Abstract
Background
Although increasing evidence indicates that an adipokine adiponectin exerts protective actions on heart, its effects on coronary angiogenesis following pressure overload have not been examined previously. Because disruption of angiogenesis during heart growth leads to contractile dysfunction and heart failure, we hypothesized that adiponectin modulates cardiac remodeling in response to pressure overload through its ability to regulate adaptive angiogenesis.
Methods and Results
Adiponectin-knockout (APN-KO) and wild-type (WT) mice were subjected to pressure overload caused by transverse aortic constriction (TAC). APN-KO mice exhibited greater cardiac hypertrophy, pulmonary congestion, left ventricular (LV) interstitial fibrosis and LV systolic dysfunction after TAC surgery compared with WT mice. APN-KO mice also displayed reduced capillary density in the myocardium after TAC, which was accompanied by a significant decrease in expression of vascular endothelial growth factor (VEGF) and phosphorylation of AMP-activated protein kinase (AMPK). Inhibition of AMPK in WT mice resulted in aggravated LV systolic function, attenuated myocardial capillary density and decreased VEGF expression in response to TAC. The adverse effects of AMPK inhibition on cardiac function and angiogenic response following TAC were diminished in APN-KO mice relative to WT mice. Moreover, adenovirus-mediated VEGF delivery reversed the TAC-induced deficiencies in cardiac microvessel formation and ventricular function observed in the APN-KO mice. In cultured cardiac myocytes, adiponectin treatment stimulated VEGF production, which was inhibited by inactivation of AMPK signaling pathway.
Conclusions
Adiponectin deficiency can accelerate the transition from cardiac hypertrophy to heart failure during pressure overload through disruption of AMPK-dependent angiogenic regulatory axis.
Keywords: adiponectin, AMPK, cardiac angiogenesis, pressure overload, heart failure
Introduction
Sustained cardiac hypertrophy is associated with an increased risk of cardiovascular events and death [1]. Cardiac hypertrophy in response to pathological stimuli contributes to maladaptive cardiac remodeling and ultimately leads to the development of contractile dysfunction and heart failure [2, 3]. The molecular mechanisms underlying the maladaptive features of cardiac hypertrophy remain incompletely understood. Recent reports show that incongruent cardiac angiogenesis and myocyte hypertrophy plays a role in the transition from compensated to decompensated cardiac hypertrophy [4, 5]. Thus, understanding the mechanisms by which myocardial angiogenesis is maintained during cardiac growth could lead to a better understanding of the processes that contribute to heart failure.
Adiponectin is an adipocyte-derived plasma protein with anti-diabetic and anti-inflammatory properties [6, 7]. Plasma adiponectin levels are decreased in obese subjects [8] and patients with type 2 diabetes [9], coronary artery disease [10, 11] and hypertension [12]. Accumulating evidence indicates that adiponectin plays a protective role in the development of myocardial remodeling under various pathological conditions [13–15]. We and others have shown that adiponectin-knockout (APN-KO) mice develop increased myocardial damage and systolic dysfunction in response to ischemic insult [16–20]. APN-KO mice also exhibit severe concentric hypertrophy during pressure overload, which is associated with reduced activation of AMP-activated protein kinase (AMPK) and impaired glucose metabolism in the heart [21, 22]. Adiponectin also exhibits vascular protective activities, and low adiponectin levels lead to impaired angiogenic responses to ischemia [21, 23–27]. However, the role of coronary angiogenesis in adiponectin-mediated cardiac remodeling has not been examined previously.
We hypothesized that adiponectin affects the transition from cardiac hypertrophy to heart failure during pressure overload through modulation of adaptive cardiac angiogenesis. Therefore, in this present study we investigated the role of adiponectin in regulation of cardiac vessel recruitment that is coupled to heart growth during pressure overload. Our observations indicate that adiponectin deficiency leads to impaired angiogenic response to pressure overload via an AMPK-dependent mechanism, thereby contributing to exacerbated cardiac dysfunction.
Methods
Materials
Antibodies against phospho-AMPK (Thr172), pan-α-AMPK, phospho-LKB1 (Ser428) and β-actin were purchased from Cell Signaling Technology (Beverly, MA). Vascular endothelial growth factor (VEGF) and LKB1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). AdipoR1 and AdipoR2 antibodies were purchased from Abcam Inc. (Cambridge, MA). Tubulin antibody and compound C were from Calbiochem (Madison, WI). Recombinant adiponectin protein produced in COS-7 cells was obtained from Novo Nordisk (Copenhagen, Denmark). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Invitrogen (San Diego, CA).
Transverse aortic constriction (TAC) protocol
Studies using adiponectin-knockout (APN-KO) and wild-type (WT) littermate mice in a C57BL/6 background [28] were approved by the Institutional Animal Care and Use Committee in Nagoya University and Boston University where these surgeries were performed. Mice, at the ages of 7–9 weeks, were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally). The chest was opened and the thoracic aorta was identified after blunt dissection through the intercostal muscles. A 7-0 silk suture was placed around the transverse aorta and tied around a 26-gauge blunt needle, which was then removed as previously described [22]. Sham-operated mice underwent a similar surgical procedure without constriction of the aorta. Animals were anesthetized and then killed, and the hearts and the lungs were weighed. In some experiments, heart and blood samples were extracted after 14 days. In another experiment, compound C (20mg/kg) as an AMPK inhibitor was dissolved in dimethylsulphoxide (DMSO) and intraperitoneally injected into abdomen of WT mice 1 day before the operation until sacrifice as described previously [29]. Control mice received vehicle alone. In other experiments, seven to nine week old male mice were intravenously administered the adenoviral vectors Ad-VEGF (encoding VEGF-A165) or Ad-βgal (encoding β-galactosidase as control) at 1.0 × 109 plaque forming units/mouse [30] via the external jugular vein. Serum VEGF was assayed by ELISA (R&D systems) three days after adenovirus delivery. At this time point mice also were underwent TAC surgery.
