Abstract
Objective
– To evaluate the efficacy at 6-, 12-, and 24-month follow-up of Keep Active Minnesota (KAM), a telephone and mail-based intervention designed to promote physical activity (PA) maintenance among currently active adults age 50 to 70.
Method
- Participants who reported having recently increased their MVPA to a minimum of 2d/wk, 30 mins/bout, (N=1,049) were recruited in 2004 and 2005 from one large managed care organization in Minnesota, and randomly assigned to either treatment (KAM; N=523), or Usual Care (UC; N=526) with PA assessed using the CHAMPS questionnaire, and expressed as kcal/wk energy expenditure.
Results
– We find a sustained, significant benefit of the intervention at 6, 12 and 24 months. Kcal/wk expenditure in moderate or vigorous activities was higher at 6 (p<.03, Cohen’s d6m = .16), 12 (p<.04, d12m = .13) and 24 months (p<.01, d24m = .16) for KAM participants, compared to UC participants.
Conclusions
- The KAM telephone- and mail-based PA maintenance intervention was effective at maintaining PA in both the short-term (6 months) and longer-term (12 and 24 months) relative to usual care.
Keywords: intervention studies, exercise/physical activity behavioral research, health maintenance organizations, randomized controlled trial
Introduction
Substantial evidence documents the health benefits of regular physical activity (PA) (Physical Activity Guidelines Advisory Committee, 2008). Many of the beneficial effects of PA are particularly salient for mid-life and older adult populations (Angevaren, et al., 2008, Blair, et al., 1992, Brach, et al., 2004, Colbert, et al., 2004, Cox, et al., 2004, Elavsky, et al., 2005, Feskanich, et al., 2002, Hughes, et al., 2004, King, et al., 2008, Liu-Ambrose, et al., 2008, Lord, et al., 1995, Mayer-Davis, et al., 1998, McAuley, et al., 2006, Pescatello, et al., 2004, Tuomilehto, et al., 2001, Vallance, et al., 2007, Weuve, et al., 2004). Unfortunately, mid-life and older adults in the U.S. remain relatively sedentary. The 2007 Behavioral Risk Factor Surveillance System (BRFSS) documents that, more than half of adults age 45–54 years (52%) were obtaining less than recommended levels of PA, with the same being true for 53% of adults age 55–64 years and for 61% of adults ages 65 and over (Centers for Disease Control and Prevention, 2007). To increase US population PA levels, health plans and public health policy makers are seeking low-cost intervention strategies that produce long-term behavior changes and have potential to reach a broad spectrum of the population.
Complementary routes to reach national PA goals include increasing the number of sedentary individuals who initiate PA and increasing the long term maintenance of beneficial levels of PA. This is underscored by evidence from a number of PA intervention programs targeted to midlife and older adults that sustaining recommended PA levels is difficult for this population. Attrition rates in the first year of such studies range from about 27% to 50% (Jacobsen, et al., 2003, Jancey, et al., 2007, Prohaska, et al., 2000, Schmidt, et al., 2000, Tu, et al., 2004) with the most rapid attrition typically occurring within the first three months (Jancey, et al., 2007, Schmidt, et al., 2000, Tu, et al., 2004). These data, coupled with the observation that prevalence of sedentary behavior increases with age, (Centers for Disease Control and Prevention, 2005) suggest that population levels of PA may be substantially increased by preventing the currently active, particularly those with recently increased PA levels, from becoming sedentary.
The optimal intervention delivery method is also an important question. The efficacy of clinic-based approaches to increasing PA is equivocal (Bull, et al., 1999, Eaton and Menard, 1998, Goldstein, et al., 1999, The Writing Group for the Activity Counseling Trial Research, 2001, Walsh, et al., 1999). More recent studies linking brief primary care based advice/counseling with referrals to telephone-based counseling are more promising (Anderson, et al., 2005, Harrison, et al., 2005, Kerse, et al., 2005, Pinto, et al., 2005, van Sluijs, et al., 2005). However, such approaches are relatively expensive, difficult to implement in busy practice settings, and have variable reach to community populations. A recent literature review documents that home- and group-based interventions can increase PA in the short-term, suggesting that community based interventions may be viable alternatives to clinic-based approaches and have greater potential for broad population reach (Van der Bij, et al., 2002).
We designed the Keep Active Minnesota (KAM) project to evaluate the efficacy of a population-based approach to promoting PA maintenance among currently active mid-life and older adults who reported an increase in PA within the past year. Participants were randomized to an interactive telephone and mail-based PA support program (KAM) or usual care (UC) and followed for a two year period. This report presents the results of the a priori study hypotheses:
Hypothesis 1
KAM intervention participants will maintain higher absolute estimated kcal energy expenditure from baseline to 6, 12, and 24 months relative to the kcal expenditure observed among the UC group.
Hypothesis 2
PA maintenance, defined as kcal expenditure at 6, 12, and 24 months relative to one’s baseline expenditure, will be higher among KAM participants than among the UC group.
Methods
Target Population
The study was conducted among 50–70 year old members of the HealthPartners health plan in the Minneapolis/St. Paul metropolitan area.
Eligibility, Sampling, and Recruitment
We have discussed eligibility, sampling and recruitment in detail elsewhere (Martinson, et al., 2008). Briefly, we used health plan administrative data to identify age-eligible members who had been enrolled in the health plan for at least 11 of the 12 months prior to eligibility screening. Recruitment was initiated through direct mailings to random samples of individuals not meeting study exclusion criteria based on initial clinical records review. We supplemented this direct mail approach with study advertisements to facilitate “self referrals” to the study. Following an initial phone-based eligibility screening, an institutionally approved consent form was mailed to interested individuals. When completed consent forms were received, a baseline telephone interview was scheduled, upon completion of which the subject was randomized to either the treatment or control arm. Recruitment occurred over 15 months from July to August 2004 and December 2004 through December 2005.
We considered as study-eligible those who reported accumulating at least 30 minutes of moderate or vigorous PA a day at least 2 days per week on average over the past four weeks, and who reported that this represented an increase in PA within the past 12 months. Individuals were excluded who had a modified Charlson comorbidity score > 3, a standard index of comorbidity calculated using prior year diagnoses of a broad range of serious medical conditions (Charlson, et al., 2008, Charlson, et al., 1994, Deyo, et al., 1992, Rush, et al., 2000), or had diagnoses of coronary heart disease (CHD), congestive heart failure (CHF), atrial or ventricular arrhythmias, cardiac arrest, or had an implantable defibrillator.
