Abstract
Objective
Epstein-Barr virus (EBV) infection has been linked to systemic lupus erythematosus (SLE) as demonstrated by the presence of increased seroprevalence and elevated viral loads, but the mechanism of this linkage has not been elucidated. Increased IFN-α levels and signatures, associated with innate immune responses, have been found in patients with SLE. Plasmacytoid dendritic cells (pDC) are innate immune cells that mediate viral immunity by producing large quantities of interferon alpha (IFN-α), but the role they play during infection with EBV remains unclear. To address this issue, we investigated the ability of EBV to promote IFN-α production by pDC in healthy subjects.
Methods
Human pDC were sorted and cultured in the presence of EBV, EBV small RNA (EBER), and EBV double-stranded DNA (dsDNA). IFN-α production by pDC was measured by enzyme-linked immunosorbent assay (ELISA), with activation of these cells measured by flow cytometry.
Results
We demonstrate that EBV DNA and RNA promote IFN-α production by human pDC through engagement of Toll-like receptor (TLR) 9 and TLR7, respectively, with initial viral recognition by pDC mediated by binding to major histocompatibility (MHC) class II molecules.
Conclusion
These data demonstrate that MHC class II-specific engagement by virus and subsequent viral nucleic acid recognition mediates IFN-α production by pDC. Our results suggest that elevated levels of IFN-α found in lupus patients may be a result of aberrantly controlled chronic viral infection.
Epstein-Barr virus (EBV) is a γ-herpes virus that infects 95% of adults in the United States (1) and is the pathogen responsible for mononucleosis, Burkitt’s lymphoma, nasopharyngeal carcinoma, and Sezary disease (2). This virus and SLE have been linked for 40 years (3–7). Increased prevalence of viral infections and elevated viral loads are found in patients with lupus (8–11) with reports demonstrating SLE-like disease developing after EBV-induced mononucleosis (12). Children and young adults with lupus have increased EBV seroprevalence compared to healthy controls; by contrast, there is no such correlation between SLE and other herpes viruses, including cytomegalovirus (CMV) (8, 9). One explanation for this observation is that molecular mimicry between the EBV antigen, EBNA-1 and self-ribonucleoproteins, may play a role in the expansion of the humoral autoimmune response in lupus (13, 14). Further evidence of linkage is the finding of elevated numbers of EBV-infected B cells in the peripheral blood of patients with SLE (10); an observation supported by others (11), and one that is perhaps explained by alterations in B cell maturation.
EBV infection of B cells requires engagement of complement receptor 2 (CR2) and the MHC class II molecule (15). The viral gp350 protein binds CR2 mediating viral attachment (16); whereas, the envelope protein gp42 binds the MHC class II β1 chain (17) mediating membrane fusion (17–19). After initial infection, the virus enters a latent phase in mature B cells with periodic reactivation and production of new virus. During latency, large quantities of EBV small RNAs (EBER) are made (20).
The adaptive immune system is critical for control of EBV infection. However, recent studies have demonstrated the importance of the innate immune system in control of other viral infections through engagement of toll-like receptors (TLR) and production of IFN-α (21–23). TLR7 expressed on pDC is critical in the anti-viral response against influenza, a single-stranded RNA virus (23), whereas TLR9-mediated production of IFN-α by pDC is necessary for the host response to herpes simplex virus (HSV) I and HSV II, double-stranded DNA α-herpes viruses closely related to EBV (21, 22). Less is known about the role of pDC in controlling EBV; supernatants from EBV-infected cell lines promote IFN-α production by pDC, but neither the antigens in this supernatant nor the mechanism behind IFN-α production were characterized (24).
IFN-α promotes anti-viral responses and immune system activation, including T cell activation and an increase in B cell survival, maturation, and activation (25). It also appears to play a pathogenic role in systemic lupus erythematosus (SLE) (26–28). Induction of IFN-α targeted gene transcription, the so-called IFN-α signature, and elevated levels of this cytokine, have been demonstrated in patients with lupus, with an elevated signature correlating with increased disease activity (26, 29). Single nucleotide polymorphisms of interferon regulatory factor 5 region also are associated with SLE (30, 31). Further evidence for the importance of IFN-α in SLE is demonstrated by the development of lupus-like disease as a side effect of treatment of some tumors with exogenous administration of this cytokine (27).
To further explore the link between EBV infection and SLE, we asked if and how the virus might contribute to enhanced IFN-α production. Specifically, we determined if EBV and its nucleic acids could drive IFN-α by human plasmacytoid dendritic cells (pDC), the cell principally responsible for the production of this cytokine. To test this hypothesis we sorted pDC from healthy controls and cultured them in the presence of different EBV antigens. We found that viral binding to MHC class II molecules, followed by endosomal uptake and TLR9 engagement, a process that did not require viral replication, were required for IFN-α production by pDC following challenge by intact virus. TLR7 also was required for the production of IFN-α in response to EBER made during chronic latent infection. In contrast, CR2 was not involved in IFN-α production by pDC. These results demonstrate that EBV nucleic acids can induce IFN-α production by pDC through at least two mechanisms, engagement of TLR9 and TLR7, suggesting an additional mechanism for the association of this infection with SLE.
Materials and Methods
Preparation of stocks of infectious EBV and mock inoculum
Viral stocks were prepared from culture supernatants of the EBV-positive B95-8 cell line (courtesy of George Miller, Yale School of Medicine), as previously described (32). B95-8 cells were seeded at 2×105 cells/ml in complete RPMI 1640 medium and serum starved for 14 days or induced with phorbol 12-myristate 13-acetate for 5 days in sealed flasks at 37° C. After centrifugation of cells, supernatants were collected and EBV precipitated in 8% (wt/vol) polyethylene glycol (PEG-8000; Sigma-Aldrich, St. Louis, MO) and 1M NaCl. The viral particles were then collected by centrifugation, resuspended in RPMI at 1/50 starting volume and frozen at −70° C. Mock inocula were prepared in an identical manner from the culture supernatant of the EBV-negative B cell lymphoma line, BJAB. Non-infectious EBV was made by incubation at 95° C for 5 minutes or UV irradiation at 1 J using a Stratalinker 2400 (Stratagene, La Jolla, CA). Viral infectivity was tested by measuring immortalization of B cells in the presence or absence of FK506 (Sigma-Aldrich) 14 days after infection (32).
