Abstract
The immune response to stroke is comprised of inflammatory and regulatory processes. One cell type involved in both innate and adaptive immunity is the dendritic cell (DC). A DC population residing in the healthy brain (bDC) was identified using a transgenic mouse expressing enhanced yellow fluorescent protein (EYFP) under the promoter for the DC marker, CD11c (CD11c/EYFP Tg). To determine if bDC are involved in the immune response to cerebral ischemia, transient (40min) middle cerebral artery occlusion (MCAO) followed by 6, 24, or 72hr reperfusion was conducted in CD11c/EYFP Tg mice. Our results demonstrated that DC accumulated in the ischemic hemisphere at 24hr post-MCAO-reperfusion, particularly in the border region of the infarct where T lymphocytes accrued. To distinguish resident bDC from the infiltrating peripheral DC, radiation chimeras [1. wild type (WT) hosts restored with CD11c/EYFP Tg bone marrow (BM) or 2. CD11c/EYFP Tg hosts restored with WT BM] were generated and examined by immunocytochemistry. These data confirmed that DC populating the core of the infarct at 72hr were of peripheral origin, whereas those in the border region were comprised primarily of resident bDC. The brain resident (CD45 intermediate) cells of CD11c/EYFP Tg mice were analyzed by flow cytometry. Compared to microglia, bDC displayed increased major histocompatibility class II (MHC II) and co-stimulatory molecules following MCAO-reperfusion. High levels of MHC II and the co-stimulatory molecule CD80 on bDC at 72hr corresponded to peak lymphocyte infiltration, and suggested a functional interaction between these two immune cell populations.
Keywords: brain dendritic cells, stroke, microglia, major histocompatibility class II, co-stimulatory molecules, radiation bone marrow chimeras, middle cerebral artery occlusion
INTRODUCTION
It was common conception that the central nervous system (CNS) was an immune-privileged site and did not contain a resident population of the professional antigen presenting cells (APC), dendritic cells (DC). However, brain origin cells that express mature DC markers in response to damage or disease have engendered speculation that a resident immune cell population may serve as a DC precursor (Butovsky et al., 2007; Butovsky et al., 2006; Fischer and Reichmann, 2001; Santambrogio et al., 2001). Most mature DC subsets display the surface protein CD11c (Integrin alpha x, p150/90 chain) as a heterodimer with CD18, thus CD11c is considered a pan DC marker. A transgenic (Tg) mouse expressing enhanced yellow fluorescent protein (EYFP) under the promoter for CD11c (CD11c/EYFP Tg) was developed to track DC during the steady state. This mouse revealed a population of resident CD11c/EYFP-expressing DC in the healthy central nervous system (CNS), termed brain (b)DC, that were characterized by Bulloch and colleagues (2008). The bDC were located in discrete neuroanatomic regions and were prominent in the fiber tracts, circumventricular organs, regions accessible to intranasal antigen exposure, and sites of postnatal neurogenesis. Furthermore, these cells accumulated in regions of damage following kainic acid-induced seizure and displayed morphologies distinct from that of brain resident microglia (MG). Recent studies from our laboratory have demonstrated that resident bDC can be further distinguished from MG by their potent APC capabilities and TH1 cytokine production (Gottfried-Blackmore et al., in press), suggesting they may be involved in inflammatory processes in the CNS.
One model of brain damage that induces immune activation is cerebral ischemia. This immune response to ischemic stroke is characterized by early inflammatory and later regulatory components, the outcome of which contributes to the extent of damage and functional recovery (Doyle et al., 2008; McColl et al., 2009; Offner et al., 2009). Given the role of DC in bridging innate and adaptive immunity in the periphery (Steinman and Banchereau, 2007), the bDC may be involved in the balance between inflammation and protection following stroke in the brain. As a resident DC population, the bDC are poised to orchestrate early local immune responses and to interact directly with infiltrating lymphocytes later in stroke progression. In fact, brain resident cells displaying a DC phenotype are observed as part of protective immune responses to experimental models of autoimmune and neurodegenerative diseases (Butovsky et al., 2006; Chiu et al., 2008). In these models, CD11c+ cells increase in response to interleukin (IL)-4 released by infiltrating T cells, up-regulate protective factors such as insulin-like growth factor-1, and are associated with improved functional outcome. Other studies examining the presence of DC in rodent models of cerebral ischemia have reported CD11c+ cells in the ipsilateral hemisphere, peaking at 72 hrs post-ischemia (Gelderblom et al., 2009; Kostulas et al., 2002; Reichmann et al., 2002). Using fluorescent-activated cell sorting (FACS) analysis, Gelderblom et. al. (2009) reported the infiltration of CD11c+ cells of peripheral origin into the ischemic hemisphere, however histological data from the previous studies (Kostulas et al., 2002; Reichmann et al., 2002) indicate that CD11c+ cells in the ischemic hemisphere may be comprised of two populations, one peripheral and one brain resident.
To determine the time course, distribution, and activation state of the bDC in response to stroke, we employed a model of transient middle cerebral artery occlusion (MCAO)-reperfusion in the CD11c/EYFP Tg mouse. The bDC were distinguished from peripheral DC in ischemic brains by the use of radiation bone marrow (BM) chimeras for histology, and gating on intermediate CD45 (CD45Int) fluorescence intensity for FACS analysis of the EYFP/CD11c-Tg brain. Results demonstrated that bDC do not contribute to the early (6 hr) response to MCAO, but that they accumulated over days in parallel to the time course of infiltrating leukocyte populations. Moreover, bDC were located primarily in the border regions of the infarct where T lymphocytes accrued, and contained high levels of major histocompatibility class II (MHC II) and the co-stimulatory molecule CD80. These findings are consistent with a functional interaction between bDC and T lymphocytes which may play a role in ischemic brain injury or repair.
METHODS
Animals
The Itgax CD11c/EYFP Tg (C57BL/6 background) was developed at the Rockefeller University to visualize CD11c-expressing cells by cloning EYFP venus DNA (Nagai et al., 2002) into the CD11c-pDOI-5 vector (Brocker et al., 1997) so that EYFP was expressed under CD11c promoter activity (Lindquist et al., 2004). Animals were bred by back crossing heterozygous CD11c/EYFP Tg male mice with wild type (WT) females purchased from Jackson Laboratory (Bar Harbor, Maine). All mice were genotyped by polymerase chain reaction and WT animals served as controls for FACS experiments and as donors/recipients for chimeras. Only male mice were used in this study. All animals were maintained in Rockefeller University facilities under 12:12 light:dark cycle with free access to chow and water. All experimental procedures were approved by The Rockefeller University and Weill Cornell Medical College Animal Care and Use Committees.
Chimeras
Radiation chimeras restored with syngeneic BM were generated by methods developed from previously published protocols (Kennedy and Abkowitz, 1997; Priller et al., 2001). Host WT or CD11c/EYFP Tg mice were maintained for 2 weeks on antibiotics (Sulfatrim, Test Diet, Richmond, IN) before lethal cobalt irradiation with 2x 550 cGy delivered over 4 minutes spaced by 3 hr, and reconstituted with 7-10 million BM cells from CD11c/EYFP Tg or WT age and sex matched donors by tail vein injection. Mice were maintained on antibiotics for 2 weeks and subjected to MCAO or sham conditions 4-6 weeks after BM restoration.