Echocardiography
After 21 days, we subjected surviving mice to transthoracic echocardiography to determine cardiac function and structure. To measure left ventricular (LV) systolic function and chamber dimensions, echocardiogram analysis was performed with an Acuson Sequoia C-256 machine using a 15-MHz probe. We quantified LV end diastolic diameter (LVEDD) and % LV fractional shortening (%FS) from M-mode images.
Histology
LV tissues were obtained 3 weeks after TAC procedure, embedded in OCT compound (Elkhart, IN) and frozen in liquid nitrogen. To determine the capillary density, we stained tissue sections (8 um) with anti-CD31 antibody (Becton Dickinson; Franklin Lakes, NJ) and measured the number of CD31-positive cells per a cardiac myocyte in twenty randomly chosen microscopic fields from three different sections in each tissue block. Hematoxylin was used for counter-staining. To determine the cross-sectional area of cardiac myocytes and the level of myocardial interstitial fibrosis, sections were stained with Masson’s trichrome (MT) as previously described [4].
Cultures of neonatal rat ventricular myocytes
Primary culture of neonatal rat ventricular myocytes (NRVMs) were prepared as described previously [31]. NRVMs were incubated in DMEM supplemented with 7% Fetal Calf Serum (FCS) for 18 hours after preparation and subsequently treated with an adenoviral vector expressing a dominant-negative mutant of AMPK (Ad-dnAMPK) or β-galactosidase (Ad-βgal) at a multiplicity of infection (MOI) of 50 for 16 hours in DMEM [22]. Thereafter the media were replaced with fresh DMEM without adenovirus. Serum-deprived NRVMs were incubated with recombinant adiponectin protein (30 μg/ml) for 24 hours. In some experiments, NRVMs were treated with compound C (20 μM) prior to adiponectin protein or vehicle treatment. In other experiments, NRVMs were transfected with rat small interfering RNAs (siRNAs) targeting AdipoR1 and LKB1, or unrelated siRNAs (Dharmacon Inc., Lafayette, CO) by Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocol. Forty-eight hours after transfection, NRVMs were incubated with recombinant adiponectin protein for indicated length of times.
Western blot analysis
Tissue samples were obtained on the postoperative day 7 or 14 and homogenized in lysis buffer (Cell Signaling Technology) with protease inhibitor cocktail (Sigma Chemical Co.; St. Louis, MO). Protein content was determined by the Bradford method. The same amounts of protein (80 μg) were separated with denaturing SDS 10% polyacrylamide gels. The membranes were immunoblotted with the primary antibodies at a 1:1000–5000 dilution followed by treatment with secondary antibody conjugated with horseradish peroxidase (HRP) at a 1:2000–10000 dilution. Bands were visualized using ECL Western Blotting Detection kit (Amersham Pharmacia Biotech; Piscataway, NJ).
Quantification of mRNA expression levels
Total RNA from mouse heart was extracted by using FastPrep System (Savant Instruments; Farmingdale, NY) as described previously [32], and total RNA from cultured cells was prepared by using RNA isolation kit (Qiagen; Valencia, CA) according to manufacturer’s protocols. Complementary DNA (cDNA) from 500 ng of total RNA was synthesized by using SuperScript RT-PCR Systems (Invitrogen) according to the manufacturer’s instructions. Quantitative Real-Time PCR (QRT-PCR) analysis was performed on an iCycler system (Bio-Rad; Hercules, CA) using SYBR GREEN 1 as a double-stranded DNA-specific dye according to the manufacture’s instruction (Applied Biosystems; Foster, CA). Primers were designed as follows: 5′-CTGTAACGATGAAGCCCTGGAG-3′ and 5′-TGGTGAGGTTTGATCCGCAT-3′ for mouse VEGF-A; 5′-CCCTTATTGACCTCAACTACATGGT-3′ and 5′-GAGGGGCCATCCACAGTCTTCTG-3′ for mouse GAPDH; 5′-CAAAGCCAGCACATAGGAGA-3′ and 5′-ATTTCTTGCGCTTTCGTTTT-3′ for rat VEGF; 5′-TCAAGAAGGTGGTGAAGCAG-3′ and 5′-AGGTGGAAGAATGGGAGTTG-3′ for GAPDH. The expression levels of examined transcripts were compared to that of GAPDH and normalized to the mean value of controls.
Statistical Analysis
Data are presented as mean ± SEM. Group differences were analyzed by two-tailed Student’s t test or analysis of variance (ANOVA). To compare multiple groups, Mann-Whitney U-test with Bonferroni correction was used. Survival was determined by Kaplan-Meier analysis with the log-rank test. A value of P < 0.05 was considered statistically significant.
Results
APN-KO mice show enhanced pathological responses to pressure overload
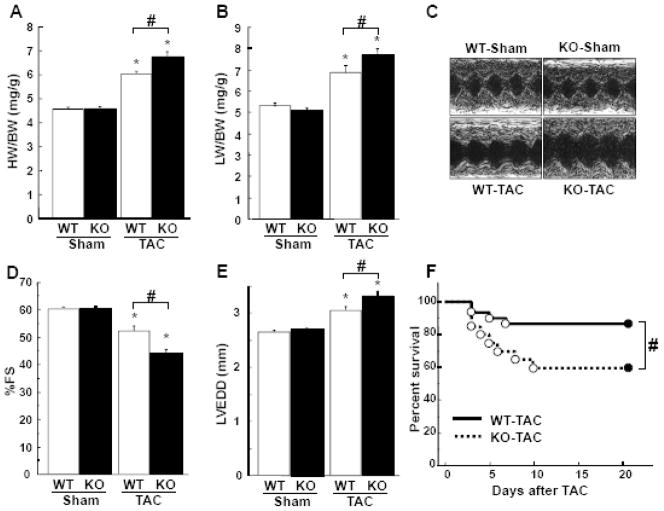
In our previous study, APN-KO and WT hearts were examined 7 days after TAC [22]. In the current study, a longer time point was utilized to examine the transition from compensated to decompensated hypertrophy. The ratio of heart weight to body weight (HW/BW) and lung weight to body weight (LW/BW) did not differ under sham-operated conditions between APN-KO and WT mice (Figure 1A and B). APN-KO mice developed greater increases in HW/BW and LW/BW ratios at 3 weeks after TAC as compared with WT mice (Figure 1A and B). Echocardiographic analysis demonstrated that APN-KO mice had impaired LV systolic dysfunction, as assessed by a greater reduction of %FS (Figure 1C and D) and an increase in LVEDD (Figure 1C and E) at 3 weeks after TAC, compared to WT mice. No significant difference was observed in %FS and LVEDD following sham operation between two strains. The heart rate was not significantly different between WT and APN-KO groups (data not shown). Furthermore APN-KO mice had a higher mortality after TAC than WT mice (Figure 1F).