Isolation of viral DNA and determination of genome copy number
DNA was amplified using primers for the EBV EBER-1 gene using a Bio-Rad iQ5 Real-time PCR Detection System (10), with a standard curve generated using the EBV-containing Raji cell line, allowing determination of viral genome number (10). EBV genomic and mock viral DNA were prepared using the DNAeasy Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s instruction for Blood and Body Fluid Spin Protocol. The concentration of purified DNA was determined by absorption at OD260.
Isolation of pDC and mDC
Human subjects research was in compliance with the Helsinki Declaration and was approved by the Yale University Human Investigation Committee. pDC were purified from Leukopaks (NY Blood Center, Long Island City, NY) by collecting peripheral blood mononuclear cells by centrifugation on Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden). pDC were enriched using the Stem Sep Human Dendritic Cell Enrichment Kit (Stem Cell Technologies, Vancouver, BC, Canada). pDC were identified on a FacsAria cell sorter (Becton Dickinson, Franklin Lakes, NJ) as lineage negative (Lin−, using anti-CD3, -CD14, -CD16, -CD19, -CD56, and –glycophorin A; all from BD Pharmingen, San Diego, CA), HLA-DR+, CD11c− (both from BD Pharmingen), and CD123+ (Miltenyi Biotech, Auburn, CA) with mDC identified as HLA DR+, CD11c+, and CD123−. Cells were greater than 99% pure, as assessed by flow cytometry. pDC were then cultured in RPMI 1640 supplemented with 10% fetal calf serum, 2mM L-glutamine, 110 µg/ml sodium pyruvate, antibiotics, and 10 ng/ml IL-3 (R&D Systems, Minneapolis, MN); mDC were cultured in RPMI with 10% fetal calf serum, 2mM L-glutamine, 110 µg/ml sodium pyruvate, antibiotics, and 2 ng/ml GM-CSF (R&D Systems, Minneapolis, MN).
Stimulation of pDC and mDC
pDC or mDC were incubated in the presence of antigens for 18 hours at 37°C and 5% CO2, including HSV (provided by Jennifer Lund and Akiko Iwasaki, Yale School of Medicine), EBV, UV-treated EBV, and heat-treated EBV at a concentration of 15,000 virions as measured by quantitative PCR, as well as with EBV DNA or mock virus DNA. In some experiments, before addition of virus or nucleic acids, cells were preincubated for 15 min with the highly inhibitory anti-CR2 mAb 171, known to block gp350 binding and infection of primary B lymphocytes (33), anti-MHC I (eBioscience, San Diego, CA), anti-MHC II (BD Pharmingen), anti-HLA DR-α (Biolegend, San Diego, CA), anti-HLA DR-β (DA2; Abcam, Cambridge, MA), GpC 2006 (phospho-thiolated TGCTGCTTTTGTGCTTTTGTGCTT) or CpG 2006 (TCGTCGTTTTGTCGTTTTGTCGTT; both from the W.M. Keck Oligosynthesis Facility, Yale University School of Medicine), or hydroxychloroquine (Sigma-Aldrich). Viability of pDC (post-stimulation) was assessed by trypan blue staining where indicated. IFN-α production was measured by ELISA (PBL Biomedical Labs, Piscataway, NJ). The activation markers CD86 (BD Pharmingen) and CR2 were measured by flow cytometry (FACS Calibur, Pharmingen).
Labeling of EBV
EBV was purified over dextran gradients and incubated for 10 min in the presence of 5µM CFSE at 37° C. Unincorporated CFSE was removed by ultrafiltration with Amicon ultra-15 centrifugal filter unit (100,000 MW cutoff spin column; Millipore, Billerica, MA).
TLR7 stimulation
A plasmid containing the full-length EBER sequence under the control of T7 promoter (courtesy of Joan Steitz, Yale School of Medicine) was linearized with Dra I. RNA transcripts were generated with a MEGAscript T7 kit (Ambion, Austin, TX) according to the manufacturer’s protocol. Full-length EBER were visualized by UV shadowing and excised from an 8% urea-acrylamide gel. RNA was purified by phenol chloroform extraction and loaded onto DOTAP (N- [1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate) Liposomal Transfection Reagent (Roche Pharmaceutical, Nutley, NJ) per the manufacturer’s protocol. pDC were pre-incubated with or without TLR7, TLR9, and TLR7/9 inhibitors (oligonucleotides IRS 661, 869 and 954 (34)(34); a kind gift from Franck Barrat, Dynavax Technologies) and then incubated with EBER-DOTAP, EBER alone or carrier alone overnight 37° C.
Statistical analysis
Statistical analysis was performed using Mann-Whitney or 1-Way ANOVA followed by Dunnett’s comparison test using Prism (GraphPad, La Jolla, CA).