Transient middle cerebral artery occlusion
Procedures were conducted as previously published (Cho et al., 2005; Kawano et al., 2006; Kunz et al., 2008; Kunz et al., 2007). Briefly, mice were anesthetized with a mixture of isoflurane (1.5–2%), oxygen, and nitrogen. A fiber optic probe was glued to the right parietal bone 2 mm posterior and 5 mm lateral to bregma, and connected to a laser-Doppler flowmeter (Periflux System 5000; Perimed, Jarfalla, Sweden) for continuous monitoring of cerebral blood flow (CBF). A second probe was placed at 0 mm posterior and 2 mm lateral from bregma to monitor the CBF reduction at the periphery of the ischemic territory. For MCAO, a heat-blunted monofilament surgical suture (6-0) was inserted into the exposed external carotid artery, advanced into the internal carotid artery, and wedged into the circle of Willis to obstruct the origin of the right MCA. The filament was left in place for 40 min and then withdrawn. Only animals that exhibited a reduction in CBF >85% during MCAO and a CBF recovery by >80% after 10 min of reperfusion were included in the study (Cho et al., 2005; Kunz et al., 2008; Kunz et al., 2007). Rectal temperature was monitored and kept constant (37.0 +/− 0.5°C) during the surgical procedure and in the recovery period until the animals regained full consciousness.
Perfusion and tissue collection
For histology, 15 CD11c/EYFP Tg mice and 18 chimeras were deeply anaesthetized with sodium pentobarbital and transcardially perfused with 0.9% saline containing heparin 1 U/ml followed by 4% paraformaldehyde at 6, 24, or 72 h post-MCAO-reperfusion. Brains were removed and post-fixed for 6-12 hour, sunk in 30% sucrose in 0.1 M phosphate buffer (PB), and stored at −80° C until sectioning. Thirty micron brain sections were collected either by a Reichter-Jung 1800 Cryocut (Lieca, Bannockburn, IL) or a freezing microtome (Microm 400E, Thermo-Fisher, Pittsburgh, PA) at the same rostrocaudal levels used for assessment of infarct volume (see below). Sections were stored in cryoprotectant solution at −20°C until further processing.
Infarct volume measurement
The procedure was adapted from previously published protocols (Cho et al., 2005; Kawano et al., 2006; Kunz et al., 2008; Kunz et al., 2007). A series of sections from 15 brain regions, spaced 600 microns apart from bregma −3.7 to 3.5, were collected at 600 micron intervals throughout the ischemic lesion and stained with cresyl violet. Infarct volume was determined using an image analyzer (MCID; Imaging Research, St. Catharines, Ontario, Canada). To eliminate the contribution of post-ischemic edema to the volume of injury, values were corrected for swelling according to the method of Lin et al. (1993) as previously described (Zhang and Iadecola, 1994).
Immunofluorescence, immunohistochemistry, and brain map generation
Free-floating sections were rinsed in Phosphate-Buffered Saline (pH 7.6; PBS) and then blocked in 0.5% bovine serum albumin (BSA) in PBS for 1hr. Sections were then incubated overnight at 4°C with primary antibodies (see Supplementary Materials, Table 1) in 0.1% BSA, 0.1% Triton PBS. Sections were then rinsed and incubated for 1hr at room temperature with secondary fluorescent antibodies of the appropriate species labeled with Alexa-647 or Alexa-633 (Invitrogen, Carlsbad, CA) or anti-chicken biotin-labeled antibody (Vector, Burlingame, CA). For diaminobenzidine (DAB)-immunohistochemistry (IHC) detection of EYFP protein, sections were then incubated with peroxidase-avidin complex (ABC; Vector), followed by detection with a DAB peroxidase substrate kit (Vector). Detection of CD11c signal was enhanced using a tyramine signal amplification method adapted from the protocols of Adams, JC (1992) and Berghorn et al. (1994). Briefly, following incubation with biotinylated secondary antibody and ABC, staining was revealed using biotinylated tyramine (Sigma, St. Louis, MO) in borate buffer followed by Alexa-633 conjugated streptavidin (Invitrogen). Spleen tissue was used as a positive control for all antibodies. Brain or spleen tissue incubated without the primary antibody but processed with all secondary and tertiary reagents served as the negative control. Immunofluorescence histochemistry (IFC) sections were counterstained with DAPI or a comparable nuclear stain (Invitrogen). Sections were mounted and cover-slipped with Fluoromount (Sigma) for IFC or mounted, dehydrated, and cover-slipped with DPX mounting medium (Sigma) for IHC. Light microscopy images were acquired using a Nikon Optiphot Microscope or light box with a Coolpix Digital camera. Fluorescent microscopy images were acquired on a LSM510 confocal Zeiss Axioplan microscope with a kripton/argon laser and a HeNe laser (Rockefeller University Bioimaging Facility). Brain maps were generated by adapting the methods of Bulloch et al. (2008) using the Atlas Navigator (2000) and Adobe Illustrator 10 (Adobe) systems. Briefly, the number of DAB stained EYFP+DC per 40 μm section were estimated and denoted by marks representing approximately 2 cells. Four levels were chosen corresponding to neighboring sections from cresyl violet-stained sections used for infarct volume calculation. Maps are not intended to represent an exact number, and the pattern of EYFP+DC varied depending on infarct dimensions. For IFC, images were analyzed by collapsing 1 μm serial Z stacks into a single image using Image J software (NIH, Bethesda, MD). Sections were rotated in the orthogonal plane to confirm double labeling. Photomicrographs were collected and assembled from digital images for which colors were assigned and optimal levels, contrast, and brightness were adjusted in Adobe Photoshop 7.0.
Leukocyte isolation for FACS analysis
Slight variations on previously reported methods to obtain a single population of brain leukocytes by FACS were used. Briefly, naïve control, sham operated, and 24 or 72 hr post-MCAO CD11c/EYFP Tg mice were rapidly decapitated, the brains removed and placed on ice in Hank’s balanced salt solution (HBSS; Gibco, Carlsbad, CA). Ischemic brains were examined for the visual appearance of an infarct and the spleen weights were recorded for control and MCAO animals to confirm sufficient stroke (Offner et al., 2006b) see Supplementary Materials, Fig. S1). The meninges, blood vessels and choroid plexus were carefully removed, and the cerebellum and olfactory bulb were dissected away. Under a dissecting microscope, the brains were divided into two cerebral hemispheres, the right containing the ischemic lesion. Both the contralateral and ipsilateral hemispheres were then separately dissociated by gentle triturating with frosted glass-slides, and incubated with type II-S Collagenase (600U; Sigma), DNAse (450U; Invitrogen, Carlsbad, CA), and Dispase II (Roche Diagnostics, Indianapolis, IN) for 30 min at 37°C in 10ml HBSS (w/ CaMg2+). After digestion, brains were homogenized by repetitive gentle pipetting with fire-polished Pasteur pipettes on ice. Cells were washed by centrifugation and subjected to a 70%-37% Percoll gradient centrifugation. Cells were collected from the 37/70 interface for the ipsilateral and contralateral sides, washed in PB and re-suspended in 5% fetal bovine serum in PBS (FACS buffer) for FACS analysis. A series of 3 experiments including naïve or sham controls, and experimental mice were conducted yielding a total of 6 hemispheres for each condition.
