Abstract
Epidemiological and experimental reports have linked mild-to-moderate wine and/or grape consumption to a lowered incidence of cardiovascular, cerebrovascular, and peripheral vascular risk. This study revealed that resveratrol, an enriched bioactive polyphenol in red wine, selectively induces heme oxygenase 1 (HO1) in a dose- and time-dependent manner in cultured mouse cortical neuronal cells and provides neuroprotection from free-radical or excitotoxicity damage. This protection was lost when cells were treated with a protein synthesis or heme oxygenase inhibitor, suggesting that HO1 induction is at least partially required for resveratrol’s prophylactic properties. Furthermore, resveratrol pretreatment dose-dependently protected mice subjected to an optimized ischemic-reperfusion stroke model. Mice in which HO1 was selectively deleted lost most, if not all, of the beneficial effects. Together, the data suggest a potential intracellular pathway by which resveratrol can provide cell/organ resistance against neuropathological conditions.
Keywords: Cerebral ischemia, Neuroprotection, Polyphenol, Red wine, Stilbene
A number of epidemiological studies have shown that natural dietary components can help prevent various pathological conditions ranging from cardiovascular diseases to age-related neurodegenerative conditions (Gordon, 1996; Penumathsa and Maulik, 2009). Furthermore, reports have linked moderate wine consumption to a lowered incidence of cardiovascular disease—the so-called French Paradox (Renaud and de Lorgeril, 1992; Zhuang et al., 2003; Liu et al., 2008). The French Paradox (i.e., low incidence of cardiovascular events despite a diet high in saturated fat) was attributed to the regular drinking of red wine in southern France (Zhuang et al., 2003).
During the making of red wine, the sugar in grapes is fermented into alcohol, which, over several days, acts as a solvent for polyphenol extraction in the presence of the skins and seeds. Ample opportunity exists for extraction of many of the polyphenols. Among the polyphenols found in red wines, the stilbene resveratrol has been shown to provide multiple antioxidant-related beneficial effects (Frankel et al., 1993; Formica and Regelson, 1995; Pace-Asciak et al., 1995; de Lorimier, 2000). Resveratrol can mediate a wide range of biological activities with multi-system benefits. For example, it has been shown to protect the heart from ischemia in an isolated rat heart model (Sato et al., 2000). The heart and the brain are organs especially sensitive to free-radical damage. Although various reports have documented the anti-oxidant and anti-inflammatory properties of resveratrol, the levels reached in blood or in cells are likely not sufficient to be responsible for such heath benefits; consequently, we propose that resveratrol activates an indirect endogenous cellular and organ protective system.
Previously, we showed that resveratrol can induce the endogenous enzyme heme oxygenase 1 (HO1) in isolated cultured neurons (Zhuang et al., 2003). Heme oxygenase has two isoforms, HO2, which is constitutively expressed, and HO1, an inducible form. The main function of heme oxygenase is to degrade pro-oxidant heme into biliverdin/bilirubin, both known to be antioxidants (Doré et al., 1999b). Free heme can come from extracellular sources, such as by degradation of hemoglobin, and from intracellular sources, such as by metabolism of heme-containing proteins. During hypoxic and excitotoxic conditions, hemoprotein turnover accelerates, and since heme cannot be recycled, it must be degraded by heme oxygenase. The brain is particularly sensitive to free radical damage because it has low levels of natural antioxidants. Here, we tested the hypothesis that resveratrol can induce endogenous HO1 in the brain to provide resistance against free radicals and the cascade of events that leads to infarct brain damage after ischemic reperfusion injury.