Design
The study coordinator randomized 1,049 subjects to either the PA treatment condition (KAM) or a usual care control group (UC). Subjects were allocated equally in blocks of 20 according to a schedule prepared by the study statistician using a random numbers table and unobservable to the study coordinator. All participants self-reported their PA levels at baseline and at 6, 12, and 24 month follow-ups. Primary study outcomes were PA expressed as estimated kcal/wk of energy expenditure and maintenance of PA levels relative to baseline. Telephone interviewers collecting self-report data were blind to study condition.
KAM Intervention Description
Participants randomized into the intervention were offered a 24-month interactive telephone and mail-based PA support program, based primarily on the principles of Social Cognitive Theory (SCT) (Bandura, 2004, Bandura, 1986) and relapse prevention theory (Marlatt and Gordon, 1985). Intervention strategies were weighted toward maintenance focused self-management, including cognitive (goal setting, identification of barriers, and problem solving), behavioral (self-monitoring using pedometers and log-books), and environmental (telephone coaching support, leveraging participants’ social support networks) strategies. The core component of the intervention was a seven session course delivered approximately bi-weekly over the telephone by PA coaches with exercise science backgrounds and training in behavior change theory. Participants then received monthly follow-up calls for the remainder of the first year and bi-monthly calls for the second year. Additional intervention components included motivational campaigns and a lending library of PA resources. Details of the intervention are provided in Table 1 with further details available elsewhere (Sherwood, et al., 2008).
Table 1.
Overview of all Keep Active Minnesota intervention contacts and topics addressed
| Orientation Session | Group in-person or individual phone session Introduce intervention team Review intervention goals |
| Core Phone Course | |
| Session 1: Getting Started | Baseline pedometer steps Individual PA history Individual PA benefits PA precautions Setting realistic goals |
| Session 2: Active Living | Tracking sedentary activity PA versus exercise How to maintain or increase PA Stretching and strength training |
| Session 3: Overcoming Barriers | Barriers to PA and exercise Problem solving to overcome barriers Strategies for staying active (e.g., successful recovery from illness & injury, dealing with pain, dressing for weather, keeping exercise gear handy, time management) |
| Session 4: Social Support | Involving friends and family in your active lifestyle When friends and family become barriers Community support for your active lifestyle |
| Session 5: Healthy Eating | How dietary intake can affect PA Tracking food and eating patterns Health advantages of colorful fruits and vegetables Eating patterns and PA Portion size, eating out Liquid calories |
| Session 6: Relapse Prevention | “Lapse” versus “relapse” Preparing for situations that could trigger a lapse Sress and its effect on relapse |
| Session 7: Putting It All Together | Update PA benefits Review PA goals Re-examine barriers to PA Assess support from family and friends Identify dietary changes Describe relapse prevention plans Reflect on success Plan future goals |
| Monthly calls (Month 5–12) | Review and revise PA goals Problem-solve barriers to exercise Provide encouragement for maintaining PA |
| Bi-monthly calls (Month 13–24) | Review and revise PA goals Problem-solve barriers to exercise Provide encouragement for maintaining PA |
| Motivational Campaigns | Optional “motivational c1hallenges” for participants to take part in Self-monitoring forms to record effort Small prizes for participation, for the most engaging story about a campaign experience, and for the recruitment “wave” with highest percentage of participants completing the campaign. |
| Walk the North Shore: a virtual walk to a popular and well-known Minnesota destination, through tracking of distances accumulated via pedometer steps or time spent walking or doing other PA | |
| Mix it Up to Keep it Up: try new “cross training” activities | |
| Multiply your Benefits: try new healthy eating and/or stress reduction activities | |
| Group Events | Four group sessions featuring outside guest-speakers during the second year of the study Topics included sports medicine, healthy eating, staying active in the winter and bicycling. |
| Lending Library | Resources (books, videos and DVD s) offered to participants who wanted to try a new type of exercise or in need of motivation |
Study conducted in participants recruited from one health plan in Minnesota during 2004 and 2005.
Usual Care Description
Participants randomized to the UC arm received information about the 10,000 steps PA program offered by the health plan and 4 newsletters focused on general health and wellness during their two years of study participation.
Measures
All outcome measures were collected during a 45-minute telephone interview administered prior to randomization (baseline) and 6, 12, and 24 months later.
Outcomes of Interest
The outcome variables of interest were kilocalories expended per week in a range of physical activity (AllPA kcal), and specifically in moderate and vigorous intensity activities (MVPA kcal), calculated at baseline and 6, 12, and 24 months, and maintenance of PA at 6, 12, and 24 months relative to baseline (maintenance). Both kcal expenditure measures were computed using the CHAMPS instrument, designed to quantify relative kcal expenditure in adult populations based on self-reported frequency and duration of a range of common physical activities (Stewart, et al., 2001). CHAMPS-calculated kcal expenditure has demonstrated acceptable reliability, with ICCs for moderate intensity activities of 0.67, 0.76, and 0.81–0.88 at six months, two weeks, and one week, respectively (Stewart, et al., 2001, Cyarto, et al., 2006). The instrument has also demonstrated adequate discriminant and construct validity, correlates well with other measures of PA, and is sensitive to change (Stewart, et al., 2001). Two types of routine activities, “Do heavy work around the house (washing windows, cleaning gutters, shoveling snow),” “Do heavy gardening (spading, raking, pushing a lawnmower)” were reported at unrealistically high levels consistently over time and across study groups. Similar high reporting on these two items has been found by the instrument authors in other studies of mid-life and older adults (Castro, et al., 2008, King, et al., 2009). Similar to these studies, we removed the two over-reported items from all kcal expenditure calculations, while two items pertaining to light housework and gardening remained. We identified participants as maintaining PA if their MVPA kcal expenditure at a follow-up measurement was at least 80% of their baseline expenditure and at least 1500kcal/wk. These lower bounds ensured that participants classified as maintaining PA were engaging in about the same amount of activity as they had been at baseline, and that this activity level approximated the recommended 5 bouts of 30 minutes of moderate activity per week.
