Abstract
Serotonin plays an important role in the regulation of anxiety states and physiological responses to aversive stimuli. Intracerebroventricular (i.c.v.) injection of the stress- and anxiety-related neuropeptide urocortin 2 (Ucn 2) increases c-Fos expression in serotonergic neurons in the dorsal (DRD) and caudal (DRC) parts of the dorsal raphe nucleus. These regions contain a subset of serotonergic neurons that projects via the dorsal raphe periventricular tract to periventricular structures, including the subfornical organ and ependymal layer, and to the ventricular system. To determine if Ucn 2 activates ventricle/periventricular-projecting serotonergic neurons in the midbrain raphe complex we made i.c.v. injections of the retrograde tracer Fluoro-Gold into the lateral ventricle, followed 7 days later by i.c.v. injection of Ucn 2. The DRD at −8.18 mm and the DRC at −8.54 mm and −9.16 mm bregma were analyzed using a combined brightfield and immunofluorescence technique. Approximately 40% of the ventricle/periventricular-projecting neurons in the subdivisions sampled were serotonergic. Urocortin 2 increased c-Fos expression in ventricle/periventricular-projecting serotonergic neurons in the DRC and in non-ventricle/periventricular-projecting serotonergic neurons in the DRD and DRC. Of the total population of ventricle/periventricular-projecting serotonergic neurons in the DRC at −8.54 and −9.16 mm bregma, 35% expressed c-Fos following Ucn 2 injections. These data are consistent with previous studies showing that i.c.v. injection of Ucn 2 activates subpopulations of serotonergic neurons restricted to the mid-rostrocaudal DRD and DRC, and further demonstrate that these include both subsets of serotonergic neurons that do and do not project to the ventricle/periventricular system.
Keywords: 5-HT, 5-hydroxytryptamine, cerebral aqueduct, cerebrospinal fluid, corticotropin-releasing factor, CRF, CRH, lateral ventricle, serotonin, third ventricle
Urocortin 2 (Ucn 2) is a 38-amino acid neuropeptide and a member of the corticotropin-releasing factor (CRF) family of neuropeptides, a group of stress and anxiety-related neuropeptides, that also includes CRF, urocortin 1 (Ucn 1), and urocortin 3 (Ucn 3) (Reyes et al, 2001). Urocortin 2 binds selectively and with high affinity to the type 2 CRF receptor (Reul and Holsboer, 2002) and has been implicated in a variety of behaviors including feeding (Hsu and Hsueh, 2001;Ohata and Shibasaki, 2004;Reyes et al, 2001;Zorrilla et al, 2004), motor behavior (Valdez et al, 2002) and anxiety-related behaviors (Pelleymounter et al, 2004). In Ucn 2 knockout mice, females, but not males, show increased basal corticosterone and adrenocorticotropic hormone concentrations at the transition from the light to the dark phase and an antidepressant-like phenotype in the forced swim test and tail suspension test (Chen et al, 2006). Urocortin 2 may exert some of its behavioral and physiological effects through interactions with midbrain and brainstem neuromodulatory systems such as the serotonergic system.
Corticotropin-releasing factor and the urocortins alter the neuronal activity of midbrain and pontine serotonergic systems. Microinfusions of low doses of CRF-related peptides directly into the dorsal raphe nucleus, a region with high numbers of serotonergic neurons projecting to limbic forebrain sites, decrease neuronal firing rates of serotonergic neurons while microinfusion of high doses increase them (Kirby et al, 2000;Pernar et al, 2004;Price et al, 1998). Consistent with these findings, direct microinjection of low doses of CRF or CRF-related neuropeptides into the dorsal raphe nucleus decreases extracellular concentrations of serotonin, while high doses increase them, in forebrain sites, including the lateral striatum (Price et al, 1998;Price and Lucki, 2001), lateral septum (Price and Lucki, 2001), basolateral nucleus of the amygdala (BLA; Amat et al, 2004), central nucleus of the amygdala (CE; Forster et al, 2006), hippocampus (de Groote et al, 2005), and nucleus accumbens (Lukkes et al, 2008). Consistent with these electrophysiological and neurochemical studies, we have previously shown that intracerebroventricular (i.c.v.) injection of Ucn 2 activates subpopulations of serotonergic neurons in the dorsal part of the caudal dorsal raphe nucleus, including the dorsal raphe nucleus, dorsal part (DRD) and caudal part (DRC; Staub et al, 2005;Staub et al, 2006).
Although the DRD and DRC regions are both sensitive to Ucn 2, these regions are anatomically and functionally different subdivisions of the dorsal raphe complex each with distinct patterns of afferent and efferent projections. The DRD region preferentially receives afferents from regions involved in the regulation of emotional behavior, including the lateral and ventral orbitofrontal and infralimbic cortices, the CE, the bed nucleus of the stria terminalis (BNST), and the dorsal, dorsomedial, lateral and posterior hypothalamic nuclei (Peyron et al, 1998); meanwhile, it sends efferent projections to the dorsolateral periaqueductal gray (DLPAG; Stezhka and Lovick, 1997)), BLA and CE (Abrams et al, 2005;Hale et al, 2008;Ottersen, 1981), BNST (Petit et al, 1995), nucleus accumbens and medial prefrontal cortex (mPFC; Van Bockstaele et al, 1993) and ventral hippocampus (Imai et al, 1986). The DRC is distinct from the DRD and preferentially receives afferents from the mPFC, hypothalamic regions including the preoptic area, arcuate nucleus, and perifornical and lateral hypothalamic areas, and midbrain and brainstem regions including the interpeduncular nucleus, laterodorsal tegmental area and area postrema (Lee et al, 2003); meanwhile, it sends efferents to the hippocampus and midline thalamic nuclei. The DRC is also the major source of serotonergic projections to the subfornical organ (a circumventricular organ with a reduced blood-brain barrier) as well as the ependymal lining and the cerebral ventricles including the lateral, third and fourth ventricles (Mikkelsen et al, 1997;Simpson et al, 1998). Although i.c.v. administration of Ucn 2 increases c-Fos expression within the DRD and DRC (Staub et al, 2005;Staub et al, 2006), it is unclear whether it activates the subpopulation of ventricle/periventricular-projecting serotonergic neurons there.
In this experiment we tested the hypothesis that the midbrain raphe complex contains a functional subset of ventricle/periventricular-projecting serotonergic neurons that are activated by stress and anxiety-related stimuli. In order to identify ventricle/periventricular-projecting serotonergic neurons in the dorsal raphe nucleus, the retrograde tracer Fluoro-Gold (FG) was injected into the lateral ventricle, followed 7 days later by i.c.v. injection of vehicle or Ucn 2. Immunohistochemistry was used to detect c-Fos (the protein product of the immediate-early gene, c-fos; as a marker of cellular responses to afferent stimulation) while immunofluorescence was used to detect tryptophan hydroxylase (TPH; as a marker of serotonergic neurons) and autofluorescence was used to detect FG. The combined immunohistochemistry/immunofluoresence/autofluorescence procedure was used to detect colocalization.
Materials and Methods
Animals
Adult male Wistar rats (B&K Universal, Hull, UK; N = 20) weighing 250–275 g, were housed in groups of 4 for one week prior to the surgery (cage dimensions (L×W×H): 56×38×15 cm). Rats were handled for 2 min per day for 5 days prior to surgery and were handled and weighed each day for seven days following the surgery to monitor health status. Following surgery rats were individually housed and remained single housed for the duration of the experiment (cage dimensions (L×W×H): 40×24×20 cm). Rats were kept under a 14/10 h light/dark cycle (lights on at 0500 h) in an experimental room with standard temperature (22°C) and humidity (50%). Experimental procedures were performed during the light cycle. Food and water were available ad libitum. All procedures were approved by the Ethical Review Group at the University of Bristol and were conducted in accordance with the UK Home Office Guidelines and the Scientific (Animal Procedures) Act, 1986.
Cannula Implantation
The procedures for implantation of i.c.v. cannulae have been described previously (Staub et al, 2005;Staub et al, 2006). Briefly, rats were anesthetized with sodium pentobarbital i.p. (65 mg/kg at 65 mg/mL dissolved in sterile saline; Sigma-Aldrich, P3761, St Louis, MO, USA). Following induction of anesthesia, rats were placed in a stereotaxic frame (SAS-4100, ASI Instruments, Warren, MI, USA) and i.c.v. guide cannulae (C315G, Plastics One, Roanoke, VA, USA) were implanted using coordinates AP, −1.0 mm; DV, −2.5 mm; ML, +1.5 mm (right) with reference to bregma (the tip of the guide cannula was placed 1 mm dorsal to the lateral ventricle) using coordinates from a standard rat brain stereotaxic atlas (Paxinos and Watson, 1998). The cannula assembly was secured to the skull using two stainless steel screws and fixed with dental acrylic. A dummy cannula (C315DC, Plastics One) of the same length as the guide cannula was placed inside the guide cannula.