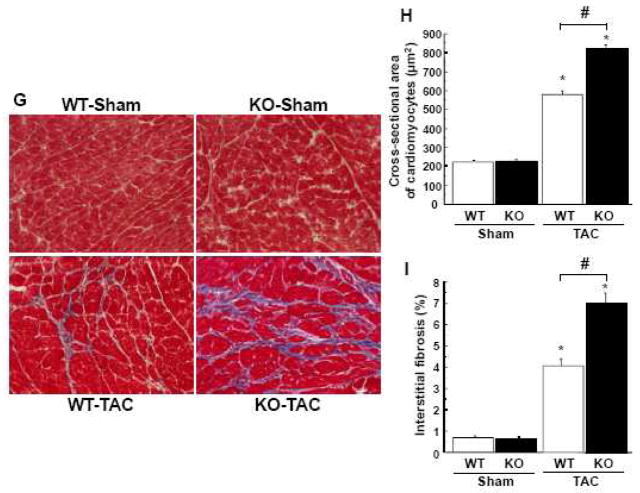
Figure 1. Loss of adiponectin results in exacerbation of pressure overload induced cardiac hypertrophy and heart failure.
A and B, The ratio of heart weight to body weight (HW/BW)(A) and lung weight to body weight (LW/BW)(B) in wild-type (WT) and adiponectin-knockout (KO) mice at 3 weeks after sham operation or TAC. C, Representative M-mode echocardiograms for WT or KO mice at 3 weeks after sham or TAC surgery. D and E, Echocardiographic analysis of the fractional shortening (FS)(D) and LV end systolic diameter (LVEDD)(E) for WT and KO mice at 3 weeks after sham operation or TAC. F, Kaplan-Meier survival analysis of WT and KO mice following TAC. G, Representative histological LV sections from WT and KO mice stained with Masson Trichrome. Heart samples were collected at 3 weeks after sham operation or TAC. Magnification, ×400. H and I, Quantitative analysis of the cross-sectional area of cardiomyocytes (H) and the intestinal fibrosis area (I) from each group. Results are presented as mean ± SEM (n=10 to 15 per each group). *P<0.05 versus corresponding sham and #P<0.05 versus corresponding WT mice.
Hearts were also processed for histological analyses. TAC resulted in an increase in cross-sectional area (CSA) of cardiomyocytes and LV interstitial fibrosis in WT mice (Figure 1G, H and I). APN-KO mice developed a greater increase in CSA and LV interstitial fibrosis following TAC compared with WT mice (Figure 1G, H and I). There were no significant differences in CSA and LV interstitial fibrosis after sham operation between APN-KO and WT mice.
Reduced myocardial capillary density and VEGF induction in APN-KO mice subjected to pressure overload
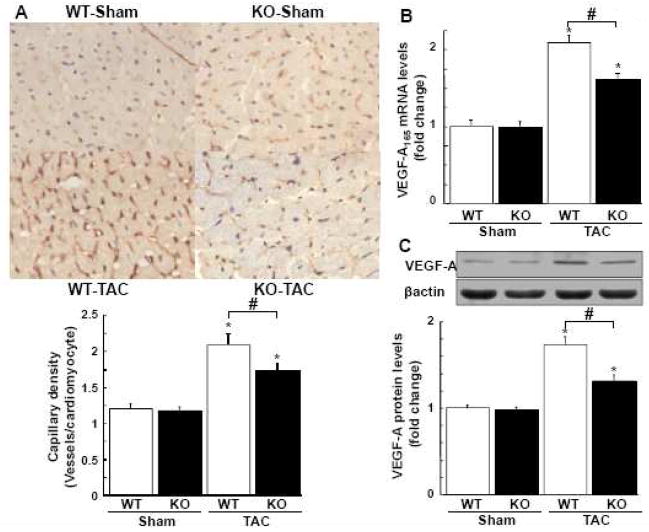
To investigate the extent of cardiac microvessel formation following pressure overload, capillary density was measured by staining for CD31, an endothelial cell marker, in histological sections harvested from the hearts. Representative photomicrographs of CD31-stained myocardium are shown in Figure 2A. Quantitative analysis of CD31-positive cells revealed that the capillary density was increased in WT mice at 3 weeks after TAC and that this induction was significantly attenuated in APN-KO mice (Figure 2A). No significant difference was observed in capillary density after sham operation between two strains.
Figure 2. Adiponectin-deficiency causes decreased cardiac angiogenesis and VEGF expression following pressure overload.
A, Representative images of CD31-stained heart sections from WT and KO mice at 3 weeks after sham operation or TAC in. Brown indicates CD31-positive cells, whereas light purple indicates nuclei in the hematoxylin stain. Magnification, ×400. Lower panel shows quantitative analysis of capillary density by measuring the number of CD31-positive cells per cardiomyocyte from each group. B, VEGF-A165 mRNA levels in the myocardium of WT and KO mice at 2 weeks after sham operation or TAC. VEGF-A165 mRNA levels were quantified by QRT-PCR analysis and presented relative to levels of GAPDH. C, Myocardial expression of VEGF-A protein in WT and KO mice at 2 weeks after sham operation or TAC. VEGF protein levels were determined by western blot analysis. Representative VEGF-A bands with corresponding β-actin bands are shown. Band intensities were normalized to sham operated WT mice. Lower panel shows quantitative analysis of VEGF-A protein levels. Results are shown as mean ± SEM (n=8 in each group). *P<0.05 versus corresponding Sham and #P<0.05 versus WT mice.