Results
EBV promotes IFN-α production by pDC but not mDC
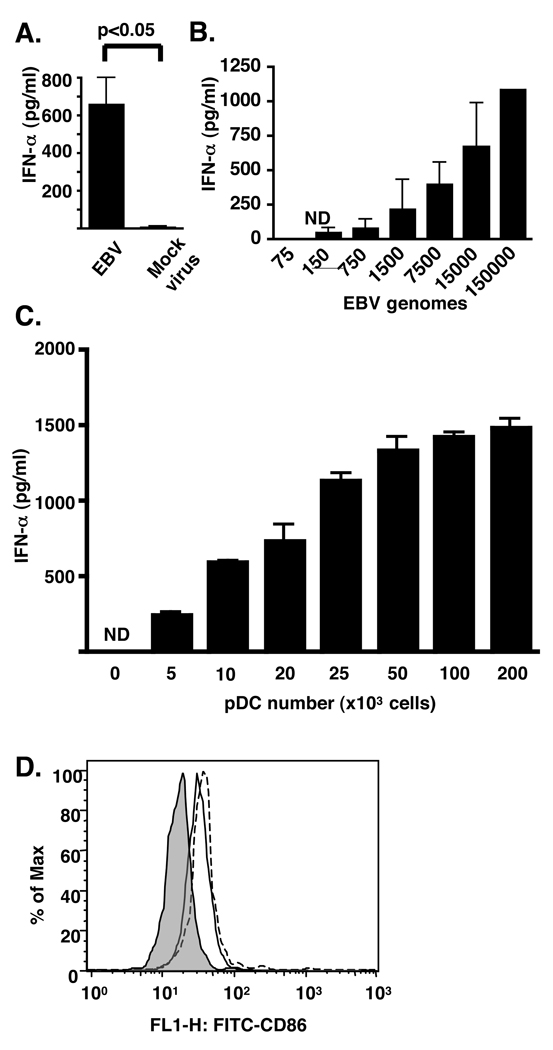
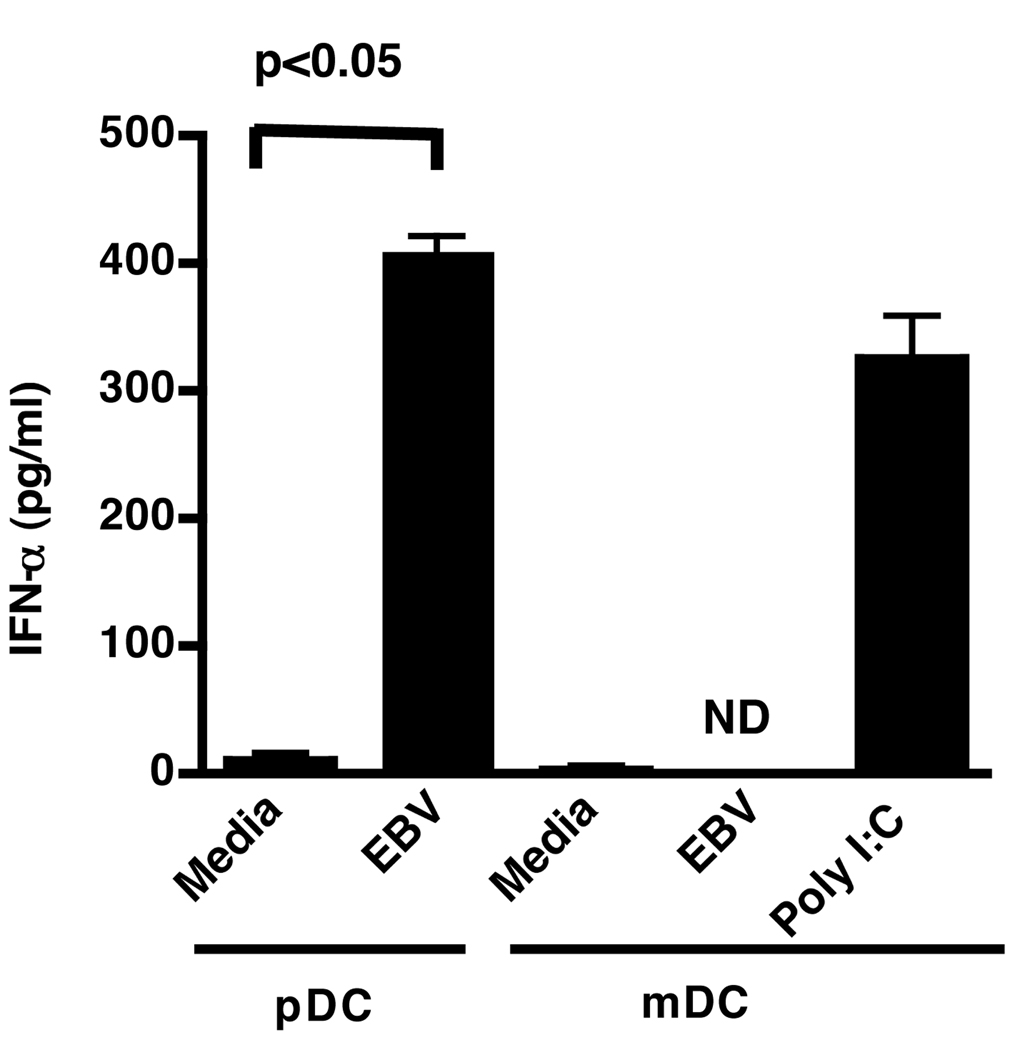
pDC sorted by flow cytometry were stimulated with purified virus and the IFN-α produced in the supernatants was measured by ELISA (Fig. 1A). EBV stimulated the production of IFN-α whereas mock virus from a non-EBV carrying lymphoma cell line, made in parallel with the EBV, did not. Fifty thousand pDC were activated in a dose-dependent manner by as few as 150 to as many as 150,000 viral genome copies as measured by IFN-α production (Fig. 1B). IFN-α production increased when viral genome copies were kept constant (15,000) and pDC numbers increased (Fig. 1C). Additional evidence of pDC activation by EBV (Fig. 1D, solid line) and CpG 2006 (Fig. 1D, dotted line) was evidenced by the upregulation of CD86 as measured by flow cytometry. By contrast, mDC which do not express TLR8 or 9 (35, 36) did not produce IFN-α following challenge (Fig. 2) suggesting that EBV can stimulate pDC, but not mDC.
Figure 1. EBV promotes IFN-α production and CD86 upregulation by pDC.
Fifty thousand pDC sorted by flow cytometry were incubated with (A) purified EBV (15,000 genomes) or mock virus for 18 h, or (B) increasing numbers of viral genomes. (C) Increasing numbers of pDC were incubated with 15,000 genomes of EBV. (D) pDC were cultured with EBV (solid line, 15,000 genomes), CpG DNA (broken line, 63nM) or with buffer control (shaded line), and CD86 expression determined by flow cytometry. Supernatants and cells were harvested 18 h after beginning incubations. In A–C, IFN-α in supernatants was measured by ELISA. In A, error bars represent SD, with experiments done in triplicate. In B and C, error bars represent SD of duplicate wells. ND (not detected) represents IFN-α amounts of less than 12.5 pg/ml. Statistics performed by Mann-Whitney testing.
Figure 2. EBV does not promote IFN-α production by mDC.
pDC (50,000 cells) and mDC (50,000 cells) sorted by flow cytometry were incubated with media alone, EBV (15,000 genomes) or polyriboinosinic:polyribocytidylic acid (poly I:C; 1.5 µg/ml) as a positive control. Supernatants were harvested 18 h after beginning incubations. Error bars represent SD, with experiments done in duplicate. ND (not detected) represents IFN-α amounts of less than 12.5 pg/ml. Statistics performed by Mann-Whitney testing.