FG injection
Twenty-four hours following surgery each rat received an i.c.v. injection of FG (Fluorochrome LLC, Denver, CO, USA). For the FG injection rats received injections while they remained in their home cages. An injection cannula (C315I, Plastics One) was connected to a 10 μl Hamilton syringe with PE20 tubing (PE-20, Braintree Scientific Inc, Braintree, MA, USA) and backfilled with FG. The injection cannula was inserted into the guide cannula and 0.5 μl of 0.9% sterile saline, followed by 2 μl of a 1% solution of FG in 0.9% sterile saline and then a further 0.5 μl of saline (total volume 3 μl) was injected (i.c.v.) using a Harvard Apparatus PHD 2000 Infusion pump (Cat. No. 70-2000, Harvard Apparatus, Holliston, MA, USA) at a rate of 1 μl/min. The injection cannula remained in place for 10 min following the injection. The guide cannula was then replaced.
Drug administration
Seven days following the FG injection, each rat received an i.c.v. injection of either Ucn 2 (n = 10) or vehicle (n = 10). The procedure for injection of Ucn 2 has been described previously (Staub et al, 2005;Staub et al, 2006) and is the same as that described for FG injection except that rats received injection of either 2 μl of 0.5 μg/μl mouse Ucn 2 in 0.9% sterile saline (1 μg/rat; Cat. No. 2019-24, Lot No. 417539, Phoenix Pharmaceutical, Karlsruhe, Germany) or 2 μl of 0.9% sterile saline. The injection cannula remained in place for 30 sec. The guide cannula was then replaced.
Tissue processing
Two hours following i.c.v. injection of either Ucn 2 or vehicle, rats were anesthetized with 0.5 ml of 200 mg/kg sodium pentobarbital (Euthatal, Merial, Harlow, UK) and transcardially perfused with 0.05 M phosphate buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde solution (prepared using 160 g paraformaldehyde, 60 g sucrose, 1616 ml 0.2 M Na2HPO4·7H2O, 384 ml 0.2 M NaH2PO4·H2O, and 2 L distilled H2O (dH2O), brought to pH 7.4 using sodium hydroxide pellets). Following fixation, the brains were removed and post-fixed in the 4% paraformaldehyde solution for 24 h at 4 °C and were then rinsed in 0.1 M sodium phosphate buffer (PB; 80.8% 0.1 M Na2HPO4·7H2O and 19.2% 0.1 M NaH2PO4·H2O) twice for 12 h. The brains were then placed in 30% sucrose in 0.1 M PB for 2–3 days (until brains had sunk). Brains were then blocked into two pieces with a cut in the coronal plane at the caudal border of the mammillary bodies (approximately −5.30 mm bregma) using a rat brain matrix (RBM-4000C, ASI Instruments) and rapidly frozen in isopentane chilled on dry ice. The brains were then stored at −80 °C. Brain sections (30 μm) were then made using a cryostat and stored as 6 alternate sets of sections. The tissue was stored at −20 °C in cryoprotectant (prepared using 270 ml ethylene glycol, 160 ml glycerol, 202 ml 0.2 M Na2HPO4·7H2O, 48 ml 0.2 M NaH2PO4·H2O, and 320 ml dH2O) until immunohistochemical procedures were conducted.
Immunohistochemistry
Fluoro-Gold immunohistochemistry
To examine the injection site, one set of forebrain sections was used for immunohistochemistry using an antibody directed against FG (rabbit anti-FG, Cat. No. AB153, Lot No. 25060005, Chemicon (Millipore), Billerica, MA, USA). Immunohistochemistry was conducted on free-floating brain sections in 12-well plates (Corning Life Sciences, Lowell, MA, USA) and sections were gently shaken on an orbital shaker throughout the procedure. The length of all washes, rinses and pre-incubations was 15 min. Tissue was first washed twice in 0.05 M phosphate buffered saline (PBS), then rinsed in 1% H2O2 in 0.05 M PBS, followed by washing in 0.05 M PBS and pre-incubation in 0.05 M PBS containing 0.3% Triton X-100 (0.3% PBST); sections were then incubated overnight at room temperature (RT) with the rabbit anti-FG antibody (1:10000) in 0.05 M PBS containing 0.1% Triton X-100 (0.1% PBST). After a 16 h incubation, the tissue was washed twice in 0.05 M PBS followed by incubation with a biotinylated donkey anti-rabbit secondary antibody (1:500, Cat. No. 711-065-152, Lot No. 84855, Jackson ImmunoResearch, West Grove, PA, USA) in 0.05 M PBS for 90 min. Tissue was washed twice in 0.05 M PBS followed by incubation with an avidin–biotin–peroxidase complex (Elite ABC reagent, Cat. No. PK-6100, 1:200; Vector Laboratories, Burlingame, CA, USA) in 0.05 M PBS for 90 min. Last, tissue was washed twice in 0.05 M PBS, and incubated in a peroxidase chromogen substrate solution consisting of 0.01% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Cat. No. D9015, Lot No. 85897LJ, Sigma-Aldrich, St. Louis, MO, USA) and 0.0015% H2O2 in 0.05 M PBS for 20 min. Finally, sections were washed twice in 0.05 M PBS to stop the reaction. Brain sections were rinsed briefly in 0.15% gelatin in distilled water then mounted on microscope slides (VistaVision, Cat No. 16004-382, VWR, West Chester, PA, USA), dehydrated through an alcohol series and cleared with xylene. The slides were then mounted with cover slips using Entellan mounting medium (Electron Microscopy Science, Hatfield, PA, USA). The color reaction of the FG immunostaining was orange–brown and localized in fibers and cell bodies.
c-Fos/TPH immunohistochemistry
A second set of sections, including the midbrain raphe complex, was used for double immunostaining using primary antibodies directed against the protein product of the immediate-early gene c-fos (rabbit anti-c-Fos polyclonal antibody, Cat. No. PC38, Lot No. D00015268, 1:3000; Calbiochem (EMD Chemicals) Gibbstown, NJ, USA), and against tryptophan hydroxylase (TPH; sheep anti-TPH antibody, Cat. No. T8575, Lot No. 096K1026, 1:10000; Sigma-Aldrich). The procedure for double immunostaining for c-Fos and TPH was essentially the same as described for immunostaining for FG. Tissue was washed twice in 0.05 M PBS, then rinsed in 1% H2O2 in 0.05 M PBS, followed by washing in 0.05 M PBS and pre-incubation in 0.3% PBST; sections were then incubated overnight at RT with rabbit anti-c-Fos antibody (1:3000) in 0.1% PBST. After a 16 h incubation, the tissue was washed twice in 0.05 M PBS followed by incubation with a biotinylated donkey anti-rabbit secondary antibody (1:200, Cat. No. E0353, Lot No. E035301028501-0802, DAKO, Carpinteria, CA, USA) in 0.05 M PBS for 90 min. Tissue was washed twice in 0.05 M PBS followed by incubation with an avidin–biotin–peroxidase complex (Elite ABC reagent, Cat. No. PK-6100, 1:200; Vector Laboratories) in 0.05 M PBS for 90 min. Tissue was then washed twice in 0.05 M PBS, and incubated in a peroxidase chromogen substrate (Cat. No. SK4700; Vector SG; Vector Laboratories; diluted as recommended by the vendor) in 0.05 M PBS for 20 min. After the chromogen reaction, tissue was immediately washed in 0.05 M PBS, then in 1% H2O2 in 0.05 M PBS, and twice in 0.05 M PBS. Then, slices were incubated with sheep anti-TPH antibody (1:10000) in 0.1% PBST overnight at room temperature. All subsequent steps were identical to those described above for the immuno-peroxidase localization of c-Fos immunoreactivity, except for the secondary antibody and chromogen reaction steps; these used a rabbit anti-sheep secondary antibody (Cat. No. PK- 6106, 1:200, Vector Laboratories), and a peroxidase chromogen substrate solution consisting of 0.01% 3,3′-diaminobenzidine tetrahydrochloride (DAB) and 0.0015% H2O2 in 0.05 M PBS (20 min). Finally, sections were washed twice in 0.05 M PBS to stop the reaction. Brain sections were then mounted on microscope slides and mounted with cover slips in the same manner as described for sections immunostained for FG. The color reaction of the c-Fos immunostaining was blue–black and localized to the nucleus while TPH immunostaining was orange–brown and localized to the cytoplasm.
c-Fos/TPH/FG combined immunohistochemistry/immunofluorescence/autofluorescence
A third set of sections was used for the combined detection of c-Fos, TPH and FG in the dorsal raphe nucleus. Fluoro-Gold was visualized as autofluorescence using a wide band ultraviolet excitation filter (Excitation, 356 nm, Emission, 458 nm; DAPI). The procedure for the brightfield immunohistochemical detection of c-Fos was essentially the same as that described above, except that a different lot of rabbit anti-c-Fos primary antibody was used (Cat. No. PC38, Lot No. D00058535, 1:3000; Calbiochem (EMD Chemicals)) and the peroxidase chromogen substrate solution was 0.01% DAB and 0.0015% H2O2 in 0.05 M PBS (20 min), which resulted in a brown/orange nuclear stain. The immunofluorescence procedure for TPH was then conducted as follows. Immediately following the DAB reaction, tissue was washed twice in 0.05 M PBS for 15 min each time and then incubated with sheep anti-TPH antibody (1:1000) in 0.1% PBST overnight at RT (TPH; sheep anti-TPH antibody, Cat. No. T8575, Lot No. 047K1223, 1:1000; Sigma-Aldrich). After 16 h, tissue was rinsed twice in 0.05 M PBS and then incubated with a secondary antibody conjugated to Cy5 (donkey anti-sheep Cy5, 1:200, Cat No. 713-176-147, Lot No. 80271, Jackson ImmunoResearch). Tissue was then washed twice in 0.05 M PBS and mounted on glass microscope slides using Vectashield Mounting Medium for fluorescence (H-1000, Vector Laboratories). Nail polish was used to seal the outer edges of the cover slips.