Because VEGF plays an important role in regulation of angiogenesis following TAC [4], we assessed cardiac expression of VEGF-A at 2 weeks after TAC or sham operation by QRT-PCR and Western blot analyses. TAC surgery significantly increased cardiac VEGF-A165 expression at both mRNA and protein levels in WT mice, but the increase in VEGF-A165 in the heart following TAC was diminished in APN-KO mice (Figure 2B and C). VEGF-A165 transcript and protein levels in the heart did not differ after sham operation between WT and APN-KO mice.
Myocardial LKB1 and AMPK phosphorylation in response to TAC
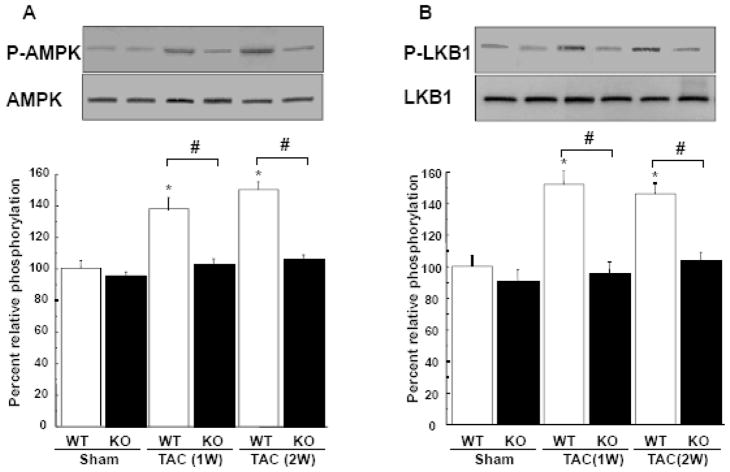
AMPK functions as a negative regulator of cardiac hypertrophy and protects against the development of heart failure [17, 18]. The LKB1 protein kinase functions as an upstream regulator of AMPK signaling in the heart [19, 20]. Thus, to examine the possible participation of this signaling axis in TAC-induced heart failure, the phosphorylation of AMPK and LKB1 in the heart was assessed by Western blot analysis. The cardiac expression of total AMPK and LKB1 protein did not differ between KO and WT mice after TAC. However, the activating phosphorylation of AMPK at Thr172 and LKB1 at Ser428 was increased in response to TAC in WT mice, but this induction was significantly reduced in APN-KO mice (Figure 3A and B). There were no differences in phosphorylation of AMPK and LKB1 in sham-operated hearts between APN-KO and WT mice.
Figure 3. Loss of adiponectin contributes to attenuated phosphorylation of AMPK and LKB1 after TAC.
A and B, Changes in phosphorylation of AMPK (P-AMPK)(A) and LKB1 (P-LKB1)(B) in the hearts of WT and KO mice at 1 or 2 weeks after sham operation or TAC. Representative blots of phosphorylated and total LKB1 and AMPK are shown. Lower panels show quantitative analysis of LKB1 and AMPK phosphorylation. Relative phosphorylated levels of LKB1 and AMPK were normalized to control values in sham-WT mice (n=4–8 hearts in each group). *P<0.05 versus corresponding sham and #P<0.05 versus WT mice.
Effect of AMPK inhibition on TAC-induced cardiac remodeling
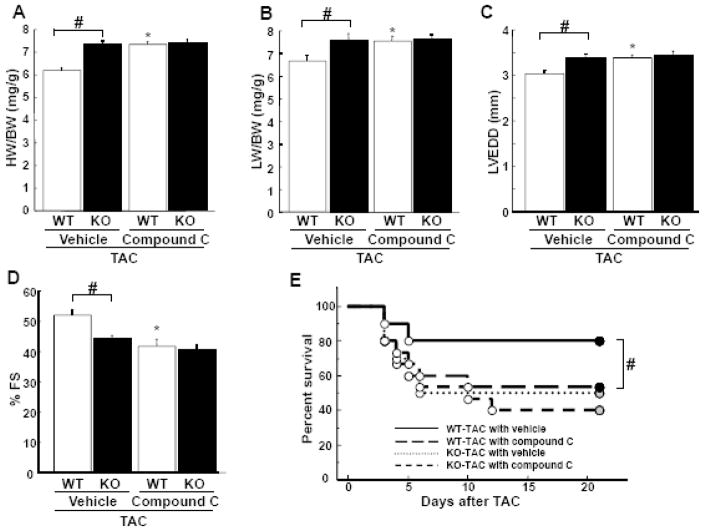
To further analyze the involvement of AMPK signaling in pathological cardiac remodeling caused by TAC, the AMPK inhibitor compound C was intraperitoneally administered (20 mg/kg 3 times per week) to WT and APN-KO mice starting 1 day before TAC and maintained until sacrifice. Treatment of WT mice with compound C resulted in increase in HW/BW and LW/BW ratios and LVEDD, and decrease in %FS and survival following TAC compared with vehicle treatment (Figure 4A–E), suggesting inhibition of AMPK exacerbates systolic heart failure after pressure overload. In contrast, treatment of compound C with APN-KO mice had no additive effects on HW/BW and LW/BW ratios, LVEDD, %FS and mortality after TAC as compared with vehicle (Figure 4A–E). In the presence of compound C, no statistically significant differences were observed in TAC-induced changes in HW/BW and LW/BW ratios, LVEDD, %FS and mortality between WT and APN-KO mice.
Figure 4. Effect of AMPK inhibition on cardiac remodeling in WT and APN-KO mice subjected to TAC.