Viral integrity is important for the production of IFN-α by pDC
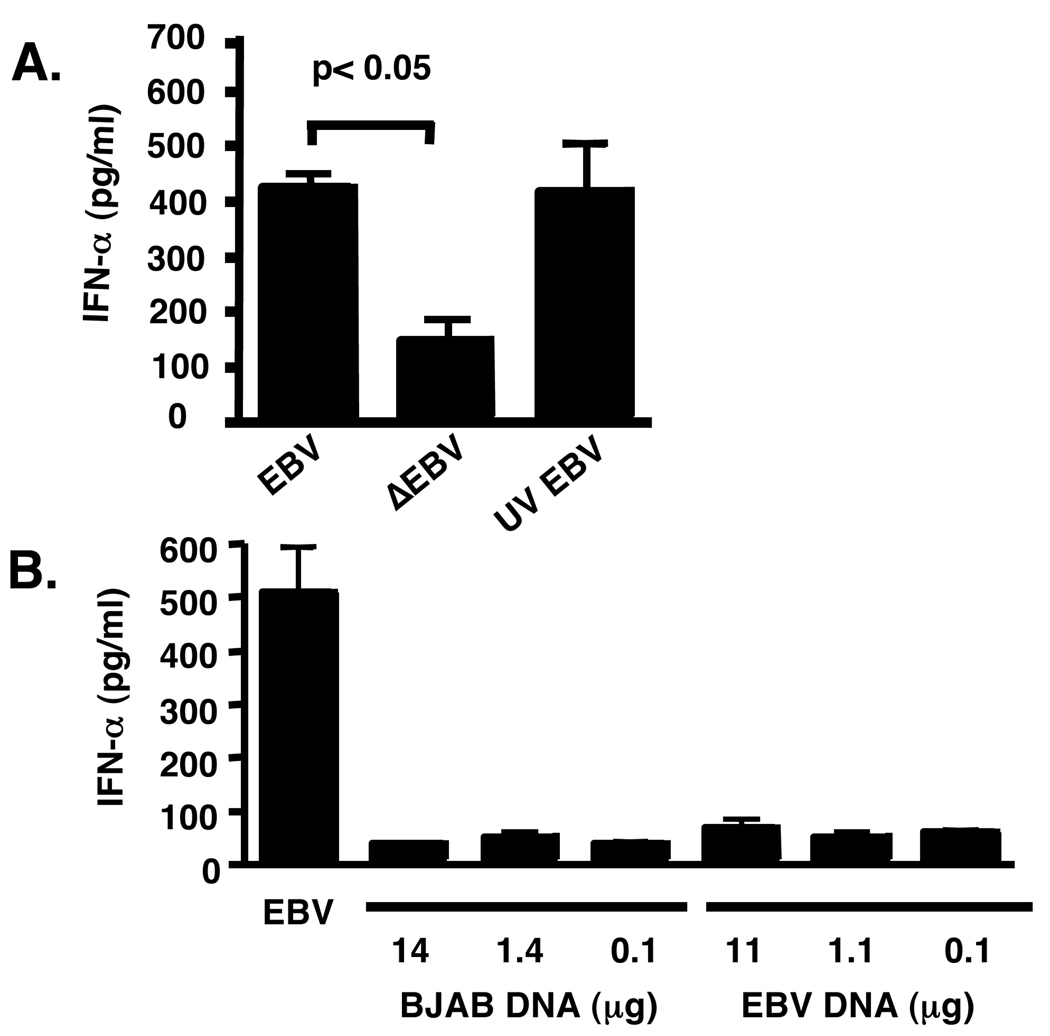
We next determined if infection was essential to promote IFN-α production by pDC. We found that EBV treated with UV light, which renders the virus non-replicating by DNA cross linking, still activated pDC as measured by IFN-α production (Fig. 3A); however, heat inactivation of EBV (95° C for 5 min) stimulated pDC less compared to viable virus (Fig. 3A). In addition, EBV DNA incubated alone with pDC was unable to promote IFN-α production, even at a concentration 200-fold higher than that of intact virus (Fig. 3B), suggesting that intact virions, but not viral replication are required for IFN-α production.
Figure 3. Intact EBV is required for IFN-α production by pDC.
pDC (50,000) sorted by flow cytometry were incubated (A) in the presence of intact EBV, heat-inactivated (ΔEBV), or UV-treated EBV (UV EBV; 15,000 genomes used in each condition), or (B) in the presence of increasing dilutions of purified EBV DNA (11 µg/ml, 1.1 µg/ml, 0.1 µg/ml) or as a control, BJAB DNA (14 µg/ml, 1.4 µg/ml, 0.1 µg/ml). In A and B supernatants and cells were harvested 18 h after beginning incubations. Statistics performed by 1-Way ANOVA followed by Dunnett’s comparison. Error bars represent SD, with experiments done in duplicate.