Cell counting
Brightfield analysis of c-Fos/TPH immunohistochemistry
Cell counting for the c-Fos/TPH immunohistochemistry analysis using brightfield microscopy was conducted as previously described (Staub et al, 2005;Staub et al, 2006). The numbers of c-Fos-immunoreactive (c-Fos-ir) serotonergic neurons (i.e. c-Fos-ir/TPH-ir neurons), the numbers of c-Fos-ir, non-serotonergic cells (i.e. c-Fos-ir/TPH-immunonegative cells), and the total numbers of TPH-ir neurons sampled (i.e., both c-Fos-ir/TPH-ir and c-Fos-immunonegative/TPH-ir neurons) were counted in different regions of the DR and MnR at five rostrocaudal levels (−7.46, −8.00, −8.18, −8.54, and −9.16 mm bregma) using brightfield microscopy. The subdivisions of the DR studied included the dorsal raphe nucleus, dorsal part (DRD) and dorsal raphe nucleus, ventral part (DRV) at −7.46 mm bregma, the DRD, DRV and dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG) at −8.00 mm bregma, the DRD, DRV, DRVL/VLPAG, dorsal raphe nucleus, interfascicular part (DRI) and the median raphe nucleus (MnR) at −8.18 mm bregma, the DRI, MnR, and dorsal raphe nucleus, caudal part (DRC) at −8.54 mm bregma, and the DRC at −9.16 mm bregma. The DRVL and adjacent VLPAG contain a distinct cluster of multipolar serotonergic neurons (Johnson et al, 2004), together defined as the “lateral wings” of the DR (Steinbusch, 1981;Steinbusch, 1984). As serotonergic neurons in the DRVL and VLPAG appear to be functionally related (Johnson et al, 2004), cells in these structures were counted as a single population, using the abbreviation DRVL/VLPAG. Cells were counted in both the left and the right DRVL/VLPAG and the cell counts were summed to give a total number of cells in the DRVL/VLPAG. For the remaining subdivisions, which are all located on the midline, cells were counted on both the left and right sides of the midline and the cell counts were summed to give a total number of cells in each subdivision. One section from each rat at each anatomical level was sampled. Cell counts were conducted using brightfield microscopy using a 40x objective lens (400× total magnification) by an investigator blind to the assignment of treatment groups.
Brightfield/fluorescence analysis of combined c-Fos/TPH immunohistochemistry/immunofluorescence and FG autofluorescence
Cell counting for the combined brightfield/fluorescence imaging was conducted in a manner similar to that previously described (Hale et al, 2008). Photomicrographs (200× total magnification) were generated for the DRD at −8.18 mm bregma and the DRC at −8.54 mm and −9.16 mm bregma using a Nikon 90i microscope and a Photometrics CoolSNAP ES digital camera linked to a computer with NIS Elements 3.00 imaging software (A.G. Heinze Inc., Lake Forest, CA, USA). For each rat, photographs were taken using DAPI (to visualize FG autofluorescence), FITC (to visualize c-Fos, as nuclear c-Fos immunoreactivity occludes background autofluorescence) and Cy5 (to visualize TPH immunofluorescence) filters. Two sets of images were required, either side of the midline, to capture the entire subdivision. Additional photographs using brightfield microscopy were taken to confirm c-Fos-ir cells. Separate layers for c-Fos-ir, FG autofluorescent (FG+) and TPH-ir photomicrographs were created using Adobe Photoshop 6.0.1 (Adobe Systems Incorporated, San Jose, CA, USA) and the numbers of c-Fos-ir, FG+ and TPH-ir cells were quantified by placing dots over each c-Fos-ir, FG+ or TPH-ir profile in additional cell counting layers. The cell counting layers were superimposed and the numbers of single-labeled c-Fos-ir, single-labeled FG+, single-labeled TPH-ir, double-labeled (c-Fos-ir/TPH-ir; c-Fos-ir/FG+ and FG+/TPH-ir), and triple-labeled (c-Fos-ir/FG+/TPH-ir) neurons were counted. Only full cell body profiles were counted. Double-labeled and triple-labeled neurons were confirmed with the slides themselves using a 40x objective lens (400× total magnification).
Image capture
Photomicrographs were taken using a Nikon 90i microscope and a Photometrics CoolSNAP ES digital camera (for fluorescence) or a Nikon DS-Fi1 digital camera, both of which were linked to a computer with NIS Elements 3.00 imaging software (A.G. Heinze Inc., Lake Forest, CA, USA). Confocal images were generated using a Leica TCS SP2 AOBS laser scanning confocal microscope using DAPI and Cy5 filter cubes and Leica Confocal Software (v. 2.00, Leica Microsystems, Heidelberg, Germany), and presented as 21 μm-thick z-stack projections. Photographic plates were prepared in CorelDraw for Windows 12.0 (Viglen Ltd., Wembley, UK).
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) with repeated measures followed, when appropriate, by planned pairwise comparisons using Fisher’s Protected Least Significant Difference (LSD) tests using PASW Statistics (17.0.2 for Windows, SPSS Inc., Chicago, IL, USA). A Greenhouse-Geisser correction epsilon (ε) was used for repeated measures analysis to correct for potential violation of the sphericity assumption.
The brightfield cell counts for the numbers of c-Fos-ir/TPH-ir (serotonergic) neurons, the numbers of c-Fos-ir/TPH-immunonegative (non-serotonergic) neurons, and the total numbers of TPH-ir neurons sampled were analyzed separately using treatment group (two levels: i.c.v. saline and i.c.v. Ucn 2) as a between-subjects factor and brain region (14 levels) as a within-subjects factor.
For the combined brightfield/fluorescence cell counts several populations of labeled cells were analyzed, 1) the numbers of single-labeled c-Fos-ir cells, 2) the total numbers of FG+ neurons, 3) the total numbers of TPH-ir neurons, 4) the numbers of c-Fos-ir/TPH-ir/FG− (non-ventricle/periventricular-projecting, c-Fos-expressing, serotonergic) neurons, 5) the numbers of c-Fos-ir/TPH-immunonegative/FG+ (ventricle/periventricular-projecting, c-Fos-expressing, non-serotonergic) neurons, 6) the prevalence of FG+/TPH+ neurons (the proportion of ventricle/periventricular-projecting neurons that were serotonergic), and 7) the numbers of c-Fos-ir/TPH-ir/FG+ (triple-labeled) neurons. These populations of cells were analyzed separately using treatment group (two levels: i.c.v. saline and i.c.v. Ucn 2) as a between-subjects factor and brain region (3 levels) as a within-subjects factor. Significance was accepted for the ANOVAs and post hoc Fisher’s Protected LSD tests when P < 0.05.
Outliers (2.6% (brightfield); 2.8% (combined brightfield/fluorescence)) were identified by Grubbs test (Grubbs, 1969) and excluded. Replacement data used for the repeated measures ANOVAs were calculated using the Petersen method (Petersen, 1985). Replacement data were not included in post hoc analyses and are not represented in the table or in graphical representation of the data.
Results
FG injection site and uptake
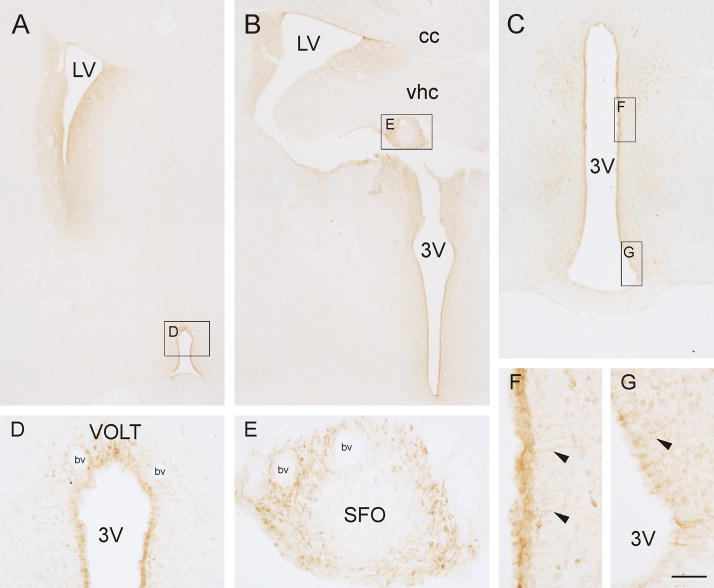
To examine the localization of the i.c.v. injection site and uptake of FG, immunohistochemistry for FG was conducted on forebrain sections (Fig. 1). The criteria for inclusion of individual rats in the analysis of retrogradely labeled cells in the midbrain raphe nuclei were based on Mikkelsen et al (1997). Rats were included in the analysis if FG was taken up by the ependyma of the lateral (Fig. 1A, B) and third ventricles (Fig. 1B, C, D, F) and if there was staining in fibers (presumed tanycytes) close to the 3V (Fig 1F, G). Six rats (3 from each treatment group) were excluded from the analysis based on these criteria.