FACS Analysis
Cells were pipetted into a 96-well microtiter plate then blocked for 15 min at 4°C with anti-FC block (BD). Cells were then stained with fluorophore-conjugated primary antibodies (see Supplementary Materials, Table 2) for 15 min at 4°C and rinsed 3x with FACS buffer. Dead cells were excluded with DAPI and live cells were evaluated using a BD LSR-II FACS analyzer using FACS Diva software. Spleen DC and lymphocytes were used as positive controls for AB titrations and compensations. Data was analyzed using FlowJo software (Treestar, Ashland, OR). Uniform gates were set using isotype controls and fluorescence minus one antibody combinations and applied to all samples within an experiment.
Statistics
All statistical analyses were performed using SPSS or SigmaStat Software (Aspire, Ashburn, VA). Students T tests were used for comparison of a single factor between two groups. For comparison of a single factor across multiple conditions (naïve control, sham, ipsilateral and contralateral ischemic hemispheres), one-way Analysis of Variance (ANOVA) was conducted. For data sets comparing 2 factors across multiple conditions, two-way ANOVA was performed. In order to compare brain resident versus cells of peripheral origin within the ischemic hemisphere, two-way repeated measures ANOVAs were employed. Subsequent post hoc analyses were conducted using Tukey’s multiple comparison procedures. For data sets that failed equal variance tests, non-parametric Kruskal-Wallis one-way ANOVA on ranks followed by Dunn’s Method of multiple comparisons were performed. Non-normal data sets were transformed using standard procedures to achieve normality (log, natural log, or square root). All tests of significance were 2-tailed with an alpha level of significance p < 0.05. Data were summarized as the mean +/− the standard error of the mean (SEM) for graphic representation.
RESULTS
EYFP+DC accumulate within the ipsilateral (right) ischemic hemisphere at 24 and 72 hr post-MCAO-reperfusion
The model of 40 min MCAO-reperfusion employed in this study resulted in reproducible, progressive cerebral infarct volumes revealed by cresyl violet staining, ranging from an average of 22+/−5 mm3 at 6 hr to 59+/−4 mm3 at 72 hr post-MCAO-reperfusion (Fig. 1; n=3-9 per group). These findings are consistent with previous studies from this laboratory (Kunz et al., 2008; Kunz et al., 2007).
Fig. 1.
Infarct volume after MCAO-reperfusion. Mice were sacrificed by PFA perfusion and brains were collected for histology at 6, 24, or 72 hr following MCAO-reperfusion. Representative serial sections collected every 600 μm from bregma −3.7 to 3.5 were stained with cresyl violet for infarct volume determination. This MCAO-reperfusion protocol produced consistent, progressive infarcts of the ipsilateral (right) hemisphere.
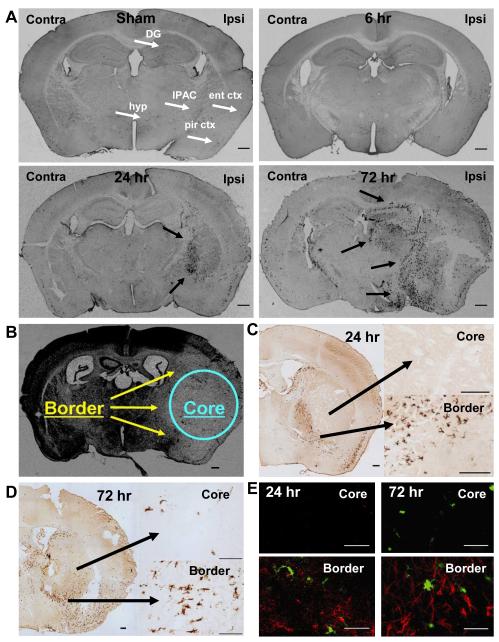
Tissue sections were processed by DAB-IHC to detect EYFP+ cells in CD11c/EYFP Tg mice following sham surgery or 6, 24, and 72 hr MCAO-reperfusion (Fig. 2A). Sham operated animals demonstrated a similar distribution of EYFP+bDC as observed in young naïve male mice (Bulloch et al., 2008), with EYFP+bDC observed in the dentate gyrus of the hippocampus, the interstitial nucleus of the posterior limb of the anterior commissure, fiber tracts, hypothalamic nuclei, the piriform and entorhinal cortices (white arrows). At 6 hr post-MCAO reperfusion, no increase in EYFP+ cells was observed, whereas at 24 hr post-MCAO, EYFP+ cells began to accumulate in the ischemic hemisphere and appeared to line the edges of the infarct (black arrows). At 72 hr post-MCAO, EYFP+ cells were prevalent in the ipsilateral hemisphere and formed clusters in the regions surrounding the infarct (black arrows).
Fig. 2.
EYFP+DC accumulated in the ischemic hemisphere in response to MCAO-reperfusion. Representative brain sections from CD11c/EYFP Tg mice subject to sham surgery or MCAO-reperfusion were processed for DAB-IHC (A). Sham operated animals demonstrated a similar quantity and distribution of EYFP+ cells as in young naïve male mice, with cells observed primarily in the dentate gyrus of the hippocampus (DG), the interstitial nucleus of the posterior limb of the anterior commissure (IPAC), fiber tracts, hypothalamic nuclei (hyp), the piriform (pir ctx) and entorhinal cortices (ent ctx), as indicated by white arrows. At 6 hr post-MCAO reperfusion, no increase in EYFP+ cells was observed in the ipsilateral or contralateral hemispheres. At 24 and 72 hr-post MCAO-reperfusion, EYFP+ cells accumulated in the ischemic hemisphere and formed clusters in regions surrounding the cerebral infract (black arrows). The distribution of EYFP+DC in the ischemic hemisphere was compared to cresyl violet stained sections used for infarct volume determination (B), demonstrating that EYFP+DC were found in high densities in the border regions as compared to the core of the infarct. Higher magnification images demonstrated that at 24 hours post-MCAO-reperfusion, DC were observed in the border region, whereas the core of the infarct was devoid of EYFP+ cells (C). At 72 hr, DC were present in the core and border of the infarct, although cells in the core adopted an ovoid morphology compared to the ramified cells of the infarct border (D). To verify the location of sampling from core and border regions of the infarct, IFC staining for GFAP was conducted in neighboring serial sections and examined for presence (border) or absence (core) of astrocytes (E). Scale bars A, B = 400, C = 200, D = 200 (small), 100 (large), E = 50 microns; red = IFC for GFAP, green = EYFP+DC
A comparison of the distribution of EYFP+DC in the ischemic hemisphere to the cresyl violet stained sections used for infarct volume determination (Fig. 2B) confirmed that the cells were found in high densities in the border regions of the infarct as compared to the core (Fig. 2C-D). Higher magnification images of the DAB-IHC at 24 hours post-MCAO demonstrated that EYFP+DC were observed only in the border region, whereas the core of the infarct was devoid of EYFP+ cells (Fig. 2C). At 72 hr, ovoid EYFP+ cells were present in the core compared to the ramified EYFP+ cells that populated the infarct border region (Fig. 2D). The location of the core and border region of the infarct was verified by IFC staining for glial fibrillary acid protein (GFAP; (Ito et al., 2001) on parallel serial sections and examined for presence (border) or absence (core) of astrocytes (Fig. 2E). Brain maps were generated to illustrate the time course and location of EYFP+ cell accumulation in the ischemic hemisphere (Fig. 3).