IFN-α induction by pDC is dependent on viral uptake, endosomal acidification, and TLR9 engagement
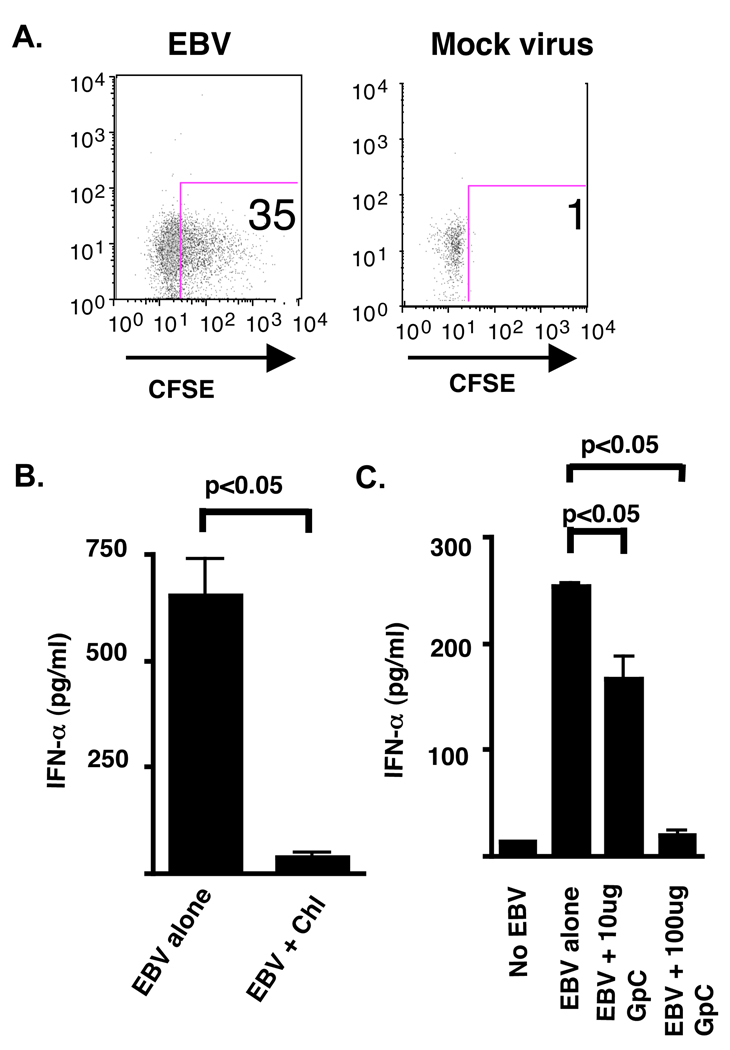
HSV, an α-herpes virus, engages the innate immune system via activation of TLR9 by viral dsDNA after viral uptake (21). Thus, we examined the importance of endosomal acidification and TLR9 engagement on EBV-induced IFN-α production. We first determined that CFSE-labeled EBV was efficiently taken up by pDC compared to CFSE-labeled mock viral preparation (Fig. 4A). Pretreatment of pDC with chloroquine, an inhibitor of the endosomal acidification, significantly decreased production of IFN-α (Fig. 4B). While addition of exogenous EBV dsDNA alone did not stimulate IFN-α secretion (Fig. 3B), EBV after internalization did engage TLR9, as determined by blockade of the TLR9 receptor with increasing amounts of an antagonist deoxynucleotide, GpC 2006 (Fig. 4C), demonstrating a concentration-dependent blockade of IFN-α production. Thus, production of IFN-α by pDC following EBV exposure is dependent on viral uptake, endosomal acidification, and TLR9. This is consistent with the observation that endosomal acidification is essential in the innate immune response against the related herpes family virus member, HSV (21), and for the uncoating of EBV in B cells (37).
Figure 4. IFN-α production by pDC is dependent upon viral entry, endosomal acidification, and TLR9 engagement.
pDC were stimulated for 18 h in the presence of CFSE-labeled EBV (15,000 genomes) or mock virus with (A) viral interaction assessed by flow cytometry. (B) pDC (50,000 cells) sorted by flow cytometry were stimulated for 18 h in the presence of EBV (15,000 genomes) with or without pre-incubation with chloroquine (chl; 10 µm) or (C) in the presence of increasing amounts of the inhibitory deoxynucleotide GpC 2006. IFN-α levels were measured from the supernatants by ELISA. Greater than 90% of cells were viable after stimulation as determined by trypan blue staining. Error bars represent SD for experiments done in duplicate. ND (not detected) represents IFN-α less than 12.5 pg/ml. In (B) statistical analysis was performed by Mann-Whitney testing. Statistics in (C) performed by 1-Way ANOVA followed by Dunnett’s comparison.
Anti-MHC class II Abs inhibit IFN-α production by EBV in pDC
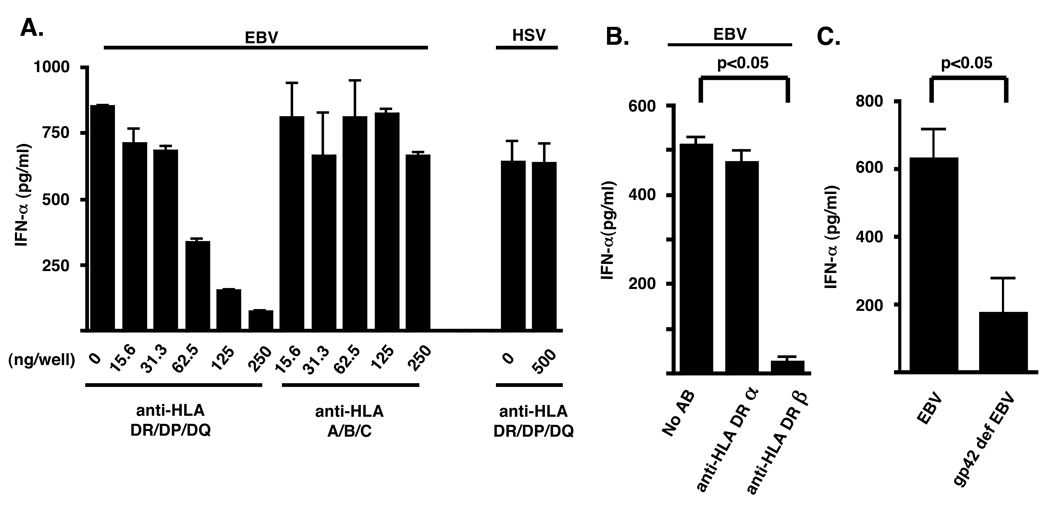
The binding of EBV gp42 protein to MHC class II molecules is necessary for the viral fusion to the cell membrane, as EBV deficient in this glycoprotein is unable to infect B cells (38). This binding can inhibit T cell activation by interfering with interaction of the T cell receptor with HLA-DR interaction of antigen presenting cells (39). Given the lack of CR2 expression on pDC (data not shown), we asked if EBV binds to MHC class II on pDC via gp42. We tested this hypothesis by pretreating purified pDC with anti-MHC class II antibodies before activation by EBV. IFN-α production by these cells decreased in a dose-dependent manner with increasing concentrations of blocking antibody (0.78 µg/ml–1.25 µg/ml) (Fig. 5A), an effect mediated largely by specific block of HLA DRβ (Fig. 5B). By contrast, IFN-α synthesis by pDC was not inhibited by addition of a blocking antibody to class I MHC (Fig. 5A). Importantly, we demonstrated that pretreatment of pDC with anti-class II MHC did not inhibit cell activation by HSV (Fig. 5A), consistent with studies demonstrating that this virus enters cells through nectins, heparins, and Herpes Virus Entry Mediators in addition to MHC class II binding (15). As a further control we demonstrated that, gp42-deficient EBV virions (19) did not promote IFN-α production by pDC (Fig. 5C). Taken together, these data suggest viral entry into pDC is dependent on MHC class II engagement.