Figure 1.
Photomicrographs illustrating Fluoro-Gold (FG) immunoreactivity following intracerebroventricular (i.c.v.) injection of FG into the lateral ventricle in Rat #135 (see also Figs. 5 and 6). A) Low power photomicrograph of a rat brain section from approximately −0.36 mm bregma showing FG-like immunoreactivity in the lateral ventricle (LV) and the rostral part of the third ventricle (3V) including the vascular organ of the lamina terminalis (VOLT). Black box in A indicates region shown at higher magnification in D. B) Low power photomicrograph of a rat brain section from approximately −1.00 mm bregma, close to the injection site, including the subfornical organ (SFO). Black box in B indicates the region shown at higher magnification in E. C) Low power photomicrograph showing FG-like immunoreactivity in the 3V from approximately −3.30 mm bregma. Boxes in C indicate regions shown at higher magnification in F and G. Arrowheads in F and G show FG-like immunoreactivity in fibers (presumed tanycytes) close to the 3V. Abbreviations; 3V, third ventricle; bv, blood vessel, cc, corpus callosum; LV, lateral ventricle; SFO, subfornical organ; vhc, ventral hippocampal commissure; VOLT, vascular organ of the lamina terminalis. Scale bar, 500 μm (A,B), 250 μm (C), 100 μm (D,E), 50 μm (F,G).
Fluoro-Gold immunoreactivity was also observed in circumventricular organs including the vascular organ of the lamina terminalis (VOLT; Fig. 1A, D) and subfornical organ (SFO; Fig. 1B, E). Both of which are known to contain serotonin-immunoreactive fibers (Takeuchi and Sano, 1983) and therefore may contribute to the pattern of retrograde labeling in the midbrain raphe nuclei following i.c.v. injection of FG.
c-Fos/TPH immunohistochemistry
c-Fos expression in serotonergic neurons in subdivisions of the midbrain raphe complex
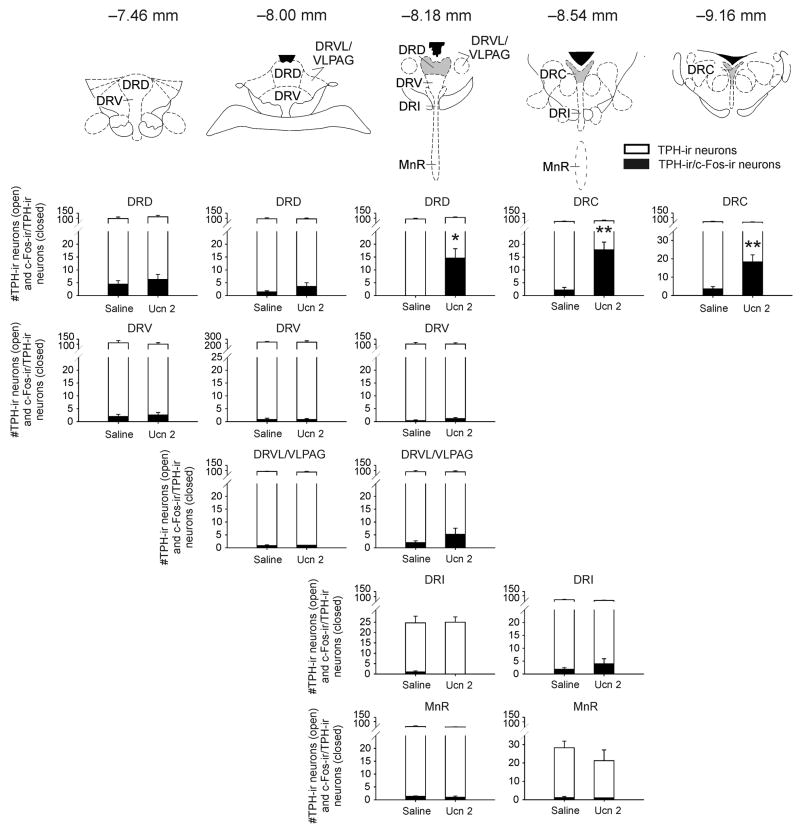
Consistent with our previous reports (Staub et al, 2005;Staub et al, 2006), i.c.v. injection of Ucn 2 increased c-Fos expression in serotonergic neurons within subdivisions of the midbrain raphe complex (repeated measures ANOVA, treatment × region interaction effect, F(13,156) = 7.61, p < 0.001, ε = 0.23; treatment main effect, F(1,12) = 8.70, p = 0.012; region main effect, F(13,156) = 16.24, p < 0.001, ε = 0.23; Fig. 2, closed bars; Fig. 3). Post hoc Fisher’s Protected LSD tests showed increased c-Fos expression in serotonergic neurons of the DRD at −8.18 mm bregma, the DRC at −8.54 mm bregma, and the DRC at −9.16 mm bregma. Unlike our previous report (Staub et al, 2005), post hoc tests failed to detect an increase in the DRD at −7.46 mm, the DRV at −8.18 mm and the DRI at −8.54 mm bregma. These results are however consistent with our more recent findings, suggesting a more regionally specific effect of Ucn 2 (Staub et al, 2006). It is possible that the differences between the findings may be due to differences in the experimental procedures (for discussion, see Staub et al, 2006).
Figure 2.
Intracerebroventricular (i.c.v.) injection of mouse urocortin 2 (Ucn 2) increased c-Fos expression in specific subpopulations of tryptophan hydroxylase-immunoreactive (TPH-ir) neurons within the midbrain raphe complex. Closed bars represent the numbers of c-Fos-immunoreactive (c-Fos-ir)/TPH-ir neurons within each subdivision. Open bars represent the total numbers of TPH-ir neurons within each subdivision. The subdivisions and their rostrocaudal levels (mm bregma) are illustrated in line drawings from a standard stereotaxic atlas of the rat brain (Paxinos and Watson, 1998) above each column of graphs. Grey shaded areas in the line drawings highlight regions that showed increased c-Fos expression in serotonergic neurons following Ucn 2 injection. *p < 0.05, **p < 0.01 versus saline-treated controls, Fisher’s Protected LSD tests, (n = 7 for both groups). Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; MnR, median raphe nucleus; Ucn 2, mouse urocortin 2; VLPAG, ventrolateral part of the periaqueductal gray.
Figure 3.
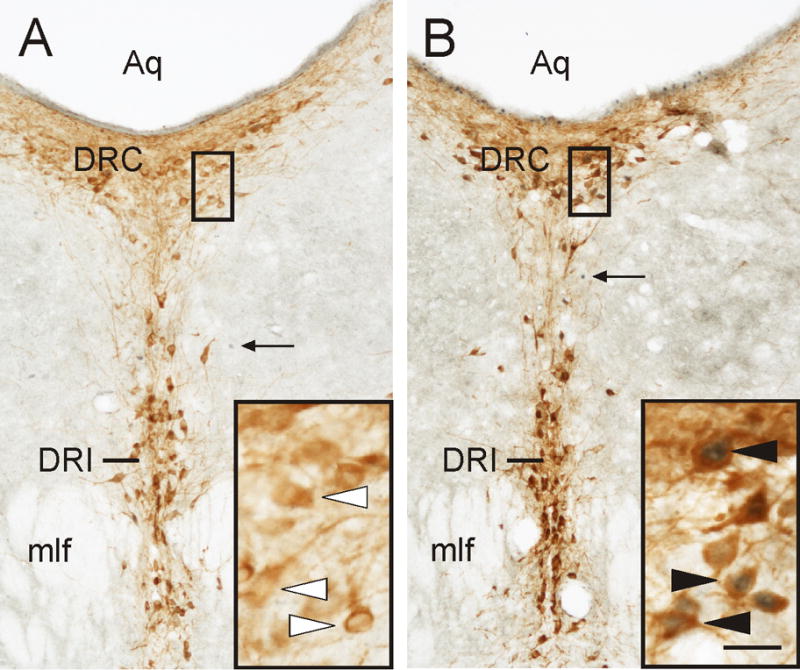
Photomicrographs illustrating c-Fos expression in serotonergic neurons in the caudal part of the dorsal raphe nucleus (DRC; −8.54 mm bregma) following i.c.v. injection of Ucn 2. A) Representative photomicrograph of a section from an i.c.v. saline-injected control rat. B) Representative photomicrograph of a section from an i.c.v. Ucn 2-injected rat. Black boxes indicate regions shown at higher magnification in insets in the lower right corner of each image. Arrows indicate examples of c-Fos-ir cells (blue/black nuclear staining); white arrowheads indicate TPH-ir/c-Fos-immunonegative (serotonergic) neurons (brown/orange cytoplasmic staining); filled arrowheads indicate TPH-ir/c-Fos-ir (double-immunostained) neurons. Abbreviation: Aq, cerebral aqueduct; DRC, dorsal raphe nucleus, caudal part; DRI, dorsal raphe nucleus, interfascicular part; mlf, medial longitudinal fasciculus. Scale bar, 100 μm (A,B), 25 μm (insets).
c-Fos expression in non-serotonergic cells in subdivisions of the midbrain raphe complex
Intracerebroventricular injection of Ucn 2 increased c-Fos expression in non-serotonergic cells within subdivisions of the midbrain raphe complex (treatment × region interaction effect, F(13,156) = 3.04, p = 0.015, ε = 0.40; region main effect, F(13,156) = 17.44, p < 0.001, ε = 0.40; the treatment main effect did not reach statistical significance; Table 1). Post hoc Fisher’s Protected LSD tests showed increased c-Fos expression in non-serotonergic cells in Ucn 2-injected rats compared with saline-injected controls in the DRD at −8.18 mm bregma, the DRC at −8.54 mm bregma, and the DRC at −9.16 mm bregma.