In the current study, all mice (C57BL/6) were used in accordance with protocols and guidelines approved by the Johns Hopkins University Animal Care and Use Committee. Cortical neuronal cells were isolated from 17-day-old embryos of timed pregnant C57BL/6 mice and cultured in serum-free conditions (Doré et al., 1999b). Cells were grown for 10–14 days and then pretreated with resveratrol, which was freshly dissolved in ethanol into a 250× stock solution and then dissolved in culture medium. Vehicle control was prepared identically but without the resveratrol. Cells were then washed with medium with or without various other treatments before being processed for Western blot analysis or viability assays. For Western blot analysis, polyacrylamide gels were stained with Ponceau S Solution to verify that equal amounts of protein were loaded in each lane. Polyclonal antibodies to HO1 and HO2 were obtained from StressGen Inc. (Victoria, BC) and anti-actin was obtained from Sigma Co. (St. Louis, MO) and used at dilutions of 1:3500, 1:2500, and 1:5000 respectively. In the excitotoxicity protocols, cell survival was monitored after 23 h by phase-contrast microscopy with the Trypan Blue exclusion assay and quantified with the MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)] colorimetric assay. Unless specified otherwise, all reagents were from Sigma Co.
For the in vivo experimental protocol, the resveratrol was freshly dissolved in ethanol, mixed into 2% methylcellulose, and administered to adult male mice (6–8 weeks old) orally at a constant volume, either one time 2 h before the experimental stroke procedure (acute regimen) or once daily for 7 days (chronic regimen). The experimental stroke protocol was carried out as described before (Shah et al., 2006). The middle cerebral artery (MCA) was occluded for 90 min with a nylon filament while efficacy was monitored by laser-Doppler flowmetry (LDF). To initiate reperfusion, the mice were placed under halothane anesthesia, and the filament was removed. Twenty-three hours later, the brain was removed and processed for infarct size quantification. Unpaired t-test was used to compare the treatment groups. Multiple comparisons were analyzed by ANOVA and Tukey’s test. Values are represented as mean ± sem.
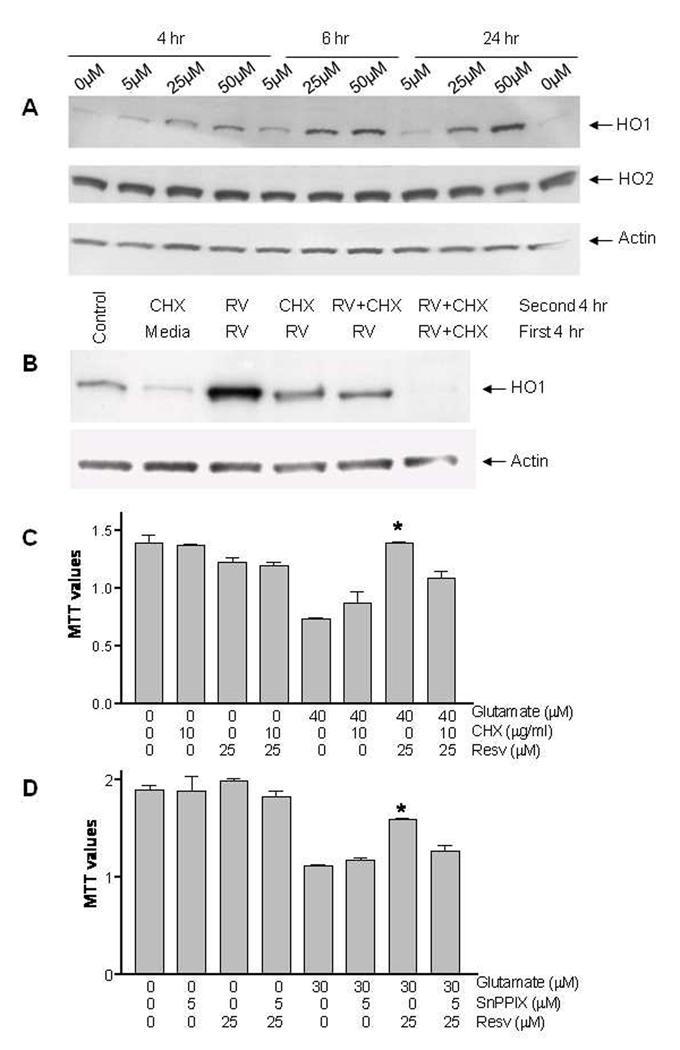
To address whether resveratrol is sufficient to induce de novo HO1 protein expression, we treated primary neuronal cells with different concentrations of resveratrol. Western blot analysis revealed a dose- and time-dependent increase in HO1 protein levels, but no changes in HO2 or actin levels were detected (Fig. 1A). For subsequent experiments, the 25 µM concentration of resveratrol was selected. Next we investigated whether the increased HO1 protein level was due to new HO1 protein synthesis or to another pathway, such as decreased HO1 protein degradation. We treated the cells with resveratrol or vehicle alone, or together with the protein synthesis inhibitor cycloheximide (CHX) for the first 4 h and then with resveratrol and CHX for another 4 h. Our data revealed that CHX can block the HO1 induction (Fig. 1B). Next, we investigated whether CHX could block resveratrol’s protective effect on neurons. Neuronal cultures were pretreated for 1 h with CHX or vehicle (control), rinsed with PBS, and then treated with resveratrol for 6 h. The cells then were rinsed and incubated with fresh medium containing glutamate or vehicle for an additional 24 h (Fig. 1C). The results show that the protective effect of resveratrol against the glutamate-induced toxicity was significantly reduced by the protein synthesis inhibitor.