Figure 5. IFN-α production by pDC requires MHC class II engagement.
pDC (50,000 cells) sorted by flow cytometry were incubated with EBV (15,000 genomes) or HSV-2 (5×10^6 pfu/ml) in the presence or absence of increasing amounts of (A) an anti-HLA DR, DP, DQ or an anti-HLA A, B, C blocking antibody, or (B) an anti-HLA DRα or HLA DRβ chain antibody for 15 minutes. (C) pDC were stimulated with gp42-deficient EBV (15,000 genomes) . After 18 h IFN-α ELISA measured levels in the supernatant. Error bars represent SD for experiments done in triplicate. ND (not detected) represents IFN-α less than 12.5 pg/ml. In (B), statistical analysis was performed by 1-Way ANOVA followed by Dunnett’s comparison. In (C), statistical analysis was performed by Mann-Whitney testing.
EBER induce IFN-α by pDC via TLR7 engagement
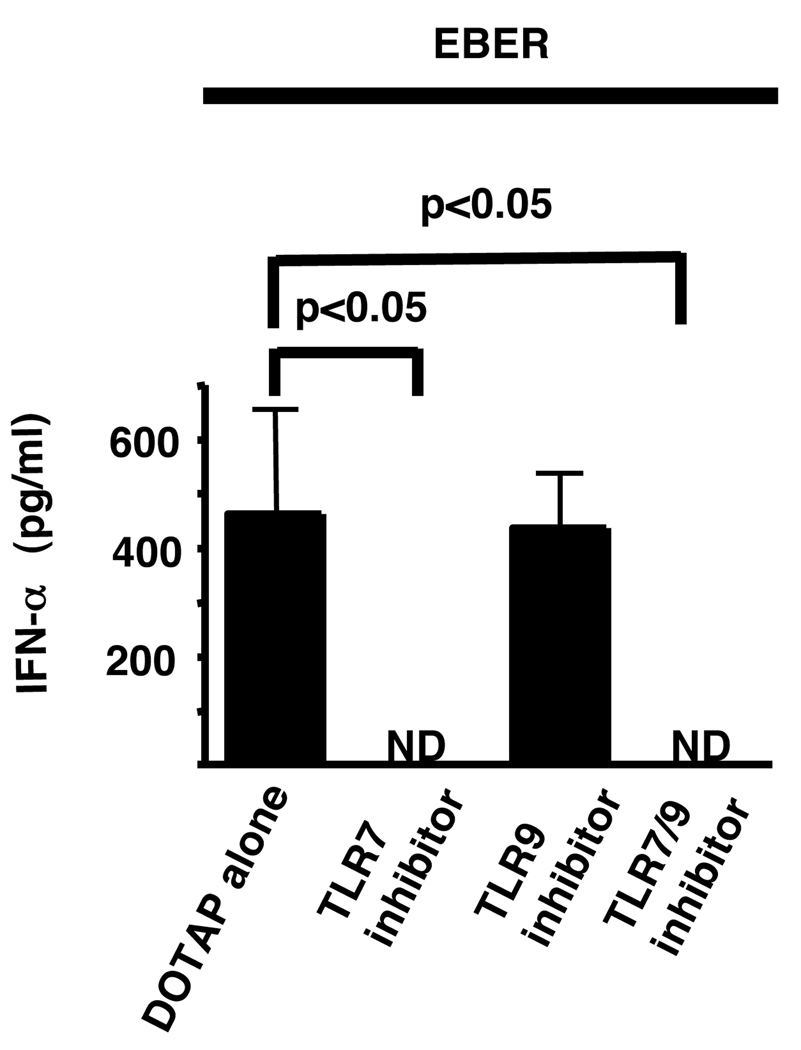
Since ssRNA are known to engage TLR7 (23, 40), we examined the effect of pDC stimulation by EBER which have ssRNA motifs throughout their structure (Fig. 6). EBER are small RNA molecules made in large quantities (107 copies/infected B cell) during latent infection of B cells (20). EBER transfected using DOTAP, a liposomal transfection system, promoted IFN-α production by pDC, an effect that was blocked by the TLR7 inhibitory oligonucleotide, IRS 661 and 954, but not by the TLR9–specific inhibitory oligonucleotide, IRS 869.
Figure 6. The stimulatory effect of EBER on pDC is TLR7 dependent.
In vitro transcribed and gel purified EBER were loaded onto DOTAP and incubated with sorted pDC (50,000 cells), with or without preincubation for 15 minutes with inhibitors of TLR7 (IRS 661), TLR9 (IRS 869), and TLR7/9 (IRS 954) . After 18 h IFN-α levels were measured in the supernatants by ELISA. Greater than 90% of cells were viable after stimulation as determined by trypan blue staining. ND (not detected) represents IFN-α less than 12.5 pg/ml. Statistical analysis was performed by 1-Way ANOVA followed by Dunnett’s comparison.
Discussion
We examined the interaction of EBV with human pDC, specifically demonstrating that upon viral challenge, these cells robustly make IFN-α. After initial replication in the host, EBV becomes a chronic latent infection with intermittent activation into a lytic phase (2). During the latter, new virus is produced, whereas during latency, large amounts of small RNAs known as EBER are synthesized. We took both phases of the viral life cycle into account demonstrating that both products, dsDNA viral core and EBER composed of ssRNA, promote IFN-α production by pDC via TLR9 and TLR7 engagement, respectively. In addition the unique association of EBV with SLE compared to other herpes viruses maybe due to its ability to bind MHC class II on pDC.
The role that MHC class II plays in EBV interaction with pDC may provide a mechanism by which these cells control viral infection. CR2 is a receptor for IFN-α on B cells, and the inhibition of this receptor diminishes the induction of IFN-α responsive genes (7). By contrast, CR2 is not expressed on pDC, which may explain why we did not find evidence of EBV infection of pDC when these cells were challenged with virus; in culture the pDC died within 2–4 days. We posit that the lack of viral infectivity of pDC is the absence of CR2 that is needed for binding to B cells and the inability to respond to B cell specific maturation signals initiated by EBV (11). In any case, the effect of IFN-α on pDC to resist infection needs to be further examined.
