Abstract
Cerebral periventricular white matter injury stands as a leading cause of cognitive, behavioral and motor impairment in preterm infants. There is epidemiological and histopathological evidence demonstrating the role of prenatal or neonatal inflammation in brain injury in preterm infants. In order to define the effect of an inflammatory insult in the developing brain on magnetic resonance (MR) imaging, we obtained high resolution conventional and diffusion MR images of the brain of rat pups after an inflammatory injury. Rat pups were subjected on postnatal day 5 (P5) to a stereotaxic injection of lipopolysaccharide in the corpus callosum and then imaged at 11.7 Tesla on days 0, 2 and 4 following the injury. They were subsequently sacrificed for immunohistochemistry. Diffusion tensor imaging (DTI) acquired at high spatial resolution showed an initial reduction of the apparent diffusion coefficient (ADC) in the white matter. This was followed by an increase in ADC value and in T2 relaxation time constant in the white matter, with an associated increase of radial diffusivity of the corpus callosum, and a 10-fold increase in ventricular size. On histology, these MR changes corresponded to widespread astrogliosis, and decreased proportion of the section areas containing cresyl violet positive stain. The increase in radial diffusivity, typically attributed to myelin loss, occurred in this case despite the absence of myelin at this developmental stage.
Keywords: periventricular leukomalacia, lipopolysaccharide, diffusion tensor imaging, radial diffusivity, high field MRI, myelin, white matter injury, inflammation, apparent diffusion coefficient
Introduction
Cerebral periventricular white matter injury stands as the predominant cause of cognitive and motor delay in the preterm infant (Blumenthal, 2004). The hypoxic-ischemic and the inflammatory injury pathways have been identified as two main causes of periventricular leukomalacia (PVL). Epidemiological studies have described an association between inflammation and brain injury in preterm infants (Dammann and Leviton, 1997; Dammann et al., 2001; Hagberg et al., 2005). Inflammatory cytokines such as TNFα, interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) have been shown to be elevated in cerebral white matter of infants with PVL (Deguchi et al., 1996; Deguchi et al., 1997; Yoon et al., 1997b). Further support for the role of inflammation comes from data showing a high incidence of severe white matter injury and poor outcome in infants with necrotizing enterocolitis or sepsis (Shah et al., 2008).
Human data regarding acute changes in the apparent diffusion coefficient (ADC) measured by MRI in association with white matter injury, and PVL in particular, are relatively sparse. A case report has described a decrease in the ADC in the first days of life in the cerebral white matter that correlated with subsequent severe periventricular leukomalacia (Inder et al., 1999). When imaged at term equivalent age, MR images of preterm infants with PVL show hyperintensity of the white matter on T2 weighted imaging (Maalouf et al., 1999; Woodward et al., 2006) which, in some cases, is associated with an increase in ADC (Counsell et al., 2003). Infants with such injury, when evaluated at 2 years of age, have impaired neurocognitive performance (Domizio et al., 2005).
A variety of animal models employ lipopolysaccharide (LPS) or bacterial inoculation during the prenatal or newborn period to induce PVL. The agent may be administered by prenatal exposure or postnatal stereotaxic injection in the rat (Cai et al., 2000; Cai et al., 2003), by intrauterine Escherichia coli infection in the rabbit (Debillon et al., 2003; Debillon et al., 2000; Yoon et al., 1997a), or by intraperitoneal injection in neonatal cats (Gilles et al., 1977) and dogs (Young et al., 1982). A robust model of inflammatory white matter injury in the immature rat brain has been developed using stereotactic injection of lipopolysaccharide (LPS) into the corpus callosum (Cai et al., 2003). This model has been shown to induce a major cytokine release (TNFα as well as IL-1β, IL-6 and inducible nitric oxide synthase (iNOS)) by activated microglia and macrophages. The strong inflammatory reaction has been shown to induce a reduction of preoligodendrocytes followed by hypomyelination and ventricular dilatation (Cai et al., 2003; Pang et al., 2003). In addition, this animal model has also been shown to be associated with motor deficits in the first weeks of life (Fan et al., 2005).
Animal models of PVL present an opportunity to obtain a better understanding of the early MRI changes associated with this condition. It is well known that water ADC values decrease following a variety of forms of brain injury, particularly hypoxic-ischemic injury. In animal models, ADC values initially fall for a period of days, pass through normal (pseudonormalization), and then rise to greater than normal (Welch et al., 1995). A similar pattern is found for ADC changes following brain injury in term-born human infants (McKinstry et al., 2002). In addition to showing injury via changes in ADC values, DTI can be used to assess white matter integrity through the measurement of diffusion anisotropy. In healthy white matter, water diffusion values measured orthogonal to white matter tracts are a factor of three smaller than those measured parallel to them, a condition known as diffusion anisotropy. This likely reflects the fact that there is greater hindrance to water displacement orthogonal to axons (for which water has to move around or through axons and myelin) than parallel to them (for which water can move between axons or myelin layers without crossing lipid membranes). DTI data can be analyzed in terms of overall anisotropy, or in terms of axial (parallel to axons) and radial (orthogonal to axons) diffusivities. Disruption of myelin is associated with an increase in radial diffusivity in mouse models (Song et al., 2002; Song et al., 2005). Axonal injury, in contrast, is associated with a reduction in axial diffusivity (Sun et al., 2006). While these and other changes are well described for hypoxic-ischemic injury, the changes associated with inflammatory white matter injury in neonates are less well known.