Table 1.
Intracerebroventricular (i.c.v.) injection of Ucn 2 increased c-Fos expression in non-serotonergic neurons within the midbrain raphe complex.
| Rostrocaudal level (mm bregma) | Saline | Ucn2 | |
|---|---|---|---|
| DRD | − 7.46 mm | 16.3 ± 2.8 | 20.7 ± 3.5 |
| DRV | − 7.46 mm | 5.7 ± 2.1 | 4.8 ± 1.1 |
| DRD | − 8.00 mm | 9.1 ± 2.1 | 12.0 ± 2.8 |
| DRVL | − 8.00 mm | 12.4 ± 2.5 | 17.4 ± 2.9 |
| DRV | − 8.00 mm | 4.6 ± 0.9 | 3.2 ± 0.9 |
| DRD | − 8.18 mm | 2.0 ± 1.7 | 16.7 ± 3.7* |
| DRVL | − 8.18 mm | 16.8 ± 3.8 | 18.0 ± 3.1 |
| DRV | − 8.18 mm | 4.2 ± 1.6 | 3.9 ± 0.9 |
| DRI | − 8.18 mm | 2.3 ± 1.3 | 0.6 ± 0.3 |
| MnR | − 8.18 mm | 3.2 ± 0.9 | 1.7 ± 0.7 |
| DRC | − 8.54 mm | 6.7 ± 2.6 | 19.0 ± 3.1* |
| DRI | − 8.54 mm | 4.7 ± 0.8 | 4.8 ± 2.0 |
| MnR | − 8.54 mm | 6.0 ± 1.3 | 3.0 ± 0.7 |
| DRC | − 9.16 mm | 10.0 ± 2.2 | 21.6 ± 4.7* |
p < 0.05, Fisher's protected LSD test.
Total TPH-ir neurons in subdivisions of the midbrain raphe complex sampled
The numbers of TPH-ir neurons varied across the subdivisions of the midbrain raphe complex (region main effect, F(13,156) = 59.38, p < 0.001, ε = 0.25; Fig 2, open bars). However there was no difference in the numbers of TPH-ir neurons across treatments.
c-Fos/FG/TPH combined brightfield/fluorescence analysis
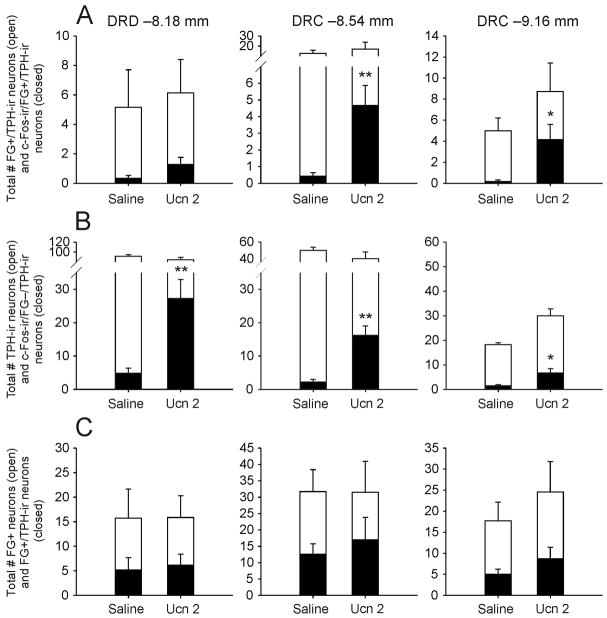
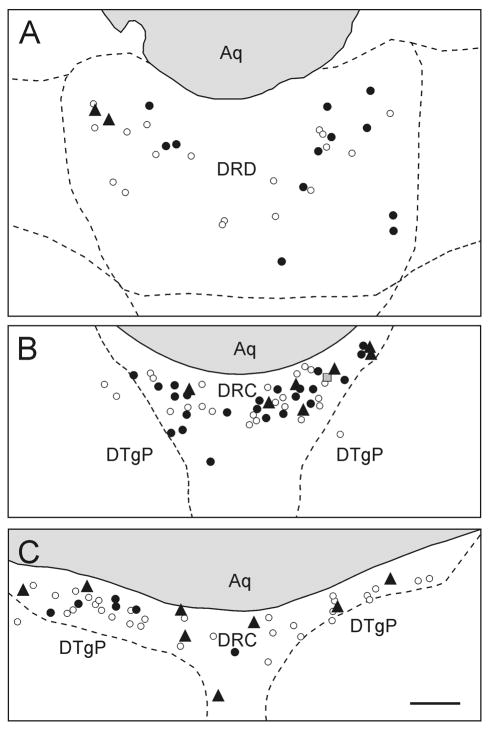
Intracerebroventricular injection of Ucn 2 increased c-Fos expression in ventricle/periventricular-projecting serotonergic neurons (c-Fos-ir/FG+/TPH-ir; triple-labeled) in the dorsal raphe nucleus (region × treatment interaction, F(2,24) = 4.07, p = 0.037, ε = 0.87; treatment main effect, F(1,12) = 13.57, p = 0.003; region main effect, F(2,24) = 5.58, p = 0.014, ε = 0.87; Fig. 4A, Fig. 5, Fig. 6). Post hoc Fisher’s Protected LSD tests showed an increase in triple-labeled neurons in the Ucn 2-injected group compared with the saline-injected control group in the DRC at −8.54 mm bregma (Fig 4A, Fig. 6) and the DRC at −9.16 mm bregma (Fig. 4A, Fig. 5, Fig. 6). Of the population of ventricle/periventricular-projecting serotonergic neurons, 12.9 ± 4.9% in the DRD at −8.18 mm bregma, 31.5 ± 9.7% in the DRC at −8.54 mm bregma and 38.7 ± 13.0% in the DRC at −9.16 mm bregma were activated (c-Fos-ir) following i.c.v. Ucn 2 injection. Of the total population of ventricle/periventricular-projecting serotonergic neurons in the regions that showed statistically significant increases, the DRC at −8.54 mm bregma and DRC at −9.16 mm bregma, 35.4 ± 8.0% expressed c-Fos following Ucn 2 injection.
Figure 4.
Graphs illustrating the effects of i.c.v. Ucn 2 on c-Fos expression in ventricle/periventricular-projecting and non-ventricle/periventricular-projecting serotonergic neurons in the dorsal part of the dorsal raphe nucleus (DRD; −8.18 mm bregma; left column) and the caudal part of the dorsal raphe nucleus (DRC; −8.54 mm bregma; middle column; −9.16 mm bregma; right column). A) i.c.v. injection of Ucn 2 increased c-Fos expression in FG+ (ventricle/periventricular-projecting) neurons that were immunopositive for TPH (serotonergic neurons) in the DRC (−8.54 mm and −9.16 mm bregma) (closed bars). Open bars show the total numbers of FG+/TPH-ir neurons, closed bars show the numbers c-Fos-ir/FG+/TPH-ir (triple-labeled) neurons. B) i.c.v. injection of Ucn 2 increased c-Fos expression in FG− (non-ventricle/periventricular-projecting) serotonergic neurons in the DRD and DRC (closed bars). Open bars represent the total numbers of TPH-ir neurons. C) Graphs illustrating the proportion of FG+ (ventricle/periventricular-projecting) neurons that were serotonergic. Open bars show the total numbers of FG+ neurons, closed bars show the numbers of FG+/TPH-ir neurons. Note differences in y-axis scales. *p < 0.05, **p < 0.01 versus saline-injected controls, Fisher’s Protected LSD tests (n = 7 for both groups).
