Abstract
Brain iron deficiency leads to altered dopaminergic function in experimental animals, which can provide a mechanistic explanation for iron deficiency-related human sensory-motor disorders, such as Restless Legs Syndrome (RLS). However, mechanisms linking both conditions have not been determined. Considering the strong modulation exerted by adenosine on dopamine signaling, one connection could involve changes in adenosine receptor expression or function. In the striatum, presynaptic A2A receptors are localized in glutamatergic terminals contacting GABAergic dynorphinergic neurons and their function can be analyzed by the ability of A2A receptor antagonists to block the motor output induced by cortical electrical stimulation. Postsynaptic A2A receptors are localized in the dendritic field of GABAergic enkephalinergic neurons and their function can be analyzed by studying the ability of A2A receptor antagonists to produce locomotor activity and to counteract striatal ERK1/2 phosphorylation induced by cortical electrical stimulation. Increased density of striatal A2A receptors was found in rats fed during three weeks with an iron-deficient diet during the post-weaning period. In iron-deficient rats, the selective A2A receptor antagonist MSX-3, at doses of 1 and 3 mg/kg, was more effective at blocking motor output induced by cortical electrical stimulation (presynaptic A2A receptor-mediated effect) and at enhancing locomotor activation and blocking striatal ERK phosphorylation induced by cortical electrical stimulation (postsynaptic A2A receptor-mediated effects). These results indicate that brain iron deficiency induces a functional up-regulation of both striatal pre- and postsynaptic A2A receptor, which could be involved in sensory-motor disorders associated with iron deficiency such as RLS.
Introduction
Restless Legs Syndrome (RLS) is a common sensory–motor disorder that affects about 7.5% of the population (Allen et al., 2005). The core feature of the syndrome is an overwhelming urge to move the legs that is triggered by rest and relieved with movement (Allen et al., 2003). Iron deficiency was first noted by Nordlander in the 1950s to be associated with symptom development and by treating the iron deficiency symptoms would improve or completely resolve (Nordlander, 1954). Subsequent studies using cerebrospinal fluid analysis of iron proteins (Earley et al., 2000), MRI-determined brain iron levels (Earley et al., 2006) and autopsy studies (Boyer et al., 2000; Connor et al., 2004) have all indicated the presence of low brain iron in RLS subjects, even in those with normal blood levels of iron. The most effective treatment for RLS has been the use of L-dopa and, based on this finding, the dopaminergic system has been implicated in RLS pathology along with iron deficiency (Allen, 2004). One of the most consistent and best characterized functional abnormalities associated to post-weaning iron deficiency in the rodent is altered dopaminergic neurotransmission. In fact, the distribution of dopamine and iron in the brain are closely correlated, with particularly high concentrations of both in the adult striatum (Beard and Connor, 2003). In the experimental animal, iron deficiency has been associated with a decrease in the striatal density of the dopamine transporter and dopamine D1 and D2 receptors and with an increase in striatal dopamine levels and nigral tyrosine hydroxylase (Youdim et al., 1983; Nelson et al., 1997; Erikson et al., 2000, 2001; Unger et al., 2008; Connor et al., 2009). As a confirmation of the predictive nature of the iron-deficiency rodent model as a tool for exploring the iron-dopamine relation in RLS, a recent autopsy study in RLS and control cases showed decreases in D2 receptor density and increases in the putaminal and nigral tyrosine hydroxylase (Connor et al., 2009). However, the mechanisms linking brain iron deficiency and the alterations in the dopaminergic function still need to be determined.
Adenosine exerts a tight antagonistic control of the dopaminergic system. One of the main mechanisms involved in that control depends on the existence of intermolecular interactions between adenosine A2A and dopamine D2 receptors in the striatum (Ferré et al., 1997, 2008). In SH-SY5Y cells in culture, a step-wise decrease in iron induced by progressively increased concentrations of desferroxamine led to an inversely proportional increase in A2A receptor density (Gulyani et al., 2009). Dietary iron-deficiency in mice also led to a decrease in striatal iron and an increase in striatal A2A receptor density (Gulyani et al., 2009). Furthermore, when mice strains are selected on the basis of their natural variation in striatal iron concentration, strains with the lower striatal iron concentrations had significantly higher striatal A2A receptor density (Gulyani et al., 2009).
In the striatum, presynaptic A2A receptors are localized in the glutamatergic terminals that contact GABAergic dynorphinergic neurons and their function can be analyzed by the ability of A2A receptor antagonists to block the motor output induced by cortical electrical stimulation. Postsynaptic A2A receptors are localized in the dendritic field of GABAergic enkephalinergic neurons and their function can be analyzed by studying the ability of A2A receptor antagonists to produce locomotor activity and to counteract striatal ERK1/2 phosphorylation induced by cortical electrical stimulation (Quiroz et al., 2009). The present study was designed to further explore the iron-A2A receptor interaction by utilizing these A2A- receptor experimental paradigms.
Materials and Methods
Animals
All animal experiments were performed in accordance with the National Institutes of Health Animal care guidelines. Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), 21 days-old at the beginning of the experiments, were used. All rats had continuous access to food and water and were maintained on a 12:12 hr light/dark cycle (lights on at 0700 h), with the room temperature maintained at 25 ± 1°C. The rats were divided into 2 groups: the control group was fed with a diet containing an essential amount of iron (48 ppm iron, Catalog TD.80394, Harlan-Teklad, Madison, WI) and the iron- deficient group was fed with a diet containing a low iron concentration (4 ppm iron, Catalog TD.80396, Harlan-Teklad, Madison, WI). The other contents of the diet were the same. Diets were started immediately after weaning and continued for 21 days. In order to determine the hematocrit levels for each rat in the two groups, blood samples were obtained from retro-orbital sinus through the course of the three-week period, during surgery (in the rats that were implanted with electrodes) and immediately after the end of both locomotor activity and protein phosphorylation experiments.