In this study, we characterized the acute temporal evolution of water ADC changes following stereotaxic injection of LPS into the corpus callosum of an immature rodent. Animals underwent MR imaging, which included DTI at high spatial resolution, on the day of injection as well as at 2 and 4 days after injury. They were subsequently sacrificed for immunohistochemistry. Postnatal day 5 (P5) male rats were used for intracerebral injection. P3 rat pups are equivalent to very preterm human infants between 24 to 28 weeks gestation (Sizonenko et al., 2003). P7 rat pups have been used mainly with the Levine model as a model of newborn asphyxia. The exact human gestational age equivalent to P7 in the rat is a subject of debate. Hagberg et al. describe the term newborn to be equivalent to P7-P14 (Hagberg et al., 1997), whereas Vannucci et al. consider the P7 rat pups to be histologically similar to a 32- to 34-week gestation human fetus (Vannucci and Vannucci, 2005). P5 rat pups lie in between, with a maturation equivalence to 28 to 32 weeks gestation in humans. This is the period of high sensitivity to white matter injury in preterm infants (Back et al., 2001), and corresponds to a period during which white matter is very rich in pre-oligodendrocytes. An increased male vulnerability to injury has been reported in several outcome studies of children born preterm (Hindmarsh et al., 2000; Johnston and Hagberg, 2007; Lauterbach et al., 2001; Lodygensky et al., 2005; Reiss et al., 2004) with specific learning difficulties and academic achievement (Hack et al., 2002). Similar findings were described in hippocampal cell culture, with greater cell death induced by a GABA agonist in male- versus female-derived cultures (Nunez et al 2008). Further, a significant reduction of brain levels of NAD+ in male mouse pups as compared with female has been observed after a hypoxic-ischemic brain injury, thus favoring a stronger apoptotic response in this group (Hagberg et al., 2004). Moreover, a difference response to a neuroprotective treatment favoring female rat pups was also described (Bona et al., 1998). Thus, we limited this study to males to minimize variability in results related to the sex of the animals.
Methods
Animals and neonatal inflammatory injury
Food and water were provided to the dam ad libitum, and all care was provided in compliance with National Institute of Health guidelines on the use of laboratory animals and were approved by the Institutional Animal Studies Committee of Washington University, St Louis. P5 male Sprague-Dawley rats were used for intracerebral injection. One mg/kg of LPS suspension (Escherichia coli, serotype 055:B5, Sigma St Louis, MO) in sterile saline in a total volume of 0.5 μL or the same volume of sterile saline alone was administered under isoflurane anesthesia. Stereotaxic injections into the left corpus callosum were made at coordinates 1.0 mm posterior and 1.0 mm lateral to the bregma and 2.0 mm deep to the skull surface. Injections were made over five minutes with a titanium, 33-G needle mounted on a NanoFil Injection holder (World Precision Instruments©). The needle was left in place for an additional two minutes and then retrieved slowly (Cai et al., 2003; Pang et al., 2003). Eleven male rat pups injected with LPS and 12 with saline alone (CTR). All animals survived the injection. The location of the injection was assessed through MR imaging (Figure 1A). Injections were properly placed in 8 animals injected with LPS and 7 with saline alone. These animals were also reimaged at P7 and P9. On the 9th day of life brains were fixed by transcardiac perfusion of a saline solution followed by 4% paraformaldehyde for preparation of frozen brain sections.
Figure 1.
(A) T1-weighted image obtained following LPS injection on P5. Note the hypointensity at the site of injection (arrow). (B) ADC map with regions of interest shown for white matter at P7. (C) RGB map on P9 in which color represents the preferred direction of water diffusion. Red corresponds to medial-lateral, green to superior-inferior, and blue to rostral-caudal. Regions of interest are shown for the corpus callosum and left external capsule.
Magnetic Resonance Imaging
All MRI studies were acquired on an 11.74 Tesla Magnex Scientific magnet with a 26 cm diameter clear horizontal bore. The system was equipped with an 8-cm inner diameter, actively shielded gradient set driven by Copley high-performance gradient amplifiers and providing 120 G/cm with a rise time of 200 μs. The magnet, gradient coil and gradient power supply were run with a Varian UNITY/INOVA console (Varian Associates, Palo Alto, CA) controlled by a Sun Blade 1000 workstation.
