborns: might enhancement of developmentally regulated endogenous protection open a door for prevention? Pediatrics. 1999;104(3 Pt 1):541–50. doi: 10.1542/peds.104.3.541. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Inflammatory brain damage in preterm newborns--dry numbers, wet lab, and causal inferences. Early Hum Dev. 2004;79(1):1–15. doi: 10.1016/j.earlhumdev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A, et al. Lung and brain damage in preterm newborns, and their association with gestational age, prematurity subgroup, infection/inflammation and long term outcome. Bjog. 2005;112(Suppl 1):4–9. doi: 10.1111/j.1471-0528.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86(1):9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- Deadwyler GD, Pouly S, et al. Neuregulins and erbB receptor expression in adult human oligodendrocytes. Glia. 2000;32(3):304–12. [PubMed] [Google Scholar]
- Dordelmann M, Kerk J, et al. Interleukin-10 high producer allele and ultrasound-defined periventricular white matter abnormalities in preterm infants: a preliminary study. Neuropediatrics. 2006;37(3):130–6. doi: 10.1055/s-2006-924554. [DOI] [PubMed] [Google Scholar]
- Dudley DJ. Pre-term labor: an intra-uterine inflammatory response syndrome? J Reprod Immunol. 1997;36(1-2):93–109. doi: 10.1016/s0165-0378(97)00065-x. [DOI] [PubMed] [Google Scholar]
- Esper RM, Pankonin MS, et al. Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev. 2006;51(2):161–75. doi: 10.1016/j.brainresrev.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284(1):14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351(19):1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Frankenburg WK, Dodds J, et al. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89(1):91–7. [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378(6555):390–4. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, et al. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Mol Cell Neurosci. 2004;27(4):379–93. doi: 10.1016/j.mcn.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Gui C, Wang JA, et al. Heregulin protects mesenchymal stem cells from serum deprivation and hypoxia-induced apoptosis. Mol Cell Biochem. 2007;305(1-2):171–8. doi: 10.1007/s11010-007-9541-3. [DOI] [PubMed] [Google Scholar]
- Hanninen K, Katila H, et al. Interleukin-1 beta gene polymorphism and its interactions with neuregulin-1 gene polymorphism are associated with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007 doi: 10.1007/s00406-007-0756-9. [DOI] [PubMed] [Google Scholar]
- Krug A, Markov V, et al. Genetic variation in the schizophrenia-risk gene neuregulin1 correlates with differences in frontal brain activation in a working memory task in healthy individuals. Neuroimage. 2008;42(4):1569–76. doi: 10.1016/j.neuroimage.2008.05.058. [DOI] [PubMed] [Google Scholar]
- Kuban KC, Leviton A. Cerebral palsy. N Engl J Med. 1994;330(3):188–95. doi: 10.1056/NEJM199401203300308. [DOI] [PubMed] [Google Scholar]
- Li D, Collier DA, et al. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15(12):1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu Z, et al. Neuroprotection by neuregulin-1 in a rat model of permanent focal cerebral ischemia. Brain Res. 2007;1184:277–83. doi: 10.1016/j.brainres.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Zhu Y, et al. Progressive dopamine neuron loss following supranigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006;199(2):499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Lok J, Sardi SP, et al. Neuregulin-1 signaling in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29(1):39–43. doi: 10.1038/jcbfm.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J, Wang H, et al. Effect of neuregulin-1 on histopathological and functional outcome after controlled cortical impact in mice. J Neurotrauma. 2007;24(12):1817–22. doi: 10.1089/neu.2007.0372. [DOI] [PubMed] [Google Scholar]
- Magder S, Neculcea J, et al. Lipopolysaccharide and TNF-alpha produce very similar changes in gene expression in human endothelial cells. J Vasc Res. 2006;43(5):447–61. doi: 10.1159/000095162. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378(6555):386–90. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, et al. Isoform-specific expression and function of neuregulin. Development. 1997;124(18):3575–86. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Norton N, Williams HJ, et al. An update on the genetics of schizophrenia. Curr Opin Psychiatry. 2006;19(2):158–64. doi: 10.1097/01.yco.0000214341.52249.59. [DOI] [PubMed] [Google Scholar]
- Padmakumar VC, Abraham S, et al. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res. 2004;295(2):330–9. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Pang Y, Zheng B, et al. IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55(11):1099–107. doi: 10.1002/glia.20530. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Ravi L, et al. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature. 1998;393(6680):83–5. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, et al. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–26. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Keller A, Stark PF. Prenatal inflammatory effects on nigrostriatal development in organotypic cultures. Brain Res. 2008;1233:160–7. doi: 10.1016/j.brainres.2008.07.106. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sarginson J, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72(1):83–7. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71(4):877–92. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens BE, Vohr BR. Neurodevelopmental outcome of the premature infant. Pediatr Clin North Am. 2009;56(3):631–46. doi: 10.1016/j.pcl.2009.03.005. Table of Contents. [DOI] [PubMed] [Google Scholar]
- Sussman CR, Vartanian T, et al. The ErbB4 neuregulin receptor mediates suppression of oligodendrocyte maturation. J Neurosci. 2005;25(24):5757–62. doi: 10.1523/JNEUROSCI.4748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt M, Schneider KT, et al. [Analysis of a 1992 birth sample in Germany. 1: New percentile values of the body weight of newborn infants] Geburtshilfe Frauenheilkd. 1996;56(10):550–8. doi: 10.1055/s-2007-1023283. [DOI] [PubMed] [Google Scholar]
- Wen D, Suggs SV, et al. Structural and functional aspects of the multiplicity of Neu differentiation factors. Mol Cell Biol. 1994;14(3):1909–19. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Konrad A, et al. Association of 5′ end neuregulin-1 (NRG1) gene variation with subcortical medial frontal microstructure in humans. Neuroimage. 2008;40(2):712–8. doi: 10.1016/j.neuroimage.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Xu Z, Ford GD, et al. Neuroprotection by neuregulin-1 following focal stroke is associated with the attenuation of ischemia-induced pro-inflammatory and stress gene expression. Neurobiol Dis. 2005;19(3):461–70. doi: 10.1016/j.nbd.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Xu Z, Jiang J, et al. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophys Res Commun. 2004;322(2):440–6. doi: 10.1016/j.bbrc.2004.07.149. [DOI] [PubMed] [Google Scholar]
- Zhang D, Sliwkowski MX, et al. Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci U S A. 1997;94(18):9562–7. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Sawyer DR, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273(17):10261–9. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]