Locomotor Activity
After 21 days of iron-deficient or control diet, the rats from both groups were placed in individual, sound-proof chambers (50 × 50 centimeters; Med Associates Inc, VT) filled with wood chip bedding. A Columbus Instruments Auto-Track system (Coulbourn Instruments, Lehigh Valley, PA) was used to quantify locomotor activity by means of counting infrared beam breaks accumulated in periods of 10 minutes. To measure the overall effect of iron deficiency on motor activity, rats were placed individually in the chambers and their activity was measured during 24 hours with free access to food and water and keeping the same lights-dark period than in the housing room. Activity recording (for locomotion and stereotypic movements) started after 90 minutes of habituation. To measure the effect of iron deficiency on the locomotor activation induced by the systemic administration of the selective A2A receptor antagonist MSX-3 (3,7-dihydro-8-[(1E)-2-(3-methoxyphenyl)ethenyl]-7-methyl-3-[3-(phosphonooxy)propyl-1-(2-propynyl)-1H-purine-2,6-dione] disodium salt hydrate; Sigma, St. Louis, MO), rats were placed in individual acrylic chambers at noon on the day of testing. A lamp inside each chamber remained lit during this period. Both iron-deficient and control groups were tested during the same session to control the variability associated with testing conditions. Locomotor activity was first recorded during 90 min of habituation (exploratory activity), followed by 120 min of recording after the drug or vehicle administration. Following habituation, the rats were injected intraperitoneally (i.p.) with either 1 or 3 mg/kg of MSX-3. The drug was dissolved in sterile saline (with 3 µl/ml saline of 1 M NaOH solution, final pH 7.4).
Surgical procedures
After 17 days of iron-deficient or control diet, the rats were anesthetized with 3 ml/kg of Equithesin (4.44 g of chloral hydrate, 0.972 g of Na pentobarbital, 2.124 g of MgSO4, 44.4 ml of propylene glycol, 12 ml of ethanol and distilled H2O up to 100 ml of final solution; NIDA Pharmacy, Baltimore, MD) and implanted unilaterally with bipolar stainless steel electrodes, 0.15 mm in diameter (Plastics One, Roanoke, VA), into the orofacial area of the lateral agranular motor cortex (2.5 mm anterior, 2.5 and 3.5 mm lateral, and 3.9 mm below bregma). The electrodes and a head holder (connected to a swivel during stimulation) were fixed on the skull with stainless steel screws and dental acrylic resin. For the experiments with electromyographic (EMG) recording, electrodes were also implanted in mastication muscles (during the same surgical procedure). Two 5 mm-long incisions were made in the skin on the upper and lower jaw areas to expose the masseter and the lateral pterygoid muscles. Two silicon rubber-coated coiled stainless steel recording electrodes (Plastics One, Roanoke, VA) were slipped below the skin from the incision in the skull until the tips showed up from the incisions in the jaw. The bare tips of the electrodes were then held in contact with the masseter and the lateral pterygoid muscles and the skin was closed with surgical staples. The other end of the recording electrodes was encased in a molded plastic pedestal with a round threaded post which was attached to an electrical swivel and then to a differential amplifier (Grass LP511, Grass Instruments, Warwick, RI). The pedestal was secured to the skull with dental cement together with the stimulation electrodes.
Cortical electrical stimulation
Four days after surgery (21 days after the beginning of the iron-deficient or control diet) the animals were placed in individual bowl chambers, the implanted electrodes were attached to an electrical stimulator (Grass S88X; Grass Instruments, West Warwick, RI) and then allowed 60 minutes for habituation. The parameters of cortical stimulation were the same as those shown previously to induce phosphorylation of ERK1/2 in the projecting striatal area, the lateral caudate–putamen (Quiroz et al., 2006). Rats were injected with either MSX-3 (1 mg/kg or 3 mg/kg) or vehicle 10 minutes before the beginning of the stimulation, in which biphasic current pulse trains (pulse of 0.1 ms; 120–200 µA, 100 Hz, 160 ms trains repeating once per second) were delivered during 20 minutes using two-coupled constant current units (Grass PSIU6X; Grass Instruments, West Warwick, RI). The intensity was 150 µA for most cases or it was increased (stepwise 5 µA increases in the intensity) up to 200 µA, until small jaw movements were observed. The cases that failed to show visible somatic movements (less than 10%) were excluded from additional analysis. In no case did animals display evidence of seizure activity from the electrical stimulation.
EMG recording and power correlation analysis
Four days after surgery, rats were placed in individual bowl chambers. Both the stimulation electrodes and the recording electrodes were attached using flexible shielded cabling to a four channel electrical swivel and then the stimulation electrodes were connected to two-coupled constant current isolation units (PSIU6X, Grass Instruments West Warwick, RI) driven by an electrical stimulator (Grass S88X; Grass Instruments). The recording electrodes were connected to a differential amplifier (Grass LP511, West Warwick, RI). This configuration allows the rat to move freely while the stimulation and EMG recordings are taking place. After 60 min of habituation, biphasic current pulse trains (pulse of 0.1 ms at 120–200 µA; 100 Hz, 160 ms trains repeating once per 2 seconds) were delivered. The current intensity was adjusted to the threshold level, defined as the minimal level of current intensity allowing at least 95% of the stimulation pulses to elicit a positive EMG response. Positive EMG response was defined as at least 100% increase of the peak to peak amplitude respect to the background tonic EMG activity lasting more than 100 ms or at least 70% increase in the power of the EMG signal respect to the baseline. Positive EMG responses always matched observable small jaw movements. The threshold level was different for each animal but it was very stable and reproducible once established. The threshold level was in the 100 to 150 µA range for most cases and it reached 200 µA in a few (3) animals. Animals that failed to show a positive EMG response with electrical cortical stimulation intensities of 200 µA were discarded from the experimental procedure (less than 10%). Both stimulator monitoring and the amplified and filtered EMG signal (20,000 times gain, bandwidth from 10 to 1,000 Hz with a notch filter set at 60 Hz) were directed to an analog digital converter (NI 9215, National Instruments, Austin, TX) and digitized at a sampling rate of 10,000 samples/second. Recordings of the digitized data were made with LabVIEW SignalExpress software (National Instruments, Austin, TX). A power correlation analysis was used to quantify the correlation between the stimulation pulses of current delivered into the orofacial motor cortex (input signal; µA) and the elicited EMG response in the jaw muscles (output signal; µV). Decrease in the power correlation coefficient (PCC) between these two signals is meant to describe a decrease in the efficacy of the transmission in the neural circuit. Off-line, both signals were rectified and the root mean square (RMS) over each period of the stimulation pulses was calculated in the recorded signals using LabVIEW SignalExpress software (National Instruments, Austin TX). The transformed data (RMS) from the stimulator monitor and the EMG were then exported with a time resolution of 100 samples/second to a spreadsheet file. The stimulation signal values were used as a reference to select data in a time window of 320 ms starting at the beginning of each train of pulses. This time window was chosen to ensure the analysis of any EMG response whose occurrence or length was delayed from the onset of the stimulation trains and to maximize the exclusion from the analysis of spontaneous jaw movements not associated with the stimulation. Pearson’s correlation between the RMS values from the stimulation and EMG signals was then calculated for each experimental subject. The effect of the systemic administration (i.p.) of MSX-3, 1 mg/kg, was studied in independent experiments. PCC was calculated using data recorded during ten minutes, starting forty min after the administration of the dose of MSX-3 and saline, respectively.