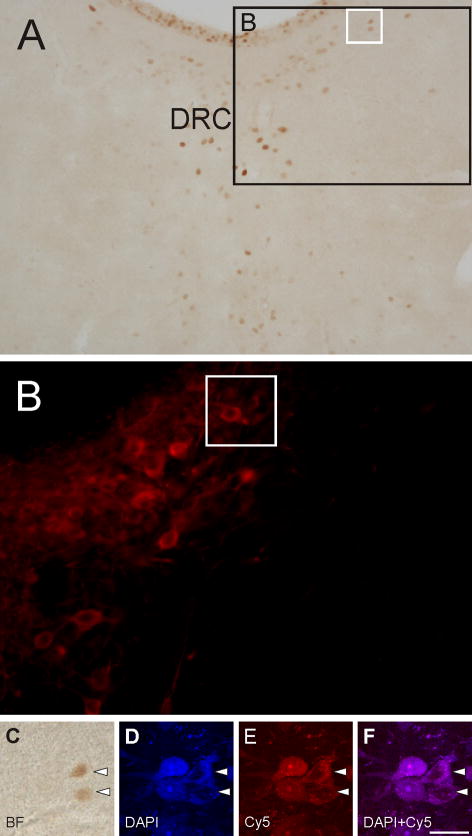
Figure 5.
Brightfield, epifluorescence and confocal fluorescence photomicrographs illustrating ventricle/periventricular-projecting serotonergic neurons activated by i.c.v. injections of Ucn 2 (Rat#135, see also Figs. 1 and 6). A) Brightfield image of c-Fos-ir nuclei (brown/orange nuclear immunostaining) in the DRC (−8.54 mm bregma). Black box in A indicates the region shown at higher magnification with epifluorescence photomicrograph in B. White box in A indicates the region shown at higher magnification with a brightfield photomicrograph in C and in confocal fluorescence photomicrographs in D, E and F. The cerebral aqueduct is located at the top of the photomicrograph. B) Epifluorescence photomicrograph showing TPH-ir neurons (red). White box in B indicates the region shown at higher magnification in C-F. C) Brightfield photomicrograph showing two cells with c-Fos-ir nuclei (white arrowheads). D) Confocal autofluorescence image of FG+ neurons (DAPI). E) Confocal immunofluorescence image of TPH-ir neurons (Cy5). F) Combined confocal image of DAPI and Cy5 showing TPH-ir/FG+ neurons. Note that nuclear c-Fos immunoreactivity occludes the DAPI/Cy5 fluorescence. White arrowheads show examples of c-Fos-ir/FG+/TPH-ir (triple-labeled) neurons. Abbreviations: BF, brightfield; DRC, dorsal raphe nucleus, caudal part. Scale bar, 72 μm (A), 37 μm (B), 25 μm (C–F).
Figure 6.
Schematic illustration showing the distribution of c-Fos expression in ventricle/periventricular-projecting serotonergic and non-serotonergic neurons in the dorsal raphe nucleus (Rat#135, see also figures 1 and 5). A) DRD (−8.18 mm bregma). B) DRC (−8.54 mm bregma). C) DRC (−9.16 mm bregma). Black circles represent c-Fos-immunonegative/FG+/TPH-ir neurons, white circles represent c-Fos-immunonegative/FG+/TPH-immunonegative neurons, grey square in B shows a c-Fos-ir/FG+/TPH-immunonegative neuron and black triangles show c-Fos-ir/FG+/TPH-ir (triple-labeled) neurons. Abbreviations; Aq, cerebral aqueduct; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DTgP, dorsal tegmental nucleus, pericentral part. Scale bar, 50 μm.
Intracerebroventricular injection of Ucn 2 also increased c-Fos expression in non-ventricle/periventricular-projecting (FG-negative) serotonergic neurons (region × treatment interaction, F(2,24) = 4.41, p = 0.046, ε = 0.62; treatment main effect, F(1,12) = 30.59 p < 0.001; region main effect, F(2,21) = 12.81, p = 0.002, ε = 0.62, Fig. 4B, closed bars). Post hoc Fisher’s Protected LSD tests showed increased c-Fos expression in non-ventricle/periventricular-projecting serotonergic neurons in the Ucn 2 group compared with saline controls in each of the 3 subdivisions of the dorsal raphe nucleus sampled (Fig. 4B, closed bars).
The total numbers of FG+/TPH-ir (i.e. ventricle/periventricular-projecting serotonergic neurons) varied among the 3 subdivisions sampled (region main effect, F(2,24) = 9.13, p < 0.009, ε = 0.53; Fig. 4A, open bars and Fig. 4C, closed bars). There was no treatment main effect or region × treatment interaction. Of the total numbers of FG+ neurons in both saline- and Ucn 2-treated rats, 35.7 ± 7.4% were TPH-ir in the DRD at −8.18 mm bregma; 46.4 ± 7.3% were TPH-ir in the DRC at −8.54 mm bregma, and 36.4 ± 6.5% were TPH-ir in the DRD at −9.16 mm bregma (Fig. 4C). Of the total population of ventricle/periventricular-projecting neurons in the sampled subdivisions, 39.5 ± 4.1% were serotonergic.
There were no differences between treatment groups in the numbers of c-Fos-ir/FG+/TPH-immunonegative (ventricle/periventricular-projecting, non-serotonergic neurons) in the dorsal raphe nucleus (data not shown). This represented a very small population of neurons in the dorsal raphe nucleus. In the DRC at −8.54 mm bregma, the mean numbers of c-Fos-ir/FG+/TPH− neurons for Ucn 2-treated rats was 0.42 ± 0.30 compared with saline controls 0 ± 0, while in the DRD at −9.16 mm bregma, the mean numbers of c-Fos-ir/FG+/TPH− neurons for Ucn 2-treated rats was 0.5 ± 0.22 compared with saline controls 0 ± 0. c-Fos-ir/FG+/TPH− neurons were never observed in the DRD at −8.18 mm bregma.
The numbers of single-labeled c-Fos-ir cells (i.e. non-ventricle/periventricular-projecting, non-serotonergic cells) varied among the 3 subdivisions of the caudal dorsal raphe nucleus sampled (F(2,24) = 21.06, p < 0.001, ε = 0.94; data not shown). However, although the treatment main effect approached statistical significance (p < 0.065) there was no difference across treatments or interaction between the region and treatment. Similarly there were region main effects but no treatment main effects or region × treatment interactions for the total numbers of TPH-ir neurons (region main effect, F(2,24) = 90.61, p < 0.001, ε = 0.89; Fig 4B, open bars), the total numbers of FG+ neurons (region main effect, F(2,24) = 20.96, p < 0.001, ε = 0.68; Fig 4C, open bars), and the total numbers of FG−/TPH-ir (i.e. non-ventricle/periventricular-projecting serotonergic neurons; region main effect, F(1,12) = 0.06, p = 0.798; data not shown).
Discussion
Intracerebroventricular injection of Ucn 2 increased c-Fos expression in a population of serotonergic neurons that gives rise to the dorsal raphe periventricular tract (Lowry et al, 2008) (hereafter referred to as ventricle/periventricular-projecting serotonergic neurons). Injection of the retrograde tracer FG into the lateral ventricle resulted in FG-like immunoreactivity in the ependymal lining of the lateral and third ventricles, in fibers (presumed tanycytes) close to the 3V and in circumventricular organs such as the VOLT and the SFO. A subregional analysis of the midbrain raphe complex suggested that i.c.v. injection of Ucn 2 increased c-Fos expression in subsets of serotonergic neurons and non-serotonergic cells in the mid-rostrocaudal DRD and in the DRC, but not in the rostral, ventral, ventrolateral or interfascicular regions of the dorsal raphe nucleus or in the median raphe nucleus. The dorsal part of the mid-rostrocaudal dorsal raphe nucleus (the DRD at −8.18 mm bregma) and the caudal dorsal raphe nucleus (the DRC at −8.54 and −9.16 mm bregma) were analyzed using combined brightfield, autofluorescence, and immunofluorescence imaging. Approximately 40% of the ventricle/periventricular-projecting neurons in the subdivisions of the dorsal raphe nucleus sampled were serotonergic. Intracerebroventricular injections of Ucn 2 increased c-Fos expression in non-ventricle/periventricular-projecting serotonergic neurons in all of the subdivisions sampled (total population: saline, 6.0 ± 1.2%; Ucn 2, 33.0 ± 4.4%) and in ventricle/periventricular-projecting serotonergic neurons in the DRC at −8.54 mm and −9.16 mm bregma (total population: saline, 6.5 ± 4.1%; Ucn 2, 35.4 ± 8.0%). These data suggest that i.c.v. injection of the stress- and anxiety-related neuropeptide, Ucn 2, activates a significant proportion (~35%) of a subpopulation of serotonergic neurons in the caudal part of the dorsal raphe nucleus that gives rise to the dorsal raphe periventricular tract, in addition to other populations of non-ventricle projecting serotonergic neurons in the same region.