A and B, The ratio of heart weight to body weight (HW/BW)(A) and lung weight to body weight (LW/BW) in WT and KO mice in the presence of compound C or vehicle at 3 weeks after TAC. C and D, Echocardiographic analysis of the fractional shortening (FS)(C) and LV end systolic diameter (LVEDD)(D) for WT and KO mice treated with compound C or vehicle at 3 weeks after sham operation or TAC. E, Kaplan-Meier survival analysis of WT and KO mice in the presence of compound C or vehicle following TAC. Results are presented as mean ± SEM (n=10 to 15 per each group). *P<0.05 versus corresponding vehicle-treated mice and #P<0.05 versus corresponding WT mice.
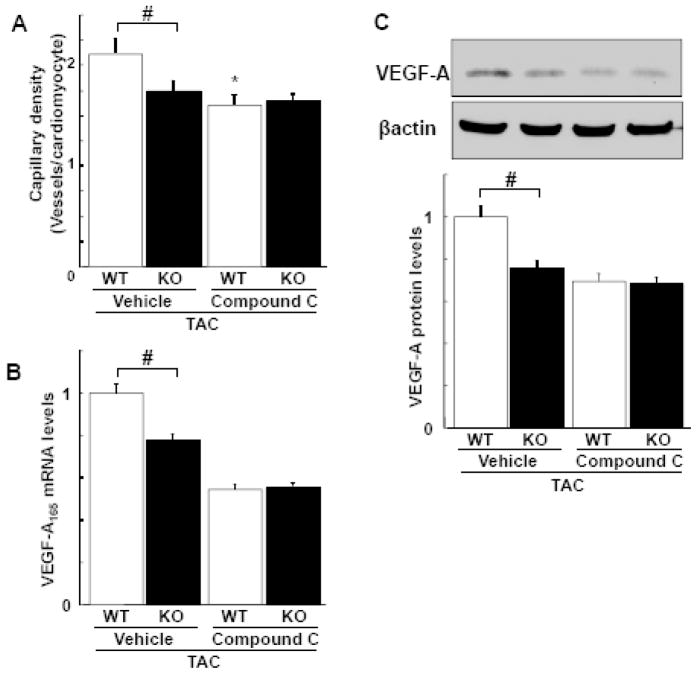
Treatment with compound C significantly suppressed capillary density in the myocardium following TAC in WT mice, whereas the AMPK inhibitor had no effect on the lower levels of capillary density in the APN-KO strain (Figure 5A). Furthermore, administration of Compound C diminished the TAC-induced increase in VEGF-A mRNA and protein expression in myocardial tissue in WT mice, but had no effect on the lower levels of VEGF expression in APN-KO mice (Figure 5B and C).
Figure 5. Effect of AMPK inhibition on capillary density and VEGF expression in response to pressure overload in WT and APN-KO mice.
A, Quantitative analysis of capillary density in the myocardium from WT and KO mice in the presence of compound C and vehicle at 3 weeks after TAC. Capillary density was determined by the number of CD31-positive cells per cardiomyocyte. B, VEGF-A165 mRNA levels in the hearts of WT and KO mice in the presence of compound C and vehicle at 2 weeks after TAC. VEGF-A165 mRNA levels were determined by QRT-PCR. C, Myocardial expression of VEGF-A protein in WT and KO mice in the presence of compound C and vehicle at 2 weeks after TAC as measured by western blot analysis. Representative blots for VEGF-A and β-actin are shown. Band intensities were normalized to vehicle-treated WT mice. Results are shown as mean ± SEM (n=8 in each group). *P<0.05 versus corresponding vehicle treatment and #P<0.05 versus WT mice.
VEGF delivery can reverse microvessel rarefaction cardiac dysfunction in APN-KO mice following pressure overload
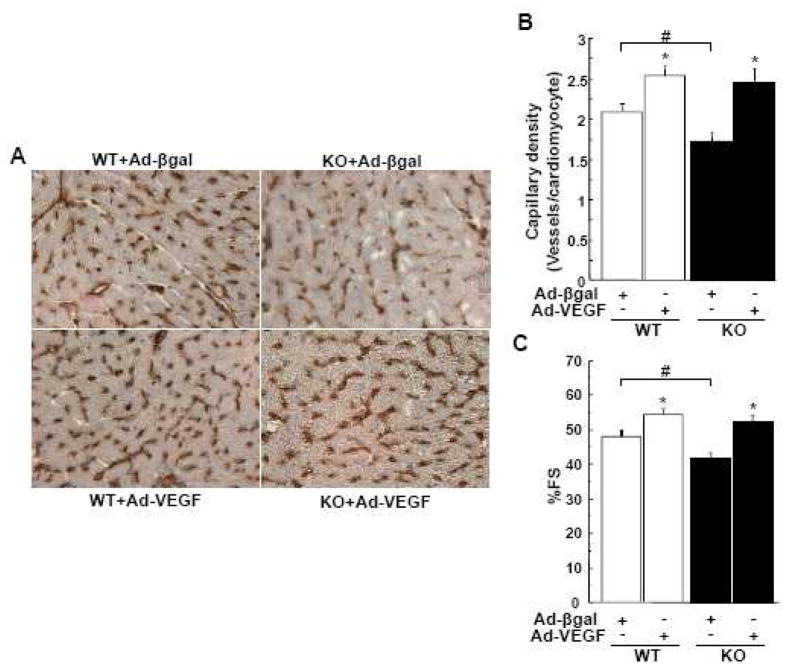
To examine the functional significance of diminished VEGF production in hearts of APN-KO mice, adenoviral vectors encoding VEGF-A165 (Ad-VEGF) or β-galactosidase (Ad-βgal) as control were delivered to WT or APN-KO mice 3 day prior to TAC. There were no differences in serum VEGF-A levels between WT and APN-KO mice at basal conditions. At the time of surgery, VEGF-A levels were significantly increased by Ad-VEGF treatment similarly in both strains (215.7±41.7 pg/ml in WT mice, 207.7±38.6 pg/ml in APN-KO mice). In TAC-treated hearts, VEGF overexpression did not affect the development of cardiac hypertrophy. However, VEGF overexpression increased cardiac capillary density (Figure 6A and 6B) and improved ventricular function by echocardiographic assessment in both strains (Figure 6C). Importantly, VEGF treatment abolished the TAC-induced differences in capillary density and ventricular performance between the WT and APN-KO mice (Figure 6). Collectively, these data indicate that the reduction of VEGF expression in TAC-treated APN-KO mice causally contributes to the greater degree of cardiac dysfunction observed in this strain.