Analysis of striatal protein phosphorylation
Immediately after the offset of 20 min of cortical stimulation, the animals were decapitated, and the brains were rapidly extracted, frozen in dry ice-cold isopentane, and stored at −80°C. Subsequently, to obtain unilateral tissue punches, the brains were cut in one millimeter -thick coronal sections using as reference the following coordinates: from +2.5 to +1.5 mm relative to bregma for the frontal cortex; +0.5 to −0.5 mm relative to bregma for the lateral caudate putamen of the striatum; and −5.5 to −6.5 mm relative to bregma for the basal mesencephalon. The tissue punches were taken from each section with a 16 gauge sampling needle, kept frozen on dry ice, and stored at −30°C until processed. The tissue punches were then sonicated for 10–15 s in 200 µL of 1% sodium dodecyl sulfate (SDS) dissolved in deionized ultrapure water. The protein concentrations of all samples were determined by light absorbance using a bicinchoninic acid assay kit (Pierce, Rockford, IL) and were further diluted with 1% SDS to equalize the protein concentrations in each sample. Loading buffer (16% glycerol, 20% mercaptoethanol, and 0.05% bromophenol blue) was added to each sample (3:1, sample to loading buffer ratio) before heating at 95°C for 10 min. The proteins in the samples were separated by SDS-PAGE (10% polyacrylamide Tris-HCl resolving precast gel, Bio-Rad, Hercules, CA) for 3–4 h at 100 V. For each electrophoresis run, 10 µL of sample (containing 4–5 µg of total protein depending on each particular run) were loaded in each well, and increasing amounts of protein pooled from the control samples were also loaded and electrophoresed to produce a five point standard curve. Proteins were transferred electrophoretically to polyvinylidene difluoride Immobilon-P or Low Fluorescence membranes, (Cat number IPVH00010, Millipore, Bedford, MA) at 0.1 A for 12 h and dried. Membranes were then rewetted and washed four times for 15 min in blocking buffer: 2% polyvinylpyrrolidone in phosphate-buffered saline plus 0.05% Tween 20 (PBST), pH 7.4 for phospho-ERK1/2 or 2% bovine albumin in PBST for total ERK1/2. Membranes were then incubated overnight at 4°C with their primary antibody diluted in the respective blocking buffers plus 0.01% sodium azide. The primary antibodies used were, mouse monoclonal anti-A2A (1:1000 dilution; Upstate, Lake Placid, NY), mouse monoclonal anti-Transferrin (1:1000 dilution; Invitrogen, Carlsbad, CA), rabbit polyclonal anti-ERK1/2 (1:2000 dilution; Cell Signaling Technology), and rabbit polyclonal anti-phospho-Thr202/Tyr204 ERK1/2 (1:5000 dilution; Cell Signaling Technology). After four washes for 15 min in blocking buffer, the blots incubated overnight with transferrin and adenosine A2A receptor antibodies were then incubated for 2 h at room temperature with horseradish peroxidase-conjugated secondary goat anti-mouse IgG antibody in blocking buffer (1:2000 dilution, PI-1000; Vector Laboratories), washed six times for 10 min in phosphate-buffered saline (PBS) and incubated during 60 s in the reagents of the enhanced chemiluminescence procedure of Amersham Biosciences. Luminescence from the blots was detected by exposing the membranes to Amersham Biosciences Hyperfilm during 30 s to 5 min, followed by digital scanning of the developed film in transparency mode. The blots incubated overnight with ERK1/2 and phospho-Thr202/Tyr204 ERK1/2 antibodies were washed four times for 15 minutes in blocking buffer and then incubated for 2 h at room temperature with goat anti-rabbit IRDye 680 (1:5000 dilution, Li-cor Biosciences, Lincoln, NE). Finally, the blots were washed six times for 10 min in PBS and scanned in an Odyssey infrared scanner (Li-cor Biosciences, Lincoln, NE). All the scanned images of the membranes and band intensities were calibrated and quantified using NIH ImageJ software (version 1.39). The amount of the protein of interest in each sample was interpolated from the band intensities of the standard curves. The band intensities for each of the test samples quantified were always within the range of the standard curve. For each animal, the values obtained from the experiments with phosphorylated and total ERK1/2 corresponded to the addition of the bands intensities of both ERK1 and ERK2. For each animal, the values of phosphorylated ERK1/2 were normalized (as percentage of control) to total ERK1/2. In each Western blot, all values were normalized (as percentage of control) with respect to the standard curve.
Statistical analysis
The effect of the iron-deficient and control diets on body weight, hematocrit, motor activity and the expression of transferrin receptor and A2A receptor was analyzed by non-paired t test. The effect of the diets on exploratory activity and MSX-3-induced locomotion was analyzed by non-paired t test. The effects of the diets and the A2A receptor antagonist on striatal protein phosphorylation and on PCC were analyzed by bifactorial ANOVA, followed by Newman–Keuls test.
Results
Effect of iron deficiency on body weight, hematocrit content and motor activity
A significant decrease (12.4%) in mean body weight was observed after 21 days of diet in iron-deficient rats (in mean ± SEM: 162.2 ± 6.4 g; n=40) compared to controls (in mean ± SEM: 185.2 ± 9.3 g; n=43) (non-paired t test, p=0.05). A significant decrease (58.1%) in hematocrit content sampled on day 21 after the beginning of the diet was detected in iron-deficient rats (in mean ± SEM: 19.4 ± 0.7%; n=41) compared to controls (in mean ± SEM: 46.5 ± 0.8%; n=39) (non-paired t test, p<0.01). There were no significant differences between the iron-deficient rats and the control group in stereotypic movements (in means ± SEM: 80,032 ± 4,732 counts and 80,475 ± 5,376 counts, respectively; n=7 in both cases; non-paired t test, p=0.94) or in locomotion (in means ± SEM: 9,998 ± 1,341 counts and 12,637 ± 1,091, respectively; n=7 in both cases; non-paired t test, p=1.57) during a 24-hours period measured on day 21 after the beginning of the diet.