Fig. 3.
The distribution of EYFP+DC accumulation in the ischemic hemisphere over time in response to MCAO-reperfusion. Brain maps were generated from sections processed for DAB-IHC to illustrate the temporal and spatial pattern of EYFP+DC in the ipsilateral ischemic hemisphere. Each mark represents approximately 2 cells; maps are not intended to display exact number. The blue dashed line defines the cerebral infarct.
To investigate the activation state of the EYFP+DC in the core versus border in response to MCAO, sections were stained for CD11c and MHC II using IFC techniques (Fig. 4). EYFP+DC immuno-positive (white arrows) for CD11c (Fig. 4A) and MHC II (Fig. 4B) were prevalent in the core but observed more rarely in the border region of the infarct.
Fig. 4.
Examination of mature DC markers on EYFP+DC within the core versus border region of the ischemic infarct at 72 hr post-MCAO-reperfusion by IFC. EYFP+DC that were immunopositive (white arrows) for CD11c (A) and MHC II (B) were frequently observed in the core of the infarct but were rare in the border region. Scale bars = 50 microns. red = IFC for CD11c or MHC II, green = EYFP+DC, yellow = merge
The use of radiation BM chimeras to determine the origin of EYFP+DC populations
Considering the difference in morphology and activation state of EYFP+ cells in the core versus border, as well as previous studies demonstrating the peak infiltration of peripheral cells at 2-3 days post-ischemia (Gelderblom et al., 2009; Stevens et al., 2002), we hypothesized that DC of different origins were present in the ischemic infarct at 72 hr post-MCAO-reperfusion.
To address this hypothesis, we generated two types of irradiated BM chimeras and subjected them to sham surgery or MCAO-reperfusion. Infarct volumes produced in both chimeras following MCAO-reperfusion did not differ from those observed in CD11c/EYFP Tg mice (55+/−5 and 57+/−4, t [7]=0.81, p>0.05). For radiation chimeras where the CD11c/EYFP Tg hosts were immunologically restored with BM from WT donors, EYFP+DC observed in the brain were considered to be derived from a radiation resistant population of brain origin cells, the bDC, as described by (Gottfried-Blackmore et al., 2009). Conversely, by irradiating WT mice and restoring their immune systems with BM from CD11c/EYFP Tg mice, EYFP+DC detected in the brain were considered peripheral cells of BM origin that infiltrated the brain, the peripheral DC. Sham operated chimeras displayed similar patterns of EYFP+ cells as age/sex-matched irradiated chimeras used in our laboratory for other studies (data not shown). Representative brain sections from these mice following 72 hr MCAO-reperfusion are depicted in Fig. 5A. In the former mice (CD11c/EYFP Tg hosts restored with WT BM), EYFP+DC were observed throughout the ischemic hemisphere and were abundant in the border regions of the infarct (white arrows). In the latter mice (WT hosts restored with CD11c/EYFP Tg BM), the EYFP+DC were primarily noted in the core regions of the infarct (black arrows). Higher magnification images were acquired to assess the morphology and precise location of EYFP+DC within the cerebral infarcts of the chimeras at 72 hr (Fig. 5B). These data confirmed that in the ischemic hemisphere of CD11c/EYFP Tg hosts restored with WT BM, EYFP+bDC that originated in the brain displayed ramified morphology, were found throughout the infarct, and accumulated in the border regions. Conversely, in the ischemic hemisphere of WT hosts restored with CD11c/EYFP Tg BM, ovoid peripheral DC populated the core of the infarct.
Fig. 5.
Radiation chimeras demonstrated the distribution of brain resident versus peripheral DC populations. Representative brain sections from radiation chimeras 72 hr post-MCAO-reperfusion were processed by DAB-IHC for detection of EYFP+ cells (A). In CD11c/EYFP Tg host with WT BM donors, EYFP+DC (considered of brain origin) were abundant in the ipsilateral hemisphere and accumulated in the border regions of the infarct (white arrows). In WT hosts with BM from CD11c/EYFP Tg mice, EYFP+DC (considered of peripheral origin) were confined to the core regions of the cerebral infarct (black arrows). Higher magnification images of DAB-IHC sections from radiation chimeras (B) confirmed the presence of bDC in the border and peripheral DC in the core regions of the cerebral infarct at 72 hr post-MCAO-reperfusion. EYFP+DC from the CD11c/EYFP Tg host mice of brain origin, bDC, displayed ramified morphology and resided in the border region of the infarct. EYFP+DC of the WT host mice of BM origin, peripheral DC, populated the core of the infarct and appeared ovoid in morphology. To verify the location of sampling from core and border regions of the infarct, IFC staining for GFAP was conducted in neighboring serial sections and examined for presence (border) or absence (core) of astrocytes (D). Scale bars A = 400 microns, B = 100 microns (black), 50 microns (white) ; red = IFC for GFAP, green = EYFP+DC
FACS analysis of bDC derived from EYFP-CD11c-Tg mice following MCAO
For FACS analysis, immune cells were extracted from the brain and identified by expression of cell surface markers. Live immune cells were considered DAPINegative(Neg) singlets that expressed CD45, and were then divided into subpopulations for analysis. The total number of CD45+ cells recovered from the hemispheres of the mice in this study was similar across conditions, although there was a trend for an increase in total CD45+ cells in the ipsilateral ischemic hemispheres of animals exposed to MCAO-reperfusion (Fig. 6A; H[5]=9.59, p=0.088). Fig. 6B summarizes the average percentages of three populations of CD45+ cells observed across the control and ischemic conditions, the CD11b+Ly6G+ neutrophils, CD11b+Ly6GNeg MG/macrophage/DC, and all others immune cells that were CD45+ but CD11bNegLy6GNeg. Whereas CD11b+Ly6GNeg cells (MG, macrophage, DC; white) comprised the majority of cells in the control and 24 hr post-MCAO-reperfusion hemispheres, at 72 hr there was a marked increase in the number of infiltrating neutrophils (black), a hallmark of the immune response following stroke, that accounted for approximately half of all CD45+ cells recovered. The FACS plot in Fig. 6C provides an example of the gating strategy used to define these three populations of CD45+ cells. In order to isolate the cell populations of interest (DC/bDC, macrophage/MG), Ly6G+ cells were excluded and the remaining CD11b+Ly6GNeg cells (Fig. 6B; white), referred to as the CD11b population, were analyzed for EYFP expression and phenotyped for mature DC markers in response to MCAO-reperfusion. The CD45+CD11bNegLy6GNeg cell population (Fig. 6B; gray) was subsequently analyzed for the presence of lymphocytes.