Injection of the retrograde tracer FG into the lateral ventricle resulted in a distinct pattern of FG-like immunoreactivity in the rat ventricular system. FG-like immunoreactivity was seen in the ependymal lining of the lateral and third ventricles and FG-like-ir fibers (presumed tanycytes) were observed close to the 3V. Circumventricular organs such as the VOLT and the SFO contained FG-ir fibers and cell bodies. These regions, like the ependyma and the cerebral ventricles, are known to contain serotonin-ir fibers (Moore, 1977;Takeuchi and Sano, 1983) and receive projections from the midbrain raphe complex (Mikkelsen et al, 1997;Simpson et al, 1998) and therefore may contribute to the pattern of retrograde labeling in the midbrain raphe nuclei following i.c.v. injection of FG. It is possible that injection of FG into the ventricular system labeled serotonergic neurons forming the dorsal raphe periventricular tract (DRPT), one of at least six different ascending serotonergic tracts innervating the forebrain, based on autoradiographic tracing studies (Azmitia and Segal, 1978). Autoradiographic evidence suggests that serotonergic neurons forming the DRPT originate in the caudal part of the dorsal raphe nucleus, ventral to the fourth ventricle. Fibers of the DRPT run ventral to the midbrain aqueduct in the ventromedial gray and project rostrally to periventricular regions of the thalamus, hypothalamus and the subfornical organ (Azmitia and Segal, 1978). Subsequent studies have confirmed that cells innervating the cerebral ventricles arise from the same region of the dorsal raphe nucleus (Mikkelsen et al, 1997;Simpson et al, 1998) and therefore it is possible that individual serotonergic neurons give rise to collateral projections to both periventricular and ventricular structures.
Intracerebroventricular injection of Ucn 2 increased c-Fos expression in a subset of serotonergic neurons in the midbrain raphe complex. Urocortin 2 injections increased c-Fos expression in serotonergic neurons in the DRD at −8.18 mm bregma and the DRC at −8.54 and −9.16 mm bregma. Most of the subdivisions of the midbrain raphe complex sampled showed no differences in c-Fos expression in serotonergic neurons between rats treated with Ucn 2 and rats treated with saline, including the DRD and DRV at −7.46 mm bregma, the DRD, DRV and DRVL/VLPAG at −8.00 mm bregma, the DRV, DRVL/VLPAG, DRI and MnR at −8.18 mm bregma, and the DRI and MnR at −8.54 mm bregma. The results from the present study are consistent with our previous report (Staub et al, 2006) and suggest that the stress- and anxiety-related neuropeptide Ucn 2 has selective actions on an anatomically distinct population of neurons in the caudal part of the midbrain raphe complex. We have previously reported that i.c.v injection of Ucn 2 has no effect on home cage behavior measured up to 2 h following injection (Staub et al, 2005), however, microinjection of Ucn 2 into the caudal dorsal raphe nucleus has been associated with poor escape learning and potentiation of fear conditioning measured 24 h following injection in a model of learned helplessness, an effect similar to the behavioral consequences of inescapable stress (Hammack et al, 2003). The role of Ucn 2 and CRF type 2 (CRF2) receptors in the regulation of anxiety-states and anxiety-related behavior is unclear, with both anxiolytic effects (Ohata and Shibasaki, 2004;Valdez et al, 2002) and anxiogenic effects (Pelleymounter et al, 2002;Pelleymounter et al, 2004) reported after i.c.v. injection of Ucn 2 (for discussion, see Staub et al, 2005).
Urocortin 2 injection increased c-Fos expression in a subset of the serotonergic neurons that give rise to the dorsal raphe periventricular tract. The proportion of ventricle/periventricular-projecting serotonergic neurons that expressed c-Fos varied across the subdivisions of the dorsal raphe nucleus sampled; 31.5 ± 9.7% in the DRC at −8.54 mm bregma and 38.7 ± 13.0% in the DRC at −9.16 mm bregma were activated (c-Fos-ir) following i.c.v. Ucn 2 injection. Of the total population of ventricle/periventricular-projecting serotonergic neurons in the DRC at −8.54 mm bregma and DRC at −9.16 mm bregma, 35.4 ± 8.0% expressed c-Fos following Ucn 2 injection. Consideration of an identified anatomical subset of serotonergic neurons increased our ability to detect treatment effects; whereas Ucn 2 increased c-Fos expression in 35.4% of FG+ serotonergic neurons, it increased c-Fos expression in only 6.5 ± 1.3% of all serotonergic neurons sampled throughout the dorsal raphe nucleus. Most of the activated ventricle/periventricular-projecting serotonergic neurons were located in the dorsal part of the dorsal raphe nucleus close to the cerebral aqueduct. Although the functional role for a Ucn 2-sensitive, ventricle/periventricular-projecting population of serotonergic neurons is not clear, this system may have important implications for stress-coping mechanisms. Serotonergic fibers project directly into the cerebrospinal fluid (Moore, 1977;Takeuchi and Sano, 1983) and serotonin acts within the cerebrospinal fluid to increase ciliary beat frequency (Nguyen et al, 2001). Ciliated ependymal cells are widely distributed throughout the ventricular system (Mathew and Singh, 1989;Mathew, 1999;Mathew, 2008). This may serve to increase clearance of bioactive molecules and metabolites following stress-related stimuli, and therefore may contribute to the stress coping functions that have been attributed to Ucn 2, Ucn 3 and CRF2 receptor activation (Bale et al, 2000;Coste et al, 2000;Hsu and Hsueh, 2001).
The Ucn 2-sensitive ventricle/periventricular-projecting serotonergic system also may have important implications in stress-related neuropsychiatric conditions. Some studies have found low cerebrospinal fluid (CSF) concentrations of the serotonin metabolites 5-hydroxyindoleacetic acid (5-HIAA) in depressed patients (Reddy et al, 1992) and low CSF concentrations of 5-HIAA are predictors of anti-social personality disorder (Constantino et al, 1997) and violent behavior (Virkkunen et al, 1995). Although the low CSF 5-HIAA concentrations in depressed patients have not been a consistent finding (Cowen, 2008), this may represent a subset of depressed patients that attempt suicide (Asberg, 1997;Lester, 1995;Placidi et al, 2001). The Ucn 2-sensitive serotonergic system identified here, particularly if it includes those serotonergic fibers that project directly into the CSF (Mikkelsen et al, 1997;Simpson et al, 1998), may play a particularly important role in the reduced 5-HIAA concentrations in depressed suicide patients. This is an intriguing possibility because total brain turnover of serotonin is elevated in depressed patients based on measurements of internal jugular venoarterial 5-HIAA plasma concentration gradients (Barton et al, 2008), suggesting that 5-HIAA concentrations in CSF and internal jugular blood compartments are differentially regulated. Ependymal cells lining the ventricles contain monoamine oxidase A (Verleysdonk et al, 2004) and may contribute to the conversion of serotonin to 5-HIAA prior to release into the cerebrospinal fluid.
The Ucn 2-sensitive serotonergic neurons may include those projecting to the subventricular zone (SVZ), a germinal layer of cells, located in the lateral walls of the lateral ventricles, that has been implicated in neurogenesis (Doetsch et al, 1999). Inhibition of serotonin synthesis or selective lesion of serotonergic neurons is associated with decreases in the numbers of newly generated cells in the SVZ (Brezun and Daszuta, 1999) while activation of 5-HT1A and 5-HT2C receptors stimulate cell proliferation in the SVZ and neurogenesis in the olfactory bulb (OB; Banasr et al, 2004). Indeed, the antidepressant-like effects of some drugs, including selective serotonin re-uptake inhibitors (SSRIs) are thought to be dependent on increased rates of hippocampal neurogenesis (Duman et al, 1999;Santarelli et al, 2003). Recent evidence suggests that the SVZ is a source of GABAergic interneurons for the OB as well as other cortical and subcortical structures (Inta et al, 2008). The question of whether the Ucn 2-sensitive serotonergic system highlighted in this study plays a role in regulation of adult neurogenesis, or the antidepressant effects of SSRIs or other antidepressant drugs requires further investigation.
In addition to its effects on ventricle/periventricular-projecting serotonergic neurons, i.c.v. injection of Ucn 2 also increased c-Fos expression in non-ventricle/periventricular-projecting serotonergic neurons in each of the three subdivision of the caudal dorsal raphe nucleus studied. Across these subdivisions, 33.0 ± 4.4% of non-ventricle/periventricular-projecting serotonergic neurons expressed c-Fos in the Ucn 2-treated group compared with 6.0 ± 1.2% in the saline-treated group. It is unclear if the effects of Ucn 2 on ventricle/periventricular-projecting and non-ventricle/periventricular-projecting serotonergic neurons is through 1) direct actions on the serotonergic neurons themselves, 2) indirect actions on the serotonergic neurons, via effects on non-serotonergic neurons in the caudal DR, 3) indirect action on the serotonergic neurons via effects on afferents to the DRC from outside the dorsal raphe nucleus, or 4) a combination of direct and indirect mechanisms. Electrophysiologic evidence suggests that activation of CRF2 receptors on serotonergic neurons in the dorsal raphe nucleus inhibits neuronal activity of serotonergic neurons, whereas activation of CRF2 receptors on non-serotonergic, possibly GABAergic, neurons in the dorsal raphe nucleus indirectly activates serotonergic neurons through the inhibition of tonic inhibitory inputs (Pernar et al, 2004). Regardless of the mechanisms, microdialysis studies using direct microinfusion of Ucn 2 into the dorsal raphe nucleus support a role for local CRF2 receptors in activation of mesolimbic serotonergic systems (Amat et al, 2004).