Effect of iron deficiency on transferrin receptor and adenosine A2A receptor density
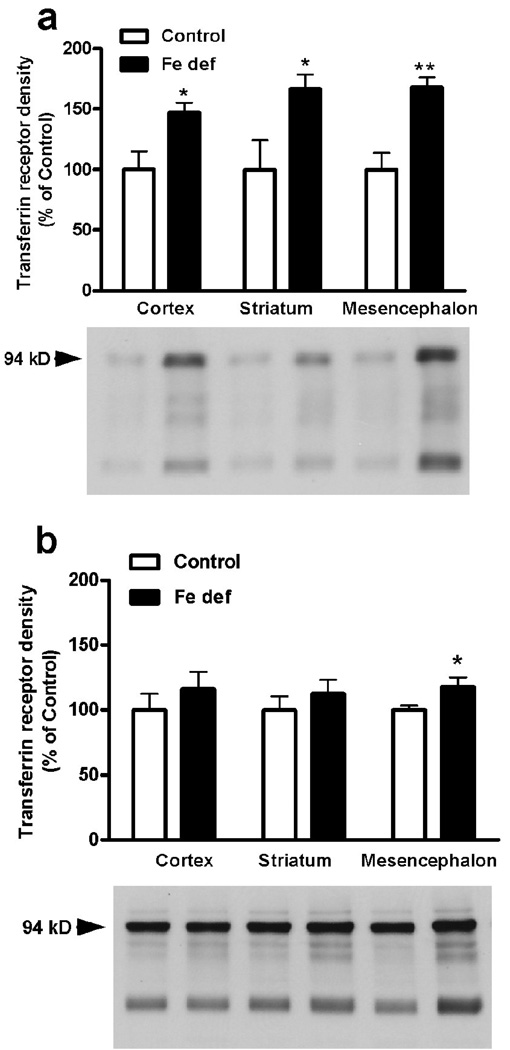
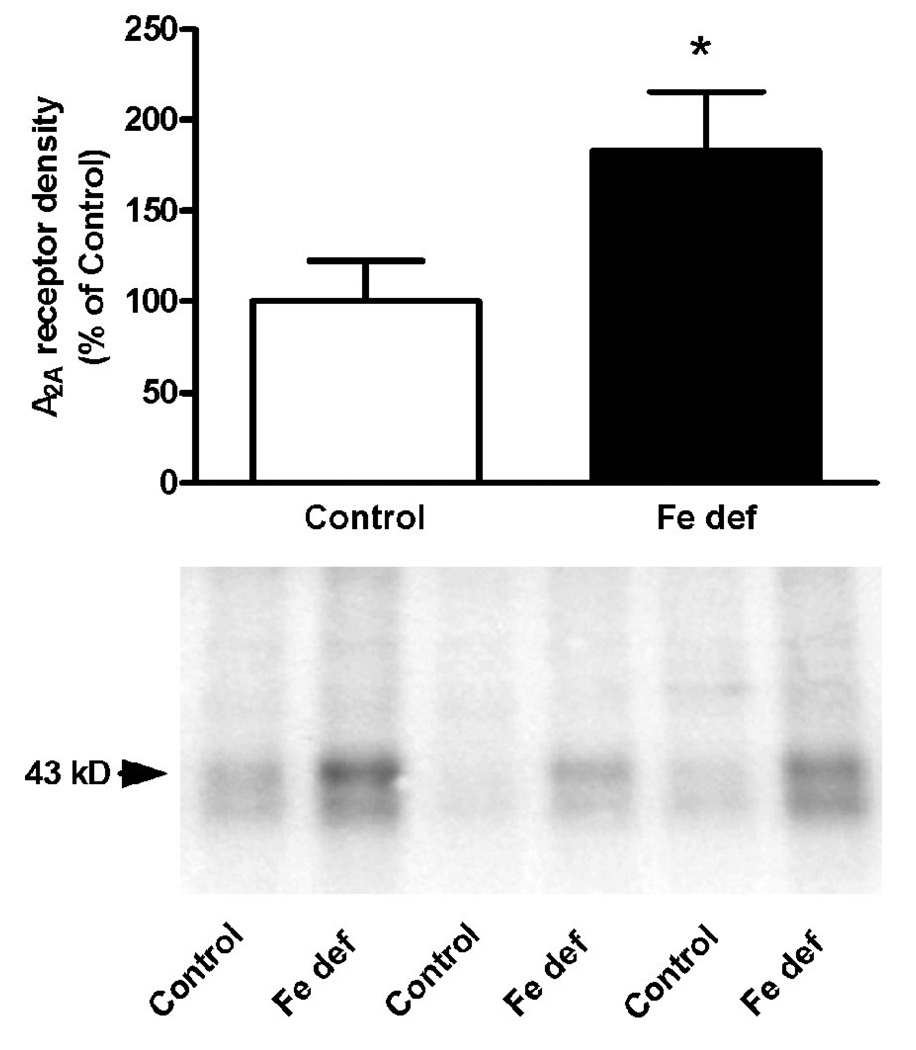
Increases in the brain density of transferrin receptor constitute a reliable indicator of chronic exposure to low levels of iron in the brain (Lok and Loh, 1998; Gulyani et al., 2009). From the three analyzed brain areas (cortex, striatum and basal mesencephalon), two weeks of iron-deficient diet only produced a significant increase in the density of transferrin receptor in the basal mesencephalon (non-paired t test: p<0.05 compared to control; Fig. 1a). After three weeks of iron-deficient diet, there was a significant increase in the density of transferrin receptor in the cortex, striatum and mesencephalon (non-paired t test: p<0.05, p<0.0.5 and p<0.01 compared to controls; Fig. 1b). Therefore, three weeks of iron-deficient diet was used in all subsequent experiments. In animals with control diet, A2A receptor density measured by Western blotting was only detected in the striatum (Fig. 2). Iron-deficient diet significantly increased A2A receptor density in the striatum (non-paired t test: p<0.05 compared to control; Fig. 2).
Figure 1.