Analysis plan
General linear mixed model (Laird and Ware, 1982) regression models were estimated using SAS PROC MIXED specifying time within participant, random participant intercepts, unspecified covariance structure and restricted maximum likelihood estimation (Littell, et al., 1996, Statistical Analysis System, 2002–2003) to test the hypothesis that KAM participants maintained kcal expenditure from baseline through the three follow-up time points relative to UC participant expenditure levels. In the primary efficacy analyses, AllPA and MVPA kcal were predicted separately from the time at which kcal was measured (baseline, 6 months, 12 months, 24 months), which varied within participants, and randomized treatment arm (KAM, UC), which varied across participants. We chose the mixed model approach because, relative to a general linear model approach (e.g., repeated measures ANOVA or ANCOVA), it accommodates variation in the number of observations per participant without reliance on imputation to replace missing observations. Preliminary examination of the kcal expenditure measures at each time point revealed outlying observations. Observations greater than 5 standard deviations from the time-specific median were excluded from the analyses (AllPA kcal n=3 at baseline, n=4 at 6 months, n=1 at 12 months, n=1 at 24 months; MVPA kcal n=3 at baseline, n=4 at 6 months, n=1 at 12 months, n=4 at 24 months) to prevent unrealistically high kcal expenditure values from resulting in the (likely) over-estimation of KAM efficacy. Additionally, kcal and maintenance outcomes were missing for time points at which participants did not complete the CHAMPS (6 months: n= 34 UC, n=28 KAM, p=.45; 12 months: n=39 UC, n=28 KAM, p=.17; 24 months: n=51 UC, n=32 KAM, p=.03). Maximum likelihood estimation ensured that all available kcal observations, excluding those greater than 5 SD above median, from all randomized participants were used to estimate model parameters. We estimated models including the outlying observations to ensure that their omission would not affect conclusions drawn from the analyses. There were no substantive differences in any of the omnibus tests or estimated model parameters, and the planned contrasts at 6, 12, and 24 months estimated the between groups differences to be inconsequentially larger than what is reported below.
The UC arm and baseline measurement were treated as reference categories for the treatment and time effects in the mixed regression models. Thus, the KAM parameter tested whether the KAM and UC groups were different at baseline. Because a separate random intercept was estimated for each participant, the 6-month, 12-month and 24-month parameters estimated how much, on average, participants in the UC arm increased or decreased kcal expenditure at each follow-up relative to their own baseline kcal expenditure. The 6-month*KAM, 12-month*KAM, and 24-month*KAM interaction parameters estimated the difference in the average change in expended kcal/wk from baseline to each follow-up among KAM compared to UC participants. Planned comparisons of kcal expenditure among KAM versus UC participants at each time point assessed whether KAM successfully helped participants maintain PA at 6, 12, and 24 months.
We estimated a generalized linear mixed model regression (GLMM) (Breslow and Clayton, 1993) using subject-specific pseudo-likelihood estimation (Wolfinger and O’Connell, 1993) in SAS PROC GLIMMIX to test the hypothesis that PA maintenance (0/1 outcome) would be higher among KAM participants than UC participants at 6, 12, and 24 months. In this model, the binary outcome was normalized using a logit link function and binary distribution, and the 6 month measurement served as the referent time point.
Sample size was based on that needed to detect a time (24 month vs. baseline) by treatment (KAM vs. UC) interaction, in which the standardized between groups difference at baseline was Cohen’s d = .00 and d = .25 at 24 months, at .80 power (two-tailed, alpha = 0.05) on AllPA kcal in a two group repeated measures ANOVA. The use of GLMM meant that more observations were included in the primary analyses than was assumed in the power analysis, so that they were better powered and more generalizable than a GLM/ANOVA approach. We assumed a common standard deviation of 1500 kcals at each of 4 time points and a first order autoregressive residual covariance structure. These parameters suggested that N=349 per study arm would be needed. Assuming non-differential 70% retention across study groups at 24 months, we targeted n=500 per arm for recruitment.
Outcome analyses were conducted after study recruitment was completed and intervention staff were blinded to results during the intervention delivery period so that neither the sample size, group assignment, nor intervention delivery could be influenced by knowledge of the impact of the intervention on PA.
Results
Sample Characteristics
With respect to age and race/ethnicity, those enrolled in the trial were reasonably representative of the recruitment pool of age eligible health plan members; themselves reflective of the local community.(Martinson, et al., 2010) Baseline demographic characteristics of participants are shown in Table 2. Our target population being individuals reporting increased MVPA within the past year to a minimum of 30 minutes on at least 2 days/week, it is not surprising that one-third reported participation in other PA “programs.” About a third of such reports were health club memberships, and another quarter being use of personal trainers. Since random group assignment of these individuals was well balanced, we have no reason to believe that their inclusion biased the primary treatment group comparisons of interest.
Table 2.
Participant Characteristics as Mean and Standard Error or Percent by Usual Care (UC) and Keep Active Minnesota (KAM) Groups
| n | UC 526 | KAM 523 | All 1049 |
|---|---|---|---|
| age at baseline | 57.1(.2) | 57.1(.2) | 57.1(.2) |
| female | 71.9 | 72.9 | 72.4 |
| BMI, kg/m2 | 27.7(.2) | 27.5(.2) | 27.6(.2) |
| White | 95.6 | 92.4* | 94.0 |
| Black | 2.8 | 3.8 | 3.3 |
| American Indian | 0.2 | 0.2 | 0.2 |
| Asian | 0.6 | 1.2 | 0.9 |
| Multiple | 0.6 | 1.2 | 0.9 |
| Other | 0.0 | 0.6 | 0.3 |
| Unknown | 0.2 | 0.8 | 0.5 |
| Hispanic/Latino | 1.1 | 2.5 | 1.8 |
| employed | 76.6 | 77.1 | 76.8 |
| 4 year degree or more | 65.8 | 67.7 | 66.7 |
| participating in another activity program | 35.2 | 34.7 | 35.0 |
| functional health status fair or poor | 5.7 | 6.7 | 6.2 |
KAM vs. UC, p<0.05
Study conducted in participants recruited from one health plan in Minnesota during 2004 and 2005.