Intracerebroventricular injection of Ucn 2 increased c-Fos expression in subpopulations of non-serotonergic cells in the midbrain raphe complex. Ucn 2 injections increased c-Fos expression in non-serotonergic cells in the DRD at −8.18 mm, and the DRC at −8.54 and −9.16 mm bregma, the same regions that showed increases in c-Fos expression in serotonergic neurons. This finding is consistent with our previous study (Staub et al, 2006). These regions of the dorsal raphe nucleus contain non-serotonergic neurons expressing the CRF2 receptor (Day et al, 2004), including GABAergic neurons, and it is possible that these populations of cells play an important role in the physiological and behavioral consequences of i.c.v. injection of Ucn 2. Further studies are required to examine the neurochemical phenotype of the c-Fos-ir non-serotonergic cells activated by Ucn 2.
The numbers of ventricle/periventricular-projecting neurons and the proportion of ventricle/periventricular-projecting neurons that are serotonergic varied across the subdivisions of the dorsal raphe nucleus sampled. Of the total numbers of FG+ neurons, 35.7 ± 7.4% were TPH-ir in the DRD at −8.18 mm bregma; 46.4 ± 7.3% were TPH-ir in the DRC at −8.54 mm bregma and 36.4 ± 6.5% were TPH-ir in the DRC at at −9.16 mm bregma. In all of the regions sampled, 39.5 ± 4.1% of the total population of FG+ neurons sampled was serotonergic. Using epifluorescence and photomicrographs for the quantification of FG+/TPH-ir neurons may have resulted in an underestimate of the numbers of FG+/TPH-ir neurons, compared with using confocal microscopy to examine each cell. Previous research using brightfield microscopy has suggested that approximately 50% of ventricle/periventricular-projecting neurons in the dorsal and ventral parts of the dorsal raphe nucleus are serotonergic (Mikkelsen et al, 1997), a slightly higher percentage than that reported here (approximately 40%).
Conclusions
The results from this study indicate that central administration of the stress- and anxiety-related neuropeptide Ucn 2 activates a subpopulation of serotonergic neurons in the caudal part of the dorsal raphe nucleus, including a subset of serotonergic neurons that gives rise to the dorsal raphe periventricular tract. The role for the specific population of serotonergic neurons giving rise to the dorsal raphe periventricular tract in the behavioral and physiological response to stress is unclear, but it may have an important role in the clearance of bioactive molecules and metabolites following exposure to stress-related stimuli and may contribute to the stress coping functions that have been attributed to Ucn 2 and related peptides. The results also identify a subpopulation of Ucn 2-sensitive serotonergic neurons that does not contribute to the dorsal raphe periventricular tract, suggesting that Ucn 2 activates at least two anatomically distinct subpopulations of serotonergic neurons. Dysregulation of these subsets of serotonergic neurons may contribute to the pathophysiology of stress-related neurologic conditions or psychiatric disorders.
Acknowledgments
We gratefully acknowledge Dr. Wilson C. Chung for assistance with preparation of laser scanning confocal images. C.A. Lowry was supported by a Wellcome Trust Research Career Development Fellowship (RCDF 068558/Z/02/Z), and a 2007 NARSAD Young Investigator Award and is currently supported by an NSF CAREER Award (NSF-IOS #0845550). C.E. Stamper was supported by a Bioscience Undergraduate Research Skills and Training (BURST) fellowship, and an Undergraduate Research Opportunities Program (UROP)/Howard Hughes Medical Institute (HHMI) Individual Grant funded by the Biological Sciences Initiative (BSI) through a grant from the Howard Hughes Medical Institute (HHMI). The project described was supported by Award Number R01MH086539 (CAL) from the NIMH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann N Y Acad Sci. 1997;836:158–181. doi: 10.1111/j.1749-6632.1997.tb52359.x. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Jr, Segal M. An autoradiographic analysis of the differential ascending projection of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:651–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C, Socratous F, Hastings J, Guo L, Wiesner G, Kaye DM, Bayles R, Schlaich MP, Lambert GW. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch Gen Psychiatry. 2008;65:38–46. doi: 10.1001/archgenpsychiatry.2007.11. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Chen A, Zorrilla E, Smith S, Rousso D, Levy C, Vaughan J, Donaldson C, Roberts A, Lee KF, Vale W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J Neurosci. 2006;26:5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Morris JA, Murphy DL. CSF 5-HIAA and family history of antisocial personality disorder in newborns. Am J Psychiatry. 1997;154:1771–1773. doi: 10.1176/ajp.154.12.1771. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Cowen PJ. Serotonin and depression: pathophysiological mechanism or marketing myth? Trends Pharmacol Sci. 2008;29:433–436. doi: 10.1016/j.tips.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha(1b) adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groote L, Penalva RG, Flachskamm C, Reul JMHM, Linthorst ACE. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor receptor type 1 and type 2 agonists. J Neurochem. 2005;94:45–56. doi: 10.1111/j.1471-4159.2005.03164.x. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA, Evans AK, Stamper CE, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience. 2008;157:733–748. doi: 10.1016/j.neuroscience.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Inta D, Alfonso J, von EJ, Kreuzberg MM, Meyer AH, van Hooft JA, Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci USA. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann N Y Acad Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Lester D. The concentration of neurotransmitter metabolites in the cerebrospinal fluid of suicidal individuals: a meta-analysis. Pharmacopsychiatry. 1995;28:45–50. doi: 10.1055/s-2007-979587. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A. Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti JM, Pandi-Perumal BL, Jacobs BL, Nutt DL, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhauser; Basel: 2008. pp. 25–68. [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew TC. Association between supraependymal nerve fibres and the ependymal cilia of the mammalian brain. Anat Histol Embryol. 1999;28:193–197. doi: 10.1046/j.1439-0264.1999.00191.x. [DOI] [PubMed] [Google Scholar]
- Mathew TC. Regional analysis of the ependyma of the third ventricle of rat by light and electron microscopy. Anat Histol Embryol. 2008;37:9–18. doi: 10.1111/j.1439-0264.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- Mathew TC, Singh DN. Morphology and distribution of tanycytes in the third ventricle of the adult rat. A study using semithin methacrylate sections. Acta Anat(Basel) 1989;134:319–321. doi: 10.1159/000146709. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Hay-Schmidt A, Larsen PJ. Central innervation of the rat ependyma and subcommissural organ with special reference to ascending serotoninergic projections from the raphe nuclei. J Comp Neurol. 1997;384:556–568. [PubMed] [Google Scholar]
- Moore RY. Organum vasculosum lamina terminalis: Innervation by serotonin neurons of the midbrain raphe. Neurosci Lett. 1977;5:297–302. doi: 10.1016/0304-3940(77)90082-9. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Chin WC, O’Brien JA, Verdugo P, Berger AJ. Intracellular pathways regulating ciliary beating of rat brain ependymal cells. J Physiol. 2001;531:131–140. doi: 10.1111/j.1469-7793.2001.0131j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata H, Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides. 2004;25:1703–1709. doi: 10.1016/j.peptides.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections of the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981;202:335–356. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor(2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145–152. doi: 10.1124/jpet.302.1.145. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RG. Design and Analysis of Experiments. Marcel Dekker, Inc; New York: 1985. [Google Scholar]
- Petit JM, Luppi PH, Peyron C, Rampon C, Jouvet M. VIP-like immunoreactive projections from the dorsal raphe and caudal linear raphe nuclei to the bed nucleus of the stria terminalis demonstrated by a double immunohistochemical method in the rat. Neurosci Lett. 1995;193:77–80. doi: 10.1016/0304-3940(95)11669-n. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50:783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PL, Khanna S, Subhash MN, Channabasavanna SM, Rao BS. CSF amine metabolites in depression. Biol Psychiatry. 1992;31:112–118. doi: 10.1016/0006-3223(92)90198-9. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Fisher TM, Waterhouse BD, Lin RC. Projection patterns from the raphe nuclear complex to the ependymal wall of the ventricular system in the rat. J Comp Neurol. 1998;399:61–72. doi: 10.1002/(sici)1096-9861(19980914)399:1<61::aid-cne5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF(2)) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat. Cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM. Serotonin-immunoreactive neurons and their projections in the CNS. In: Björklund A, Hökfelt T, editors. Classical Transmitters and Transmitter Receptors in the CNS. Elsevier Science Publishers B.V; Amsterdam: 1984. pp. 68–125. [Google Scholar]
- Stezhka VV, Lovick TA. Projections from dorsal raphe nucleus to the periaqueductal grey matter: studies in slices of rat midbrain maintained in vitro. Neurosci Lett. 1997;230:57–60. doi: 10.1016/s0304-3940(97)00464-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Sano Y. Serotonin distribution in the circumventricular organs of the rat. An immunohistochemical study. Anat Embryol (Berl) 1983;167:311–319. doi: 10.1007/BF00315669. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Verleysdonk S, Hamprecht B, Rapp M, Wellard J. Uptake and metabolism of serotonin by ependymal primary cultures. Neurochem Res. 2004;29:1739–1747. doi: 10.1023/b:nere.0000035810.08543.97. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, Goldman D, Nielsen DA, Linnoila M. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20:271–275. [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J Pharmacol Exp Ther. 2004;310:1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]