Transferrin receptor density in three brain regions from rats with control or iron-deficient (Fe def) diet for two (a) or three weeks (b). Results are expressed as means ± S.E.M. in % of control values (n=6–8 per group). * and **: statistically different versus respective control (p<0.05 and p<0.01, respectively).
Figure 2.
Adenosine A2A receptor density in the lateral striatum from rats with control or iron-deficient (Fe def) diet for three weeks. Results are expressed as means ± S.E.M. in % of control values (n= 4 per group). *: statistically different versus control (p<0.05).
Effect of iron deficiency on A2A receptor antagonist-induced locomotion and exploratory activity
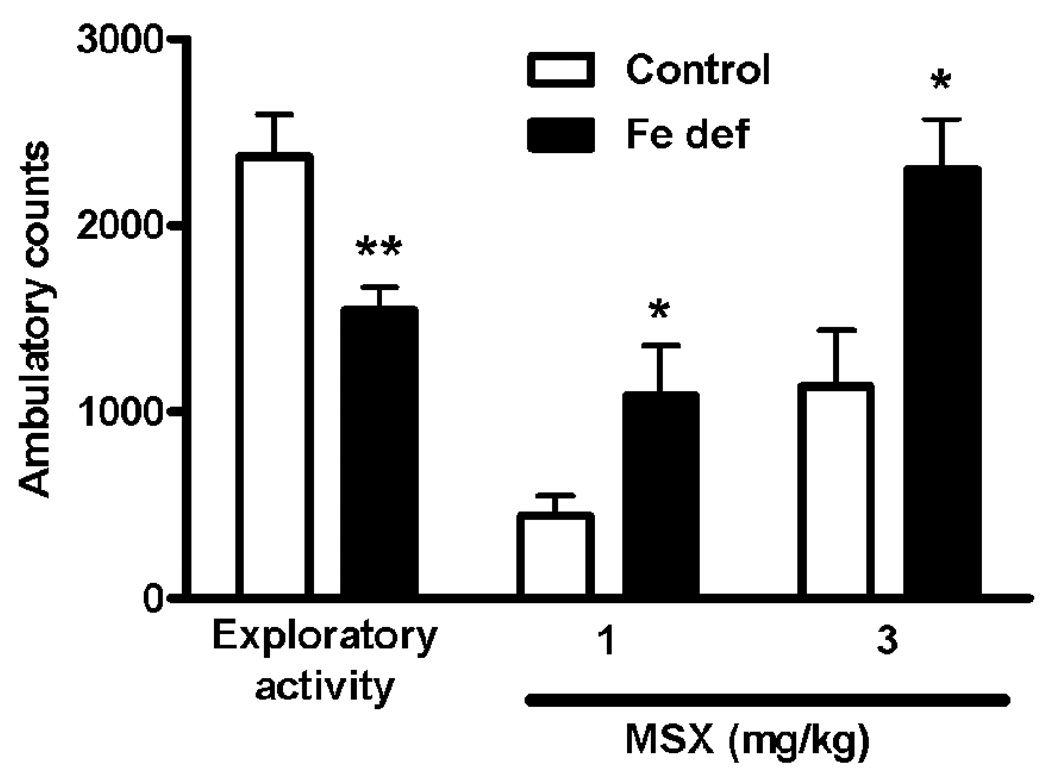
As previously described (Karcz-Kubicha et al., 2003), MSX-3 produced a dose-dependent locomotor activation. At the dose of 1 mg/kg, MSX-3 induced a very weak locomotor activation in control animals, but a significantly stronger effect in animals fed with iron-deficient diet (t test: p<0.05; Fig. 3). This effect was quantitatively the same than that obtained with the dose of 3 mg/kg in control animals. An iron-deficient diet also potentiated significantly the locomotor activation induced by the dose of 3 mg/kg of MSX-3 (t test: p<0.05; Fig. 3). Locomotor activity during the 90 min-habituation period previous to the drug administration, which measures exploratory activity to the new environment, was also determined and found to be significantly higher in the animals with control diet (t- test: p<0.05; Fig. 3).
Figure 3.
Locomotor activity during the exploratory period (first 90 min of measured locomotion), and after the administration of the A2A receptor antagonist MSX-3 (1 and 3 mg/kg, i.p.) in rats fed with control or iron-deficient (Fe def) diet for three weeks. Results are expressed as means ± S.E.M. (n= 9 per group) of the accumulated ambulatory counts during both the exploratory activity period and after the injection of the respective dose of MSX-3. *,**: statistically different versus respective control group (*: p<0.05,**: p<0.01).
Effect of iron deficiency on A2A receptor antagonist-mediated counteraction of striatal ERK1/2 phosphorylation induced by cortical stimulation
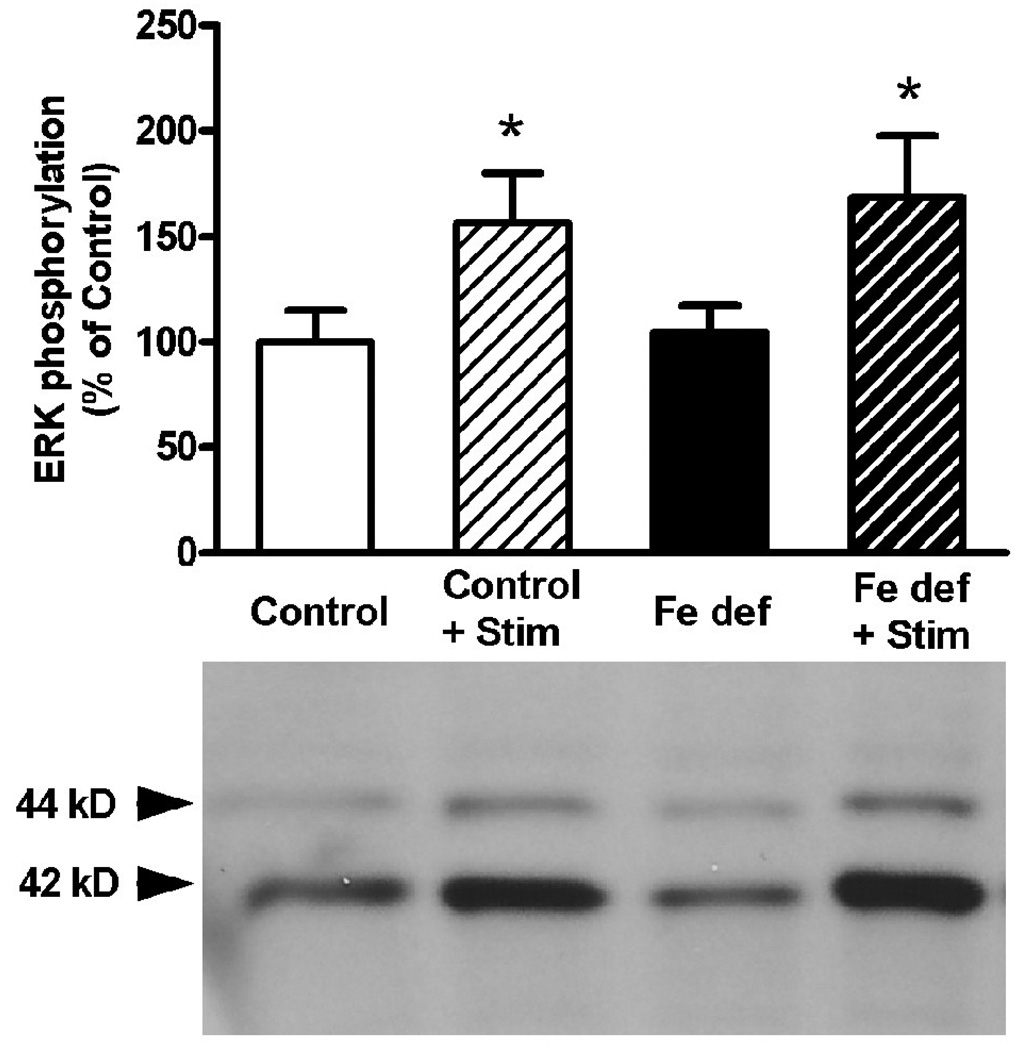