Prior to imaging, rat pups were anesthetized with isoflurane/02 (4% for induction, 0.5% to 1.5% for maintenance) and placed in a custom built, MRI compatible head holder. A birdcage coil of 2 cm of diameter (Stark Contrast, Erlangen, Germany) was used for all animals. Both groups had an MRI at 8 hours (7 h 40 min ± 50min), 2 days and 4 days following the injection. One scan 4 days after injection was excluded from the study because the animal was too big to fit into the RF coil. During MR acquisition, body temperature and respiration were monitored and kept stable using an MR compatible monitoring system (Small Animal Instruments, Inc.). Coronal T2-weighted images (T2W) were acquired using a conventional multislice spin-echo with TR = 5 s, TE = 80 ms, slice thickness = 250 μm and in-plane resolution = 140 × 140 μm. Coronal T2 maps were calculated from separate T2 weighted data sets (TR = 2s, TE = 18 or 120 ms, slice thickness = 1 mm, and in-plane resolution = 140 × 140 um). The slice thickness was chosen to provide an adequate signal to noise ratio for the long TE data set. Coronal diffusion images were acquired with a spin-echo sequence with TR = 2.2 s, TE = 40 ms, 4 averages, slice thickness = 500 μm, in-plane resolution at P5 = 240 × 240 μm zero filled to 120 × 120 μm, at P 7 and P 9 = 280 × 280 μm zero filled to 140 × 140 μm. The delay between application of the gradient pulse (Δ) = 21 ms, the gradient pulse duration (δ) = 7 ms, b value amplitudes = 0 and 753 s/mm2. Origin-symmetric diffusion gradient directions (2 × 6) were used to minimize the background magnetic field gradient effect in the diffusion measurement (Jara and Wehrli, 1994).
MRI Data Analysis
Lateral ventricles and the third ventricle cut in half were manually segmented on all T2-weighted slices containing them (250 μm thick), 4 days after injection of LPS, using Amira (Visage Imaging Inc., San Diego, CA; Figure 2A-B). T2 maps were derived pixel-by-pixel in Matlab (MathWorks, Natick, MA, USA) employing the single-exponential decay function with two parameters (relative signal intensity and T2 relaxation time constant). On a voxel-by-voxel basis, quantitative maps of the apparent diffusion coefficient, axial diffusivity and radial diffusivity were derived using software written in Matlab (MathWorks, Natick, MA, USA) as described previously (Song et al., 2003; Song et al., 2002; Song et al., 2005; Sun et al., 2003).
Figure 2.
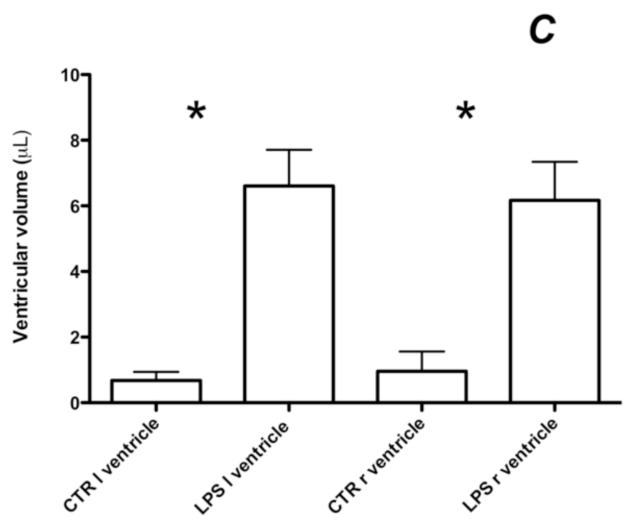
T2-weighted images with their overlaid ventricular segmentation of a CTR animal (A) and a LPS exposed animal (B). Note the significant ventricular dilatation. Bar graph (C) representing the quantification of ventricular volume on T2-weighted images in animals exposed to LPS or CTR. Note that the ventricular dilation is symmetric. (* p < 0.005). (l) left, (r) right. LPS ventricle: ventricular volume of the left ventricle of animals exposed to LPS, CTR ventricle: ventricular volume of rat pups exposed to saline alone.
Three regions of interest (ROIs) were used for the studies. The first one was a large ROI encompassing the cingulum bundle, part of the corpus callosum and the external capsule (Figure 1B) on four consecutive slices. This ROI was identified on the ADC maps and used to obtain ADC values of the white mater at P5, P7 and P9 calculated from the mean of both sides. An equivalent ROI was used to determine the T2 relaxation time constant of the white matter. The other two ROIs were smaller and identified on the RGB maps from three consecutive image slices starting at the image equivalent to bregma (− 2.5 mm on the adult atlas). They were placed on the corpus callosum and left external capsule (Figure 1C) and used to measure axial diffusivity, radial diffusivity, and ADC values for comparison with histology.
Histology and Immunohistochemistry
Extracted brains were immersion fixed in 4% paraformaldehyde for 24 hours at 4° C and then cryoprotected by immersion in 30% sucrose for at least 24 h. The brains were placed in an upright vertical position on a freezing sliding microtome. Special care was taken to ensure that the midline of the brain was absolutely perpendicular to the blade for cutting coronal sections at 50 μm. Similarly, when MRI sections were obtained the animals were carefully positioned by placing them in a custom built MRI compatible neonatal head holder in ertalyte PET. This allowed for high degree of correspondence between MRI and histology. Coronal sections that matched the MR images were chosen for histology. Brain sections 300 μm apart were stained with cresyl violet or using antibodies against GFAP: anti GFAP (1:1k, Chemicon #AB5541) or MBP: SMI94 (1:500, Covance). Sections were washed in TBS and endogenous peroxidase was quenched in 0.3% hydrogen peroxide. Non-specific binding was blocked with 2% milk in TBS. Primary antibodies were applied to free floating sections overnight at 4° C. Sections were washed and incubated in species-specific biotinylated secondary antibody. HRP was added to the biotinylated secondary antibody using the ABC Elite kit (Vector) and visualized with diaminobenzidine (Sigma).