Primary outcomes by treatment group
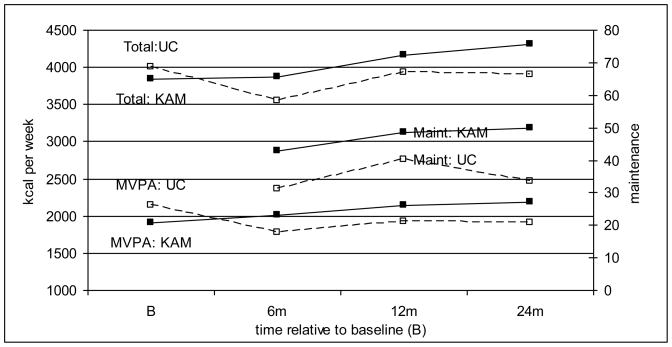
AllPA kcal expenditure was not different between KAM and UC participants at baseline (p=0.21; see Table 3). KAM participants reported significantly more activity than UC participants at the 6 (p<0.03,) and 24 month follow-ups (p<0.01), although the difference between KAM and UC participants was not significant at 12 months (p=0.08). AllPA kcal expenditure dropped markedly among UC participants between baseline and 6 months (p<0.001), but remained stable among KAM participants (p=0.71). UC participants returned to their baseline AllPA kcal expenditure at 12 months (p=0.38) while KAM participants increased their PA relative to baseline (p<0.005). At 24 months, UC participants reported a similar amount of PA relative to baseline (p=0.27) whereas KAM participants continued to increase their PA (p<0.001). Figure 2 graphically displays the model-based predicted means for the primary outcomes at baseline and each follow-up by treatment arm.
Table 3.
Descriptive statistics (mean, %, SE, n), model-estimated least squares (LS) mean differences or odds ratios with 95% confidence intervals, and significance levels for AllPA and MVPA kcal expenditure and PA maintenance by Keep Active Minnesota (KAM) or Usual Care (UC) and time
| baseline | 6 months | 12 months | 24 months | |
|---|---|---|---|---|
| AllPA kcal/wk | ||||
| KAM, Mean | 3822 | 3848 | 4163 | 4309 |
| SE | 85 | 96 | 99 | 112 |
| n | 521 | 492 | 494 | 490 |
| UC, Mean | 3998 | 3558 | 3941 | 3904 |
| SE | 95 | 102 | 94 | 102 |
| n | 525 | 491 | 487 | 475 |
| LS mean difference | −172 | 321 | 243 | 415 |
| 95% CI, lower limit | −437 | 50 | −28 | 142 |
| 95% CI, upper limit | 93 | 593 | 515 | 688 |
| p, KAM vs. UC | 0.21 | 0.03 | 0.08 | 0.01 |
| MVPA kcal/wk | ||||
| KAM, Mean | 1907 | 2008 | 2146 | 2180 |
| SE | 62 | 72 | 75 | 79 |
| n | 521 | 493 | 494 | 487 |
| UC, Mean | 2141 | 1764 | 1934 | 1903 |
| SE | 79 | 77 | 73 | 78 |
| n | 525 | 490 | 487 | 475 |
| LS mean difference | −231 | 241 | 224 | 273 |
| 95% CI, lower limit | −432 | 35 | 18 | 66 |
| 95% CI, upper limit | −29 | 447 | 430 | 481 |
| p, KAM vs. UC | 0.03 | 0.03 | 0.04 | 0.01 |
| maintenance | ||||
| KAM, % | 43.4 | 48.6 | 50.1 | |
| n | 493 | 494 | 487 | |
| UC, % | 32.0 | 40.7 | 34.5 | |
| n | 490 | 487 | 475 | |
| odds ratio | 1.66 | 1.40 | 1.98 | |
| 95% CI, lower limit | 1.24 | 1.05 | 1.48 | |
| 95% CI, upper limit | 2.23 | 1.86 | 2.66 | |
| p, KAM vs. UC | 0.001 | 0.03 | 0.001 | |
Study conducted in participants recruited from one health plan in Minnesota during 2004 and 2005.
Figure 2.
Model-Based Predicted Means for Primary CHAMPS-Based Outcomes by Treatment and Time
A similar pattern of effects was obtained for MVPA kcal expenditure. KAM participants reported less MVPA kcal expenditure than UC participants at baseline (p<0.03). However, the pattern reversed at each follow-up so that KAM participants reported significantly more kcal expenditure than UC participants at 6 (p<0.03), 12 (p<0.04) and 24 months (p<0.01). UC participants reduced their MVPA kcal expenditure over the first 6 months of study participation (p<0.001), while KAM participants reported a non-significant increase in MVPA (p=0.17). UC participants approached a return to baseline at 12 (p<0.005) and 24 months (p<0.005) while MVPA continued to increase at 12 (p<0.005) and 24 months (p<0.001) among KAM participants.
Finally, more KAM than UC participants maintained MVPA at 6 (p<0.001), 12 (p<0.03), and 24 months (p<0.001). Relative to 6 months, there was an increase in the proportion of UC (p<0.005) and KAM (p<0.001) participants who maintained activity at 12 months. At 24 months, the proportion of UC participants who maintained MVPA dropped back to approximately what was observed at 6 months (p=0.43) while the proportion maintaining among KAM participants continued to increase (p<0.05).
Satisfaction with the intervention
We obtained satisfaction ratings from 91% of those who participated in at least one of the phone-course sessions. Of these, 40% indicated that the course exceeded their expectations and 58% indicated that it met their expectations, 51% indicated that the course workbook was very helpful, 46% that it was moderately helpful, and only 3% indicated it was not helpful. Nearly two-thirds (64%) were completely satisfied with the KAM intervention, with 35% reporting being satisfied.
Discussion
This relatively low intensity telephone and mail-based PA maintenance intervention is one of the first studies to focus on maintenance of PA levels among adults ages 50 to 70 years who had recently increased their PA. Compared to UC subjects, those receiving the KAM intervention had significantly higher mean energy expenditures at 6, 12, and 24 months after randomization. The magnitude of the difference in PA between groups, roughly an additional 200kcal/wk in the KAM group, was statistically significant. While a modest effect size (d = .10 – .17), it is equivalent to about one additional hour per week of moderate intensity walking; sufficient to be clinically meaningful(Martinson, et al., 2001).