Fig. 6.
FACS analysis of CD45+ cell populations in CD11c/EYFP Tg mice following MCAO-reperfusion. The total number of CD45+ cells (mean, +/− SEM) recovered from the hemispheres of controls and mice subject to MCAO-reperfusion (A). The average percentage of the three CD45+ cell populations, CD11b+Ly6G+ neutrophils (black), CD11b+Ly6GNeg MG/macrophage/DC (white), and CD11bNegLy6GNeg other immune cells (gray), for control and ischemic conditions are represented graphically in B, and an example of the gating strategy is depicted by FACS plot in (C). The CD11b+Ly6GNeg MG/macrophage/DC population (white) was subsequently analyzed for expression of EYFP and phenotyped for activated DC markers; the other immune cell population (gray) was examined for the presence of lymphocytes.
As depicted in Fig. 7A, EYFP+DC were substantially increased in the ischemic hemispheres, and the percentage of EYFP+ cells within the CD11b population was significantly higher (F[5,30]=26.8, p<0.001) at 24 hr (p<0.01) and 72 hr (p<0.001) compared to controls. One way ANOVAs indicated that the percentage of cells within the CD11b population with upregulated mature DC markers, CD11c (F[5,30]= 21.8, p<0.001), MHC II (H[5]=22.7, p<0.001), and the co-stimulatory molecules CD86 (F[5,30]=8.1, p<0.01) and CD80 (F[5,30]=14.6, p<0.001), were increased in response to ischemic stroke (Fig. 7B). Post hoc analyses revealed that at 24 hr post-MCAO-reperfusion a significantly higher percentage of cells were positive for CD11c (p<0.01), CD86 (p<0.05), and CD80 (p<0.01) proteins compared to control hemispheres. Similarly, at 72 hr post-MCAO, the percentage of cells containing CD11c (p<0.001), CD86 (p<0.05), and CD80 (p<0.001) proteins were elevated, and this was accompanied by an increase in the percentage of cells positive for MHC II (p<0.05). The mean fluorescence intensity for each marker in the CD11b population was also compared by one way ANOVA across conditions and a significant increase in MHC class II (H[5]=19.4, p<0.01), CD86 (H[5]=14.6, p<0.05) and CD80 (H[5]=19.0, p<0.01) was observed. Post hoc tests verified an increase at 72 hr post-MCAO for MHC II (p<0.05), CD86 (p<0.05), and CD80 (p<0.05), and for CD86 (p<0.05) at 24 hr post-MCAO, as compared to control. Representative scatter plots from stroke hemispheres at 72 hr post-MCAO-reperfusion for MHC II versus CD11c, and for CD86 versus CD80, in comparison to their respective isotype controls can be found in Supplementary Materials, Fig. S2.
Fig. 7.
Cells positive for EYFP and mature DC markers were increased in the ischemic hemisphere in response to MCAO-reperfusion. The mean (+/− SEM) percentage of EYFP+DC within the CD11b population was significantly increased at 24 hr and 72 hr post-MCAO-reperfusion (A). The percentage of cells within the CD11b population positive for mature DC markers was also increased in the ipsilateral hemisphere in response to MCAO-reperfusion (B) as represented by means (+/− SEM) for CD11c, MHC II, and the co-stimulatory molecules CD86 and CD80. * = p<0.05, ** = p<0.01, *** = p<0.001 compared to control
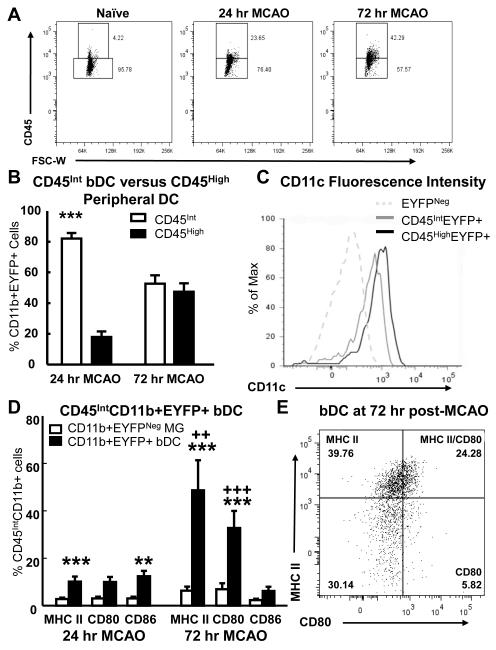
The contribution of the bDC to the observed increase in EYFP+ cells and cells positive for mature DC markers was determined by distinguishing the brain resident from peripheral CD11b+ cell population using CD45 fluorescence intensity (Fig. 8). It is generally accepted that within the immune cell populations extracted from healthy or pathological brains, CD11b+ cells with intermediate levels of CD45 (CD45Int) are of brain origin, whereas CD45HighCD11b+ cells are peripheral cells that have infiltrated the brain parenchyma or that reside in meninges and perivascular spaces (Babcock et al., 2003; D’Mello et al., 2009; Gelderblom et al., 2009; Kerfoot et al., 2006).
Fig. 8.
The resident bDC and MG populations were isolated by gating on CD45 fluorescence intensity and examined for the presence of DC markers. The percentage of CD45High cells increased in response to MCAO-reperfusion (A) as peripheral immune cells invaded the ipsilateral hemisphere. Comparison of mean (+/− SEM) percentage of CB11b+EYFP+ cells that were either CD45High (black) or CD45Int (white) showed that the majority of EYFP+DC at 24 hr were of CD45Int brain resident origin, yet at 72 hr the percentage of CD45Int and CD45High cells did not differ (B). Both the CD45IntCD11b+EYFP+ and CD45HighCD11b+EYFP+ cell populations at 72 hr were positive for CD11c compared to CD11b+EYFP-cells (C). Examination of the CD45Int resident cell populations revealed that the bDC upregulated MHC II and co-stimulatory molecules following MCAO-reperfusion (D). Compared to EYFPNeg MG (white), a higher mean (+/−SEM) percentage of bDC (black) were positive for MHC II, CD80, and CD86. Assessment of MHC II+ versus CD80+ within the bDC at 72 hr (B) demonstrated that ~25% of the bDC were positive for both MHC II and CD80. B: *** = p<0.001 compared to CD45High, D: ** = p<0.01, *** = p<0.001 compared to MG; ++ = p<0.01, +++ = p<0.001 compared to 24 hr
The percentage of CD45High cells observed in control brains was low (5% or less) but increased to over 40% in response to MCAO-reperfusion (Fig. 8A) as peripheral immune cells invaded the ischemic hemisphere. The percentage of CD11b+EYFP+ cells that were either CD45High or CD45Int were compared by two way ANOVA and a significant difference between groups (F[1,20]=61.1, p<0.001) as well as a condition by time interaction (F[2,23]=43.9, p<0.001) was indicated. Post hoc analysis at 24 hr post-MCAO reperfusion showed that the majority of EYFP+DC extracted from the brain were resident CD45Int cells (p<0.001), whereas at 72 hr post-MCAO an equivalent proportion of CD45Int and CD45High cells were observed (Fig. 8B; p>0.05). This finding indicated that the percentage of CD45High cells within the CD11b+EYFP+ population increased with time following MCAO-reperfusion, so that approximately half of the observed DC were considered of peripheral origin at 72 hr. Furthermore, both the CD45IntCD11b+EYFP+ and CD45HighCD11b+EYFP+ cell populations were positive for CD11c compared to CD11b+EYFPNeg cells at 72 hr (Fig. 8C), however the CD45HighCD11b+EYFP+ cells displayed higher fluorescence intensities than the CD45IntCD11b+EYFP+ cells (T[10]=−4.8, p<0.001).