Histology data analysis
Cresyl Violet
The mean thickness of the corpus callosum was obtained from the average of the thickness determined on 5 slices stained with cresyl violet 50 μm thick, 300 μm apart. The measures were done at the midline level. The proportion of the section areas containing cresyl violet positive stain was calculated from 3 images of 280 × 280 μm2 of stained slices, pre-processed by a rolling-ball background removal algorithm (Jacob et al., 2008; Ricard et al., 2007; Sternberg, 1983) in ImageJ (Abramoff et al., 2004) and thresholded with the same cutoff in both groups.
GFAP
The proportion of the section areas immunostained by GFAP was calculated from 3 images of 280 × 280 μm2 of stained slices in the corpus callosum and in the left external capsule, pre-processed by a rolling-ball background removal algorithm (Jacob et al., 2008; Ricard et al., 2007; Sternberg, 1983) in ImageJ (Abramoff et al., 2004) and thresholded with the same cutoff in both groups. It allowed the calculation of the percentage area covered by the cells and their processes.
Statistics
All data are presented as mean ± SEM. Comparisons between two groups were performed using a Mann-Whitney test to compare the two groups. Statistical significance was set at 0.05. Correlation analysis was done using the Spearman non-parametric correlation method. Kruskal-Wallis test with a Dunn’s post-hoc test were used to compare the evolution of the ADC of the white matter exposed to normal saline alone. All analysis was performed using GraphPad Prism (GraphPad Software).
Results
MRI
High-resolution T2-weighted images, 4 days after injection, showed a significant bilateral ventricular dilatation (total LPS 12.8 ± 2.23 μL; CTR 1.63 ± 0.85 μL, p = 0.001) with both ventricles equally dilated (Figure 2) consistent with symmetrical bihemispheric cerebral injury. Preliminary data demonstrated bilateral diffusion of the LPS injection when pups were imaged immediately after the surgery, thus exposing both hemispheres to LPS.
White matter T2 map
The quantification of T2 based on three consecutives slices showed a marked increase of the T2 relaxation time constant in rat pups 4 days after exposure to LPS (LPS 54.1 ± 0.67 ms; CTR 49.6 ± 1.04 ms, p = 0.003).
White matter ADC map
In the white matter of CTR rat pups, the ADC was found to decrease between P5 and P9 (p = 0.02) with a Dunn’s post-hoc test being significant only between P7 and P9 (p < 0.05; Figure 3). For LPS exposed rat pups, the ADC of the white matter on the same day following the LPS injection showed a significant decrease (LPS 1.10 ± 0.015 μm2/ms; CTR 1.17 ± 0.017 μm2/ms, p = 0.014). Two days after the LPS injection the ADC remained reduced (LPS 1.09 ± 0.013 μm2/ms; CTR 1.16 ± 0.017 μm2/ms, p = 0.009). In contrast, after 4 days following the LPS injection the ADC had significantly increased compared to CTR (LPS 1.16 ± 0.015 μm2/ms; CTR 1.10 ± 0.017 μm2/ms, p = 0.013).
Figure 3.
Temporal evolution of the ADC of the cerebral white matter on the same day, 2 and 4 days after the injection. Note that the reduction of water diffusion on P5 is still present at P7. At P9, the ADC is higher in the white matter in the group exposed to LPS. Note the biphasic evolution of the ADC, similar to that described following a hypoxic-ischemic injury in newborn (McKinstry et al., 2002) and in rodents (Welch et al., 1995) but with a different time course (Nedelcu et al., 1999). Note the decrease of the mean ADC of the white matter in CTR animals related to brain maturation. A Mann Whitney test was performed between groups of the same age (* p < 0.015), a Kruskal-Wallis test was performed between CTR at P5, P7 and P9 (p = 0.02) with a Dunn’s multiple comparison test significant only between P7 and P9 (γ p < 0.05).
DTI
Corpus Callosum
At 9 days of life, quantification of diffusivity showed a marked increase in the ADC (LPS 1.03 ± 0.018 μm2/ms; CTR 0.95 ± 0.02 μm2/ms, p = 0.02) associated with an increase of the radial diffusivity (LPS 0.77 ± 0.019 μm2/ms; CTR 0.65 ± 0.05 μm2/ms, p = 0.013) but no change in axial diffusivity (LPS 1.55 ± 0.03 μm2/ms; CTR 1.47 ± 0.05 μm2/ms, p = 0.18; Figure 4).
Figure 4.
Representative axial (A) and radial (B) diffusivity maps of a rat brain in vivo on P9, 4 days after a stereotaxic injection of LPS in the corpus callosum. Note at the level of the corpus callosum the intensity is high in the axial diffusivity map (A) and low in the radial diffusivity map (B). (C) Bar graph of the diffusivity in the corpus callosum. Note the significant increase in radial diffusivity and ADC in animals exposed to LPS. (D) Bar graph of the diffusivity in left external capsule. Note the significant increase in radial diffusivity and ADC as well as axial diffusivity in animals exposed to LPS. (* p ≤ 0.03).