Figure 6. Adenovirus mediated VEGF delivery reverses cardiac dysfunction in adiponectin deficient mice following pressure overload.
A, Representative images of CD31-stained heart sections from WT and KO mice treated with Ad-VEGF or Ad-βgal at 3 weeks after TAC in. Brown indicates CD31-positive endothelial cells, whereas light purple indicates nuclei in the hematoxylin stain. Magnification, ×400. B, Quantitative analysis of capillary density by measuring the number of CD31-positive cells per cardiomyocyte from each group. C, Echocardiographic analysis of the fractional shortening (FS) for WT and KO mice treated with Ad-VEGF or Ad-βgal at 3 weeks after TAC. Results are presented as mean ± SEM (n=4–7 per each group). *P<0.05 versus corresponding Ad-βgal treated mice and # P<0.05 versus corresponding WT mice.
Adiponectin stimulates VEGF production in cultured myocytes through AMPK signaling
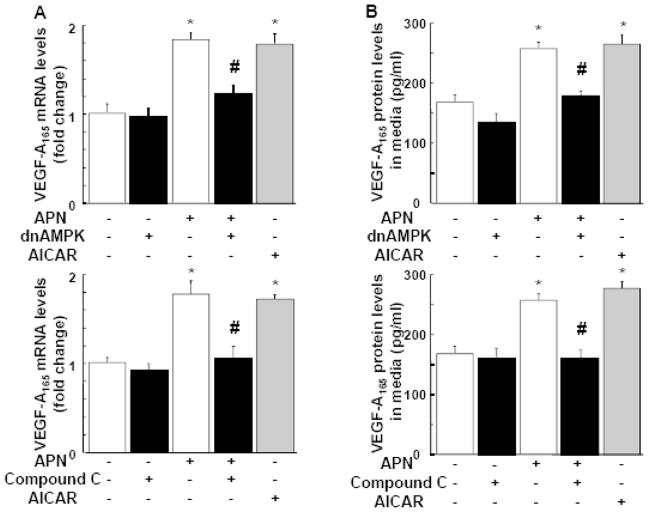
To examine whether adiponectin regulates VEGF expression in neonatal rat ventricular myocytes (NRVMs) in vitro, VEGF-A165 transcripts were measured in cultured NRVMs by QRT-PCR analysis and VEGF-A165 protein levels in the cell culture media was assessed by ELISA. Treatment with adiponectin significantly increased VEGF mRNA and protein levels in NRVMs (Figure 7A and B). Likewise, a chemical AMPK activator, aminomidazole-4-carboxamide-1-B-D-ribofuranoside (AICAR) stimulated VEGF-A165 production in NRVMs (Figure 7A and B), which is consistent with the findings in a cultured skeletal muscle cell line [33]. To examine the possible involvement of AMPK activation in regulation of VEGF-A165 expression by adiponectin, NRVMs were transduced with an adenoviral vector expressing a dominant-negative AMPK construct (Ad-dnAMPK) or Ad-βgal as a control. Transduction with Ad-dnAMPK blocked adiponectin-mediated increase in VEGF expression at mRNA and protein levels in NRVMs without affecting basal VEGF-A165 levels (Figure 7A and B). Transduction with Ad-βgal had no effects on VEGF-A165 production compared with NRVMs that were not transduced with adenovirus (data not shown). Furthermore, the AMPK inhibitor compound C blocked adiponectin-induced increase in VEGF-A165 transcript expression and protein secretion to the culture media (Figure 7A and B). These data suggest that adiponectin stimulates VEGF production in cardiac myocytes via an AMPK-dependent mechanism.
Figure 7. Adiponectin stimulates VEGF production in cultured cardiac myocytes through AMPK signaling.
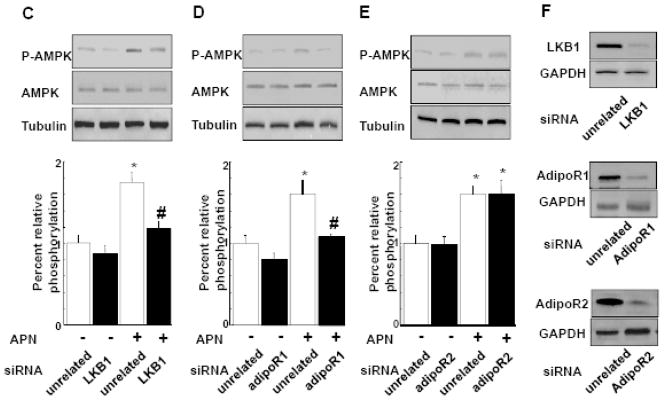
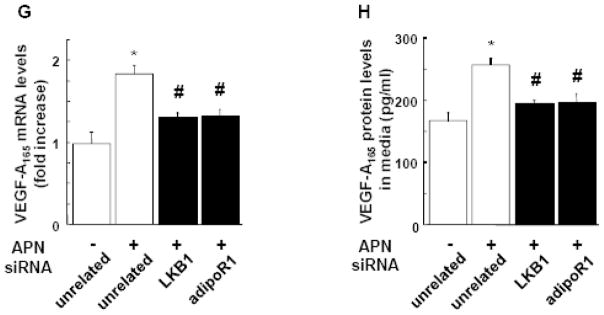
A and B, Effect of adiponectin on VEGF-A165 mRNA (A) and VEGF-A protein (B) levels in cardiomyocytes. Neonatal rat ventricular myocytes (NRVMs) were transduced with adenoviral vector expressing dominant-negative AMPK (dn-AMPK) or an adenoviral vector expressing β-galactosidase (βgal, control) for 16 hours, and then treated with adiponectin (APN, 30μg/ml) or vehicle for 24 hours. In some conditions, NRVMs were treated with compound C (20μM) prior to treatment with APN or vehicle. Treatment with AICAR (2uM) was used as a positive control. VEGF-A165 mRNA levels were measured by QRT-PCR methods. VEGF-A165 protein levels in the cell culture media were assessed by ELISA. Contribution of LKB1 (C), AdipoR1 (D) and AdipoR2 (E) to adiponectin-induced AMPK activation. NRVMs were transfected with siRNA targeting LKB1, AdipoR1 or AdipoR2, or unrelated siRNAs for 48 hours followed by stimulation with adiponectin (APN) or vehicle for 30 min. AMPK phosphorylation was assessed by western blot analysis. Representative blots are shown in upper panels. Lower panels show quantitative analysis of AMPK phosphorylation. (F) Representative immunoblots documenting knock-down of LKB1, AdipoR1 and AdipoR2 following treatment with specific and unrelated control siRNA. Effect of knockdown of AdipoR1 or LKB1 on adiponectin-induced VEGF-A165 mRNA (G) and protein (H) levels. After transfection with siRNA against LKB1 or AdipoR1, or unrelated siRNAs, NRVMs were treated with adiponectin or vehicle for 24 hours. *P<0.05 versus corresponding control and #P<0.05 versus adiponectin treatment.