After isolation of the brain resident CD45IntCD11b+ population, we then compared the percentage of cells displaying mature DC markers within the EYFPNeg brain MG and EYFP+bDC populations. Interestingly, only EYFP+bDC upregulated MHC II and the co-stimulatory molecules CD80 and CD86 following MCAO (Fig. 8D). Two-way repeated measures ANOVAs indicated a significant difference between the percentage of MG and bDC positive for MHC II (F[1,10]=165.4, p<0.001), CD80 (F[1,10]=33.9, p<0.001), and CD86 (F[1,10]= 18.7, p<0.01). A main effect and an interaction over time were also observed for MHC II (F[1,10]=10.3, p<0.01; F[2,23]=6.4, p<0.05) and CD80 (F[1,10]=7.7, p<0.05; F[2,23]=11.3, p<0.01). Post hoc analysis indicated that at 24 hr post-MCAO-reperfusion, a small number of bDC responded to MCAO by up-regulating MHC II (p<0.001) and CD86 (p<0.01) compared to the MG. In contrast, at 72 hr approximately half of the bDC displayed MHC II and one third were positive for CD80, which was significantly elevated compared to baseline levels in the MG (p<0.001 for both MHC II and CD80) and in comparison to the bDC at 24 hr (p<0.01 for MHC II, p<0.001 for CD80). Of note, the percentage of bDC positive for CD86 did not increase at 72 hr post-MCAO-reperfusion and was not significantly different from MG (p=0.11). Mean fluorescence intensities verified the observed increases in MHC II (F[1,10]=42.8, p<0.001), CD80 (F[1,10]=48.7, p<0.001), and CD86 (F[1,10]=18.5, p<0.01) in the bDC compared to the MG at 24 hr (p<0.01 for all) and 72 hr (p<0.001 for MHC II and CD80, p<0.05 for CD86) post-MCAO-reperfusion. Furthermore, comparison of MHC II and CD80 in the bDC at 72 hr demonstrated that the majority of CD80+ cells were also positive for MHC II (Fig. 8E; t[5]=4.4, p<0.01), indicating that ~25% of the bDC acquired both MHC II and co-stimulatory molecules necessary for antigen presentation to T cells.
T lymphocytes infiltrate the ischemic infarct border
To examine the anatomical distribution of lymphocytes (arrows) relative to that of the bDC, brain sections were examined by IFC for the presence of T cells and B cells (Fig. 9A). B220+ B cells were rarely observed, and the majority of lymphocytes were CD3+ T cells. Lymphocytes sparsely populated the core region of the infarct at 72 hr post-MCAO-reperfusion, but accrued in high numbers in the infarct border region in close apposition to the resident bDC population (asterisks). The time course and phenotype of infiltrating lymphocytes was assessed by FACS analysis of the CD45+CD11bNegLy6GNeg immune cell population represented by grey bars in Fig. 9B. The percentage of these cells comprised of lymphocytes (CD3+ T cells and CD19+ B cells) was compared by two-way ANOVA. This test revealed an increase in total lymphocytes (F[5,52]=4.5, p<0.01) that was significant at 72 hr post-MCAO-reperfusion (p<0.01) compared to control. Consistent with our histological findings, the majority of lymphocytes recovered from both control and stroke hemispheres were T cells (F[1,52]=151.7, p<0.001); of the T cells present in the ischemic hemisphere at 72 hr, the majority were CD4+ T cells (Fig. 9C; t[5]=4.3, p<0.05).
Fig. 9.
T lymphocytes infiltrated the ischemic hemisphere at 72 hr post-MCAO-reperfusion and accumulated in the border region near resident bDC. The neuroanatomical distribution of lymphocytes (arrows) relative to the bDC (asterisks) was examined by IFC for CD3+ T cells and B220+ B cells (A). CD3+ T cells (arrows) were found in high numbers in the infarct border region in close proximity to the resident EYFP+ bDC and their processes (asterisks). FACS analysis of the CD45+CD11bNegLy6GNeg cell population demonstrated a mean (+/− SEM) percent increase of lymphocytes at 72 hr post-MCAO-reperfusion (B). The majority of observed lymphocytes were CD3+CD4+ T cells (B, C). Scale bars = 50 microns. red = IFC for CD3 or B220, green = EYFP+DC, blue = DAPI
DISCUSSION
Summary of findings
This study is the first to provide a detailed description of the response of resident bDC to stroke using the CD11c/EYFP Tg mouse and an MCAO model of cerebral ischemia. We were able to distinguish the resident bDC population from peripheral DC by the use of radiation chimeras for histology, and also by isolating the resident CD45IntCD11b+ cell population by FACS analysis of CD11c/EYFP Tg mice. The bDC were not recruited during the early (6 hr) response to MCAO, but rather increased in the ischemic hemisphere as soon as 24 hr. As verified by histology, the bDC accumulated in the infarct border and were accompanied by an influx of peripheral DC at 72 hr that were confined mostly to the infarct core. Analysis by flow cytometry supported these findings as EYFP+ cells were increased in the ischemic hemisphere, and a significant proportion of these cells at 72 hr post-MCAO-reperfusion were of peripheral (CD45High) origin. Additionally, FACS analysis of ipsilateral ischemic hemispheres demonstrated an increase in the percentage of CD11b cells positive for CD11c, MHC II, and co-stimulatory molecules CD80 and CD86. When considering the contribution of the CD45Int resident bDC population to these observed increases, the bDC displayed a robust increase in MHC II and the co-stimulatory molecule CD80 at 72 hr-post-MCAO reperfusion, a time when T cell infiltration was observed. Moreover, histological examination revealed that invading T cells trafficked primarily to the infarct border where they co-existed with the resident bDC population.
bDC and the immune response to cerebral ischemia
The early immune response to stroke includes elevations in brain and circulating pro-inflammatory cytokines/chemokines, mobilization of circulating macrophages, increases in splenic lymphocytes, the initiation of inflammatory signaling cascades, and activation of brain MG (Doyle et al., 2008; Offner et al., 2006a; Offner et al., 2009; Wang et al., 2007). Examination of brain sections from CD11c/EYFP Tg mice exposed to 6 hr MCAO-reperfusion suggested that bDC do not participate in this initial pro-inflammatory response. Despite a relative absence of EYFP+bDC, morphologic activation of EYFPNeg MG, as detected by IFC for Iba-1, was evident in the striatum of the ischemic hemisphere at 6 hr (see Supplementary Materials, Fig. 3). The bDC were subsequently recruited over the course of stroke progression and emerged as soon as 24 hr, a time when the contribution of peripheral neutrophils and lymphocytes to the total CD45+ population was low, yet CD45High CD11b+ cells were increasing in the ischemic hemisphere. The peak of bDC activation occurred at 72 hr post-MCAO when neutrophils and lymphocytes were prevalent. This time course of bDC appearance in response to stroke raises the question of whether these cells emerged in response to signals from the periphery (via circulating cytokines or direct interactions with infiltrating cells), or if they participate in the recruitment of peripheral leukocytes into the brain. Nonetheless, the time course of accumulation suggests a role for bDC in the later immune response to ischemia that involves peripheral lymphocyte infiltration.