Fig 1. Resveratrol induces HO1 and protects against excitotoxicity in primary neuronal cultures.
(A) Effect of different concentrations of resveratrol and treatment periods on HO1 and HO2 protein expression in neuronal cultures. Mouse cortical neurons cultured in serum-free conditions for 10–14 days were harvested at different times after resveratrol treatment; proteins were analyzed by Western blot. Resveratrol induced HO1 expression dose and time dependently but did not affect HO2. Actin was used to confirm equal loading. (B) Effect of resveratrol (RV) and the protein synthesis inhibitor cycloheximide (CHX) on HO1 protein expression in neuronal cultures. Cells were treated with vehicle (control), resveratrol (25 µM) or resveratrol together with CHX for the first 4 h, then with resveratrol, CHX (10 µg/ml) or both together for the remaining 4 h before being harvested and analyzed. CHX blocked the increase in HO1 expression. (C) The protective effect of resveratrol pretreatment against excitotoxicity induced by glutamate in primary cultured neurons was significantly reduced by treatment with CHX. Neuronal cultures were first pretreated for 4 h with CHX or vehicle, rinsed once, and then treated with resveratrol for 6 h. Then, cells were rinsed and incubated with fresh medium containing 40 µM glutamate or vehicle (control). After 24 h, cell survival was estimated by MTT assay, which is an indicator of mitochondrial function. (D) The protective effect of resveratrol pretreatment against excitotoxicity induced by glutamate in cultured neurons was significantly reduced by HO inhibitor. Cells were pretreated with 25 µM resveratrol for 6 h; then cells were rinsed and incubated with fresh medium containing 30 µM glutamate or vehicle (control), with or without 5 µM of the HO inhibitor SnPPIX. After 24 h the cell survival was estimated by MTT assay. Experimental conditions were carried out in quadruplicate, and the experiment was reproduced four times with different primary culture batches.
We further tested whether resveratrol is toxic to neuronal cells and found no significant toxicity with 24-h exposure of resveratrol up to 75 µM (data not shown). Taking into consideration that resveratrol induces HO1 and provides neuroprotection, we investigated whether a heme oxygenase inhibitor can block the protective effect. Neuronal cultures were pretreated with resveratrol, rinsed with PBS, and then incubated with fresh medium containing glutamate or vehicle, with or without HO inhibitor (SnPPIX). After an additional 24-h incubation, cell survival was monitored (Fig. 1D). We found that resveratrol pretreatment protected cortical neuronal cells against the glutamate-induced toxicity but that SnPPIX blocked this protective effect.