We are aware of only one published randomized controlled trial demonstrating a long-term (2 year) improved PA outcome following a behavioral intervention approach to increase PA among middle-aged adults (Dunn, et al., 1999). In this study, sedentary healthy adults effectively increased their PA over the first 6 months of treatment and maintained this gain over the following 18 months. Participants attended 16 weekly meetings followed by 4 biweekly meetings. This relatively resource intensive approach is not likely to meet the cost and penetration criteria for a successful population-based intervention (Glasgow, et al., 1999), suggesting the need for alternative methods such as telephone-based counseling which has an increasing evidence base (Castro, et al., 2001, Eakin, et al., 2007, King, et al., 1995).
Although the majority of PA intervention trials have focused primarily on PA initiation among sedentary individuals (Harrison, et al., 2005, Kerse, et al., 2005, Ackermann, et al., 2005, Cyarto, et al., 2004, Jancey, et al., 2008, Leveille, et al., 1998, Morgan, 2005, Motl, et al., 2005, Pinto, et al., 2005, Rejeski, et al., 2005, Stewart, et al., 2001, Stewart, et al., 1998), attention to the need for longer-term PA maintenance has been growing (The Writing Group for the Activity Counseling Trial Research, 2001, Castro, et al., 2001, Cox, et al., 2003, McAuley, et al., 2007, Müller-Riemenschneider, et al., 2008). The promising 2-year results reported here represent an important advance in the overall effort to raise levels of PA in the U.S. population.
Although health care provider success at increasing PA among patient populations has been mixed, they may play an important role in maintaining PA gains by providing verbal support and encouragement to their currently active patients. Given the time pressure and demands placed on primary care physicians, implementing sustainable telephone based coaching programs will likely require the development of parallel support systems, integrated with primary care (O’Connor and Pronk, 1998, Pronk, et al., 2002, Pronk and O’Connor, 1997) such as HealthPartner’s own JourneyWell (Pronk and Kottke, 2009, Pronk, 2009) or New Zealand’s Green Prescription program (Kerse, et al., 2005). Other recent work suggests the potential for even greater efficiencies and scaling of interventions by taking the human interventionist out of the picture completely (King, et al., 2007). Clearly, additional dissemination research (Eakin, et al., 2007, Wilcox, et al., 2008) and building the evidence base to document the cost-effectiveness of such programs is needed (Anderson, et al., 2005, Jeffery, et al., 2003, Martinson, et al., 2003, Pronk, et al., 2002, Pronk, et al., 1999, Sherwood, et al., 2006). The potential payoffs of harnessing the energy of other community groups already committed to the health and well-being of mid-life and older adults should not be overlooked (Wilcox, et al., 2008, King and Sallis, 2009).
Study Strengths
We attained an excellent retention rate (92% response rate at 24 months) with no study group difference in attrition, in contrast to many previous behavioral interventions to increase PA in mid-life and older adults (Jacobsen, et al., 2003, Jancey, et al., 2007, Prohaska, et al., 2000, Schmidt, et al., 2000, Tu, et al., 2004). Likewise, we attained high intervention fidelity and high client satisfaction.
Limitations
Interpretation of the data is limited by several factors. First, generalization to other, non-research contexts should be done with caution due to the likelihood of self-selection, a threat to external validity in most clinical trials(Martinson, et al., 2010). Second, our comparison group was not conducted as a “contact-control” so some or all of the intervention effect may be attributable to the non-specific effect of “contact” with those in the intervention. Third, although this was a reasonably low-intensity intervention, costs were not negligible, and affordability should be considered for potential payers. Comparing the costs of such a phone-based program to more automated approaches (e.g, interactive voice response systems, personal data assistants) may be useful.
Despite these limitations, the results have important clinical and public health implications. Pursuing complementary strategies to help the newly active to maintain PA and fostering PA initiation among the sedentary may be the only feasible way to reach national PA goals. Thus, the demonstration of a promising approach to maintain PA among those who are already active adds a potentially efficacious strategy to the relatively few proven strategies available for increasing PA in the adult population.
Supplementary Material
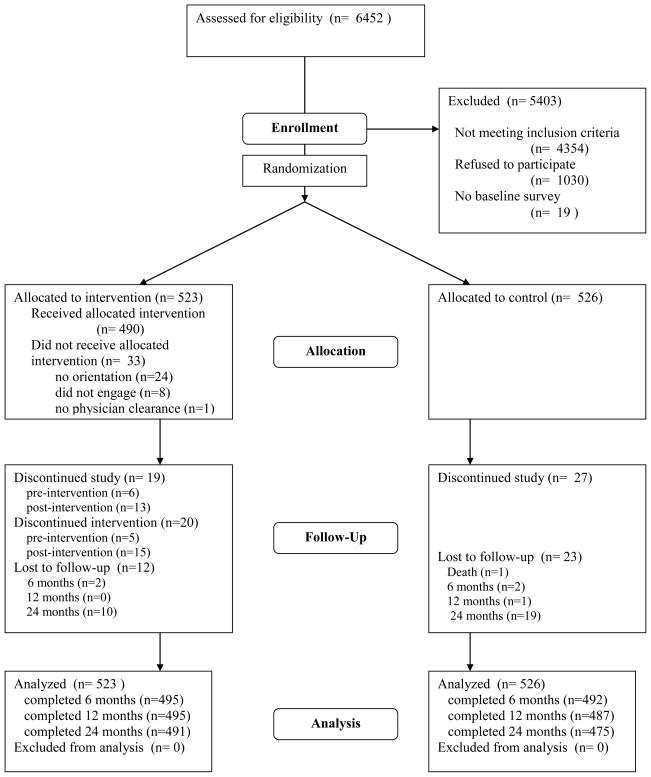
Figure 1.