External capsule
At 9 days of life, quantification of diffusivity in the left external capsule demonstrated a marked increase in the ADC (LPS 1.25 ± 0.02 μm2/ms; CTR 1.16 ± 0.02 μm2/ms, p = 0.02) associated with an increase in both radial diffusivity (LPS 1.06 ± 0.02 μm2/ms; CTR 0.97 ± 0.03 μm2/ms, p = 0.02) and axial diffusivity (LPS 1.63 ± 0.03 μm2/ms; CTR 1.53 ± 0.03 μm2/ms, p = 0.03; Figure 4).
Histology
Myelin
To investigate if LPS injection affected myelin, we stained sections with a mouse monoclonal antibody against myelin basic protein, SMI-94. We detected very little myelin at this age. However the SMI-94 antibody has been shown to detect early myelination (Fukuda et al., 2005). A qualitative and quantitative analysis of myelination with SMI-94 showed no difference (data not shown) in myelination 4 days after exposure to LPS (Figure 5), suggesting that myelination at this stage has not been affected by LPS injection in the regions studied.
Figure 5.
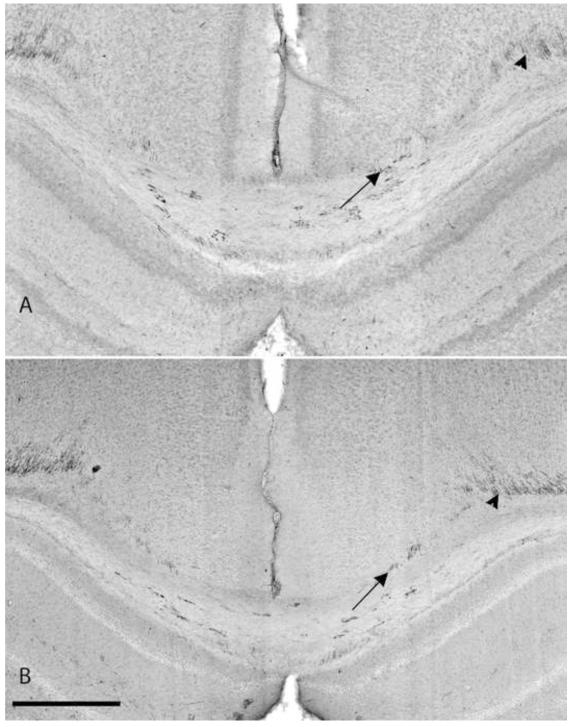
Immunohistochemistriy with SMI 94 for myelin. (A) injured rat pup with LPS 4 days after injection. (B) CTR animal injected with normal saline alone 4 days after injection. Note the paucity of myelin in the CTR animal at 9 days of age with very little staining in the corpus callosum, similar to the animal exposed to LPS. Arrow: myelin stained by SMI 94 in the corpus callosum. Arrowhead: myelin extending into the cingulum. Scale bar is 0.5 mm.
Proportion of the section areas containing GFAP positive stain
The proportion of the section areas immunostained by GFAP in the corpus callosum showed a trend towards higher GFAP expression in LPS-treated animals compared to the CTR, which did not reach statistical significance (LPS 31.2 ± 3.2 %; CTR 23.4 ± 2.2 %, p = 0.1812).
The proportion of the section areas immunostained by GFAP in the left external capsule showed a marked increase in the animals exposed to LPS (LPS 34.4 ± 2.2 %; CTR 21.8 ± 3.3 %, p = 0.013; Figure 6).
Figure 6.
Immunohistochemistry with GFAP for astrogliosis of an animal exposed to normal saline alone (CTR) (A) or LPS (B). Note the significant increase GFAP expression bilaterally in the injured animal also seen on the square images of 280 × 280 μm2 after background removal. Bar graph of the percentage area covered by GFAP expression in the corpus callosum (C) and in the left external capsule (D). Note the significant increase in GFAP expression in the left external capsule. Thick scale bar is 1 mm, thin scale bar is 0.5 mm. (* p = 0.013)
Proportion of the section areas containing cresyl violet positive stain
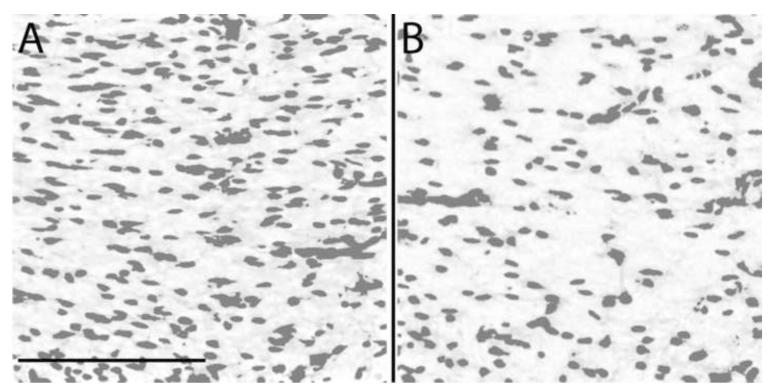
The proportion of the section areas containing cresyl violet positive stain of the corpus callosum based on cresyl violet slices was decreased 4 days after exposure to LPS (LPS 15.6 ± 0.7 %, CTR 19.5 ± 1.1 %, p = 0.014; Figure 7).