After observing that resveratrol can be cytoprotective in cultured neuronal cells, we tested whether resveratrol limits infarct size after ischemic reperfusion injury. In mice subjected to MCA occlusion (Shah et al., 2006), acute and chronic oral pretreatment with resveratrol each were sufficient to significantly attenuate infarct size in a dose-dependent manner (Figs. 2A and 2B, respectively). The optimal effect was observed at 20 mg/kg, which is consistent with a study in rats (Andrabi et al., 2004). The plasma concentration of resveratrol, measured by HPLC in samples taken from two chronically treated mice 90 min after the last oral administration of 20 mg/kg of resveratrol was 0.11 ± 0.03 µM.
Fig 2. Acute and chronic pretreatment with resveratrol protects mice subjected to ischemic-reperfusion injury.
(A) Resveratrol dose-dependently reduced infarct volume compared to vehicle when given orally 2 h before 90-min MCA occlusion and 24-h reperfusion. (B) Pretreatment with oral resveratrol once daily for 7 days reduced infarct volume caused by the transient ischemia protocol. (C) Representative 2,3,5-triphenyltetrazolium chloride-stained brain sections of mice treated with vehicle or resveratrol before being subjected to MCA occlusion. (D) The significant acute protective effect of resveratrol in WT animals (left) was lost in HO1−/− mice. (E) Acute pretreatment with resveratrol did not alter the intraischemic blood flow distribution. WT mice were treated orally with vehicle (n = 7) or 20 mg/kg of resveratrol (n = 5) 2 h before MCA occlusion. The [14C]-iodoantipyrine (4 µCi) was infused intravenously over 45 s while arterial blood was sampled to obtain the arterial input function. Brains were immediately harvested after the infusion and sectioned for autoradiographic analysis at 1-mm coronal increments from +2 through −3 mm from bregma. No significant differences were observed between vehicle and resveratrol treatment in cortical or subcortical regions ipsilateral or contralateral to the occlusion.
To determine whether resveratrol may act by improving the distribution of cerebral blood flow during focal ischemia, blood flow was measured by infusing the tracer [14C]-iodoantipyrine during MCA occlusion (Saleem et al., 2007). Acute resveratrol pretreatment did not significantly alter blood flow in the ischemic core (striatum and lateral cortex) or in the penumbra regions (dorsolateral cortex and subcortex; Fig. 2E), nor did it alter blood flow in contralateral regions. Thus, resveratrol did not decrease the severity of the ischemic insult.
We then tested whether the protective effect of resveratrol could be partially due to HO1 by using HO1 knockout (HO1−/−) mice. The physiological parameters monitored under basal conditions (body weight, rectal and temporal temperature) and the percentage decrease in the LDF during the experimental protocol did not differ between wildtype (WT) and HO1−/− mice in either the acute or chronic treatment groups (data not shown). Furthermore, we have previously shown that after a similar ischemia-reperfusion protocol, the infarct sizes of untreated WT and HO1−/− mice are not significantly different (Doré et al., 1999a). Here, we found that resveratrol had a protective effect in the WT mice that was almost completely abolished in the HO1−/− mice (Fig. 2D).
As stated, the overall objective of this study was to define a potential pathway by which resveratrol could be neuroprotective and determine the potential contribution of the HO1 enzyme. We have shown that resveratrol induces HO1 in vitro and that this induction plays a significant role in its neuroprotection. Furthermore, we showed that oral consumption of resveratrol before ischemia and reperfusion significantly limited infarct size without affecting physiological parameters or cerebral blood flow. Most interestingly, this effect was abolished in mice lacking the HO1 enzyme. We are actively pursuing this work to find the lowest dose that is effective for use as an acute or chronic pretreatment, but this work is further warranted by recent studies in which it was documented that very small amounts of resveratrol would be sufficient to significantly affect gene expression and physiological parameters (Barger et al., 2008; Mattson, 2008). The search for potential analogs is also of interest, although here it was notable that a naturally occurring biologically active compound could have such a remarkable effect.