When EBV attaches via MHC class II to a pDC (as opposed to a B cell), presumably it is degraded with TLR9 activation and subsequent IFN-α production. MHC class II is known to recycle from the cell surface where it is reloaded in the early endosomal compartment with preprocessed peptides (41). This shuttling service could serve as a mechanism by which virus is continually taken up and subsequently degraded, in essence behaving as an EBV trap. Further investigations blocking the recycling pathway will be needed to show the importance of this pathway in EBV turnover. Variation in response to the virus may also play a role in its elimination, in that different individuals have varying degrees of induction of IFN-α following EBV challenge (data not shown). Regardless, as DC mature in response to EBV stimulation, they upregulate their MHC class II (42), offering a potential means to increase uptake of virus. Even though MHC class II renders B cells susceptible to EBV infection (18, 43), it conversely may serve as a lure for EBV attachment to pDC enabling these cells to degrade viral particles with subsequent production of IFN-α.
Polymorphisms in HLA -DR2 and -DR3 are associated with lupus (44). The critical EBV gp42 binding regions are at amino acid positions 46 and 72 of the β1 chain. Although the polymorphisms associated with lupus are not associated with residue changes at these positions, they could lead to increased, or decreased, viral binding via structural alterations induced by changes at other sites in the β1 chain, a hypothesis that can be explored experimentally.
Type I IFNs, including IFN-α, produced by pDC are believed to be pivotal in promotion of the lupus phenotype (45–48). Immune complexes containing RNA drive IFN-α production by pDC following binding to TLR7 (27, 48). EBER, made during latent EBV infection, can also promote type I IFN synthesis in an analogous manner. EBER are composed of dsRNA and ssRNA stem loops and have been shown to activate TL3 in mDC presumably by its dsRNA motif (49). Likewise, we would argue that the TLR7 found in pDC is activated by the ssRNA motifs of EBER. These viral RNAs also bind with high affinity to the La protein (20), found in RNA immune complexes, offering an additional link between EBV infection and stimulation of the IFN-α response in SLE because 15% of lupus patients are seropositive for antibodies against La (50).
Our data support the work of Lim and colleagues who showed that crude supernatants from the EBV-infected B95-8 marmoset cell line stimulated IFN-α production by enriched human pDC and that TLR9 stimulation of pDC by EBV is needed to induce IFN-γ secreting T cells (24). We extend their work with the demonstration that IFN-α production by pDC is dependent on TLR9 and TLR7 for dsDNA and ssRNA engagement, respectively, and dependent upon binding to MHC class II by whole virus. We use purified EBV, as well as a control consisting of a mock viral preparation (Fig. 1A), to challenge a sorted pDC population with greater that 99% cell purity (data not shown), to eliminate the possibility that cell stimulation was due to components of the cell lysate or cytokines found in the supernatant, rather than the virus per se. Although the ability of EBV to induce IFN-α production by pDC is on the surface not surprising given the importance of this cytokine in controlling infection by other herpes family viruses such as HSV, our work demonstrates the exquisite sensitivity of pDC to as few as 150 viral genomes, as well as the requirement of viral entry upon MHC class II engagement and not upon CR2, and the engagement of TLR7 by EBV RNAs.
In summary, we have demonstrated that exogenous antigens from a pathogen specifically associated with SLE can drive IFN-α production. While nucleic acids and apoptotic cells are autoantigens demonstrated to be important mediators of IFN-α production (26–28), our data suggests that in addition, EBV could initiate systemic autoimmune responses and perpetuate the cycle of inflammation in SLE by modulating IFN-α production. Elevated IFN-α signatures found in patients with lupus could be a response in part to EBV stimulation. If so, treatment of EBV infection in lupus patients with anti-viral drugs may offer a means for disease attenuation.
Acknowledgments
This work was funded by NIH Grants AR509737, AR60405, AR40072 and AR44076, CA53615, and by support from the Arthritis Foundation, the Connecticut Chapter of the Lupus Foundation, Rheuminations, Inc., and an Abbott Scholars Award.
References
- 1.National Center for Infectious Diseases. Epstein-Barr Virus and Infectious Mononucleosis. CDC; 2009 http://www.cdc.gov/ncidod/diseases/ebv.htm.
- 2.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 3.Evans AS, Rothfield NF, Niederman JC. Raised antibody titres to E.B. virus in systemic lupus erythematosus. Lancet. 1971;1(7691):167–168. doi: 10.1016/s0140-6736(71)91937-4. [DOI] [PubMed] [Google Scholar]
- 4.Tsokos GC, Magrath IT, Balow JE. Epstein-Barr virus induces normal B cell responses but defective suppressor T cell responses in patients with systemic lupus erythematosus. J Immunol. 1983;131(4):1797–1801. [PubMed] [Google Scholar]
- 5.Kaufman KM, Kirby MY, Harley JB, James JA. Peptide mimics of a major lupus epitope of SmB/B'. Ann N Y Acad Sci. 2003;987:215–229. doi: 10.1111/j.1749-6632.2003.tb06051.x. [DOI] [PubMed] [Google Scholar]
- 6.McClain MT, Rapp EC, Harley JB, James JA. Infectious mononucleosis patients temporarily recognize a unique, cross-reactive epitope of Epstein-Barr virus nuclear antigen-1. J Med Virol. 2003;70(2):253–257. doi: 10.1002/jmv.10385. [DOI] [PubMed] [Google Scholar]
- 7.Asokan R, Hua J, Young KA, Gould HJ, Hannan JP, Kraus DM, et al. Characterization of human complement receptor type 2 (CR2/CD21) as a receptor for IFN-alpha: a potential role in systemic lupus erythematosus. J Immunol. 2006;177(1):383–394. doi: 10.4049/jimmunol.177.1.383. [DOI] [PubMed] [Google Scholar]
- 8.James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997;100(12):3019–3026. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James JA, Neas BR, Moser KL, Hall T, Bruner GR, Sestak AL, et al. Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis Rheum. 2001;44(5):1122–1126. doi: 10.1002/1529-0131(200105)44:5<1122::AID-ANR193>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Kang I, Quan T, Nolasco H, Park SH, Hong MS, Crouch J, et al. Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J Immunol. 2004;172(2):1287–1294. doi: 10.4049/jimmunol.172.2.1287. [DOI] [PubMed] [Google Scholar]
- 11.Gross AJ, Hochberg D, Rand WM, Thorley-Lawson DA. EBV and systemic lupus erythematosus: a new perspective. J Immunol. 2005;174(11):6599–6607. doi: 10.4049/jimmunol.174.11.6599. [DOI] [PubMed] [Google Scholar]