The adaptive immune response occurring within days following stroke is characterized by a systemic immunosuppression and generation of regulatory T cells. These processes are thought to protect the brain against immune reactivity to exposed “self” brain antigen (Doyle et al., 2008; Liesz et al., 2009; Offner et al., 2006b; Offner et al., 2009; Prass et al., 2003). Although regulatory T cells are detected at low levels in the ischemic brain (Gelderblom et al., 2009; Liesz et al., 2009), they confer a significant degree of protection from stroke thought to be mediated by the release of IL-10 (Frenkel et al., 2003; Liesz et al., 2009; Planas and Chamorro, 2009). Regulatory T cells that release protective cytokines (e.g. IL-4, IL-10, tumor growth factor-beta) may interact with brain resident immune cells to confer local protective immunity. Previous reports describe the emergence of CD11c+ brain resident cells as an integral part of adaptive autoimmune responses and implicate the bDC as a potential substrate for the protective actions of T cells in the brain (Butovsky et al., 2007; Butovsky et al., 2006; Chiu et al., 2008). In fact, our data showed the majority of lymphocytes that infiltrated the brain in response to MCAO-reperfusion to be CD4+ T cells that accumulated in the border region of the infarct in proximity to the resident bDC. The presence of T cells in the infarct border has been described in other stroke models, and our observation of CD4 T cells as the primary infiltrating lymphocyte is consistent with these reports (Frenkel et al., 2003; Gelderblom et al., 2009; Liesz et al., 2009).
A novel finding from this study is the pronounced up-regulation of both MHC II and CD80 protein on the bDC at 72 hr post-MCAO-reperfusion. In their recent study examining peripheral CD11c+DC and co-stimulatory molecules in response to MCAO-reperfusion, Gelderblom et al. (2009) reported a robust increase in MHC II but only a trend for increased CD80. In contrast, the current study observed an increase in the percentage of cells positive for CD80 and an increase in fluorescence intensity, both within the general CD11b+ cell population at 24 and 72 hr, and in the resident bDC population at 72 hr post-ischemia. Interestingly, few bDC were positive for CD86 at 72 hr, although CD86 was increased within the general CD11b+ population at this time. Therefore, resident bDC preferentially increased CD80 relative to CD86 at 72 hr post-ischemia. Although Gelderblom et al. (2009) concluded that an increase in MHC II with a lack of co-stimulatory molecules supports a model for anergy and regulatory T cell activation following stroke, the presence of CD80 in the absence of CD86 may also promote regulatory T cell activity. Although CD80 and CD86 share a common signaling mechanism, a permissive role for CD80 and an inhibitory role for CD86 on the suppressive activity of regulatory T cells has been described (Manzotti et al., 2002; Sansom et al., 2003; Zheng et al., 2004). In the context of this literature, our findings of increased MHC II and CD80 at 72 hr post-MCAO-reperfusion illustrate the potential for bDC to initiate and maintain regulatory T cell activity in the border region of ischemic infarct.
An understanding of signals responsible for bDC accumulation in the border region of the ischemic infarct and their potential role in the immune response to stroke may shed light on the development of novel therapeutic interventions. The infarct border, also termed penumbra, consists of hypoperfused tissue that may either be spared or subject to secondary damage following stroke, and is a sight of ongoing immune activity that serves as a prime target for immunomodulatory therapies. The use of tolerizing vaccines that have established therapeutic benefit in stroke models (Becker et al., 1997; Frenkel et al., 2003; Gee et al., 2007; Ibarra et al., 2007) might serve as a tool in the CD11c/EYFP Tg mouse to identify the possible contribution of bDC to a protective immune response. Additionally, the identification of cytokine/chemokine signals that recruit bDC to the ischemic infarct, or factors produced by the bDC in response to ischemia, would facilitate the identification of agents that promote a protective immune response in the brain.
Recently, Gottfried-Blackmore et al. (in press) discovered that the cytokine interferon gamma (IFN-γ) induces resident bDC to present antigen and to stimulate naïve T cell proliferation and secretion of TH1/TH17 cytokines. IFN-γ expression in the brain has been reported to increase following stroke (Li et al., 2001; Liesz et al., 2009; Yilmaz et al., 2006), peaking 3 to 6 days post-ischemia. Although Liesz et al. (2009) demonstrated the major source of IFN-γ in the brain to be infiltrating T cells, Yilmaz et al., (2006) conclude that other peripheral immune cell types may contribute to IFN-γ production after stroke, and increased circulating IFN-γ protein (Liesz et al., 2009) and mRNA from peripheral mononuclear cells (Li et al., 2001) has been observed at early time points (as soon as 1 hr). Regardless of the source or temporal pattern of IFN-γ production, it is clear that this cytokine can drive the maturation of resident bDC, enabling them to interact with antigen specific T cells; thus IFN-γ may serve as an important contributing factor in the accumulation and function of bDC observed in stroke.
Resident versus peripheral DC
The CD11c/EYFP Tg mouse has been used to isolate a population of resident brain immune cells that express EYFP under the promoter for CD11c in the healthy brain and in response to inflammatory challenge. These cells have been distinguished from microglia by their response to IFNγ, as well as their superiority in driving antigen specific T cell responses (Gottfried-Blackmore et al., 2009). In the present study, the resident bDC were further distinguished from peripheral macrophage and infiltrating DC following stroke using radiation chimeras. In the brains of control radiation chimeras (WT hosts restored with CD11c/EYFP Tg BM), EYFP+ cells were noted in the meninges and within some parenchymal brain regions (Kaunzner et al., 2009); whereas, in these radiation chimeras at 72 hours post-MCAO, ovoid EYFP+ cells in the ischemic hemisphere populated the core but not border region of the infarct. These results clearly indicated that the periphery cells were restricted to the core and accounted for the ovoid EYFP+ cells observed in this region in the non-chimeric CD11c/EYFP Tg mouse ischemic hemispheres at 72 hr.
Although peripheral DC were not the primary focus of this study, it is interesting to note that a significant population of peripherally derived EYFP+ cells were observed in the core region of the infarct. Whether invading EYFP+ cells contribute to protection or damage during stroke progression remains to be determined, however previous literature has described protection from stroke by the blockade of peripheral inflammatory cell infiltration by inhibition of adhesion molecules used by neutrophils and macrophages (Chopp et al., 1994; Garcia et al., 1996; Lees et al., 2003; Zhang et al., 2003). Considering that circulating mononuclear cells serve as a source of cytokines in the ischemic brain, interactions between peripheral infiltrating cells and the resident bDC may play an important role in determining the severity and outcome of stroke.