To test the possible contribution of LKB1 to adiponectin-induced AMPK signaling in cardiomyocytes, NRVMs were transfected with siRNA targeting LKB1 or unrelated siRNA followed by stimulation with recombinant adiponectin protein. Treatment of NRVMs with adiponectin resulted in a significant increase in phosphorylation of AMPK at Thr172 as determined by Western blot analysis (Figure 7C, 7D and 7E) consistent with our previous data [22]. Transfection of NRVMs with siRNA against LKB1 resulted in 85% and 74% decrease in LKB1 mRNA and protein levels, respectively (Figure 7F). Knockdown of LKB1 also blocked the adiponectin-stimulated increase in AMPK phosphorylation in NRVMs without changing basal phosphorylation levels (Figure 7C). To elucidate the receptor that mediates adiponectin-AMPK signaling in cardiac myocytes, NRVMs were transfected with siRNA targeting AdipoR1 or unrelated siRNA. Transfection with siRNA against AdipoR1 reduced AdipoR1 mRNA and protein levels by 87% and 65% in NRVMs (Figure 7F). Ablation of AdipoR1 abolished adiponectin-stimulated increases in AMPK phosphorylation caused by adiponectin in NRVMs, respectively (Figure 7D). Transfection with siRNA targeting AdipoR2 attenuated AdipoR2 mRNA and protein levels by 66 and 59% in NRVMs, respectively. However AdipoR2 depletion did not affect AMPK phosphorylation induced by adiponectin in NRVMs (Figure 7E and 7F).
To dissect the receptor-mediated signaling involved in adiponectin-VEGF regulatory axis in cardiac myocytes, NRVMs were transfected with siRNA targeting LKB1 or AdipoR1 followed by treatment with adiponectin for 24 h. The adiponectin-induced increase in VEGF-A mRNA and protein expression in NRVMs was abrogated by ablation of LKB1 or AdipoR1 (Figure 7G and 7H). Transfection with siRNA against LKB1 or AdipoR1 did not affect cardiomyocyte VEGF-A165 production in the absence of adiponectin (data not shown). These data suggest that VEGF-A165 induction by adiponectin in NRVMs is dependent on AdipoR1-LKB1 signaling.
Discussion
In this study, we show that adiponectin-deficiency leads to exacerbated cardiac hypertrophy and systolic dysfunction that is associated with attenuated myocardial capillary formation during pressure overload. These effects were accompanied by reduced AMPK signaling and VEGF expression in the heart. AMPK inhibition with compound C aggravated cardiac dysfunction and reduced capillary density and VEGF production in the heart of WT mice. However, treatment with this compound was not additive with adiponectin-deficiency, and APN-KO and WT mice treated with compound C were indistinguishable with regard to cardiac function, capillary density and VEGF expression following TAC. Notably, adenovirus-mediated VEGF delivery was shown to reverse the greater cardiac dysfunction in APN-KO mice. Consistent with these findings, it has been shown that blockade of VEGF induction disrupts the normal maintenance for myocardial capillary density during pressure overload, leading to acceleration of the transition to systolic dysfunction [4]. Therefore, we propose that AMPK inactivation caused by adiponectin-deficiency is linked to the disruption of adaptive cardiac angiogenesis during compensatory hypertrophy, thereby contributing to systolic dysfunction.
We demonstrated that knockdown of AdipoR1 or LKB1 abolished adiponectin-stimulated phosphorylation of AMPK in cardiac myocytes, but ablation of AdipoR2 did not affect AMPK phosphorylation induced by adiponectin. AdipoR1 has been shown to mediate adiponectin-induced AMPK activation in hepatocytes, skeletal muscle, cardiac cells and endothelial cells [34–37]. It has been reported that LKB1 is required for AMPK activation caused by adiponectin in hepatocytes [38]. Collectively, these findings suggest that the AdipoR1/LKB1/AMPK signaling axis can regulate various cellular responses to adiponectin. Here it is shown that this pathway regulates VEGF production by the heart. Previously, AMPK activation by hypoxia or AICAR has been shown to promote VEGF induction in cancer cell lines and skeletal myocytes [33, 39, 40]. Consistent with these findings, we show for the first time that adiponectin stimulates VEGF production through an AMPK-dependent mechanism in cardiac myocytes. We also found that pressure overload-induced LKB1 phosphorylation in the heart was diminished in APN-KO mice. Thus, adiponectin appears to control cardiac remodeling partly through modulation of cardiac angiogenesis via the AdipoR1-LKB1-AMPK pathway.