Although resveratrol has gained much attention lately, the mechanism of its action is still under intense investigation. Sinclair’s group and other teams have postulated that its protective effect is due to the role of sirtuins (Baur and Sinclair, 2006). This work is somewhat tempered by the fact that the deletion of sirtuins is often lethal to mice, and the animals who survive have significant health issues, rendering them already challenged before being used in studies (McBurney et al., 2003). Such results do stress the importance of sirtuins, though their sole and unique association with the protective effect of resveratrol remains to be fully demonstrated.
Here, although we are not concluding that HO1 is the only pathway by which resveratrol can be neuroprotective, we believe that HO1 might be a unique candidate by which resveratrol can induce an endogenous cellular pathway that leads to building cellular and/or organ resistance to stress, such as in an ischemic paradigm. Heme oxygenase activity leads to various pathways that each can be associated with cytoprotection. Interestingly, many teams are actively looking for innovative ways to induce HO1 using various chemicals and/or genetic approaches. Such approaches are being investigated for organ transplant, heart ischemia, wound healing, etc. Here, it is of interest that a single natural compound such as resveratrol can induce the protective enzyme HO1. Finally, the French paradox has been suggested to be associated with red wine consumption. Knowing that moderate alcohol consumption also has documented health benefits, is it possible that small amounts of resveratrol would have a synergistic beneficial effect with alcohol? The concept that resveratrol has pleiotropic beneficial effects is fascinating, and by elucidating the pathways and mechanisms of its action, clinical trials can be designed to specifically address its potential use for prophylaxis against stroke and neurodegenerative diseases.
Acknowledgements
This work was supported in part by grants from: National Institutes of Health [AA01911 (SD), AT002113 (SD), NS38684 (RK)], Wine Institute (SD), and ABMR Foundation (SD). The authors wish to thank all members of the Doré lab.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrabi SA, Spina MG, Lorenz P, Ebmeyer U, Wolf G, Horn TF. Oxyresveratrol (trans-2,3',4,5'-tetrahydroxystilbene) is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. Brain Res. 2004;1017:98–107. doi: 10.1016/j.brainres.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- de Lorimier AA. Alcohol, wine, and health. Am J Surg. 2000;180:357–361. doi: 10.1016/s0002-9610(00)00486-4. [DOI] [PubMed] [Google Scholar]
- Doré S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med. 1999a;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- Doré S, Takahashi M, Ferris CD, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999b;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- Gordon M. Dietary antioxidants in disease prevention. Nat Prod Rep. 1996;13:265–273. doi: 10.1039/np9961300265. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang Y, Lam KS, Xu A. Moderate wine consumption in the prevention of metabolic syndrome and its related medical complications. Endocr Metab Immune Disord Drug Targets. 2008;8:89–98. doi: 10.2174/187153008784534385. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- Penumathsa SV, Maulik N. Resveratrol: a promising agent in promoting cardioprotection against coronary heart disease. Can J Physiol Pharmacol. 2009;87:275–286. doi: 10.1139/Y09-013. [DOI] [PubMed] [Google Scholar]
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- Saleem S, Li R, Wei G, Doré S. Effects of EP1 receptor on cerebral blood flow in the middle cerebral artery occlusion model of stroke in mice. J Neurosci Res. 2007;85:2433–2440. doi: 10.1002/jnr.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Ray PS, Maulik G, Maulik N, Engelman RM, Bertelli AA, Bertelli A, Das DK. Myocardial protection with red wine extract. J Cardiovasc Pharmacol. 2000;35:263–268. doi: 10.1097/00005344-200002000-00013. [DOI] [PubMed] [Google Scholar]
- Shah ZA, Namiranian K, Klaus J, Kibler K, Doré S. Use of an optimized transient occlusion of the middle cerebral artery protocol for the mouse stroke model. J Stroke Cerebrovasc Dis. 2006;15:133–138. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Zhuang H, Kim YS, Koehler RC, Doré S. Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann N Y Acad Sci. 2003;993:276–286. doi: 10.1111/j.1749-6632.2003.tb07534.x. [DOI] [PubMed] [Google Scholar]