Flow of participants through the trial
Acknowledgments
This study was supported by a grant from the National Institute on Aging (R01 AG023410). The project was initiated and analyzed by the study investigators. At no point was the study sponsor involved in the study design; in the collection, analysis or interpretation of the data, in the writing of this report; or in the decision to submit the paper for publication. For her capable project management during the study startup period we thank Kirsten Hase. For their dedicated service as a Data Safety Monitoring Board, we thank Drs. Mark Pereira, Vincent Chen and Michael Levitt. And finally, we thank Karen Speicher, Jessica Clausen, Colleen Flattum, and Stephanie Williams for their invaluable contributions as telephone coaches and counselors on the study.
Support: NIH, National Institute on Aging
Footnotes
Human Participation Protection
This study was reviewed and approved by Regions Hospital Institutional Review Board.
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
Clinical Trial Registration Number at ClinicalTrials.gov: NCT00283452
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann RT, Deyo RA, LoGerfo JP. Prompting primary providers to increase community exercise referrals for older adults: a randomized trial. J Am Geriatr Soc. 2005;53:283–289. doi: 10.1111/j.1532-5415.2005.53115.x. [DOI] [PubMed] [Google Scholar]
- Anderson LH, Martinson BC, Crain AL, Pronk NP, Whitebird RR, Fine L, O’Connor PJ. Health Care Charges Associated with Physical Inactivity, Overweight, and Obesity. Prev Chronic Dis [serial online] 2005;2 http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=16164813. [PMC free article] [PubMed]
- Anderson RT, King A, Stewart AL, Camacho F, Rejeski WJ. Physical activity counseling in primary care and patient well-being: Do patients benefit? Ann Behav Med. 2005;30:146–154. doi: 10.1207/s15324796abm3002_7. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar H, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- Bandura A. Health Promotion by Social Cognitive Means. Health Educ Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: A social cognitive theory. Prentice Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- Blair SN, Kohl HW, Gordon NF, Paffenbarger RS., Jr How much physical activity is good for health? Annu Rev Public Health. 1992;13:99–126. doi: 10.1146/annurev.pu.13.050192.000531. [DOI] [PubMed] [Google Scholar]
- Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Clayton DG. Approximate Inference in Generalized Linear Mixed Models. J Am Stat Assoc. 1993;88:9–25. [Google Scholar]
- Bull FC, Jamrozik K, Blanksby BA. Tailored advice on exercise--Does it make a difference? Am J Prev Med. 1999;16:230–239. doi: 10.1016/s0749-3797(98)00160-3. [DOI] [PubMed] [Google Scholar]
- Castro CM, Pruitt LA, French SH, Cassayre CL, King AC. Successful peer mentoring for physical activity:12-month results of the TEAM trial. Ann Behav Med. 2008;35:S081. [abstract] [Google Scholar]
- Castro CM, King AC, Brassington GS. Telephone versus mail interventions for maintenance of physical activity in older adults. Health Psychol. 2001;20:438–444. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2007. [Google Scholar]
- Centers for Disease Control and Prevention. Trends in Leisure-Time Physical Inactivity by Age, Sex, and Race/Ethnicity --- United States, 1994–2004. MMWR. 2005 Online: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5439a5.htm.
- Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61:1234. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the health, aging and body composition study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- Cox KL, Burke V, Morton AR, Beilin LJ, Puddey IB. Independent and additive effects of energy restriction and exercise on glucose and insulin concentrations in sedentary overweight men. Am J Clin Nutr. 2004;80:308–316. doi: 10.1093/ajcn/80.2.308. [DOI] [PubMed] [Google Scholar]
- Cox KL, Burke V, Gorely TJ, Beilin LJ, Puddey IB. Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40–65 years: The S.W.E.A.T. Study (Sedentary Women Exercise Adherence Trial) Prev Med. 2003;36:17–29. doi: 10.1006/pmed.2002.1134. [DOI] [PubMed] [Google Scholar]
- Cyarto EV, Marshall AL, Dickinson RK, Brown WJ. Measurement properties of the CHAMPS physical activity questionnaire in a sample of older Australians. J Sci Med Sport. 2006;9:319–326. doi: 10.1016/j.jsams.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Cyarto EV, Moorhead GE, Brown WJ. Updating the evidence relating to physical activity intervention studies in older people. J Sci Med Sport. 2004;7:30–38. doi: 10.1016/s1440-2440(04)80275-5. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Marcus B, Kampert JB, Garcia ME, Kohl HW, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespriatory fitness. JAMA. 1999;281:327–334. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- Eaton CB, Menard LM. A systematic review of physical activity promotion in primary care office settings. Br J Sports Med. 1998;32:11–16. doi: 10.1136/bjsm.32.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MG, Pinto BM, Marcus BH, Lynn H, Jette AM, Rakowski W, McDermott S, DePue JD, Milan FB, Dube C, Tennstedt S. Physician-based physical activity counseling for middle-aged and older adults: A randomized trial. Ann Behav Med. 1999;21:40–47. doi: 10.1007/BF02895032. [DOI] [PubMed] [Google Scholar]
- Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone Interventions for Physical Activity and Dietary Behavior Change: A Systematic Review. Am J Prev Med. 2007;32:419. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Elavsky S, McAuley E, Motl RW, Konopack JF, Marquez DX, Hu L, Jerome GJ, Diener E. Physical Activity Enhances Long-Term Quality of Life in Older Adults: Efficacy, Esteem, and Affective Influences. Ann Behav Med. 2005;30:138–145. doi: 10.1207/s15324796abm3002_6. [DOI] [PubMed] [Google Scholar]
- Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA. 2002;288:2300–2306. doi: 10.1001/jama.288.18.2300. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RA, Roberts C, Elton PJ. Does primary care referral to an exercise programme increase physical activity one year later? A randomized controlled trial. J Public Health (Oxf) 2005;27:25–32. doi: 10.1093/pubmed/fdh197. [DOI] [PubMed] [Google Scholar]