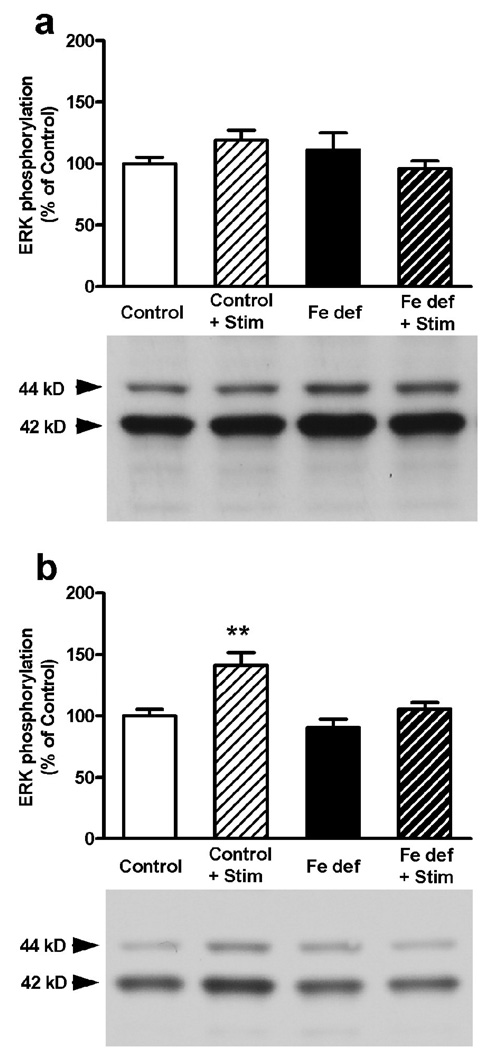
In agreement with previous studies (Gerfen et al., 2002; Quiroz et al., 2006, 2009), cortical electrical stimulation produced a significant increase in striatal ERK1/2 phosphorylation. This effect was observed in rats with control (56% increase) and iron-deficient (61% increase) diets (bifactorial ANOVA: p<0.05 in both cases; non-significant diet effect; Fig. 4). The increase in ERK1/2 phosphorylation induced by cortical stimulation was completely counteracted with 3 mg/kg of MSX-3 in both control and iron-deficient rats (Fig. 5a). Remarkably, the lower dose of MSX-3 (1 mg/kg) did not prevent ERK1/2 phosphorylation induced by cortical stimulation in rats fed with control diet, but counteracted ERK1/2 phosphorylation induced by cortical stimulation in the rats fed with iron-deficient diet (bifactorial ANOVA: p<0.05; significant diet effect; Fig. 5b). Thus, iron-deficient rats have higher sensitivity to the effects of the A2A receptor antagonist on both locomotor activation and on counteraction of ERK1/2 phosphorylation induced by cortical electrical stimulation. Since these effects are mediated by postsynaptic A2A receptors (Quiroz et al., 2009), these results indicate the existence of a functional up-regulation of postsynaptic A2A receptors.
Figure 4.
Striatal ERK1/2 phosphorylation induced by cortical electrical stimulation in rats fed with control or iron-deficient (Fe def) diet for three weeks. Results are expressed as means ± S.E.M. in % of control values (n= 5 per group) and representative Western blots. *: statistically different versus respective non-stimulated group (p<0.05).
Figure 5.
A2A receptor antagonist-induced blockade of the striatal ERK1/2 phosphorylation induced by cortical electrical stimulation in rats fed with control or iron-deficient (Fe def) diet for three weeks. (a) 3 mg/kg, i.p., of MSX-3 counteracted the effect of cortical electrical stimulation in control and iron-deficient rats. (b) 1 mg/kg, i.p., of MSX-3 did not counteracted the effect of cortical electrical stimulation in iron-deficient rats. Results are expressed as means ± S.E.M. in % of control values (n= 8 per group) and representative Western blots. **: statistically different versus respective non-stimulated group (p<0.01).