- 12.Verdolini R, Bugatti L, Giangiacomi M, Nicolini M, Filosa G, Cerio R. Systemic lupus erythematosus induced by Epstein-Barr virus infection. Br J Dermatol. 2002;146(5):877–881. doi: 10.1046/j.1365-2133.2002.04627.x. [DOI] [PubMed] [Google Scholar]
- 13.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B'-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181(2):453–461. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11(1):85–89. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 15.Spear PG, Longnecker R. Herpesvirus entry: an update. J Virol. 2003;77(19):10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemerow GR, Mold C, Schwend VK, Tollefson V, Cooper NR. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J Virol. 1987;61(5):1416–1420. doi: 10.1128/jvi.61.5.1416-1420.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen MM, Haan KM, Longnecker R, Jardetzky TS. Structure of the Epstein-Barr virus gp42 protein bound to the MHC class II receptor HLA-DR1. Mol Cell. 2002;9(2):375–385. doi: 10.1016/s1097-2765(02)00465-3. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Spriggs MK, Kovats S, Turk SM, Comeau MR, Nepom B, et al. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71(6):4657–4662. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Hutt-Fletcher LM. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J Virol. 1998;72(1):158–163. doi: 10.1128/jvi.72.1.158-163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerner MR, Andrews NC, Miller G, Steitz JA. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198(3):513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, et al. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2004;101(31):11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 24.Lim WH, Kireta S, Russ GR, Coates PT. Human plasmacytoid dendritic cells regulate immune responses to Epstein-Barr virus (EBV) infection and delay EBV-related mortality in humanized NOD-SCID mice. Blood. 2007;109(3):1043–1050. doi: 10.1182/blood-2005-12-024802. [DOI] [PubMed] [Google Scholar]
- 25.Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54(2):408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 26.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50(6):1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 28.Means TK, Luster AD. Toll-like receptor activation in the pathogenesis of systemic lupus erythematosus. Ann N Y Acad Sci. 2005;1062:242–251. doi: 10.1196/annals.1358.027. [DOI] [PubMed] [Google Scholar]
- 29.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham RR, Kyogoku C, Sigurdsson S, Vlasova IA, Davies LR, Baechler EC, et al. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc Natl Acad Sci U S A. 2007;104(16):6758–6763. doi: 10.1073/pnas.0701266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozyrev SV, Lewen S, Reddy PM, Pons-Estel B, Witte T, Junker P, et al. Structural insertion/deletion variation in IRF5 is associated with a risk haplotype and defines the precise IRF5 isoforms expressed in systemic lupus erythematosus. Arthritis Rheum. 2007;56(4):1234–1241. doi: 10.1002/art.22497. [DOI] [PubMed] [Google Scholar]
- 32.Nikiforow S, Bottomly K, Miller G. CD4+ T-cell effectors inhibit Epstein-Barr virus-induced B-cell proliferation. J Virol. 2001;75(8):3740–3752. doi: 10.1128/JVI.75.8.3740-3752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guthridge JM, Young K, Gipson MG, Sarrias MR, Szakonyi G, Chen XS, et al. Epitope mapping using the X-ray crystallographic structure of complement receptor type 2 (CR2)/CD21: identification of a highly inhibitory monoclonal antibody that directly recognizes the CR2-C3d interface. J Immunol. 2001;167(10):5758–5766. doi: 10.4049/jimmunol.167.10.5758. [DOI] [PubMed] [Google Scholar]
- 34.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37(12):3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 35.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31(11):3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 36.Kadowaki N, Liu YJ. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum Immunol. 2002;63(12):1126–1132. doi: 10.1016/s0198-8859(02)00751-6. [DOI] [PubMed] [Google Scholar]
- 37.Nemerow GR, Cooper NR. Early events in the infection of human B lymphocytes by Epstein-Barr virus: the internalization process. Virology. 1984;132(1):186–198. doi: 10.1016/0042-6822(84)90102-8. [DOI] [PubMed] [Google Scholar]
- 38.Haan KM, Longnecker R. Coreceptor restriction within the HLA-DQ locus for Epstein-Barr virus infection. Proc Natl Acad Sci U S A. 2000;97(16):9252–9257. doi: 10.1073/pnas.160171697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ressing ME, van Leeuwen D, Verreck FA, Gomez R, Heemskerk B, Toebes M, et al. Interference with T cell receptor-HLA-DR interactions by Epstein-Barr virus gp42 results in reduced T helper cell recognition. Proc Natl Acad Sci U S A. 2003;100(20):11583–11588. doi: 10.1073/pnas.2034960100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 41.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375(6532):603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 42.Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, Gaugler B. IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J Immunol. 2003;171(7):3385–3393. doi: 10.4049/jimmunol.171.7.3385. [DOI] [PubMed] [Google Scholar]
- 43.Haan KM, Kwok WW, Longnecker R, Speck P. Epstein-Barr virus entry utilizing HLA-DP or HLA-DQ as a coreceptor. J Virol. 2000;74(5):2451–2454. doi: 10.1128/jvi.74.5.2451-2454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunckley H, Gatenby PA, Serjeantson SW. DNA typing of HLA-DR antigens in systemic lupus erythematosus. Immunogenetics. 1986;24(3):158–162. doi: 10.1007/BF00364743. [DOI] [PubMed] [Google Scholar]
- 45.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of Dendritic Cell Differentiation by INF-alpha in Systemic Lupus Erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 46.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 47.Pascual V, Banchereau J, Palucka AK. The central role of dendritic cells and interferon-alpha in SLE. J Clin Invest. 2003;15(5):548–556. doi: 10.1097/00002281-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Ronnblom L, Eloranta ML, Alm GV. Role of natural interferon-alpha producing cells (plasmacytoid dendritic cells) in autoimmunity. Autoimmunity. 2003;36(8):463–472. doi: 10.1080/08916930310001602128. [DOI] [PubMed] [Google Scholar]
- 49.Iwakiri D, Zhou L, Samanta M, Matsumoto M, Ebihara T, Seya T, et al. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J Exp Med. 2009;206(10):2091–2099. doi: 10.1084/jem.20081761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]