Conclusions
This present study demonstrated that resident bDC contribute to the immune response following stroke. The bDC increased in number at 24 and 72 hr post-ischemia, up-regulated MHC II and co-stimulatory molecules, and accumulated in the infarct border in close proximity to invading T cells. Peripheral DC entering the brain were apparent at 72 hr post-MCAO-reperfusion and were confined primarily to the core of the ischemic infarct. Future studies are necessary to address the potential ability of ischemia-activated bDC to stimulate T cells, express cytokines/chemokines, and contribute to damage or recovery following stroke.
Supplementary Material
Acknowledgements
This work was supported by Woodin Dendritics, LLC and NIH grants # NS34179 and NS35806. The authors would additionally like to thank Drs. Juliana Idoyaga, Svetlana Mazel, and Alison North, and staff members Christopher Bare and Shivaprasad Bhuvanendran of the Rockefeller Flow Cytometry and Bioimaging Facilities, for intellectual and technical advice. David Einheber provided invaluable assistance in the lab. We would also like to acknowledge all of the members of the McEwen lab for their support and suggestions, and to thank the Deane family for their gracious contributions to our research program.
Footnotes
Conflict of Interest: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ, McCarron RM, Ruetzler C, Laban O, Sternberg E, Flanders KC, Hallenbeck JM. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker T, Riedinger M, Karjalainen K. Driving gene expression specifically in dendritic cells. Advances in experimental medicine and biology. 1997;417:55–57. doi: 10.1007/978-1-4757-9966-8_9. [DOI] [PubMed] [Google Scholar]
- Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, Kaunzner UW, Liu K, Lindquist R, Nussenzweig MC, Steinman RM, McEwen BS. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. The Journal of comparative neurology. 2008;508:687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Bukshpan S, Kunis G, Jung S, Schwartz M. Microglia can be induced by IFN-gamma or IL-4 to express neural or dendritic-like markers. Molecular and cellular neurosciences. 2007;35:490–500. doi: 10.1016/j.mcn.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, Schwartz M. Glatiramer acetate fights against Alzheimer’s disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Chen A, Zheng Y, Kosaras B, Tsiftsoglou SA, Vartanian TK, Brown RH, Jr., Carroll MC. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Zhang RL, Chen H, Li Y, Jiang N, Rusche JR. Postischemic administration of an anti-Mac-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in rats. Stroke; a journal of cerebral circulation. 1994;25:869–875. doi: 10.1161/01.str.25.4.869. discussion 875-866. [DOI] [PubMed] [Google Scholar]
- D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Hancock WW, Moskowitz MA, Weiner HL. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J Immunol. 2003;171:6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Bree MP. Effects of CD11b/18 monoclonal antibody on rats with permanent middle cerebral artery occlusion. The American journal of pathology. 1996;148:241–248. [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Shea C, Becker KJ. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke; a journal of cerebral circulation. 2007;38:783–788. doi: 10.1161/01.STR.0000248425.59176.7b. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke; a journal of cerebral circulation. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Kaunzner U, Idoyaga J, Felger JC, McEwen BS, Bulloch K. INF-gamma unmasks functional brain-resident dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. doi: 10.1073/pnas.0911509106. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra A, Avendano H, Cruz Y. Copolymer-1 (Cop-1) improves neurological recovery after middle cerebral artery occlusion in rats. Neuroscience letters. 2007;425:110–113. doi: 10.1016/j.neulet.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke; a journal of cerebral circulation. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- Kaunzner UW, Gottfried-Blackmore A, Bulloch K. Characterization of brain dendritic cells (bDC) in young and old radiation chimeras. Society for Neuroscience Abstracts; Chicago, IL: 2009. Program No. 138.2. [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nature medicine. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- Kerfoot SM, D’Mello C, Nguyen H, Ajuebor MN, Kubes P, Le T, Swain MG. TNF-alpha-secreting monocytes are recruited into the brain of cholestatic mice. Hepatology (Baltimore, Md. 2006;43:154–162. doi: 10.1002/hep.21003. [DOI] [PubMed] [Google Scholar]
- Kostulas N, Li HL, Xiao BG, Huang YM, Kostulas V, Link H. Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke; a journal of cerebral circulation. 2002;33:1129–1134. doi: 10.1161/hs0402.105379. [DOI] [PubMed] [Google Scholar]
- Kunz A, Abe T, Hochrainer K, Shimamura M, Anrather J, Racchumi G, Zhou P, Iadecola C. Nuclear factor-kappaB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J Neurosci. 2008;28:1649–1658. doi: 10.1523/JNEUROSCI.5205-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz A, Park L, Abe T, Gallo EF, Anrather J, Zhou P, Iadecola C. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees KR, Diener HC, Asplund K, Krams M. UK-279,276, a neutrophil inhibitory glycoprotein, in acute stroke: tolerability and pharmacokinetics. Stroke; a journal of cerebral circulation. 2003;34:1704–1709. doi: 10.1161/01.STR.0000078563.72650.61. [DOI] [PubMed] [Google Scholar]
- Li HL, Kostulas N, Huang YM, Xiao BG, van der Meide P, Kostulas V, Giedraitas V, Link H. IL-17 and IFN-gamma mRNA expression is increased in the brain and systemically after permanent middle cerebral artery occlusion in the rat. Journal of neuroimmunology. 2001;116:5–14. doi: 10.1016/s0165-5728(01)00264-8. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature medicine. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nature immunology. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- Manzotti CN, Tipping H, Perry LC, Mead KI, Blair PJ, Zheng Y, Sansom DM. Inhibition of human T cell proliferation by CTLA-4 utilizes CD80 and requires CD25+ regulatory T cells. European journal of immunology. 2002;32:2888–2896. doi: 10.1002/1521-4141(2002010)32:10<2888::AID-IMMU2888>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158:1049–1061. doi: 10.1016/j.neuroscience.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nature biotechnology. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006a;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006b;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas AM, Chamorro A. Regulatory T cells protect the brain after stroke. Nature medicine. 2009;15:138–139. doi: 10.1038/nm0209-138. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. The Journal of experimental medicine. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nature medicine. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Reichmann G, Schroeter M, Jander S, Fischer HG. Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. Journal of neuroimmunology. 2002;129:125–132. doi: 10.1016/s0165-5728(02)00184-4. [DOI] [PubMed] [Google Scholar]
- Sansom DM, Manzotti CN, Zheng Y. What’s the difference between CD80 and CD86? Trends in immunology. 2003;24:314–319. doi: 10.1016/s1471-4906(03)00111-x. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, Stern LJ, Strominger JL, Riese R. Developmental plasticity of CNS microglia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain research. 2002;932:110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. Journal of neuroimmunology. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zhang F, Iadecola C. Infarct measurement methodology. J Cereb Blood Flow Metab. 1994;14:697–698. doi: 10.1038/jcbfm.1994.88. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang RL, Lu M, Krams M, Chopp M. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke; a journal of cerebral circulation. 2003;34:1790–1795. doi: 10.1161/01.STR.0000077016.55891.2E. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172:2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.