Figure 7.
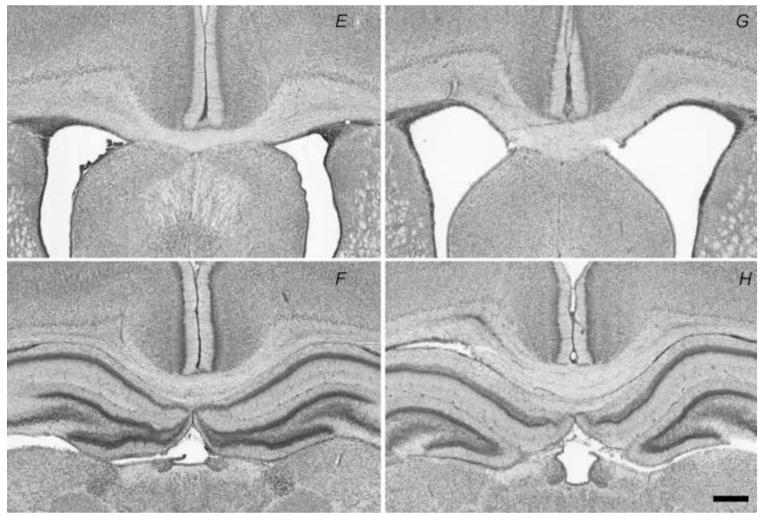
Proportion of the section areas containing cresyl violet positive stain of the corpus callosum 4 days after injection of normal saline alone (CTR) (A) or LPS (B) after a rolling-ball background removal algorithm with area covered by the cells using thresholding. (C) Bar graph of the proportion of the section areas containing cresyl violet positive stain of the corpus callosum 4 days after injection of LPS or normal saline alone (CTR). Note the significant decrease of the proportion of the section areas containing cresyl violet positive stain in animals exposed to LPS (* p = 0.014). (D) Bar graph of the mean thickness of the corpus callosum. Note the significant increase of the corpus callosum thickness in animals exposed to LPS (** p = 0.0013). Cresyl violet stain centered on the corpus callosum of a CTR animal (E-F), and an injured rat pup with LPS (G-H). Note the increase thickness of the corpus callosum. Scale bar is 0.5 mm.
Corpus callosum thickness
The thickness of the corpus callosum based on cresyl violet slices was increased when assessed 4 days after exposure to LPS (LPS 451 ± 20.8 μm, CTR 316 ± 11.5 μm, p = 0.0013; Figure 7).
Discussion
This model of inflammatory injury in the developing brain is clinically relevant in that it produces significant and consistent bilateral brain injury in surviving animals similar to that seen in preterm infant with white matter injury and poor neurodevelopmental outcome. Although inflammation is also seen after a hypoxic-ischemic insult, the injury resulting from LPS exposure is distinct from a hypoxic-ischemic lesion. The true physiopathology of periventricular leukomalacia has been debated since 1867 when Wirchow described “congenital encephalomyelitis” for the first time and proposed an acute infection as the cause because many of the mothers had syphilis or smallpox (Blumenthal, 2004). To date, there is significant epidemiological and medical evidence that inflammation alone plays a major role in preterm brain injury. This idea is supported by well-characterized animal models producing very distinctive pathological lesions mimicking what is present in preterm infants. There are no MR studies of inflammatory injury in the developing brain thus far, and very little knowledge on the interpretation of early MR (within a week from birth) in the context of inflammatory injury. It is not known if the inflammatory injury will have effects similar to those present following hypoxic-ischemic injury in the developing brain.
In this study, it was found that LPS injection into the corpus callosum in the immature rodent was associated with alterations in both the MR and histological markers of cerebral white matter. There was marked symmetrical ventricular dilatation by P9, similar to the previous results of Pang et al. (Pang et al., 2003) except that the ventricular dilatation in our study was symmetrical (Figure 2). In addition, the T2 relaxation time constant of the white matter was increased. This finding is consistent with the white matter signal abnormalities noted on T2-weighted images in premature infants (Woodward et al., 2006). In this case, high signal intensity on T2-weighted imaging was incorporated into a clinical score used to predict the neurocognitive outcome of preterm infants. The more widespread the areas of T2-hyperintensity, the higher the risk was to develop a neurocognitive delay. Using a quantitative approach, Hagmann et al found that the T2 time relaxation constant in white matter was elevated in preterm infants at risk for poor neurodevelopmental outcome (Hagmann et al., 2009).
The effect of the injection of saline itself was not tested in this study. The group that initially developed the model found no differences between saline injection rats with intact rats in terms of ventricular enlargement and inflammation (Zhengwei Cai, personal communication). In later studies, they used saline-injected rats as the control. The inflammatory response of saline injection was examined. The injection itself may have caused some acute inflammatory responses (as indicated by microglia activation), but most microglia in saline injected rat brain were at resting status and inflammatory cytokine level were quite low with an absence of O4 oligodendrocytes apoptosis quantified by caspase-3 activation (Cai et al., 2003; Fan et al., 2008; Fan et al., 2005). These data indicate that the effect of injection itself is minor.