Effect of iron deficiency on A2A receptor antagonist-mediated counteraction of the motor output induced by cortical stimulation
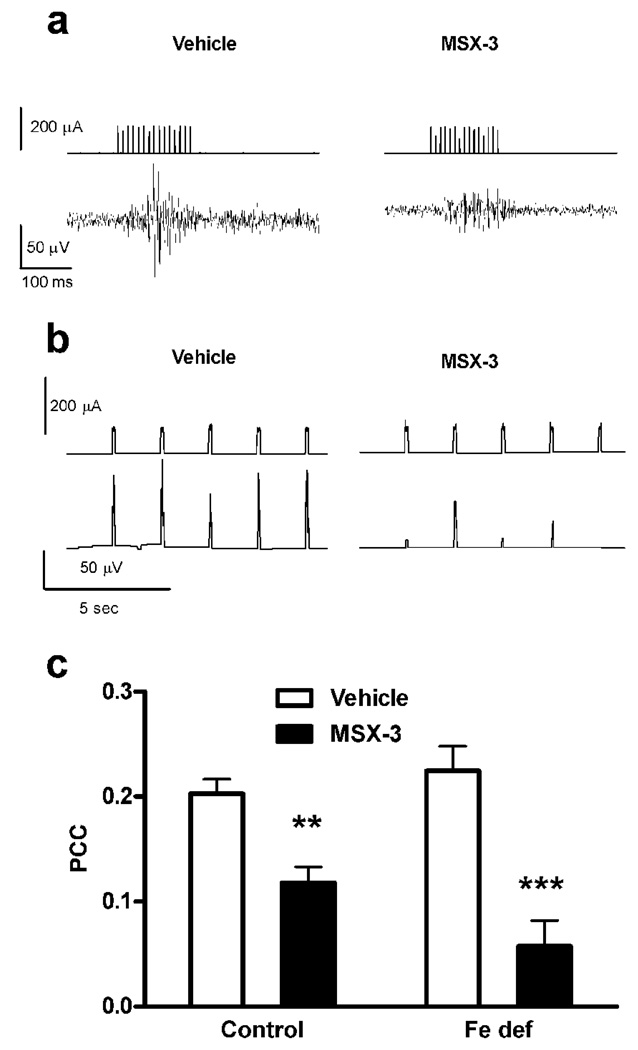
In order to determine if the iron-deficient diet also produces a functional up-regulation of presynaptic A2A receptors we analyzed the ability of MSX-3 to counteract the motor output (electromyographic activity in jaw muscles) induced by cortical electrical stimulation (in the orofacial premotor area), by measuring PCC (see methods section and Quiroz et al., 2009). As previously reported, MSX-3 (1 mg/kg) significantly decreased PCC values in controls and it did so as well in iron-deficient rats (Fig. 6c). Nonetheless, a bifactorial ANOVA demonstrated a significant drug-diet interaction effect (p<0.05), with a stronger counteractive effect of MSX-3 in iron-deficient rats compared to controls (Fig. 6c).
Figure 6.
A2A receptor antagonist-induced blockade of the motor output induced by cortical electrical stimulation in rats with control or iron-deficient (Fe def) diet for three weeks. (a) Representative recordings of the input signal (current pulses) delivered from the stimulator in the orofacial area of the motor cortex (upper traces) and the EMG output signal obtained from the jaw muscles (lower traces), after the administration of either saline (left traces) or the A2A receptor antagonist MSX-3 (3 mg/kg, i.p.; right traces). (b) Representative input stimulator signal power (time constant 0.01 sec; upper traces) and output electromyographic (EMG) signal power (time constant 0.01 sec; lower traces) after the administration of either saline (left traces) or MSX-3 (right traces). (c) The systemic administration of MSX-3 (1 mg/kg, i.p.) significantly decreased the power correlation coefficient (PCC) in rats with control or iron-deficient (Fe def) diet. Results are expressed as means ± S.E.M. n= 7–8 per group); **,***: significantly different compared to respective vehicle (p<0.01 and p<0.001, respectively). MSX-3 was significantly most effective in iron-deficient rats than in controls (bifactorial ANOVA: significant drug-diet interaction effect; p<0.05).
Discussion
Adenosine A2A receptors are expressed more densely in the striatum than anywhere else in the brain (Rosin et al., 1998; Fredholm et al., 2001; Schiffmann et al., 2007). In the striatum, A2A receptors are largely localized in the dendritic field of the D2 receptor-containing enkephalinergic medium-sized spiny neurons (MSNs), which give rise to the indirect striatal efferent pathway. In the enkephalinergic MSNs, A2A receptors establish strong functional antagonistic interactions with D2 receptors with the formation of A2A-D2 receptor heteromers (Ferré et al., 2007; Schiffmann et al., 2007; Azdad et al., 2008). These interactions have been proposed to provide a pharmacological target for the treatment of dopamine-linked movement disorders (Ferré et al., 1997, 2004). In addition to their postsynaptic localization, striatal A2A receptors are also localized presynaptically, in glutamatergic terminals that do not contact enkephalinergic, but dynorphinergic MSNs. These presynaptic A2A receptors heteromerize with A1 receptors and exert a selective modulation of cortico-striatal neurotransmission to the dynorphinergic MSN, which constitute the direct striatal efferent pathway (Ciruela et al., 2006; Quiroz et al., 2009). Striatal postsynaptic A2A receptor function can be analyzed by the ability of A2A receptor antagonists both to produce locomotor activation and to counteract striatal ERK1/2 phosphorylation induced by cortical stimulation, whereas striatal presynaptic A2A receptors function can be analyzed by the ability of A2A receptor antagonists to block the motor output induced by cortical stimulation (Quiroz et al., 2009).
The present results show that the increase in the density of striatal A2A receptors induced by brain iron deficiency is accompanied by a functional up-regulation of both pre- and post-synaptic striatal A2A receptors, as demonstrated by the increased efficacy of the A2A receptor antagonist MSX-3 to produce locomotor activation and its increased potency at blocking striatal ERK1/2 phosphorylation and motor output induced by cortical electrical stimulation. These results agree with the idea that A2A receptor up-regulation is a general cellular response to iron-deficiency which can potentially develop in any cell that can express A2A receptors. Accordingly, we have previously shown that diminished iron concentration increases A2A receptor levels in cultured human neuroblastoma cell lines (Gulyani et al., 2009). Adenosine is a potent modulator of locomotor activity. Many experimental data indicate that postsynaptic A2A receptors, by means of antagonistic A2A-D2 receptor interactions in the enkephalinergic MSNs, are the main mediators of the pronounced depressant effect of A2A receptor agonists on exploratory activity, as well as the locomotor activation induced by A2A receptor antagonists (for review, see Ferré et al., 1997; Schiffmann et al., 2007). The present results strongly suggest that a stronger tonic effect of endogenous adenosine on functionally up-regulated postsynaptic A2A receptors is involved in the well-documented decrease in exploratory activity induced by iron deficiency (Weinberg et al., 1980; Beard, 2003; Lozoff and Georgieff, 2006). We therefore postulate that the same mechanism could be involved in the reported reduced activity of iron-deficient infants when exposed to unfamiliar environments (Angulo-Kinzler et al., 2002; Lozoff and Georgieff, 2006).