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217–228. doi: 10.1093/geront/44.2.217. [DOI] [PubMed] [Google Scholar]
- Jacobsen DJ, Donnelly JE, Snyder-Heelan K, Livingston K. Adherence and Attrition With Intermittent and Continuous Exercise in Overweight Women. Int J Sports Med. 2003;24:459–464. doi: 10.1055/s-2003-41177. [DOI] [PubMed] [Google Scholar]
- Jancey JM, Lee AH, Howat PA, Clarke A, Wang K, Shilton T. The effectiveness of a physical activity intervention for seniors. Am J Health Promot. 2008;22:318–321. doi: 10.4278/ajhp.22.5.318. [DOI] [PubMed] [Google Scholar]
- Jancey J, Lee A, Howat P, Clarke A, Wang K, Shilton T. Reducing attrition in physical activity programs for older adults. J Aging Phys Act. 2007;15:152–165. doi: 10.1123/japa.15.2.152. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Sherwood NE, Brelje J, Pronk NP, Boyle RG, Boucher JL, Hase K. Mail and Phone Interventions for Weight Loss in a Managed-Care Setting: Weigh-To-Be One-Year Outcomes. Int J Obes Relat Metab Disord. 2003;27:1584–1592. doi: 10.1038/sj.ijo.0802473. [DOI] [PubMed] [Google Scholar]
- Kerse N, Elley CR, Robinson E, Arroll B. Is physical activity counseling effective for older people? A cluster randomized, controlled trial in primary care. J Am Geriatr Soc. 2005;53:1951–1956. doi: 10.1111/j.1532-5415.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- King AC, Sallis JF. Why and how to improve physical activity promotion: Lessons from behavioral science and related fields. Prev Med. 2009;49:286–288. doi: 10.1016/j.ypmed.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Castro CM, Pruitt LA, Ahn DK, Prosak C, Buman M, Hekler E. Optimizing diet and exercise changes in chronically stressed adults: Major results of the CALM trial. Ann Behav Med. 2009;37:S112. [abstract] [Google Scholar]
- King AC, Pruitt LA, Woo S, Castro CM, Ahn DK, Vitiello MV, Woodward SH, Bliwise DL. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol A Biol Sci Med Sci. 2008;63:997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Friedman R, Marcus B, Castro C, Napolitano M, Ahn D, Baker L. Ongoing physical activity advice by humans versus computers: The Community Health Advice by Telephone (CHAT) Trial. Health Psychol. 2007;26:718–727. doi: 10.1037/0278-6133.26.6.718. [DOI] [PubMed] [Google Scholar]
- King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation. 1995;91:2596–2604. doi: 10.1161/01.cir.91.10.2596. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Leveille SG, Wagner EH, Davis C, Grothaus L, Wallace J, LoGerfo J, Kent D. Preventing disability and managing chronic illness in frail older adults: a randomized trial of a community-based partnership with primary care [see comments] J Am Geriatr Soc. 1998;46:1191–1198. doi: 10.1111/j.1532-5415.1998.tb04533.x. [DOI] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R. SAS System for mixed models. SAS Institute Incorporated; Cary, NC: 1996. [Google Scholar]
- Liu-Ambrose T, Donaldson MG, Ahamed Y, Graf P, Cook WL, Close J, Lord SR, Khan KM. Otago Home-Based Strength and Balance Retraining Improves Executive Functioning in Older Fallers: A Randomized Controlled Trial. J Am Geriatr Soc. 2008;56:1821–1830. doi: 10.1111/j.1532-5415.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P, Strudwick M. The effects of a 12 month exercise trial on balance, strength and falls in older women: a randomized controlled trial. J Am Geriatr Soc. 1995;43:1198–1206. doi: 10.1111/j.1532-5415.1995.tb07394.x. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention: maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 1985. [Google Scholar]
- Martinson BC, Crain AL, Sherwood NE, Hayes M, Pronk NP, O’Connor PJ. Population Reach and Recruitment Bias in a Maintenance RCT in Physically Active Older Adults. J Phys Activ Health. 2010;7:127–135. doi: 10.1123/jpah.7.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson BC, Crain AL, Sherwood NE, Hayes M, Pronk NP, O’Connor PJ. Maintaining physical activity among older adults: Six-month outcomes of the Keep Active Minnesota randomized controlled trial. Prev Med. 2008;46:111–119. doi: 10.1016/j.ypmed.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson BC, Crain AL, Pronk NP, O’Connor PJ, Maciosek MV. Changes in physical activity and short-term changes in health care charges: a prospective cohort study of older adults. Prev Med. 2003;37:319–326. doi: 10.1016/s0091-7435(03)00139-7. [DOI] [PubMed] [Google Scholar]
- Martinson BC, O’Connor PJ, Pronk NP. Physical inactivity and short-term all-cause mortality in adults with chronic disease. Arch Intern Med. 2001;161:1173–1180. doi: 10.1001/archinte.161.9.1173. [DOI] [PubMed] [Google Scholar]
- Mayer-Davis EJ, D’Agostino R, Jr, Karter AJ, Haffner SM, Rewers MJ, Saad M, Bergman RN. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279:669–674. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- McAuley E, Konopack JF, Motl RW, Morris KS, Doerksen SE, Rosengren KR. Physical activity and quality of life in older adults: influence of health status and self-efficacy. Ann Behav Med. 2006;31:99–103. doi: 10.1207/s15324796abm3101_14. [DOI] [PubMed] [Google Scholar]
- McAuley E, Morris KS, Motl RW, Hu L, Konopack JF, Elavsky S. Long-term follow-up of physical activity behavior in older adults. Health Psychol. 2007;26:375–380. doi: 10.1037/0278-6133.26.3.375. [DOI] [PubMed] [Google Scholar]
- Morgan O. Approaches to increase physical activity: reviewing the evidence for exercise-referral schemes. Public Health. 2005;119:361–370. doi: 10.1016/j.puhe.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Motl RW, Konopack JF, McAuley E, Elavsky S, Jerome GJ, Marquez DX. Depressive Symptoms Among Older Adults: Long-Term Reduction After a Physical Activity Intervention. J Behav Med. 2005:1–10. doi: 10.1007/s10865-005-9005-5. [DOI] [PubMed] [Google Scholar]
- Müller-Riemenschneider F, Reinhold T, Nocon M, Willich SN. Long-term effectiveness of interventions promoting physical activity: A systematic review. Prev Med. 2008;47:354. doi: 10.1016/j.ypmed.2008.07.006. [DOI] [PubMed] [Google Scholar]