The diffusion studies showed an overall time course of ADC changes (initial reduction followed by an increase by P9) similar to the changes described for hypoxic-ischemic injury in animals (Aden et al., 2002; Nedelcu et al., 1999) and humans (McKinstry et al., 2002). In addition, radial diffusivity increased in the corpus callosum and external capsule. Ordinarily, this finding is interpreted as representing injury to myelin. This explanation does not hold for this case, however, as there is very little myelin present at this developmental stage (Figure 5). A possible alternative explanation for this finding is increased spacing between axons due to vasogenic edema. The finding of longer T2 relaxation time constants is consistent with edema, as is the histologic finding of increased volume and reduced proportion of the section areas containing cresyl violet positive staining.
We detected an increase in GFAP expression in the corpus callosum and the external capsule of rat pups exposed to LPS. This increase reached significance in the external capsule but was only a trend in the corpus callosum (Figure 6C and D). Prior to embarking on this study, we obtained MR images immediately after injection that showed LPS tracking in the external capsule bilaterally. Comparable findings have been described in the same model by Cai. et al using fluorescein-labeled LPS (Cai et al., 2003) explaining the widespread injury of the white matter. It is possible that this increased astrocyte reactivity does also plays a role in changes in micro-architecture detected by DTI including which included the increase in radial diffusivity in the corpus callosum and the external capsule and an increase in axial diffusivity in the external capsule.
Overall, MRI is sensitive to the white matter injury associated with inflammation. However, the methods employed in this study do not provide a ready means by which to distinguish hypoxic-ischemic injury from inflammatory injury. Nevertheless, these MRI alterations can assist in identifying infants that may be eligible for neuroprotective approaches for the cerebral white matter, whether its pathogenesis is inflammatory or ischemic.
Acknowledgments
This work was supported by the Green Fund, NIH Grant P50-NS35902 and the Reuter Foundation. The authors are grateful to Professor S.-K. Song for his helpful discussions, Professor Alpay Ozcan and M.D. Budde for their help in advanced computational signal post-processing, C.G. Hamontree for his contribution in building an MRI compatible neonatal rodent head holder and M. Parsadanian for her help and advice regarding histology.
Footnotes
Conflict of Interest Statement All authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Aden U, Dahlberg V, Fredholm BB, Lai LJ, Chen Z, Bjelke B. MRI evaluation and functional assessment of brain injury after hypoxic ischemia in neonatal mice. Stroke. 2002;33:1405–1410. doi: 10.1161/01.str.0000014608.78503.db. [DOI] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal I. Periventricular leucomalacia: a review. Eur J Pediatr. 2004;163:435–442. doi: 10.1007/s00431-004-1477-y. [DOI] [PubMed] [Google Scholar]
- Bona E, Hagberg H, Loberg EM, Bagenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res. 1998;43:738–745. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112:1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Dammann O, Phillips TM, Allred EN, O’Shea TM, Paneth N, Van Marter LJ, Bose C, Ehrenkranz RA, Bednarek FJ, Naples M, Leviton A. Mediators of fetal inflammation in extremely low gestational age newborns. Cytokine. 2001;13:234–239. doi: 10.1006/cyto.2000.0820. [DOI] [PubMed] [Google Scholar]
- Debillon T, Gras-Leguen C, Leroy S, Caillon J, Roze JC, Gressens P. Patterns of cerebral inflammatory response in a rabbit model of intrauterine infection-mediated brain lesion. Brain Res Dev Brain Res. 2003;145:39–48. doi: 10.1016/s0165-3806(03)00193-7. [DOI] [PubMed] [Google Scholar]
- Debillon T, Gras-Leguen C, Verielle V, Winer N, Caillon J, Roze JC, Gressens P. Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr Res. 2000;47:736–742. doi: 10.1203/00006450-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Deguchi K, Mizuguchi M, Takashima S. Immunohistochemical expression of tumor necrosis factor alpha in neonatal leukomalacia. Pediatr Neurol. 1996;14:13–16. doi: 10.1016/0887-8994(95)00223-5. [DOI] [PubMed] [Google Scholar]
- Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16:296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Domizio S, Barbante E, Puglielli C, Clementini E, Domizio R, Sabatino GM, Albanese A, Colosimo C, Sabatino G. Excessively high magnetic resonance signal in preterm infants and neuropsychobehavioural follow-up at 2 years. Int J Immunopathol Pharmacol. 2005;18:365–375. doi: 10.1177/039463200501800218. [DOI] [PubMed] [Google Scholar]
- Fan LW, Mitchell HJ, Tien LT, Zheng B, Pang Y, Rhodes PG, Cai Z. alpha-Phenyl-n-tert-butyl-nitrone reduces lipopolysaccharide-induced white matter injury in the neonatal rat brain. Dev Neurobiol. 2008;68:365–378. doi: 10.1002/dneu.20591. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Tien LT, Ma T, Rhodes PG, Cai Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J Neurosci Res. 2005;82:71–82. doi: 10.1002/jnr.20623. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Fukuda H, Jonsson M, Swanpalmer J, Hertzman S, Lannering B, Bjork-Eriksson T, Marky I, Blomgren K. Progenitor cell injury after irradiation to the developing brain can be modulated by mild hypothermia or hyperthermia. J Neurochem. 2005;94:1604–1619. doi: 10.1111/j.1471-4159.2005.03313.x. [DOI] [PubMed] [Google Scholar]