The functional up-regulation of striatal presynaptic A2A receptors induced by iron deficiency, with an increased potency of A2A receptor antagonists to block the motor output induced by cortical stimulation, leads simultaneously to both a stronger tonic facilitatory effect of endogenous adenosine on cortico-striatal glutamatergic neurotransmission and to a reduction of cortico-striatal filtering (Quiroz et al., 2009). It is therefore possible that functionally up-regulated presynaptic A2A receptors are involved in the alterations that iron-deficient infants show on executive function tasks, which depend on prefrontal-striatal circuit function (Lozoff and Greorgieff, 2006). It is also possible that functional up-regulation of striatal presynaptic A2A receptors could be a involved in the alterations in cortico-striatal processing that has been described as part of the pathophysiological mechanisms of RLS (Tergau et al., 1999). RLS symptomatology could in fact depend on a disruption of the normal balanced activity of the direct and indirect striatal efferent pathways, with an increased glutamatergic signaling in the dynorphynergic MSN of the direct pathway (due to the presynaptic up-regulation of A2A receptors), and a decreased dopaminergic signaling in the enkephalinergic MSN of the indirect pathway (due to the postsynaptic up-regulation of A2A receptors). The efficacy of L-dopa in RLS could be in part due to an amelioration of such imbalance, and the present results suggest that selective A2A receptor antagonists could be beneficial in RLS.
Acknowledgements
Work supported by the Intramural funds of the National Institute on Drug Abuse and by NIH grant PO1-AG21190 to C.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RP, Walter AS, Montplaisir J, Hening W, Myers A, Bell TJ, Ferini-Strambi L. Restless legs syndrome prevalence and impact: REST general population study. Arch. Intern. Med. 2005;165:1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–391. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Angulo-Kinzler RM, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum. Dev. 2002;70:85–101. doi: 10.1016/s0378-3782(02)00092-0. [DOI] [PubMed] [Google Scholar]
- Azdad K, Gall D, Woods AS, Ledent C, Ferré S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology. 2008;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JL. Iron deficiency alters brain development and functioning. J. Nutr. 2003;133:1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR. Iron status and neural functioning. Ann. Rev. Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Boyer PJ, Ondo WG, Allen RP, Earley CJ, Menzies SL, Chen XL, Dellinger BB, Connor JR. Neuropathologic evaluation of the central nervous system in restless legs syndrome: case report and review of literature; Society for Neuroscience Annual Meeting; 2000; New Orleans, LA. 2000. [Google Scholar]
- Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, Earley CJ. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–2412. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Wang XS, Patton SM, Menzies SL, Troncoso JC, Earley CJ, Allen RP. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62:1563–1567. doi: 10.1212/01.wnl.0000123251.60485.ac. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Barker PB, Horska A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006;7:459–461. doi: 10.1016/j.sleep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54:1698–1700. doi: 10.1212/wnl.54.8.1698. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J. Nutr. 2000;130:2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol. Biochem. Behav. 2001;69:409–418. doi: 10.1016/s0091-3057(01)00563-9. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casadó V, Hillion J, Torvinen M, Fanelli F, Benedetti PP, Goldberg SR, Bouvier M, Fuxe K, Agnati LF, Lluis C, Franco R, Woods A. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat. Disord. 2004;10:265–271. doi: 10.1016/j.parkreldis.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Ferré S, Agnati LF, Ciruela F, Lluis C, Woods AS, Fuxe K, Franco R. Neurotransmitter receptor heteromers and their integrative role in 'local modules':the striatal spine module. Brain Res. Rev. 2007;55:55–67. doi: 10.1016/j.brainresrev.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr. Pharm. Des. 2008;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J. Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyani S, Earley CJ, Camandola S, Maudsley S, Ferré S, Mughal MR, Martin B, Cheng A, Gleichmann M, Jones BC, Allen RP, Mattson MP. Diminished iron concentrations increase adenosine A(2A) receptor levels in mouse striatum and cultured human neuroblastoma cells. Exp. Neurol. 2009;215:236–242. doi: 10.1016/j.expneurol.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Müller CE, Fuxe K, Goldberg SR, Popoli P, Ferré S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Lok CN, Loh TT. Regulation of transferrin function and expression: review and update. Biol. Signals Recept. 1998;7:157–178. doi: 10.1159/000014542. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Georgieff MK. Iron deficiency and brain development, Semin. Pediatr. Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Nelson C, Erikson K, Piñero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J. Nutr. 1997;127:2282–2288. doi: 10.1093/jn/127.12.2282. [DOI] [PubMed] [Google Scholar]
- Nordlander NB. Restless Legs. Brit. J. Phys. Med. 1954;17:160–162. [PubMed] [Google Scholar]
- Quiroz C, Gomes C, Pak AC, Ribeiro JA, Goldberg SR, Hope BT, Ferré S. Blockade of adenosine A2A receptors prevents protein phosphorylation in the striatum induced by cortical stimulation. J. Neurosci. 2006;26:10808–10812. doi: 10.1523/JNEUROSCI.1661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz C, Lujan R, Uchigashima M, Simoes AP, lerner TN, Borycz J, Kachroo A, Canas PM, Orru M, Schwarzschild MA, Rosin DL, Kreitzer AC, Cunha RA, Watanabe M, Ferré S. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. TheScientificWorldJournal. 2009;9:1321–1344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J. Comp. Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tergau F, Wische S, Paulus W. Motor system excitability in patients with restless legs syndrome. Neurology. 1999;52:1060–1063. doi: 10.1212/wnl.52.5.1060. [DOI] [PubMed] [Google Scholar]
- Unger EL, Wiesinger JA, Hao L, Beard JL. Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J. Nutr. 2008;138:2487–2494. doi: 10.3945/jn.108.095224. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Dallman PR, Levine S. Iron deficiency during early development in the rat: behavioral and physiological consequences. Pharmacol. Biochem. Behav. 1980;12:493–502. doi: 10.1016/0091-3057(80)90179-3. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Ben-Shachar D, Ashkenazi R, Yehuda S. Brain iron and dopamine receptor function. Adv. Biochem. Psychopharmacol. 1983;37:309–321. [PubMed] [Google Scholar]