It has been shown that AMPK activity is enhanced in rat hypertrophied hearts caused by pressure overload [22, 41]. A recent study using a loss-of-function genetic manipulation showed that cardiac AMPK is protective against the development of ventricular hypertrophy and dysfunction in response to pressure overload [25]. Activation of AMPK by pharmacological reagents reduces agonist-stimulated protein synthesis in cultured cardiac myocytes via the pathways involving S6 kinase and eukaryotic elongation factor-2 [41]. Similarly we and others have shown that adiponectin attenuates cellular hypertrophic response to agonists through AMPK activation in cardiomyocytes [21, 22]. Taken together, in addition to the indirect actions of adiponectin on the vasculature via AMPK-stimulated VEGF production, adiponectin can modulate cardiac remodeling by directly affecting cardiomyocyte AMPK signaling. It should also be noted that AMPK also has direct protective activities on vascular endothelial cells. We have previously reported that adiponectin promotes ischemia-induced revascularization in skeletal muscle which is partly dependent on its ability to activate AMPK signaling [26, 27, 37]. Thus, we cannot exclude the possibility that adiponectin modulates cardiac capillary formation in vivo by directly acting on the endothelium. Ultimately, to address these issues will require a better understanding of receptor-mediated signaling pathways that are involved in adiponectin’s actions in different cardiovascular tissues and the development of new mouse models that ablate different components of these pathways in specific cell types.
In the present study, we utilized compound C, a pharmacological ATP competitive inhibitor of AMPK to examine the role of AMPK in cardiac remodeling and VEGF reduction in response to pressure overload. While a number of in vivo studies indicated the usefulness of this compound for inhibition of AMPK signaling [42–44], a recent in vitro study demonstrated compound C reduces hypoxia-induced activation of hypoxia-inducible factor-1 in an AMPK-independent manner [45]. However, a number of lines of evidence suggest that AMPK mediates the actions of adiponectin in this model. First, our data show that APN-KO mice had a reduction in AMPK activation and VEGF induction in the myocardium in response to pressure overload. Similarly, mice lacking AMPK-α2 display exacerbated myocardial remodeling following pressure overload [46]. Finally, we have shown in cell culture studies that adiponectin stimulates VEGF production in an AMPK-dependent manner in myocytes.
In conclusion, the present study documents that lack of adiponectin results in the disruption of cardiac angiogenesis during compensatory hypertrophy in response to pressure overload. Impaired angiogenesis in the hypertrophic hearts is associated with a reduction of AMPK-dependent VEGF production. Thus, states of adiponectin deficiency, i.e. obesity and diabetes, may favor the transition from cardiac hypertrophy to heart failure via a disruption of normal paracrine signaling between myocytes and the coronary vasculature.
Acknowledgments
We gratefully acknowledge the technical assistance of Rie Miura and Megumi Kondo.
Finding Sources
This study was funded by National Institutes of Health grants HL77774, HL86785, AG15052 and HL81587 to K. Walsh. R. Shibata was supported from the Grant-in-Aid for Young Scientists A. M. Shimano was supported by the Banyu Fellowship Program sponsored by Banyu Life Science Foundation International.
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiography determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–9. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 3.Luedde M, Katus HA, Frey N. Novel molecular targets in the treatment of cardiac hypertrophy. Recent Pat Cardiovasc Drug Discov. 2006;1:1–20. doi: 10.2174/157489006775244290. [DOI] [PubMed] [Google Scholar]
- 4.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–93. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–18. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep. 2003;3:207–13. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 8.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 9.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 10.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 11.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 12.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–23. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–6. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duda MK, O’Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, et al. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–10. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–74. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata R, Sato K, Kumada M, Izumiya Y, Sonoda M, Kihara S, et al. Adiponectin accumulates in myocardial tissue that has been damaged by ischemia-reperfusion injury via leakage from the vascular compartment. Cardiovasc Res. 2007;74:471–9. doi: 10.1016/j.cardiores.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–16. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, et al. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835–44. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–13. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R, Wang WQ, Zhang H, Yang X, Fan Q, Christopher TA, et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–E8. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–91. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 26.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–9. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–4. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 28.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 29.Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–24. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, et al. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002;22:4803–14. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, et al. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res. 2001;89:453–60. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 32.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, et al. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–6. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 33.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–46. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 34.Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O’Brien PE, Dixon JB, et al. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005;90:3665–72. doi: 10.1210/jc.2004-1980. [DOI] [PubMed] [Google Scholar]
- 35.Fujioka D, Kawabata K, Saito Y, Kobayashi T, Nakamura T, Kodama Y, et al. Role of adiponectin receptors in endothelin-induced cellular hypertrophy in cultured cardiomyocytes and their expression in infarcted heart. Am J Physiol Heart Circ Physiol. 2006;290:H2409–16. doi: 10.1152/ajpheart.00987.2005. [DOI] [PubMed] [Google Scholar]
- 36.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, Herschman HR, et al. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol. 2009;29:3487–99. doi: 10.1128/MCB.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, et al. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 2009;382:51–6. doi: 10.1016/j.bbrc.2009.02.131. [DOI] [PubMed] [Google Scholar]
- 39.Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, et al. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003;278:39653–61. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- 40.Yun H, Lee M, Kim SS, Ha J. Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. J Biol Chem. 2005;280:9963–72. doi: 10.1074/jbc.M412994200. [DOI] [PubMed] [Google Scholar]
- 41.Tian R, Musi N, D’Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–9. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 42.Izumi Y, Shiota M, Kusakabe H, Hikita Y, Nakao T, Nakamura Y, et al. Pravastatin accelerates ischemia-induced angiogenesis through AMP-activated protein kinase. Hypertens Res. 2009;32:675–9. doi: 10.1038/hr.2009.77. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Han Y, Pang W, Li C, Xie X, Shyy JY, et al. AMP-activated protein kinase promotes the differentiation of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1789–95. doi: 10.1161/ATVBAHA.108.172452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 45.Emerling BM, Viollet B, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett. 2007;581:5727–31. doi: 10.1016/j.febslet.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, et al. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52:918–24. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]