- Gilles FH, Averill DR, Jr., Kerr CS. Neonatal endotoxin encephalopathy. Ann Neurol. 1977;2:49–56. doi: 10.1002/ana.410020108. [DOI] [PubMed] [Google Scholar]
- Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Bona E, Gilland E, Puka-Sundvall M. Hypoxia-ischaemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl. 1997;422:85–88. doi: 10.1111/j.1651-2227.1997.tb18353.x. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(Suppl 1):16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- Hagmann CF, De Vita E, Bainbridge A, Gunny R, Kapetanakis AB, Chong WK, Cady EB, Gadian DG, Robertson NJ. T2 at MR imaging is an objective quantitative measure of cerebral white matter signal intensity abnormality in preterm infants at term-equivalent age. Radiology. 2009;252:209–217. doi: 10.1148/radiol.2522080589. [DOI] [PubMed] [Google Scholar]
- Hindmarsh GJ, O’Callaghan MJ, Mohay HA, Rogers YM. Gender differences in cognitive abilities at 2 years in ELBW infants. Extremely low birth weight. Early Hum Dev. 2000;60:115–122. doi: 10.1016/s0378-3782(00)00105-5. [DOI] [PubMed] [Google Scholar]
- Inder T, Huppi PS, Zientara GP, Maier SE, Jolesz FA, di Salvo D, Robertson R, Barnes PD, Volpe JJ. Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J Pediatr. 1999;134:631–634. doi: 10.1016/s0022-3476(99)70251-9. [DOI] [PubMed] [Google Scholar]
- Jacob RE, Minard KR, Laicher G, Timchalk C. 3D 3He diffusion MRI as a local in vivo morphometric tool to evaluate emphysematous rat lungs. J Appl Physiol. 2008;105:1291–1300. doi: 10.1152/japplphysiol.90375.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara H, Wehrli FW. Determination of background gradients with diffusion MR imaging. J Magn Reson Imaging. 1994;4:787–797. doi: 10.1002/jmri.1880040608. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm birth infants: the influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology. 2001;15:411–420. [PubMed] [Google Scholar]
- Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, Lazeyras F, Groenendaal F, de Vries LS, Huppi PS. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116:1–7. doi: 10.1542/peds.2004-1275. [DOI] [PubMed] [Google Scholar]
- Maalouf EF, Duggan PJ, Rutherford MA, Counsell SJ, Fletcher AM, Battin M, Cowan F, Edwards AD. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr. 1999;135:351–357. doi: 10.1016/s0022-3476(99)70133-2. [DOI] [PubMed] [Google Scholar]
- McKinstry RC, Miller JH, Snyder AZ, Mathur A, Schefft GL, Almli CR, Shimony JS, Shiran SI, Neil JJ. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology. 2002;59:824–833. doi: 10.1212/wnl.59.6.824. [DOI] [PubMed] [Google Scholar]
- Nedelcu J, Klein MA, Aguzzi A, Boesiger P, Martin E. Biphasic edema after hypoxic-ischemic brain injury in neonatal rats reflects early neuronal and late glial damage. Pediatr Res. 1999;46:297–304. doi: 10.1203/00006450-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Kesler SR, Vohr B, Duncan CC, Katz KH, Pajot S, Schneider KC, Makuch RW, Ment LR. Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr. 2004;145:242–249. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Ricard C, Vial JC, Douady J, van der Sanden B. In vivo imaging of elastic fibers using sulforhodamine B. J Biomed Opt. 2007;12:064017. doi: 10.1117/1.2821421. [DOI] [PubMed] [Google Scholar]
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. e171. doi: 10.1016/j.jpeds.2008.02.033. 175. [DOI] [PubMed] [Google Scholar]
- Sizonenko SV, Sirimanne E, Mayall Y, Gluckman PD, Inder T, Williams C. Selective cortical alteration after hypoxic-ischemic injury in the very immature rat brain. Pediatr Res. 2003;54:263–269. doi: 10.1203/01.PDR.0000072517.01207.87. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sternberg SR. Biomedical Image Processing. Computer. 1983;16:22–34. [Google Scholar]
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006;32:1195–1204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- Sun SW, Neil JJ, Song SK. Relative indices of water diffusion anisotropy are equivalent in live and formalin-fixed mouse brains. Magn Reson Med. 2003;50:743–748. doi: 10.1002/mrm.10605. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci. 2005;27:81–86. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- Welch KMA, Windham J, Knight RA, Nagesh V, Hugg JW, Jacobs M, Peck D, Booker P, Dereski MO, Levine SR. A model to predict the histopathology of human stroke using diffusion and T2-weighted magnetic resonance imaging. Stroke. 1995;26:1983–1989. doi: 10.1161/01.str.26.11.1983. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, Chi JG. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997a;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997b;177:406–411. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- Young RS, Hernandez MJ, Yagel SK. Selective reduction of blood flow to white matter during hypotension in newborn dogs: a possible mechanism of periventricular leukomalacia. Ann Neurol. 1982;12:445–448. doi: 10.1002/ana.410120506. [DOI] [PubMed] [Google Scholar]