- O’Connor PJ, Pronk NP. Integrating population health concepts, clinical guidelines and ambulatory medical systems to improve diabetes care. J Ambulatory Care Manage. 1998;21:67–73. doi: 10.1097/00004479-199801000-00009. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. U.S. Department of Health and Human Services; Washington, DC: 2008. [Google Scholar]
- Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23:3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- Pinto BM, Goldstein MG, Ashba J, Sciamanna CN, Jette A. Randomized controlled trial of physical activity counseling for older primary care patients. Am J Prev Med. 2005;29:247–255. doi: 10.1016/j.amepre.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Prohaska TR, Peters KE, Warren JS. Sources of Attrition in a Church-based Exercise Program for Older African-Americans. Am J Health Behav. 2000;14:380–385. doi: 10.4278/0890-1171-14.6.380. [DOI] [PubMed] [Google Scholar]
- Pronk NP, Kottke TE. Physical activity promotion as a strategic corporate priority to improve worker health and business performance. Prev Med. 2009;49:316–321. doi: 10.1016/j.ypmed.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Pronk NP. Physical Activity Promotion in Business and Industry: Evidence, Context, and Recommendations for a National Plan. J Phys Activ Health. 2009;6:S220–S235. doi: 10.1123/jpah.6.s2.s220. [DOI] [PubMed] [Google Scholar]
- Pronk NP, Boucher JL, Gehling E, Boyle RG, Jeffery RW. A platform for population-based weight management: description of a health plan-based integrated systems approach. Am J Manag Care. 2002;8:847–857. [PubMed] [Google Scholar]
- Pronk NP, O’Connor PJ, Martinson BC. Population health and active living: Economic potential of physical activity promotion. American Journal of Medicine and Sports. 2002;4:51–57. [Google Scholar]
- Pronk NP, Goodman MJ, O’Connor PJ, Martinson BC. Relationship between modifiable health risks and short-term health care charges. JAMA. 1999;282:2235–2239. doi: 10.1001/jama.282.23.2235. [DOI] [PubMed] [Google Scholar]
- Pronk NP, O’Connor PJ. Systems approach to population health improvement. J Ambulatory Care Manage. 1997;20:24–31. doi: 10.1097/00004479-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Schmidt JA, Gruman C, King MB, Wolfson LI. Attrition in an exercise intervention: a comparison of early and later dropouts. J Am Geriatr Soc. 2000;48:952–960. doi: 10.1111/j.1532-5415.2000.tb06894.x. [DOI] [PubMed] [Google Scholar]
- Sherwood NE, Martinson BC, Crain AL, Hayes MG, Pronk NP, O’Connor PJ. A new approach to physical activity maintenance: Rationale, design, and baseline data from the Keep Active Minnesota trial. BMC Geriatr. 2008;8:17. doi: 10.1186/1471-2318-8-17. (Online serial) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NE, Jeffery RW, Pronk NP, Boucher JL, Hanson A, Boyle R, Brelje K, Hase K, Chen V. Mail and phone interventions for weight loss in a managed-care setting: weigh-to-be 2-year outcomes. Int J Obes. 2006;30:1565. doi: 10.1038/sj.ijo.0803295. [DOI] [PubMed] [Google Scholar]
- Statistical Analysis System, 2002–2003. SAS for Windows; Cary, NC: [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, Sepsis PG, King AC, McLellan BY, Roitz K, Ritter PL. Evaluation of CHAMPS, a physical activity promotion program for older adults. Ann Behav Med. 1998;19:353–361. doi: 10.1007/BF02895154. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Verboncoeur CJ, McLellan BY, Gillis DE, Rush S, Mills KM, King AC, Ritter P, Brown BW, Jr, Bortz WM., 2nd Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M465–470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Fielding RA, Blair SN, Guralnik JM, Gill TM, Hadley EC, King AC, Kritchevsky SB, Miller ME, Newman AB, Pahor M. The lifestyle interventions and independence for elders (LIFE) pilot study: Design and methods. Contemp Clin Trials. 2005;26:141–154. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Rush WA, O’Connor PJ, Goodman MJ. Validation of a modified Charlson score for using health plan claims data. Fourth annual Minnesota Health Services Research Conference.2000. [Google Scholar]
- The Writing Group for the Activity Counseling Trial Research, G. Effects of physical activity counseling in primary care: the Activity Counseling Trial: a randomized controlled trial. JAMA. 2001;286:677–687. doi: 10.1001/jama.286.6.677. [DOI] [PubMed] [Google Scholar]
- Tu W, Stump TE, Damush TM, Clark DO. The Effects of Health and Environment on Exercise-Class Participation in Older, Urban Women. Journal of Aging & Physical Activity. 2004;12:480–496. doi: 10.1123/japa.12.4.480. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Aunola S, Cepaitis Z, Moltchanov V, Hakumaki M, Mannelin M, Martikkala V, Sundvall J, Uusitupa M the Finnish Diabetes Prevention Study, G. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- Van der Bij AK, Laurant MGH, Wensing M. Effectiveness of physical activity interventions for older adults: A review. Am J Prev Med. 2002;22:120–133. doi: 10.1016/s0749-3797(01)00413-5. [DOI] [PubMed] [Google Scholar]
- van Sluijs EMF, van Poppel MNM, Twisk JWR, Chin A Paw MJ, Calfas KJ, van Mechelen W. Effect of a Tailored Physical Activity Intervention Delivered in General Practice Settings: Results of a Randomized Controlled Trial. Am J Public Health. 2005;95:1825–1831. doi: 10.2105/AJPH.2004.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JME, Swangard DM, Davis T, McPhee SJ. Exercise counseling by primary care physicians in the era of managed care. Am J Prev Med. 1999;16:307–313. doi: 10.1016/s0749-3797(99)00021-5. [DOI] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, Grodstein F. Physical Activity, Including Walking, and Cognitive Function in Older Women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Wilcox S, Dowda M, Leviton LC, Bartlett-Prescott J, Bazzarre T, Campbell-Voytal K, Carpenter RA, Castro CM, Dowdy D, Dunn AL, Griffin SF, Guerra M, King AC, Ory MG, Rheaume C, Tobnick J, Wegley S. Active for Life: Final Results from the Translation of Two Physical Activity Programs. Am J Prev Med. 2008;35:340. doi: 10.1016/j.amepre.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Wolfinger R, O’Connell M. Generalized linear models: A pseudo-likelihood approach. J Stat Comput Simulat. 1993;48:233–243. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.